Abstract
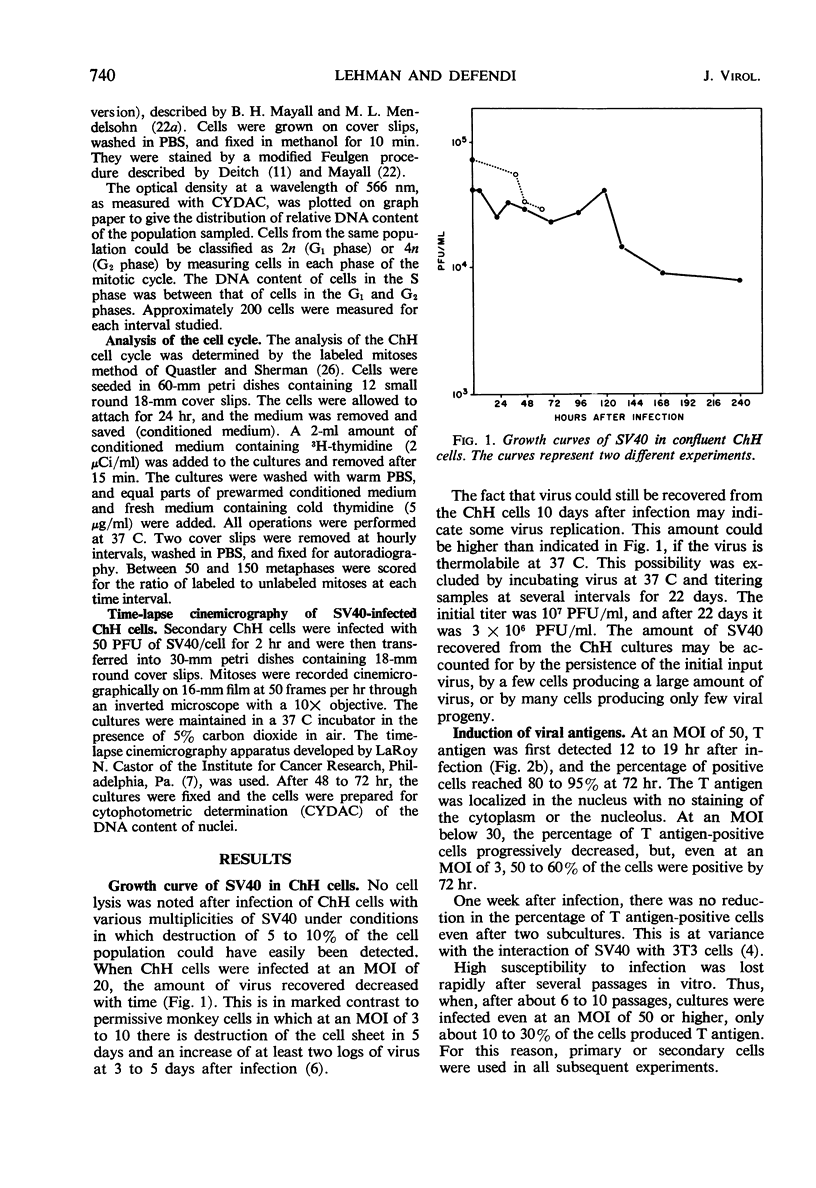
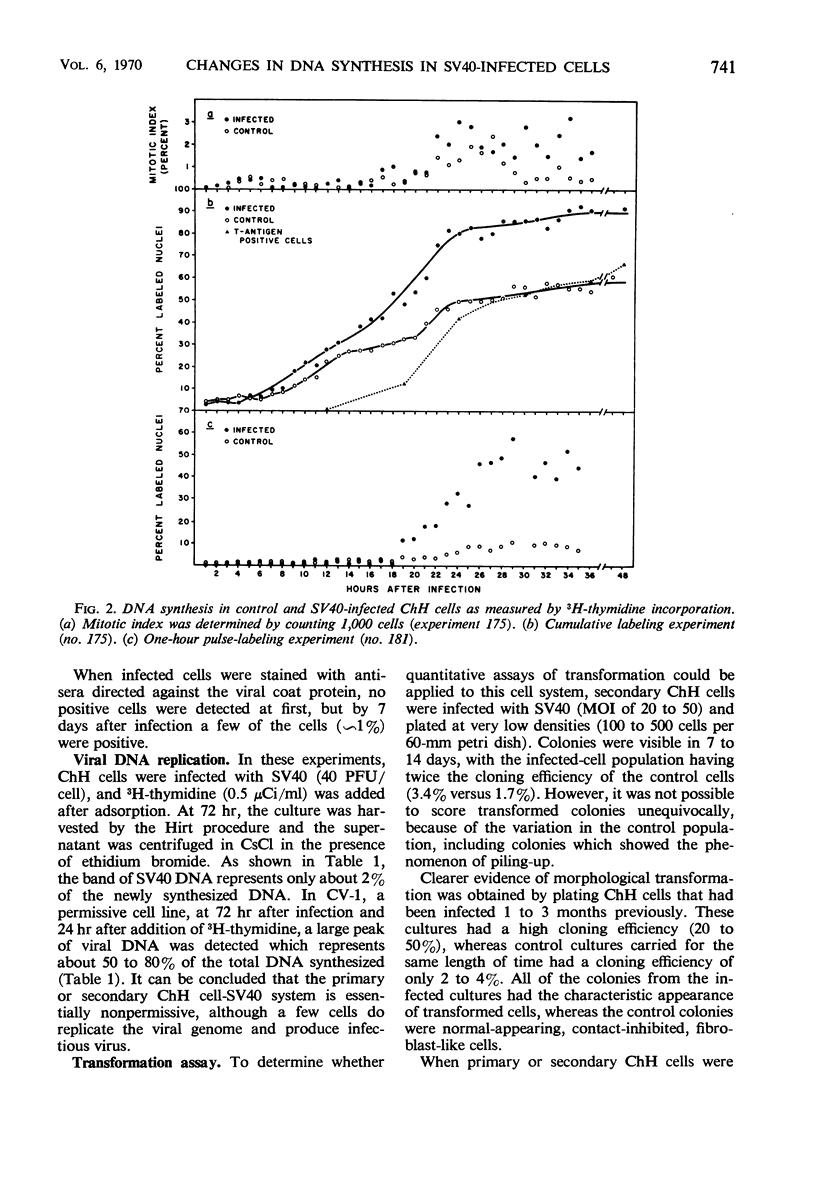
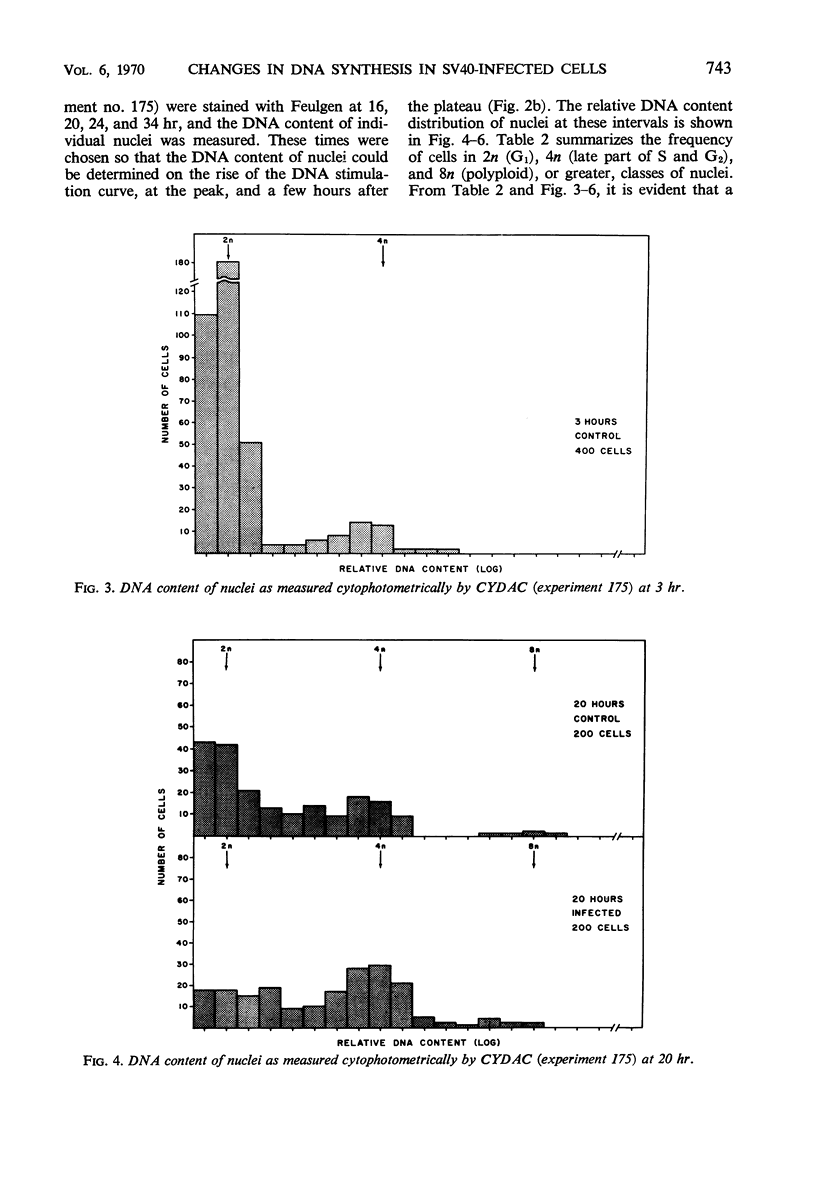
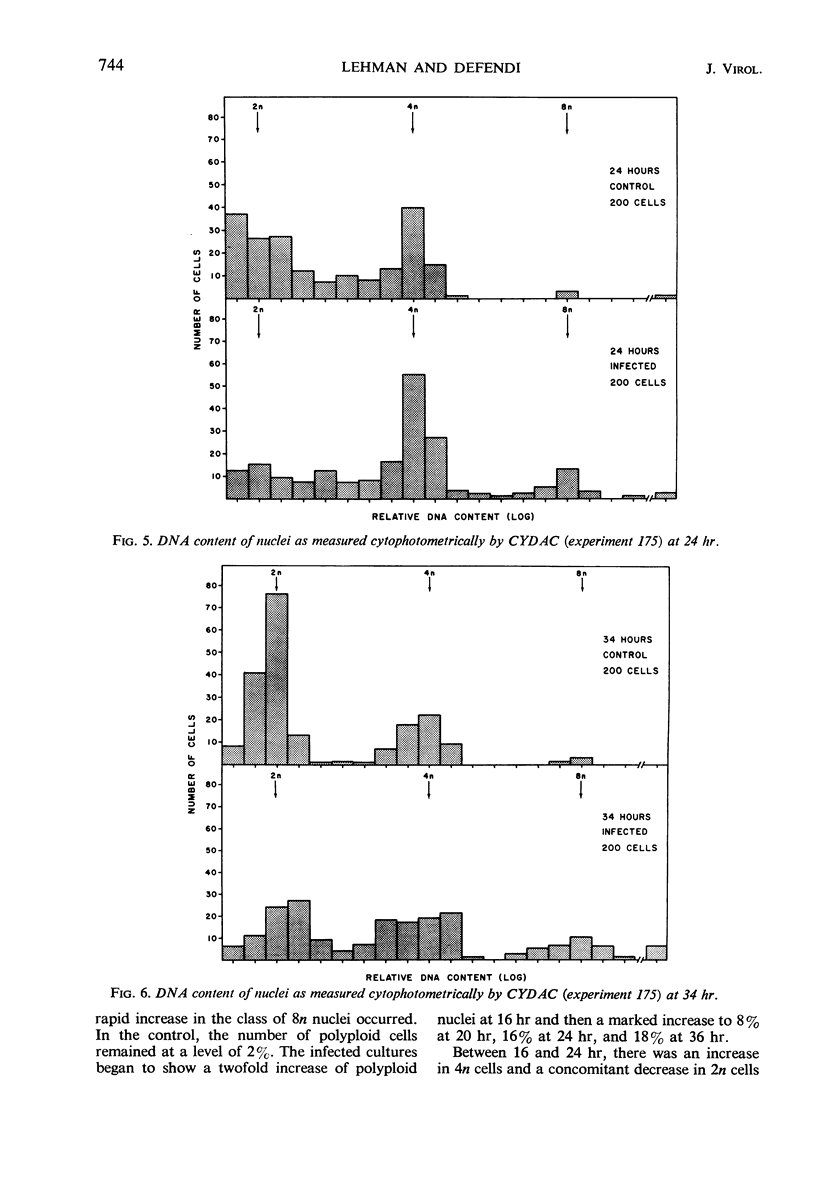
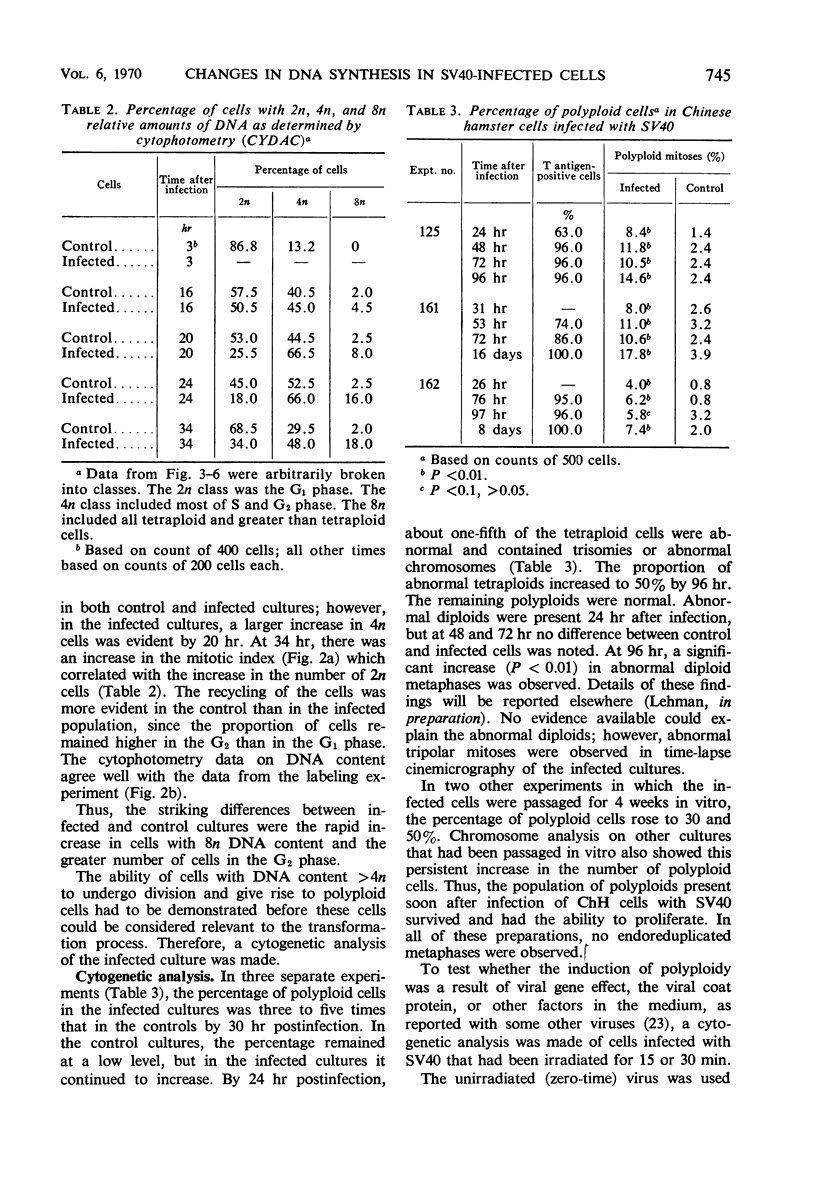
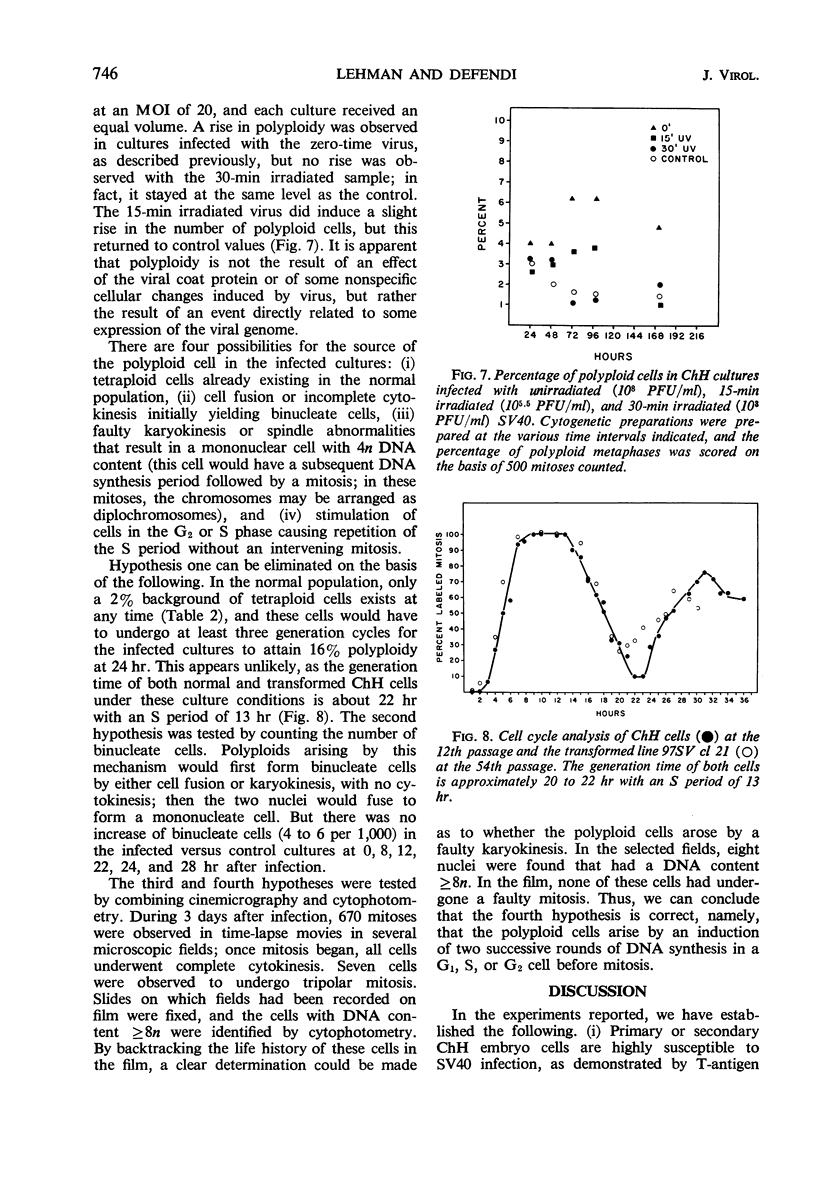
Infection of primary or secondary cultures of Chinese hamster embryo cells with simian virus 40 at a multiplicity of 20 to 50 induced synthesis of the virus-specific intranuclear T antigen in 80 to 90% of the cells within 48 to 72 hr. In the infected cultures, 30 to 50% more cells were recruited into deoxyribonucleic acid (DNA) synthesis than in the controls, whether or not the cultures were confluent. The newly synthesized DNA was mostly cellular, since little virus was produced (as shown by various techniques: immunofluorescence for viral antigen, virus growth curves, and isolation of viral DNA from infected cultures). Transformed cells could be detected a few weeks after infection and produced tumors when inoculated into irradiated animals. Chromosomal changes were observed soon after infection (24 hr). Initially, there was a marked increase in the proportion of polyploid cells (8 to 14%), most of which were chromosomally normal. In a few weeks, a large majority of the infected population was polyploid (30 to 50%). Thus, the polyploid cells have the ability to proliferate. Evidence is presented to suggest that polyploid cells arise by stimulation of cells in the G1, G2, or S phases to undergo two or more successive periods of DNA synthesis without an intervening mitosis. With a subsequent loss or redistribution of chromosomal material, this may lead eventually to a biologically transformed cell; thus, it is suggested that the initial event(s) relevant to transformation occurs at the level of control of cellular DNA synthesis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Coto C., Kaplan A. S. Unstable DNA synthesized by polyoma virus-infected cells. Virology. 1966 Sep;30(1):74–81. doi: 10.1016/s0042-6822(66)81011-5. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Kaplan A. S., Tennant R. W. Effect of 5-fluorouracil on the multiplication of a virulent virus (pseudorabies) and an oncogenic virus (polyoma). Virology. 1967 Jul;32(3):445–456. doi: 10.1016/0042-6822(67)90296-6. [DOI] [PubMed] [Google Scholar]
- Black P. H. Transformation of mouse cell line 3T3 by SV40: dose response relationship and correlation with SV40 tumor antigen production. Virology. 1966 Apr;28(4):760–763. doi: 10.1016/0042-6822(66)90262-5. [DOI] [PubMed] [Google Scholar]
- COOPER H. L., BLACK P. H. CYTOGENETIC STUDIES OF THREE CLONES DERIVED FROM A PERMANENT LINE OF HAMSTER CELLS TRANSFORMED BY SV40. J Cell Physiol. 1964 Oct;64:201–219. doi: 10.1002/jcp.1030640206. [DOI] [PubMed] [Google Scholar]
- Carp R. I., Gilden R. V. A comparison of the replication cycles of simian virus 40 in human diploid and African green monkey kidney cells. Virology. 1966 Jan;28(1):150–162. doi: 10.1016/0042-6822(66)90316-3. [DOI] [PubMed] [Google Scholar]
- Castor L. N. Contact regulation of cell division in an epithelial-like cell line. J Cell Physiol. 1968 Dec;72(3):161–172. doi: 10.1002/jcp.1040720304. [DOI] [PubMed] [Google Scholar]
- Crawford L. V. Nucleic acids of tumor viruses. Adv Virus Res. 1969;14:89–152. doi: 10.1016/s0065-3527(08)60558-8. [DOI] [PubMed] [Google Scholar]
- Defendi V., Lehman J. M. Transformation of hamster embryo cells in vitro by polyoma virus: morphological, karyological, immunological and transplantation characteristics. J Cell Physiol. 1965 Dec;66(3):351–409. doi: 10.1002/jcp.1030660313. [DOI] [PubMed] [Google Scholar]
- Fogel M., Defendi V. Infection of muscle cultures from various species with oncogenic DNA viruses (SV40 and polyoma). Proc Natl Acad Sci U S A. 1967 Sep;58(3):967–973. doi: 10.1073/pnas.58.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILDEN R. V., CARP R. I., TAGUCHI F., DEFEND V. THE NATURE AND LOCALIZATION OF THE SV 40-INDUCED COMPLEMENT-FIXING ANTIGEN. Proc Natl Acad Sci U S A. 1965 Mar;53:684–692. doi: 10.1073/pnas.53.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon D., Sachs L., Winocour E. The induction of cellular DNA synthesis by simian virus 40 in contact-inhibited and in x-irradiated cells. Proc Natl Acad Sci U S A. 1966 Sep;56(3):918–925. doi: 10.1073/pnas.56.3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R., Weil R. Biochemical evidence for induction by polyoma virus of replication of the chromosomes of mouse kidney cells. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1144–1150. doi: 10.1073/pnas.63.4.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- JENSEN F. C., GIRARDI A. J., GILDEN R. V., KOPROWSKI H. INFECTION OF HUMAN AND SIMIAN TISSUE CULTURES WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1964 Jul;52:53–59. doi: 10.1073/pnas.52.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHMAN J. M., MACPHERSON I., MOORHEAD P. S. KARYOTYPE OF THE SYRIAN HAMSTER. J Natl Cancer Inst. 1963 Sep;31:639–650. [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Maio J. J., Schildkraut C. L. Isolated mammalian metaphase chromosomes. II. Fractionated chromosomes of mouse and Chinese hamster cells. J Mol Biol. 1969 Mar 14;40(2):203–216. doi: 10.1016/0022-2836(69)90469-0. [DOI] [PubMed] [Google Scholar]
- Mayall B. H. Deoxyribonucleic acid cytophotometry of stained human leukocytes. I. Differences among cell types. J Histochem Cytochem. 1969 Apr;17(4):249–257. doi: 10.1177/17.4.249. [DOI] [PubMed] [Google Scholar]
- Mayall B. H., Mendelsohn M. L. Deoxyribonucleic acid cytophotometry of stained human leukocytes. II. The mechanical scanner od CYDAC, the theory of scanning photometry and the magnitude of residual errors. J Histochem Cytochem. 1970 Jun;18(6):383–407. doi: 10.1177/18.6.383. [DOI] [PubMed] [Google Scholar]
- Norrby E., Levan A., Nichols W. W. The correlation between the chromosome pulverization effect and other biological activities of measles virus preparations. Exp Cell Res. 1966 Mar;41(3):483–491. doi: 10.1016/s0014-4827(66)80099-x. [DOI] [PubMed] [Google Scholar]
- Odell T. T., Jr, Jackson C. W. Polyploidy and maturation of rat megakaryocytes. Blood. 1968 Jul;32(1):102–110. [PubMed] [Google Scholar]
- Ossovski L., Sachs L. Temperature sensitivity of polyoma virus, induction of cellular DNA synthesis, and multiplication of transformed cells at high temperature. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1938–1943. doi: 10.1073/pnas.58.5.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUASTLER H., SHERMAN F. G. Cell population kinetics in the intestinal epithelium of the mouse. Exp Cell Res. 1959 Jun;17(3):420–438. doi: 10.1016/0014-4827(59)90063-1. [DOI] [PubMed] [Google Scholar]
- Roberts P. A., Kimball R. F., Pavan C. Response of Rhynchosciara chromosomes to microsporidian infection. Increased polyteny and generalized puffing. Exp Cell Res. 1967 Aug;47(1):408–422. doi: 10.1016/0014-4827(67)90244-3. [DOI] [PubMed] [Google Scholar]
- Sauer G., Defendi V. Stimulation of DNA synthesis and complement-fixing antigen production by SV40 in human diploid cell cultures: evidence for "abortive" infection. Proc Natl Acad Sci U S A. 1966 Aug;56(2):452–457. doi: 10.1073/pnas.56.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinin R. DNA synthesis in rat embryo cells infected with polyoma virus. Virology. 1966 May;29(1):167–170. doi: 10.1016/0042-6822(66)90206-6. [DOI] [PubMed] [Google Scholar]
- Smith B. J. Light satellite-band DNA in mouse cells infected with polyoma virus. J Mol Biol. 1970 Jan 14;47(1):101–106. doi: 10.1016/0022-2836(70)90405-5. [DOI] [PubMed] [Google Scholar]
- Stoker M. Abortive transformation by polyoma virus. Nature. 1968 Apr 20;218(5138):234–238. doi: 10.1038/218234a0. [DOI] [PubMed] [Google Scholar]
- TORREY J. G. Kinetin as trigger for mitosis in mature endomitotic plant cells. Exp Cell Res. 1961 Mar;23:281–299. doi: 10.1016/0014-4827(61)90038-6. [DOI] [PubMed] [Google Scholar]
- Winocour E., Kaye A. M., Stollar V. Synthesis and transmethylation of DNA in polyoma-infected cultures. Virology. 1965 Oct;27(2):156–169. doi: 10.1016/0042-6822(65)90155-8. [DOI] [PubMed] [Google Scholar]
- Winocour E., Robbins E. Histone synthesis in polyoma- and SV40-infected cells. Virology. 1970 Feb;40(2):307–315. doi: 10.1016/0042-6822(70)90406-x. [DOI] [PubMed] [Google Scholar]
- Winocour E. Some aspects of the interaction between polyoma virus and cell DNA. Adv Virus Res. 1969;14:153–200. doi: 10.1016/s0065-3527(08)60559-x. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Gershon D. Multinucleated muscle fibres: induction of DNA synthesis and mitosis by polyoma virus infection. Nature. 1967 Jul 22;215(5099):421–424. doi: 10.1038/215421a0. [DOI] [PubMed] [Google Scholar]



