Abstract
We determined whether ultra-runners in a multi-stage ultra- endurance run lose body mass, fat mass, skeletal muscle mass or total body water in a descriptive field study at the ‘Deutschlandlauf’ 2007 a 1,200 km run within 17 consecutive days with 10 male non-professional Caucasian ultra-runners (mean ± SD, 43.8 ± 6.2 years, 73.8 ± 6.0 kg body mass, 1.77 ± 0.05 m body height, BMI 23.3 ± 1.8 kg·m-2). Body mass, fat mass, skeletal muscle mass, lean body mass and percent total body water were determined using bioelectrical impedance analysis and the anthropometric method before the race and after each stage. In addition, urinary specific gravity was measured in order to quantify hydration status. Fat mass (bioelectrical impedance analysis) decreased by 3.9 kg (p < 0.05), skeletal muscle mass (anthropometric method) decreased by 2.0 kg (p < 0.05) whereas percent total body water increased by 6.1 % (p < 0.05) by the end of the race. Ultra-runners in a multi-stage ultra-endurance event over 1,200 km, with 17 consecutive stages, showed a cumulative increase in percent total body water, a decrease in skeletal muscle mass and a decrease in fat mass, depending upon the method used. We presume that the eccentric component of running leads to damage of skeletal muscle, leading to rhabdomyolysis, with impaired renal function.
Key points.
Ultra-runners in a multi-stage ultra-endurance run over 1,200 km in 17 consecutive stages suffered a decrease in fat mass, skeletal muscle mass and an increase in total body water, whereas overall body mass showed no change.
Key words: Body fat, body mass, dehydration, skeletal muscle mass
Introduction
Running is one of the most popular endurance sport disciplines and marathon running in particular has increased over recent years. Apart from marathon running, ultra-marathon running is becoming more and more attractive. Within running there is a difference between ultra-distance events, of more than the marathon distance, and multi-day runs. Abundant literature is available about single ultra-distance running, but little is known about the effects on the human body after running hundreds or even thousands of kilometres within a few days or weeks (Knechtle and Kohler, 2007; Raschka and Plath, 1992; Raschka et al., 1991).
In a run over 1,000 km within 20 days, all skin-fold thicknesses and fat mass showed a tendency to decrease; only the thigh skin-fold initially increased, and then further decreased from the 4th day onwards in the study reported by Raschka and Plath, 1992. In a further study (Raschka et al., 1991) on a run over the same distance, muscle mass initially decreased from 59.3 to 58.9 kg on day 11 and increased at the end of the run to 59.9 kg, which was higher than the muscle mass at the start. As a result of the initially decreased muscle mass, all muscle circumferences were reduced with the exception of the thigh (Raschka et al., 1991). The authors concluded that high mechanical stresses experienced by the lower extremities had a positive effect on skeletal muscle mass.
In contrast to these findings, in a recent investigation during a multi-stage ultra-endurance run over 338 km in 5 days, skeletal muscle mass was significantly reduced after the first day and then remained stable (Knechtle and Kohler, 2007). The difference between these findings could be the longer performance (1,000 versus 338 km) with different exercise intensities since in both races, anthropometric techniques were used to estimate muscle mass.
The aim of the present investigation was to study the effects of a multi-stage ultra-endurance run of 1,200 km in 17 days, where athletes had to run on average more than 70 km per day. In addition to the anthropometrical measurements used in the shorter multi-day run (Knechtle and Kohler, 2007), the change of total body water, percent body fat and lean body mass using bioelectrical impedance analysis was determined. Urinary specific gravity was additionally measured after every stage in order to determine hydration status. The hypothesis was to investigate any change in body mass, skeletal muscle mass and/or fat mass.
Methods
Subjects
The organisers of the 3rd ‘Deutschlandlauf’ contacted all participants of the race 3 months before the race in 2007 with a separate newsletter, in which they were asked to participate in the study. 41 ultra-runners (36 male and 5 female athletes) from 8 countries entered the ‘Deutschlandlauf’ 2007, a multi-stage ultra- endurance run over 1,200 km to be covered within 17 days. The stages of the race are shown in Table 1. Twenty-four male runners agreed to participate in the investigation. They all gave informed written consent in accordance with the guidelines established by the local Institutional Ethics Committee. No criteria for inclusion/exclusion were used. Ten runners recruited to the study (mean ± SD; 43.8 ± 6.2 years, 73.8 ± 6.0 kg body mass, 1.77 ± 0.05 m body height, BMI 23.3 ± 1.8 kg·m-2) finished the race successfully. The other 14 runners retired from the race due to orthopaedic problems such as shin splints.
Table 1.
The stages of the race with daily distances, ascents, average speed and weather conditions. Results of speed in the race are presented as mean ± SD.
| Stage | Start | Finish | Distance (km) |
Ascent (m) |
Speed (km/h) |
Weather | Temperature at the start (° Celsius) |
Temperature at the finish (° Celsius) |
|---|---|---|---|---|---|---|---|---|
| 1 | Kap Arkona | Stralsund | 64.9 | 180 | 8.8 ± 1.2 | rain | 12 | 15 |
| 2 | Stralsund | Stavenhagen | 85.4 | 250 | 8.5 ± 1.3 | clouds | 8 | 16 |
| 3 | Stavenhagen | Pritzwalk | 93.4 | 450 | 7.8 ± 1.3 | clouds | 12 | 12 |
| 4 | Pritzwalk | Jerichow | 81.6 | 215 | 7.5 ± 1.4 | clouds | 13 | 19 |
| 5 | Jerichow | Schönebeck | 73.3 | 240 | 7.3 ± 1.3 | sunny | 11 | 22 |
| 6 | Schönebeck | Eisleben | 66.9 | 605 | 7.7 ± 1.4 | sunny | 12 | 21 |
| 7 | Eisleben | Sömmerda | 70.5 | 580 | 7.6 ± 1.2 | sunny | 7 | 24 |
| 8 | Sömmerda | Ilmenau | 80.6 | 1,030 | 7.3 ± 1.2 | clouds, rain | 12 | 23 |
| 9 | Ilmenau | Trappstadt | 64.8 | 720 | 7.6 ± 1.0 | rain | 9 | 15 |
| 10 | Trappstadt | Prosselsheim | 74.4 | 605 | 7.4 ± 1.0 | sunny | 2.5 | 12 |
| 11 | Prosselsheim | Assamstadt | 85.9 | 1,000 | 6.9 ± 0.9 | sunny | 3 | 15 |
| 12 | Assamstadt | Biberach | 72.7 | 680 | 7.5 ± 1.2 | sunny | 7 | 24 |
| 13 | Biberach | Malmsheim | 63 | 900 | 7.5 ± 1.4 | sunny | 9 | 25 |
| 14 | Malmsheim | Horb | 62.2 | 450 | 7.7 ± 0.9 | sunny | 7 | 25 |
| 15 | Horb | St. Georgen | 57.5 | 730 | 7.4 ± 0.8 | sunny | 8 | 25 |
| 16 | St. Georgen | Feldberg | 51.7 | 760 | 9.0 ± 1.0 | clouds, rain | 7 | 15 |
| 17 | Feldberg | Lörrach | 59.9 | 400 | 8.6 ± 1.0 | sunny | 8 | 19 |
The race
The 3rd ‘Deutschlandlauf’ was held from 10th to 27th of September 2007. It is a multi-stage ultra-endurance run where runners have to run across Germany, from the north-east (Kap Arkona on the island of Rügen) to the south-west (Lörrach). The average daily distances were 70.9 km (Table 1). The race director, together with his staff organised the accommodation in halls, and also the food before, during and after each stage.
Measurements and calculations
The day before the start of the race and after every stage, within one hour after arrival at the finish line, every participant underwent anthropometric measurements, bioelectrical impedance analysis (BIA), and the collection of urinary samples in order to determine body mass (BM), skeletal muscle mass (SM), percent body fat (% BF), lean body mass (LBM), and percent total body water (% TBW). Samples of urine were collected for immediate determination of urinary specific gravity (USG) in order to quantify hydration status.
The anthropometric method as well as the BIA determined body fat as % BF, and body water was also determined with BIA as % TBW. However, BM and LBM as well as SM were determined in absolute values in kg. For comparison between 2 measured parameters (BIA versus anthropometry) either relative or absolute values were used. Absolute fat mass (FM) was calculated with % BF derived from BM and volume of total body water (TBW) was calculated using % TBW and BM.
BM was measured with the BIA balance Tanita BC-545 (Tanita, Arlington Heights, IL, U.S.A.) to the nearest 0.1 kg. SM was calculated using the following formula:
| SM = Ht x (0.00744 x CAG2 0.00088 x CTG2 + 0.00441 x CCG2) + 2.4 x sex - 0.048 x age + race + 7.8 |
where Ht = height, CAG = skin-fold-corrected upper arm girth, CTG = skin-fold-corrected thigh girth, CCG = skin-fold-corrected calf girth, sex = 1 for male, race = 0 for white, according to Lee et al., 2000.
% BF was calculated using the following formula:
| %BF = 0.465 0.180(Σ7SF) - 0.0002406(Σ7SF)2 0.0661(age) where Σ7SF = sum of skin-fold thickness of chest, midaxillary, triceps, subscapular, abdomen, suprailiac and thigh mean, according to Ball et al., 2004. |
Circumference of the upper arm, thigh and calf were measured at the largest circumference of the limb to the nearest 0.1 cm. At the thigh, circumference was determined 20 cm above the upper pole of the patella. Skin-fold thicknesses of chest, midaxillary (vertical), triceps, subscapular, abdominal (vertical), suprailiac (at anterior axillary), thigh and calf were measured with a skin- fold calliper (GPM-Hautfaltenmessgerät, Siber & Hegner, Zurich, Switzerland) to the nearest 0.2 cm at the right side, according to Lee et al., 2000. Every measurement using anthropometric techniques was taken by the same person 3 times, and the mean value used for calculation. % TBW, LBM and % BF were measured with the BIA balance (Tanita BC- 545). Impedance measurements were performed with the athletes standing in an upright position, barefoot in running wear, on foot-electrodes on the platform of the instrument, with the legs and thighs not touching, and the arms not touching the torso. The subjects stood on the 4 foot- electrodes: 2 oval-shaped and 2 heel-shaped electrodes, and gripped the 2 palm-and-thumb electrodes in order to yield 2 thumb- and 2 palm-electrodes. The skin and the electrodes were pre-cleaned and dried. USG was determined with the Combur10 Test® (Roche Diagnostics, Mannheim, Germany). The test detects the ion concentration of urine. In the presence of cations, protons are released by a complexing agent and produce a colour change in the indicator bromthymol from blue via blue-green to yellow.
Statistical analysis
Statistical analysis was performed with SPSS version 14.0 (SPSS Inc., Chicago, USA). For all statistical tests significance was set at the 0.05 level. A one sample Wilcoxon signed rank test was used to determine any significant change in body mass, skeletal muscle mass, lean body mass, total body water and fat mass (anthropometry and bioelectrical impedance analysis) during the complete run, during the first stage (pre-race and day 1) and during the last 17 stages (day 1 and day 17). Bonferroni correction was used to compensate for multiple testing effects (9 pairwise tests of 9 parameters).
Results
BM, FM determined using anthropometric techniques (Ant), FM and SM using BIA, did not change after the first stage (Figures 1 and 2). However, SM (Ant) decreased by 2.0 kg and FM (BIA) reduced by 3.9 kg (p < 0.05) during the race (Figure 1); in comparison FM (Ant) did not change (Figure 2). % TBW did not change after the first stage, however, it increased significantly (p < 0.05) during the race (Figure 1). Calculated TBW increased by 2.3 Litres. USG, prerace, was 1.013 ± 0.007 and increased to 1.023 ± 0. 005 at day 6. Then USG values dropped slowly to recover to a second peak at day 13 (1.021 ± 0.005). However, the changes did not reach statistical significance (Figure 3).
Figure 1.
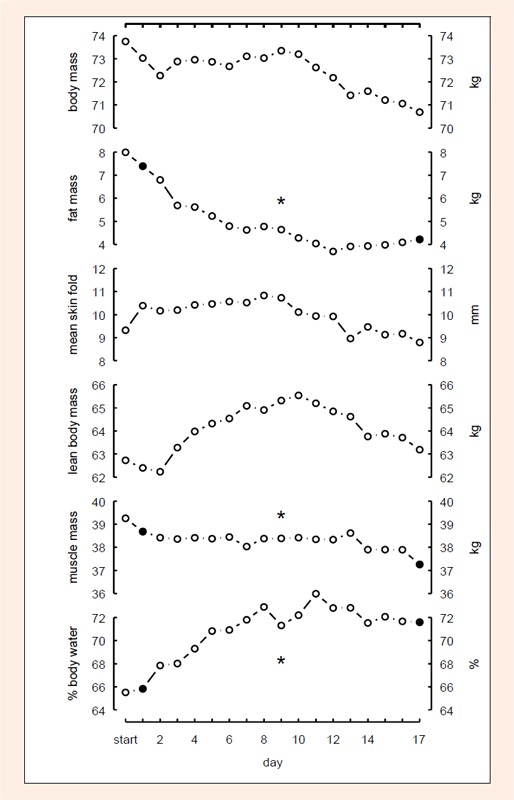
Changes of body mass, fat mass (BIA), mean skin-fold thickness (Ant), lean body mass (BIA); skeletal muscle mass (Ant) and percent total body water (BIA) during the race. Fat mass and skeletal muscle mass decreased, percent total water increased significantly during the race.
BIA = bioelectrical impedance analysis, Ant = anthropometric method. * = p<0.05.
Figure 2.
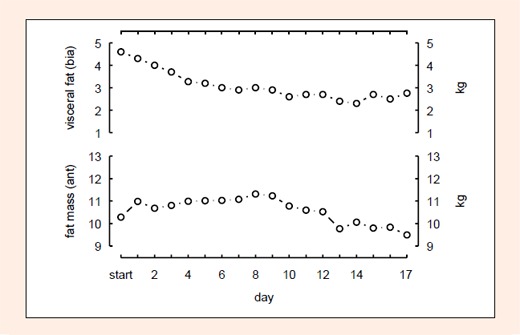
No significant change in visceral fat mass (BIA) or fat mass (Ant).
BIA = bioelectrical impedance analysis, Ant = anthropometric method.
Figure 3.
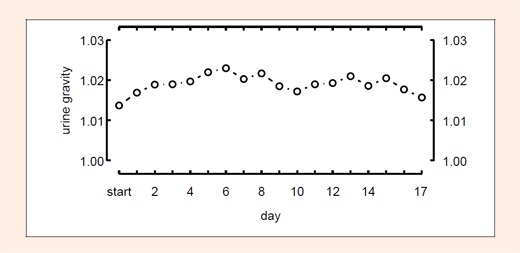
Changes of urinary specific gravity during the race. No significant differences have been found.
Discussion
The main finding of this field study was that FM - depending upon the method used (anthropometry versus BIA) - as well as SM (determined using anthropometric techniques) decreased during the run. In addition, % TBW increased. Interestingly, BM and USG showed no change.
Decrease of body mass
In general, a decrease of BM is observed during ultra-running (Rehrer et al., 1992; Skenderi et al., 2006). However, in the present study, this finding could not be confirmed. During the race there was no decrease in BM (Figure 1) as already shown in another multi- stage ultra-endurance run (Knechtle and Kohler, 2007). % TBW increased continuously (Figure 1) for 11 stages and USG (Figure 3) - as a sign of dehydration and possible renal impairment - also continuously increased in the race.
However, changes in USG were not significant. The BIA demonstrated a continuous increase of % TBW until stage 11 (Figure 1). This increase of TBW seems to be distributed also in the SM, as the change in LBM parallels the % TBW increase (Figure 1). Presumably, the increase of % TBW also led to subcutaneous oedemas and therefore the skin- fold thickness increased at some regions of the body so that the determination of FM using the anthropometric method showed no change (Figure 2). The increase of % TBW could be the result of different mechanisms: Protein catabolism with hypoproteinemic edema, increased protein synthesis with increased plasma volume (PV), increase in PV due to sodium retention, based on an increased aldosterone concentration and impairment of the antidiuretic hormone and dehydration and impaired renal function due to skeletal muscle damage.
Protein catabolism with hypoproteinemic edema?
Protein catabolism and the consequent fluid shifts might occur in an ultra-endurance performance. Lehmann et al., 1995 postulated an exercise-related intra-extra-cellular water shift after a Double Iron Triathlon. They found a decrease in intra-cellular water and an extra-cellular fluid increase, and reported the decrease in cellular hydration state as a protein-catabolic signal. In the present study, SM determined using anthropometry decreased, whereas LBM determined with BIA showed no significant change (Figure 1). Parameters to detect skeletal muscle damage were not performed. However, SM decreased significantly and % TBW increased so that hypoproteinemic edema might be a possible cause.
Increased protein synthesis with increased plasma volume?
A further explanation for the increase of % TBW could be an increase in circulating proteins inducing an increase in plasma oncotic pressure. An increase of plasma protein concentration - especially albumin - might explain an increase in PV. Maughan et al. , 1985 found after a marathon an increase of total protein and albumin, as also reported by Mischler et al. , 2003 after a multi-stage ultra-endurance trail. In contrast, Wu et al., 2004 reported in a 24-hour-run that 2 days after the race there was a significant decrease of total protein and Fellmann et al., 1989 found a reduced concentration of plasma protein after a 24-hour-run, with a continuous drop in the days after the race. Presumably, an increase in circulating proteins inducing an increase in plasma oncotic pressure mechanism was not responsible for the increase of % TBW in this present investigation.
Change of plasma volume due to hormonal changes?
A further possible explanation for the increase of % TBW could be an increase in PV due to sodium retention, as a consequence of increased aldosterone activity. Transient expansion of PV is commonly reported after endurance events (Fellmann, 1992; 1999; Maughan et al., 1985; Milledge et al., 1982). Immediately after a 24-hour-run, PV was initially reduced, but started to increase after the race with a peak on day 2 (Fellmann et al., 1989). Prolonged exercise causes an increased loss of TBW by sweating and respiration (Deuster et al., 1992). The resulting activation of the renin-angiotensin-aldosterone system (RAAS) leads to retention of sodium, causing water retention within the circulation. After intense exercise, antidiuretic hormone and aldosterone are increased (Freund et al., 1987; Melin et al., 1980) and both hormones increase in concentration with increasing exercise intensity (Freund et al., 1991). Increased aldosterone production during intense exercise helps the body to maintain sodium by increasing its reabsorption from the filtered tubular fluid (Poortmans, 1984). Physical exercise also leads to an elevation of antidiuretic hormone concentration (Fellmann et al. , 1989), probably due to an increase in plasma osmolality and a decrease in PV. Antidiuretic hormone is involved in the conservation of body water by facilitating the reabsorption of solute-free water (Fellmann, 1992). Water balance is regulated by the adjustment of antidiuretic hormone, which reduces water excretion, and by the feeling of thirst, which induces water intake (Ramsay, 1989). The activation of the RAAS and of the antidiuretic hormone system leads to an enhanced retention of sodium and free water, consequently resulting in an increase of PV (Neumayr et al., 2005).
Dehydration and impaired renal function due to skeletal muscle damage in running
We presume that the continuous increase of % TBW (Figure 1) could also be a result of the impairment of the kidney due to the rhabdomyolyis occurring in ultra- endurance performances (Kim et al., 2007). As already shown in the ‘Isarrun’ (Knechtle and Kohler, 2007), a significant decrease of SM was demonstrated.
Rhabodmyolysis during ultra-endurance running has been demonstrated (Skenderi et al., 2006; Uberoi et al., 1991) and an association between skeletal muscle damage and impaired renal function has been postulated. Strenuous exercise including running leads to damage of skeletal muscle cells (Koller et al., 1998). Marathon running, especially, has also been demonstrated to alter renal function (Gault et al., 1992; Neviackas and Bauer, 1981). It is known from marathon running that the eccentric loads of long-term running may cause acute renal failure through dehydration, haemolysis and rhabdomyolysis (Dancaster et al., 1969; Irving et al., 1990; MacSearraigh et al., 1979). In cases of severe muscle damage, creatine kinase and myoglobin from skeletal muscle cells will be released into the blood and myoglobinuria (Uberoi et al., 1991) can result. Myoglobin can precipitate in the kidneys and thereby result in acute renal failure (Uberoi et al., 1991). There is one study of an ultra-endurance race which presumed that an increase of plasma volume was due to impaired renal function. Gastmann et al., 1998 found an increase in plasma volume and serum urea as well as a decrease of haemoglobin and haematocrit. These changes could not be explained by haemoconcentration but were related to a suppressed renal function with diminished renal blood flow, decreased glomerular filtration rate and increased hyperaldosteronemia-related renal sodium-re-uptake as well as to proteolysis during prolonged exercise. Regarding the present results with a decrease of SM and an increase of % TBW (Figure 1), would lead to the assumption that impaired renal function was responsible for the increase of % TBW.
The problem of timing of determination of body mass after endurance performance
The determination of fat free mass (FFM) corresponding to LBM by BIA is dependent upon the measurement of TBW. Fat mass (FM) is calculated by subtracting FFM from BM. Therefore, assessment of body composition using BIA is dependent upon the correct determination of TBW. Since BIA assumes a constant hydration of FFM, the calculation of FFM from TBW is affected by changes in TBW. An increase of TBW is perceived as an increase of FFM with underestimation of FM, and in contrast, a decrease of TBW results in an overestimation of FM (Pialoux et al., 2004). Therefore, FM will be falsely high or low depending upon the hydration status of the individual. Fluid shifts cannot be determined with skin-fold measurements. However, BIA seems also to be limited regarding detection of small changes of TBW. Even small fluid changes may be incorrectly interpreted as changes in an athlete’s BF content (Saunders et al., 1998). In addition, it is difficult to determine low and high % BF correctly (Kaminsky and Whaley, 1993). As a consequence during ultra-endurance performance with retention of TBW and loss of BF, BIA may not be reliable in a correct estimation of FM (Pialoux et al., 2004). Hydration status is of vital importance in determining FM and FFM correctly using BIA (Montagnani et al., 1998). Changes of FM and FFM - when determined with BIA - are significantly correlated with changes of TBW. BIA is a useful method for estimation of TBW when fluid shifts are equilibrated and electrolyte concentrations remain unchanged (Pialoux et al., 2004). However, BIA may not provide correct estimates of TBW and FM when hydration status is altered. The timing of measuring body composition after performance using BIA might be of importance, as % BF could be measured incorrectly after physical performance due to fluid shift. Ultra-endurance leads to an increased shift of fluid from the intracellular to the extracellular space with an increase in leg volume (Milledge et al., 1982). One problem is that PV can be increased after ultra-endurance performances; therefore, TBW also increases. On stopping performance there is a return of PV to control values that may take up to several days (Milledge et al., 1982; Pialoux et al., 2004). Immediately after a 24-hour-run, PV was reduced, but started to increase after the race with a peak on day 2 (Fellmann et al., 1989). Regarding a stable BM (Figure 1) and stable USG (Figure 3) of our runners through out the race, we presume a constant hydration status (Kavouras, 2002) during the 17 stages and therefore BIA can be used in order to determine TBW and FM their respective changes.
What was the importance of stage 11?
Regarding Figure 1, the present results show that after stage 11 the increase of LBM and % TBW turned into a decrease, similarly a decrease in FM turned into an increase for the rest of the race. Stage 11 covered a distance of 86 km and the athlete’s lowest speed was at this stage (Table 1). At the beginning of the race, stages 2 to 4 were 80 km long and more, but flat. Then stages 8 and 11 were 80 km and longer, reaching an altitude of more than 1,000 m. After stage 11, stages became shorter and athletes had more time for recovery. From stage 1 to stage 11, the distance per stage was 70 km or more. After stage 11, the distance was below 70 km. This might also have had an effect on FM and the decrease of % TBW (Figure 1). In the 1,000 km run described by Raschka and Plath, 1992, FM decreased in the male runners by 8.8 kg (– 11.9 %) after 500 km and at the end of the run by 7.7 kg (– 10.6 %). In the present study a significant decrease of FM occurred after day 11.
Nutrient and energy intake
A limitation of this study is the fact that we did not determine energy turnover as it has been reported in a case report of ultra-endurance, where a considerable energy deficit has been reported (Bircher et al., 2006; Knechtle et al., 2005; Knechtle et al., 2008). It would be interesting to determine in future field-studies in multi-stage ultra-endurance runs, whether an increased protein intake during ultra-endurance performance (Knechtle et al. 2007) is associated with a decrease in skeletal muscle mass. The nutrient intake should be of quantitative analysis, not only qualitative (Knechtle and Schulze, 2008).
Conclusion
Ultra-runners in a multi-stage ultra-endurance run over 1,200 km in 17 stages suffered a decrease of skeletal muscle mass and fat mass - when determined with bioelectrical impedance analysis - as well as an increase of total body water. We presume that the continuous eccentric exercise leads to damage of skeletal muscle, a continuous rhabdomyolysis and an impairment of renal function, thus leading to a continuous accumulation of body water. In future studies, the continuous and increasing damage of skeletal muscle with associated impairment of renal function should be documented with indirect biochemical parameters such as metabolites in blood and urine. In a follow-up study the enzyme creatine kinase should be measured (CK) in order to provide evidence for the hypothesis of rhabdomyolysis. In addition, hormones of fluid and electrolyte metabolism should be measured to give / allow further insights in the regulation of water metabolism during ultra-endurance performance. With dual-energy X-ray absorptiometry the continuous decrease of skeletal muscle mass could be better determined. In future investigations, the performance level of the runners should be included as a potential factor to explain different results in some anthropometric parameters in ultra-endurance athletes. Additionally, in future studies in ultra-endurance performance, energy intake and energy expenditure should be directly quantified in order to investigate the relationship between an increased protein intake in the face of a reduced skeletal muscle mass.
Acknowledgments
We thank Prof. Dr. med. Hans G. Drexler, Braunschweig, Germany, for his constructive criticism during the race. For their help in translation, we thank Matthias Knechtle, Lausanne, Switzerland and Mary Miller from Stockton-on-Tees, Cleveland in England, member of an ultra-endurance support crew.
Biographies

Beat Knechtle
Employment
Gesundheitszentrum St. Gallen and Department of General Practice, University of Zurich, Switzerland.
Degree
MD
Research interests
Anthropometry and change of body composition in ultra-endurance.
E-mail: beat.knechtle@hispeed.ch

Brida Duff
Employment
Nurse, Gesundheitszentrum St. Gallen, Switzerland
Research interests
Anthropometry and body composition in ultra-endurance.
E-mail: bridaduff@hotmail.com

Ingo Schulze
Employment
Race director Deutschlandlauf and Trans-Europe-Foot-Race, Deutschlandlauf, Germany.
Research interests
Nutrition, anthropometry and body composition in ultra-endurance.
E-mail: ultralauf@ischulze.de

Götz Kohler
Employment
Biozentrum, University of Basel, Switzerland.
Degree
PhD
Research interests
Muscle metabolism, cycling pedalling rate.
E-mail: goetz.kohler@unibas.ch
References
- Ball S.D., Altena T.S., Swan P.D. (2004) Comparison of anthropometry to DXA: a new prediction equation for men. European Journal of Clinical Nutrition 58, 1525-1531 [DOI] [PubMed] [Google Scholar]
- Bircher S., Enggist A., Jehle T., Knechtle B. (2006) Effects of an extreme endurance race on energy balance and body composition - a case report. Journal of Sports Science and Medicine 5, 154-162 [PMC free article] [PubMed] [Google Scholar]
- Dancaster C.P., Duckworth W.C., Roper C.J. (1992) Nephropathy in marathon runners. South African Medical Journal 43, 758-759 [PubMed] [Google Scholar]
- Deuster P.A., Singh A., Hofmann A., Mosses F.M., Chrousos G.C. (1969) Hormonal responses to ingesting water or a carbohydrate beverage during a 2 h run. Medicine and Science in Sports and Exercise 24, 72-79 [PubMed] [Google Scholar]
- Fellmann N., Bedu M., Giry J., Pharmakis-Amadieu M., Bezou M.J., Barlet J.P., Coudert J. (1989) Hormonal, fluid, and electrolyte changes during a 72-h recovery from a 24-h endurance run. International Journal of Sports Medicine 10, 406-412 [DOI] [PubMed] [Google Scholar]
- Fellmann N. (1992) Hormonal and plasma volume alterations following endurance exercise: a brief review. Sports Medicine 13, 37-49 [DOI] [PubMed] [Google Scholar]
- Fellmann N., Ritz P., Ribeyre J., Beaufrère B., Delaître M., Coudert J. (1999) Intracellular hyperhydration induced by a 7-day endurance race. European Journal of Applied Physiology 80, 353-359 [DOI] [PubMed] [Google Scholar]
- Freund B.J., Claybaugh J.R., Dice M.S., Hashiro G.M. (1987) Hormonal and vascular fluid responses to maximal exercise in trained and untrained males. Journal of Applied Physiology 63, 669-675 [DOI] [PubMed] [Google Scholar]
- Freund B.J., Shizuru E.M., Hashiro G.M., Claybaugh J.R. (1991) Hormonal, electrolyte, and renal responses to exercise are intensity dependent. Journal of Applied Physiology 70, 900-906 [DOI] [PubMed] [Google Scholar]
- Gastmann U., Dimeo F., Huonker M., Böcker J., Steinacker J.M., Petersen K.G., Wieland H., Keul J., Lehmann M. (1998) Ultra-triathlon-related blood-chemical and endocrinological responses in nine athletes. The Journal of Sports Medicine and Physical Fitness 38, 18-23 [PubMed] [Google Scholar]
- Gault M.H., Longerich L.L., Harnett J.D., Wesolowski C. (1992) Predicting glomerular function from adjusted serum creatinine. Nephron 62, 249-256 [DOI] [PubMed] [Google Scholar]
- Irving R.A., Noakes T.D., Raine R.I., Van Zyl Smit R. (1990) Transient oliguria with renal tubular dysfunction after a 90 km run. Medicine and Science in Sports and Exercise 22, 756-761 [DOI] [PubMed] [Google Scholar]
- Kaminsky L.A., Whaley M.H. (1993) Differences in estimates of percent body fat using bioelectrical impedance. The Journal of Sports Medicine and Physical Fitness 33, 172-177 [PubMed] [Google Scholar]
- Kavouras S.A. (2002). Assessing hydration status. Current Opinion in Clinical Nutrition & Metabolic Care 5, 519-524 [DOI] [PubMed] [Google Scholar]
- Kim H.J., Lee Y.H., Kim C.K. (2007) Biomarkers of muscle and cartilage damage and inflammation during a 200 km run. European Journal of Applied Physiology 99, 443-447 [DOI] [PubMed] [Google Scholar]
- Knechtle B., Enggist A., Jehle T. (2005) Energy turnover at the Race across America (RAAM). International Journal of Sports Medicine 26, 499-503 [DOI] [PubMed] [Google Scholar]
- Knechtle B., Kohler G. (2007) Running 338 kilometres within five days has no effect on body mass and body fat but reduces skeletal muscle mass - the Isarrun 2006. Journal of Sports Science and Medicine 6, 401-407 [PMC free article] [PubMed] [Google Scholar]
- Knechtle B., Pitre J., Chandler C. (2006) Food habits and use of supplements in extreme endurance cyclists - the Race Across AMerica (RAAM) 2006. Schweizerische Zeitschrift für Sportmedizin und Sporttraumatologie 55, 102-106 [Google Scholar]
- Knechtle B., Schulze I. (2008). Nutritional behaviours in ultra-endurance runners - Deutschlandlauf 2006. PRAXIS 97, 243-251 [DOI] [PubMed] [Google Scholar]
- Knechtle B., Knechtle P., Schück R., Andonie J.L., Kohler G. (2008) Effects of a Deca Iron Triathlon on body composition - a case study. International Journal of Sports Medicine 29, 343-351 [DOI] [PubMed] [Google Scholar]
- Koller A., Mair J., Schobersberger W., Wohlfarter T., Haid C., Mayr M., Villiger B., Frey W., Puschendorf B. (1998) Effects of prolonged strenuous endurance exercise on plasma myosin heavy chain fragments and other muscular proteins. Cycling vs running. The Journal of Sports Medicine and Physical Fitness 38, 10-17 [PubMed] [Google Scholar]
- Lee R.C., Wang Z., Heo M., Ross R., Janssen I., Heymsfield S.B. (2000) Total-body skeletal muscle mass: development and cross-validation of anthropometric prediction models. American Journal of Clinical Nutrition 72, 796-803 [DOI] [PubMed] [Google Scholar]
- Lehmann M., Huonker M., Dimeo F. (1995) Serum amino acid concentrations in nine athletes before and after the 1993 Colmar Ultra Triathlon. International Journal of Sports Medicine 16, 155-159 [DOI] [PubMed] [Google Scholar]
- MacSearraigh E.T., Kallmeyer J.C., Schiff H.B. (1979) Acute renal failure in marathon runners. Nephron 24, 236-240 [DOI] [PubMed] [Google Scholar]
- Maughan R.J., Whiting P.H., Davidson R.J. (1985) Estimation of plasma volume changes during marathon running. British Journal of Sports Medicine 19, 138-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melin B., Eclache J.P., Geelen G., Annat G., Allevard A.M., Jarsaillon E., Zebidi A., Legros J.J., Gharib C. (1980) Plasma AVP, neurophysin, rennin activity, and aldosterone during submaximal exercise performed until exhaustion in trained and untrained men. European Journal of Applied Physiology 44, 141-151 [DOI] [PubMed] [Google Scholar]
- Milledge J.S., Bryson E.I., Catley D.M., Hesp R., Luff N., Minty B.D., Older M.W., Payne N.N., Ward M.P., Withey W.R. (1982) Sodium balance, fluid homeostasis and the renin-aldosterone system during the prolonged exercise of hill walking. Clinical Science (London, England: 1979) 62, 595-604 [DOI] [PubMed] [Google Scholar]
- Mischler I., Boirie Y., Gachon P., Pialoux V., Mounier R., Rousset P., Coudert J., Fellmann N. (2003) Human albumin synthesis is increased by an ultra-endurance trial. Medicine and Science in Sports and Exercise 35, 75-81 [DOI] [PubMed] [Google Scholar]
- Montagnani M., Montomoli M., Mulinari M., Guzzo G., Scopetani N., Gennari C. (1998) Relevance of hydration state of the fat free mass in estimating fat mass by body impedance analysis. Applied Radiation and Isotopes 49, 499-500 [DOI] [PubMed] [Google Scholar]
- Neumayr G., Pfister R., Hoertnagl H., Mitterbauer G., Prokop W., Joannidis M. (2005) Renal function and plasma volume following ultramarathon cycling. International Journal of Sports Medicine 26, 2-8 [DOI] [PubMed] [Google Scholar]
- Neviackas J.A., Bauer J.H. (1981) Renal function abnormalities induced by marathon running. Southern Medical Journal 74, 1457-1460 [DOI] [PubMed] [Google Scholar]
- Pialoux V., Mischler I., Mounier R., Gachon P., Ritz P., Coudert J., Fellmann N. (2004) Effect of equilibrated hydration changes on total body water estimates by bioelectrical impedance analysis. British Journal of Nutrition 91, 153-159 [DOI] [PubMed] [Google Scholar]
- Poortmans J.R. (1984) Exercise and renal function. Sports Medicine 1, 125-153 [DOI] [PubMed] [Google Scholar]
- Ramsay D.J. (1989) The importance of thirst in maintenance of fluid balance. Baillière’s Clinical Endocrinology and Metabolism, 3, 371-389 [DOI] [PubMed] [Google Scholar]
- Raschka C., Plath M., Cerull R., Bernhard W., Jung K., Leitzmann C. (1991). The body muscle compartment and its relationship to food absorption and blood chemistry during an extreme endurance performance. Zeitschrift für Ernährungswissenschaft 30, 276-288 [DOI] [PubMed] [Google Scholar]
- Raschka C., Plath M. (1992) Body fat compartment and its relationship to food intake and clinical chemical parameters during extreme endurance performance. Schweizerische Zeitschrift für Sportmedizin und Sporttraumatologie 40, 13-25 [PubMed] [Google Scholar]
- Rehrer N.J., Brouns F., Beckers E.J., Frey W.O., Villiger B., Riddoch C.J., Menheere P.P., Saris W.H. (1992) Physiological changes and gastro-intestinal symptoms as a result of ultra-endurance running. European Journal of Applied Physiology 64, 1-8 [DOI] [PubMed] [Google Scholar]
- Saunders M.J., Blevins J.E., Broeder C.E. (1998) Effects of hydration changes on bioelectrical impedance in endurance trained individuals. Medicine and Science in Sports and Exercise 30, 885-892 [DOI] [PubMed] [Google Scholar]
- Skenderi K.P., Kavouras S.A., Anastasiou C.A., Yiannakouris N., Matalas A.L. (2006) Exertional rhabdomyolysis during a 246-km continuous running race. Medicine and Science in Sports and Exercise 38, 1054-1057 [DOI] [PubMed] [Google Scholar]
- Uberoi H.S., Dugal J.S., Kasthuri A.S., Kolhe V.S., Kumar A.K., Cruz S.A. (1991) Acute renal failure in severe exertional rhabdomyolysis. The Journal of the Association of Physicians of India 39, 677-679 [PubMed] [Google Scholar]
- Wu H.J., Chen K.T., Shee B.W., Chang H.C., Huang Y.J., Yang R.S. (2004) Effects of 24 h ultra-marathon on biochemical and haematological parameters. World Journal of Gastroenterology 10, 2711-2714 [DOI] [PMC free article] [PubMed] [Google Scholar]


