Abstract
Epilepsy imposes a significant clinical, epidemiologic and economic burden on societies throughout the world. Despite the development of more than ten new antiepileptic drugs over the past 15 years, approximately a third of patients with epilepsy remain resistant to pharmacotherapy. Individuals who fail to respond, or respond only partially, continue to have incapacitating seizures. Managing patients with medically refractory epilepsy is challenging and requires a structured multidisciplinary approach in specialized clinics. If the problems related to drug resistance could be resolved, even in part, by improving the pharmacokinetic profile of existing drugs, the economic savings would be remarkable and the time required to design drugs that achieve seizure control would be shorter than the discovery of new targets and molecules was required. A promising approach is the use of corticosteroids that may have a dual beneficial effect. Resective brain surgery remains the ultimate and highly successful approach to multiple drug resistance in epileptic patients.
Keywords: combination therapy steroids, dexamethasone, epileptic seizures, multiple drug resistance, neurological surgery
The proportion of patients with refractory seizures varies across studies between 6 and 35% [1–4]. The variability in reported drug resistance depends on the criteria adopted for definition (e.g., number of ineffective drugs, persistence of acceptable number of seizures vs seizure freedom), on the characteristics of the recruited population (e.g., population-based study vs patients referring to tertiary center; children vs adults, patients with homogeneous vs heterogeneous epileptic syndromes) and on the study design (e.g., prospective vs retrospective). In the last two decades, the introduction of several new drugs, which are often better tolerated and manageable than the older ones, has certainly improved our ability to treat epilepsy. Recent studies reported that 12–17% of treatment-resistant patients became seizure-free with the addition of a previously untried, in most cases new-generation, antiepileptic drugs (AEDs) [5,6].
Nevertheless, drug resistance still remains the main determinant of low quality of life in a high number of patients with epilepsy who account – in what is probably an optimistic view – for 15% of cases.
Among these patients, a proportion of cases may find a valuable alternative treatment in epilepsy surgery; nevertheless, surgery is not feasible for all patients either because removal of the epileptic area would result in unacceptable deficits (e.g., when motor or language areas are involved) or because seizures have multifocal origin.
A further issue to be considered is that of the economic weight of drug resistance. Interest in the economic aspects of epilepsy has been growing both in rich and poor countries, and it is known that the cost of epilepsy care is directly correlated with the severity of illness. An Italian prospective study carried out on both children and adults reported that the direct annual cost of patients with epilepsy is €412 for seizure-free patients, €2198 for drug-resistant patients and reaches €945 for surgical candidates [7]. Further studies confirmed that patients with intractable seizures incur a cost many times higher than those with controlled epilepsy [8,9]. In the wealthier nations, an ongoing debate continues regarding how to curb rapidly rising healthcare costs. State-financed healthcare systems are facing the problem of an inflation of needs and, therefore, are forced to find ways to limit the expenditures for healthcare. Consequently, there is an urgent need for solutions that are therapeutically effective yet fiscally conservative.
The development of new drug treatments is expensive and often fraught with various unexpected problems. In fact, if the problems related to drug resistance could be resolved, even in part, by improving the pharmacokinetic profile of existing drugs, the overall savings would be remarkable and the time required to produce drugs that achieve seizure control would be shorter than if the discovery of new targets and molecules was required. For example, if a pharmacological treatment was to improve response to AEDs, the result would consist in fewer surgeries, monitoring sessions and invasive procedures. A promising approach is the use of corticosteroids, which may have a dual beneficial effect. Based on laboratory data, the use of corticosteroids appears to be effective in promoting the BBB penetration of AEDs rather than targeting a focus and operating by a specific epileptogenic mechanism. Newer research, particularly in pharmacogenomics, holds promise for therapy that more closely suits an individual's profile and type of epilepsy.
New (or improved) therapeutic approaches to epilepsy management
Resective brain surgery
There is little question that surgery is, and will for the foreseeable future remain, the ‘drug of choice’ for multiple drug resistance, and the effectiveness of the surgical approach has been supported by meta-analysis of literature [10,11], as well as, for temporal lobe epilepsy, by a randomized, controlled study (Box 1 & Table 1) [12]. The relative success of neurosurgery in patients refractory to treatment suggests that more scientific efforts ought to be directed towards the improvement of resective techniques, mapping and monitoring. The reality, however, has shown that the main laboratory focus remains indifferent to these quests and that the pursuit of ‘mechanisms’ is still deemed more important than the search for better therapies.
Box 1. Mechanisms and clinical management of refractoriness to antiepileptic drugs.
There are at least three broad mechanisms of refractoriness to antiepileptic drugs:
Pharmacodynamic (drugs fail to exert its effects)
Pharmacokinetic (transporters and P450 enzymes; drug fails to reach the brain parenchyma)
Altered brain homeostasis (free drug partitions poorly with the CNS despite increased passage of total drug)
The following clinical approaches are currently used to deal with multiple-drug resistance in epileptic individuals:
Surgery
Blockade of MDR1
Change of antiepileptic drug target (e.g., Keppra)
Anti-inflammatory drug therapies
Table 1.
Strategies to manage the refractory patient.
| Strategy | Current and future treatments | Ref. |
|---|---|---|
| Polytherapy | Add-on traditional AED | |
| Change drug target (e.g., from GABA to NMDA or vice versa) | ||
| Increase CNS levels by manipulation of MDR1 (e.g., verapamil) or by BBB healing (e.g., dexamethasone) | ||
|
| ||
| Surgery | Temporal or extratemporal resections | [83] |
| Removal of drug-resistant brain after diagnosis with PET/verapamil | ||
|
| ||
| Ketogenic diet | Endogenous or exogenous molecules that mimic the effects of the diet without compliance issues | |
|
| ||
| Anti-inflammatory agents | If target is well defined (BBB? Drug passage across the BBB?) develop specific molecules to treat seizures, resistance to drugs and/or multiple-drug resistance in epileptic individuals | [54–56] |
AED: Antiepileptic drug.
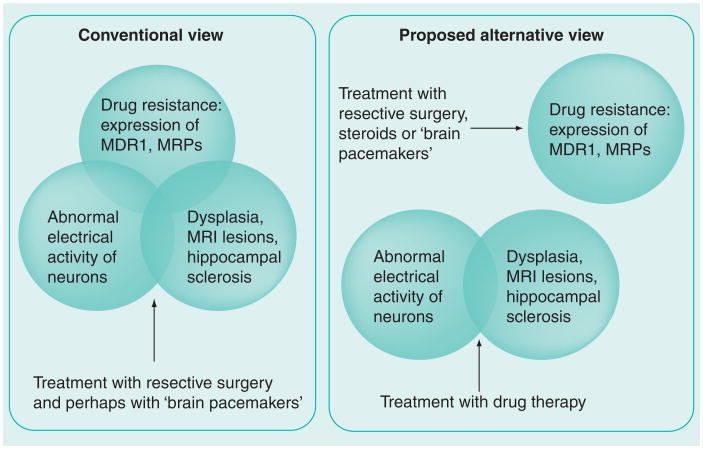
However, long-term follow-up studies have demonstrated that seizure freedom after surgery in many cases depends on drug maintenance and that a number of patients relapse with seizures, albeit at low frequency, despite the continuation of AEDs [13–16]. These data suggest that, at least in a subset of patients, surgery does not cure epilepsy (if so, drugs could be discontinued safely), but rather treats drug resistance and transforming refractory epilepsy in a drug-responsive epilepsy (Figures 1 & 2). If this hypothesis is at least in part true, then we may well accept that multiple drug resistance to AEDs is a dual disease, one resulting from abnormal neuronal firing and a pathological neuronal substrate, and the other owing to insufficient drug efficacy or levels.
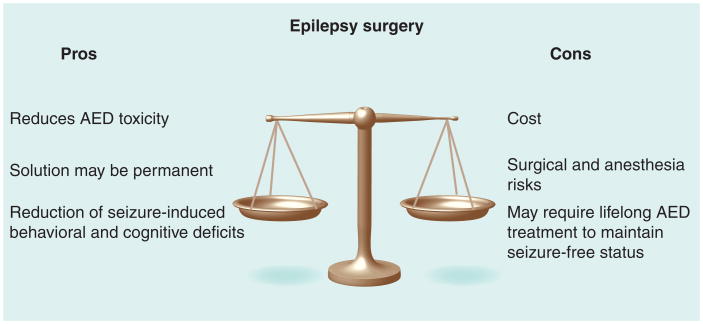
Figure 1. To cut or not to cut? The decision-making process for resective surgery.
AED: Antiepileptic drug.
Figure 2. Is drug-resistant epilepsy a dual disease?
MRPs: Multidrug resistance-associated proteins.
In support of this hypothesis is the fact that MDR1 and other ‘drug resistance genes’ are almost invariably found in the resected portion of the epileptic brain. In fact, most, if not all, human studies are based on resected brain, with comparatively little attention devoted to the majority of epileptic pathologies that respond to AEDs. It is currently unknown whether the epileptic brain in drug-responsive patients expresses elevated levels of drug transporters, but indirect evidence has shown that these are primarily associated to epileptogenic regions [17,18]. In other words, pre- and intra-operative mapping of neuronal activity has allowed the pinpointing of overexpressed transporters to highly active regions, while the surrounding brain (which is also ‘epileptic’ but to a lesser extent) is virtually devoid of abnormal patterns of expression. There is also evidence to show that seizures cause abnormal MDR1 expression, but since this was reported from animal models the clinical translation is not entirely straightforward [19]. Interestingly, the function of MDR1 is decreased by specific patterns of electrical activity [20]. This may imply that different seizures impact drug extrusion in different ways, and perhaps that AC current (as the one used for the experiments described in [20]) may become a treatment options for epileptic patients who develop drug resistance.
Palliative surgery
Palliative treatments, including vagus nerve stimulation and deep brain stimulation (DBS), have been increasingly used in drug-resistant patients who are not candidates for resective surgery. Vagus nerve stimulation was introduced in the 1990s as an adjunctive therapy in patients with intractable epilepsy. In controlled trials, vagus nerve stimulation has been reported to be effective in reducing seizure frequency in 23–30% of patients, but only a minority of patients experience complete seizure control [21,22].
Deep brain stimulation with ‘brain pacemakers’ similar to those used to treat movement disorders were performed in the hope that the epileptic brain could be reset to a preseizure status or permanently disallowed from re-entry into a seizure-producing modality. Different subcortical structures have been chosen as targets for stimulations, including anterior and centromedian thalamic nuclei; subthalamic and caudate nuclei; and the cerebellum. Limited experience is also available for hippocampal stimulation. A significant but not spectacular decrease in seizure frequency in a subpopulation of patients has been reported by almost all authors, regardless of the target used (for a review see [23]), but the true efficacy of DBS is difficult to judge since most studies have been carried out on a small series of patients, affected by different types of epilepsies, who were treated in the context of open-label trials. However, if the preliminary positive results of a recent, double-blind study including 110 patients affected by focal seizures and treated with anterior thalamic nucleus stimulation are confirmed [24], a subset of drug-resistant patients will benefit from this therapeutic advancement. In addition, if the same trials were to be repeated to treat drug resistance as a separate phenomenon, a different electrical stimulation pattern may be used to mimic the reversal of drug resistance obtained by traditional surgery.
As also noted for the surgical approach and the use of anti-inflammatory drugs (see later), the failure of current therapeutic advancements may relate to the fact that we are treating a poorly defined disease, or rather a cluster of diseases with unknown components.
The failure of available antiepileptic medications to adequately control seizures in a substantial number of patients underscores the need to focus on new and better understood therapeutic targets with an eye on the development of novel AEDs. Furthermore, advancements in technology and neuroscience, and the success of neuromodulation in ameliorating a variety of neurological disorders (e.g., Parkinson's disease) have spurred interest to explore promising therapeutic alternatives, such as electrical stimulation, local drug delivery, cell transplantation and gene-based therapies [25]. Future targeted therapies, for example, could be coupled to seizure forecasting systems to create ‘smart’ implantable devices that will detect/predict and pre-emptively treat the patient's seizures. Thus, targeted therapies can be employed in a closed-loop fashion to prevent or suppress seizures before they become clinically manifest.
New AEDs
In the last 15 years, several new-generation AEDs have been made available to patients. However, the impact of these new drugs in the management of patients with intractable epilepsy has been quite limited. If one examines the percentage of patients with uncontrolled epilepsy who became seizure-free during the brief period of randomization as part of the regulatory studies that led to medication approval, this percentage ranges from 1% up to a maximum of 8% across the newer AEDs. This observation reinforces the belief of most investigators that when adequate trials of two or three appropriate AEDs have failed, the likelihood of attaining future sustained control of seizures in these patients is approximately 5% at best, and certainly below 10%.
Rational polypharmacy using specific combinations of available AEDs may be beneficial even when they fail to control seizures as monotherapy, although existing data are insufficient to make firm recommendations. Some authors believe that the use of drugs with different mechanisms of action appears to be more effective than combining drugs with similar mechanisms. A computational approach, which factors in genomic- and epilepsy-related variables in addition to the drugs' specific mechanisms of action, may perhaps be employed in the future to assist clinicians in the decision-making process.
Current AEDs exert their action through a limited range of molecular targets, which include sodium and calcium channels, as well as inhibitory (GABA receptors and transporters) and, to a lesser degree, excitatory (glutamatergic) neurotransmission. If we are to tackle the hitherto medically intractable epilepsies, we are clearly in need of novel AEDs that can attack a wider range of molecular targets to counteract neuronal excitability or modify the impaired, seizure-sustaining microenvironment.
Some of the new AEDs in the pipeline represent modifications of already available parent AED compounds, the development of which is geared towards improved safety (e.g., fluorofelbamate, which is an analog of felbamate developed to avoid the serious life-threatening adverse effects of the parent compound, including aplastic anemia and hepatic failure), tolerability (e.g., eslicarbazepine, which possesses a minor molecular variation from the basic chemical structure of carbamazepine and oxcarbazepine, and is therefore associated with a more favorable metabolic process and with lesser neurological impairment in animal models) and/or effectiveness (e.g., pregabalin, which is structurally and functionally related to gabapentin, similarly exhibiting high affinity binding to the α2-δ auxiliary subunit of voltage-gated calcium channels, and possesses not only a more favorable pharmacokinetic profile but also a higher degree of anticonvulsant efficacy in clinical studies).
Other drugs have novel or previously unidentified mechanisms of action, and these drugs are usually discovered through screening in animal models. Levetiracetam represents a good example of an AED that came to the market based on its unique antiepileptic profile in animal models. Unlike all previous AEDs, levetiracetam showed no activity in the two standard acute seizure models used to screen potentially new AED agents (the maximal electroshock and subcutaneous pentylenetetrazol seizure models). At the same time levetiracetam was found to be effective in animal models of chronic epilepsy and exhibited a broad spectrum of action against various audiogenic, chemo-convulsive and kindled seizure models [26]. At a molecular level, levetiracetam did not exhibit activity against the traditional AED molecular targets. Its mechanism of action was elucidated after the medication became available for clinical use, when it was observed that it specifically binds to a synaptic vesicle protein (SV2A). A positive correlation has been shown between binding of levetiracetam and related compounds to SV2A and their antiseizure potency in rat brain [27]. These postmarketing discoveries led investigators to carefully examine the hitherto unknown role of SV2A, and its potential as a novel target for current and future AED development. Levetiracetam analogs are currently in various stages of development.
Another new AED, rufinamide, was recently approved by the US FDA as an orphan-drug designation to be used as adjunctive therapy in patients with Lennox–Gastaut syndrome – a highly intractable population of patients – based on the positive results in a randomized, placebo-controlled trial performed in this population [28]. Rufinamide is structurally unrelated to other AEDs, and is suspected to exert its action by limiting high-frequency firing of sodium-dependent action potentials [29].
Lacosamide, the most recent AED to be introduced into the European and US markets in 2009 is a new drug discovered by high-throughput animal screens. In animal models this compond appears to have a profile similar to AEDs that inhibit sustained, repetitive sodium channel activity, such as the voltage-gated sodium channel (VGSC) blockers, phenytoin and carbamazepine. These two commonly prescribed old-generation AEDs exert their action on the VGSC fast inactivation, which follows within milliseconds of depolarization, thereby reducing the likelihood of further generation of action potentials. Lacosamide, on the other hand, is the first AED that selectively facilitates the process of slow inactivation of the VGSCs, a process occurring over a period of seconds to minutes (approximately a 100–1000-fold greater time-frame compared with fast inactivation) [30]. This effect is unique to lacosamide and is not shared by the other currently available VSGC blockers. Although experience with lacosamide is limited at this time, this new drug appears promising as an add-on therapy in patients with focal epilepsies.
Other novel drugs in various stages of development include the first potassium-channel-acting AED, retigabine, which has a stabilizing effect on hyperexcitable cells via its action on KCNQ2/3 and 3/5 channels, and has shown efficacy in a broad-spectrum of animal models, and talampanel, which is the first AED in development with a mechanism of AMPA receptor antagonism [31].
Lastly, ganaxalone is a neuroactive steroid that, similarly to the endogenous steroid hormone progesterone, exhibits high-affinity GABA-A receptor allosteric modulation, which includes anticonvulsant activity in animal models [32]. Early clinical trials have been conducted and promising results in intractable patients with infantile spasms have been reported [33]. The use of other steroids is discussed later.
It becomes apparent that although the currently used paradigms for AED development and animal model screening have made important contributions in the pharmacotherapy of epilepsy, new pharmacological paradigms are needed to tackle the problem of intractable epilepsy. A better understanding of the basic mechanisms that govern epileptogenesis, seizure initiation/termination and development of intractability are essential, as is the development of animal models that approximate the human condition of epilepsy refractoriness.
Disease modification is a highly desirable goal in patients at risk for later development of epilepsy following an identifiable initial precipitating injury, with resultant changes in the functional circuitry leading to later development of an abnormal hyperexcitable, epileptogenic network. Thus, primary and secondary prevention may effectively abort the later occurrence of refractory epilepsy. Finally, genetic stratification and the development of individualized treatment that takes into account the underlying genetic substrate offer promising and challenging paths of scientific exploration.
Nonpharmacological treatment options for pharmaco-resistant epilepsy: the ketogenic diet
The ketogenic diet is an important nonpharmacological alternative, usually reserved for young patients with any type of difficult-to-control seizures (Table 1). The diet was originally developed almost a century ago, and is designed to mimic the biochemical changes associated with starvation. It consists of a strict dietary regimen that is high in fat and low in carbohydrate and protein content. The typical ratio of fats to carbohydrates and protein is 4:1 (classic ketogenic diet) or 3:1 (often used in adolescents, and in very young children below 2 years of age). Such a strict regimen is difficult to implement and maintain, and requires close supervision of the child by a trained dietician and by the treating physician(s). In addition to the practical complexities, concerns also exist regarding the long-term effects of the diet on the child's growth. For these reasons, the ketogenic diet is restricted to a small group of young patients with pharmaco-resistant epilepsy, and does not usually represent a long-term therapy. The diet is usually initiated with in the hospital setting. Thereafter, regular follow-up appointments with the ketogenic diet team are recommended to monitor the patient's growth and laboratory studies, the nutritional and neurological status, as well as the overall quality of life and effectiveness of the diet. Most children benefiting from the diet will show a substantial decrease in seizures during the first 3 months. As expected, children and families are more likely to comply with the diet if there is evidence of response. Adherence to the regimen can be followed with a simple and cost-effective laboratory (urine) measurement that can be performed at home (i.e., ketonuria).
No randomized, controlled clinical trials are available [34]; nonetheless, the efficacy of the diet in selected patients have been reported in several large observational studies. The first multi-center study examining the efficacy of the ketogenic diet in pharmaco-resistant epilepsy was completed in 1998 [35]. A total of 51 children, aged 1–8 years, with an average seizure frequency of 230 seizures per month, were recruited at seven epilepsy centers. A 4:1 ketogenic diet was initiated in-hospital, and all children were subsequently followed up for at least 6 months, at which time 55% of the children initiating the diet had shown evidence of response, defined as at least 50% reduction of seizure frequency in comparison with baseline. Not all children were able to tolerate the diet throughout the period of observation: 88% remained on the diet for at least 3 months, 69% for at least 6 months and only 47% remained on it at 1 year. Notably, five patients (10%) were free of seizures at 1 year. Recent meta-analysis describes an estimated rate for obtaining seizure control of 15%, with 33% of patients obtaining a reduction greater than 50% in seizure frequency and that the ketogenic diet is particularly effective in intractable generalized epilepsies (for systematic review and meta-analysis see [36,37]). It should be noted, however, that only half of patients following a ketogenic diet were able to maintain it for a long period; the most frequent reasons for discontinuation included insufficient control of seizures, inability to tolerate the diet owing to medical intolerance or concurrent medical illness and inability to tolerate the strict dietary regimen. There are only a few studies indicating when it is appropriate to terminate the ketogenic diet. Most clinicians wean the patient from the diet after a 2–3-year period. A recent study reports a long-term experience in 28 patients (now aged 7–23 years) among a group of 386 treated patients who continued the diet for at least 6 years: 24 out of 28 patients experienced more than a 90% decrease in seizures. The side effects included slowed growth, kidney stones and fractures, whereas the lipid profile was not significantly affected [38,39]. Reports on the use of the ketogenic diet in adults are scarce, although a benefit was seen in a small series of adults with pharmaco-resistant epilepsy. No long-term follow-up data exist for adults on the ketogenic diet, especially regarding the future risk of atherosclerosis.
Although the ketogenic diet has been employed as a treatment of intractable epilepsies for almost a century, its mechanism of action remains poorly understood. While no single mechanism can account for the observed clinical effects, it is believed that the diet exerts its action by activating several endogenous metabolic and genetic processes, and thereby stabilizes and/or enhances cellular metabolism [40].
Complementary & alternative medical therapies
In the industrialized world, homeopathy, acupuncture, psychological techniques and herbal treatments are often undertaken by patients with epilepsy, particularly when conventional treatments fail. On the other hand, a large proportion of epileptic patients around the world are not receiving AEDs, either for economic reasons or cultural attitudes. These patients often refer to what we consider ‘alternative’ medicine. Well-designed clinical trials of herbal therapies in patients with epilepsy are scarce and methodological issues prevent any conclusions of their efficacy or safety (for review see [41]).
Unorthodox efficacy of anti-inflammatory drugs in the treatment of drug-resistant epilepsy: preliminary findings & proposed mechanisms
The use of steroids and adrenocorticotropic hormone in the treatment of infantile spasms has been established since the mid-20th Century. Since that time, despite the fact that solid evidence on efficacy or safety are still lacking for epilepsy other than West syndrome [42], these drugs have also been utilized in other epileptic syndromes including Lennox–Gastaut, Landau–Kleffner, continuous spike and waves during slow sleep (CSWSS) and Rasmussen's encephalopathy [43]. The aforementioned pathologies are termed ‘epileptic encephalopathies’ and share some common features: high frequency of seizures and/or of epileptic EEG abnormalities, arrest or regression of psychomotor development or of acquired skills (e.g., aphasia in Landau–Kleffner patients) and, at least in some instances, evidence of inflammation (for review see [44]). More interestingly, some observational studies suggest that corticoids could also be effective in other forms of generalized and focal epilepsies [45,46]. A study aimed at evaluating the impact of steroids on cerebral swelling and seizures during presurgical subdural grid EEG monitoring in 37 drug-resistant children demonstrated a reduced seizure frequency and a statistically significant longer time to obtain seizures recording in patients given dexamethasone (2–10 days) compared with untreated patients (1–5 days) [47].
Recently, a retrospective study identified 29 pediatric patients (aged 18 years or younger), who had received a steroid course (methylprednisolone, adrenocorticotropic hormone, hydrocortisone or dexamethasone) for the treatment of medically intractable epilepsy. It should be noted that patients with a history of epileptic syndromes known to respond to steroids (e.g., West, Landau– Kleffner and Rasmussen's symndromes) were excluded. Efficacy was defined as termination of status epilepticus or a reduction in seizure burden by at least 50%.
Treatment was effective in 25 out of 29 patients and observed after 32 of the 38 steroid therapies. A clinically significant reduction of partial seizures resulting in motor functional improvement was observed after ten out of 13 treatments. This positive effect persisted after withdrawal in half of the cases. Only in 5% of the patients were steroids withdrawn owing to the severity of side effects.
The question then arises as to why or how steroids ameliorate seizures. There are at least two broad mechanistic approaches to this problem. From the neurosurgical standpoint, dexamethasone is the drug of choice to treat brain edema, for example in association with neoplasms. The rationale for the use of steroids varies from hospital to hospital, but animal and in vitro data clearly show a direct effect on the BBB [48]. Thus, the results presented previously may be explained by a direct action of the drug on BBB integrity. This point of view is supported by several lines of evidence linking a leaky BBB to epilepsy [49–51] (or acute seizures [52,53]). According to the alternative hypothesis, steroids act as anti-inflammatory agents; this is the most common rationale for their use. In support of this hypothesis is the fact that inflammation has been linked to seizures in several recent papers [44,54–56]. However, it is in our opinion quite unlikely that these two mechanisms may actually exist in isolation. In other words, it is currently apparent that inflammation causes seizures by a direct action on BBB endothelial cells [53,54,56] and, therefore, it is likely that white blood cell activation and BBB damage coexist in patients.
As stated previously, the current rationale for the use of steroids in ‘noninfammatory’ seizure disorders is dual and scientifically sound. There are indirect observations that support the data produced in the limited retrospective trail by Granata and colleagues. For example, it has been shown that dexamethasone reduces the frequency and intensity of electrographic seizures measured by ECG [47]. In addition, at least two recent papers have shown a marked efficacy of anti-inflammatory agents in seizure models [54,56]. However, these data are confounded by other reports where the efficacy of anti-inflammatory therapies was not shown (e.g., in the same pilocarpine model [57]). We have recently tested dexamethasone's efficacy in pilocarpine-induced seizures and have essentially failed to measure any effects in preventing status epilepticus. How these data are related to the clinical experience remains to be understood. However, the lack of efficacy of dexamethasone on seizures induced chemically in otherwise naive animals paired with the curative effect in patients begs an alternative explanation of its mechanism of action.
A parsimonious explanation of these contrasting data is that, as in the case of lobectomies, the effects of steroids are on the disease of drug resistance rather than on neurons or other CNS cells participating in EEG abnormalities. Thus, a possible mechanism of action may imply an effect on the BBB that favors chemotherapeutic access to the brain. This again leads to a paradox whereby a leaky BBB has to be healed to allow drug penetration. The rationale for this way of thinking was recently provided by in vivo animal studies [58]. These findings suggested that free drug penetration in epileptic brain is hampered by a leaky, protein-permeant BBB. Thus, by acting either directly (targeting the endothelium) or indirectly (targeting white blood cells), dexamethasone may improve free drug passage by improving the BBB.
Drug resistance in patients with epilepsy: scientific & clinical background
Current orthodoxy predicts that epilepsy is a neuronal disease and most therapeutic approaches thus attempt to modify neuronal excitability. This approach has the drawback of pharmacokinetic barriers for CNS delivery. Surprisingly, while the field of epilepsy research has primarily targeted neurons or other brain cells (astrocytes), the epidemiology of acute seizures reveals a striking role for cerebrovascular- or BBB-related factors.
A major challenge facing epilepsy treatment is the effective delivery of AEDs across the BBB (Box 1). The rationale for this mini-review stems from the urgent need to address the issue of CNS drug delivery in view of recent contradictory findings and the continuous debate on the role of drug transporters in determining the refractory phenotype. In more general terms, the issue of multiple drug resistance to CNS drugs is not a prerogative of seizure disorders. In fact, neoplasms and CNS infections are only an example of the broad impact that drug resistance has on the management of neurologic patients.
As a consequence of the molecular biology revolution, drug research has experienced an incredible growth of knowledge on the molecular mechanisms of human diseases, leading to the generation of novel therapeutic concepts. At the same time, there has been a rapid increase in research costs associated with a significant decrease in new drugs reaching the market. In the case of neurological disorders, one of the reasons for this translational crisis is the lack of reliable predictive early assessment of absorption, distribution, metabolism and excretion (ADME) parameters. Thus, many useful drugs fail late in clinical trials when their poor pharmacokinetic behavior should have been discovered earlier. Even when preclinical ADME measurements suggest that brain penetration or targeting is not an issue the diseased brain often provides new and sometimes unpredictable challenges, such as the overexpression of multiple drug resistance efflux pumps [59]. The extent of this problem only underscores the difficulty of ‘pushing’ drugs into the CNS of patients affected by neurological disorders. Reliable drug delivery to the brain remains an unsolved task for small molecule drugs and even more so for macromolecular drugs, which become more and more available as a consequence of the progress made by the biotechnology and pharmaceutical industry sectors. The functional and protective role of the BBB is a crucial factor preventing straightforward drug therapy of CNS diseases. The last two decades have seen the field dominated by studies of multiple drug resistance genes and proteins, which have been shown to be partially responsible for poor penetration of a variety of drugs. In the case of epilepsy, early cDNA array experiments demonstrated the complexity of this phenomenon. Based on similar results in clinical trials of cancer patients, a number of investigators have attempted to link MDR1 expression and polymorphism to AED resistance. The results were encouraging at first [60,61] but failed to be duplicated [62]. In light of the complex biology of chemotherapy resistance, these findings are not surprising since MDR1 is only one of the multitudes of mechanisms that can lead to drug resistance.
Why bother if the ‘epileptic’ BBB is leaky?
A perhaps paradoxical aspect of multiple drug resistance in neoplastic disease or seizure disorders is the fact that over-expression of drug transporters has been noted on a background of impaired BBB function (for a review, see [63]). Thus, extrusion of drugs occurs under conditions of apparent free passage across leaky endothelial cells. This led to an alternative, yet not exclusive, explanation for this seemingly inconsistent phenomenon. A recent paper dealing with a rat model of brain edema described regions of impaired BBB function characterized by lower levels of free drug (phenytoin or carbamazepine) compared with regions of intact cerebral vasculature [58]. These findings confirmed that drug lipophilicity (log P) is a good predictor of drug passage across the cerebrovascular endothelium even after BBB disruption. However, these experiments revealed for the first time that protein–drug extravasation is the biggest factor controlling free drug partition in regions of BBB disruption (Box 1). The possibility thus exists that future attempts to tackle drug delivery to the epileptic focus will consists of ‘BBB drugs’ that allow better free drug distribution by re-establishing normal BBB function.
Clinical findings & possible mechanisms of multiple drug-resistant epilepsy
In most cases, patients with epilepsy are effectively treated with AEDs; however, approximately 20% of patients with primary generalized epilepsy, and up to 60% of patients with focal epilepsies, may exhibit pharmaco-resistance during the course of their frequently lifelong condition [64]. There is no generally accepted definition of intractability. No single step in the treatment defines pharmaco-resistance, but from the patient's perspective intractability refects the inability to achieve acceptable seizure control despite adequate trials with a sufficient number of medications at doses that are associated with tolerable, if any, side effects. The probability of seizure freedom becomes very low if the administration of three appropriately selected and prescribed AEDs, given alone or in combination, fails – hence the term of multiple drug resistance. In these cases, neurosurgical resection of the epileptic tissue represents the final alternative to resolve seizures in such patients. In the past 15 years, new AEDs were released but no major reduction in the overall percentage of drug-resistant patients was achieved [65].
The complexity of drug-resistant epilepsy reflects the nature of the pathophysiological process, its possible evolution over time and the different individual sensitivity to drugs, whether it be congenital or acquired. Biologic factors influencing prognosis refer almost exclusively to symptomatic epilepsy that may be the result of a structural lesion including a wide variety of pathologic conditions (e.g., inflammatory, neoplastic or metabolic) or disorders of neuronal development and migration, and other situations presumed to be symptomatic, but of unknown etiology [65–67]. Furthermore, the appearance of intractability may be quite delayed, by a median of 9 years or more in some studies of patients with symptomatic epilepsy, while in other patients intractability may be a prominent feature from the outset (de novo intractability present at the time of the first clinically manifest seizures). Keeping in mind these complex scenarios, several hypotheses have been formulated, including those discussed in the following sections.
AED neuronal target modification (pharmacodynamic hypothesis)
Changes in the electrical and synaptic properties of neurons in specific brain areas can result in decreased sensitivity to the actions of AEDs. Using brain specimens from drug-refractory patients or from chronically epileptic rats, a small number of studies demonstrated changes in ion channel assembly and reduced sensitivity to the AED in both sodium and calcium channels. Different experimental paradigms used to assess the efficacy of different AEDs in other epileptic brain areas led to miscellaneous results that sometimes appear to be difficult to interpret. For instance, while Vreugdenhil and colleagues showed diminished carbamazepine sensitivity of rat CA1 neurons in reducing burst activity [68], Remy et al. showed a normal neuronal response to the same AED when measured in dentate granule cells of kindled rats [69]. Similarly contradictory results were obtained when studying in vitro sensitivity to phenytoin of sodium and calcium currents of hippocampal CA1 neurons in the amygdala-kindled rat compared with in vivo. Neurons in the CA1 region of kindled rats are fully responsive to valproic acid treatment, while the effect of carbamazepine is reduced immediately after kindling. Remarkably, Remy et al. demonstrated a reduced sensitivity of voltage-dependent sodium channels to carbamazepine in human epileptic brain [70]. The loss of GABA-ergic responses to AEDs was reported more recently [71]. Other results suggest that these changes are not crucial in determining drug resistance in epileptic brain, since human brain slices from refractory patients responded to AEDs in a fashion similar to normal rodent brain [72].
Altered brain distribution of AEDs: overexpression of multidrug-resistant proteins (pharmacokinetic hypothesis)
Alternatively, drug resistance in epilepsy may depend on inadequate brain intraparenchymal AED concentrations (for recent updates see [73,74]). This occurs if AEDs are extruded into the vascular bed at the BBB before entry into the brain, or if insufficient plasma levels are reached. Drug transporters so far identified in brain are cell membrane proteins that play an important role in regulating the entry of a large variety of clinically relevant drugs into the CNS. These proteins have been initially discovered and characterized for their ability to confer resistance of cancer cells to chemotherapeutics. Among these drug transporters, members of the ATP binding cassette (ABC) family (e.g., P-glycoprotein [P-gp] or MDR1), multidrug resistance-associated proteins (MRPs) and breast cancer-resistance protein all raised interest in the epilepsy field because of their overexpression in human drug-resistant brain (see review in [59]). Since these transporters restrict the entry of lipophilic substrates into the brain, it was suggested that BBB overexpression in epileptic tissue could reduce the effective concentration of AEDs at their target sites. Indeed, human epileptic brain transporter proteins are upregulated at the BBB [17,75–78]. Experimental models of seizures mimicking the neuropathological features of temporal lobe epilepsy have been used to study the molecular mechanisms of intractability. In rodents, MDR1, MRP1–2 and the breast cancer-resistance protein are overexpressed in endothelial cells, astrocytes and neurons after induction of sustained limbic seizures or in malformed cortical regions [19,79].
Molecular and functional studies have implicated several drug transport proteins at the BBB and blood–cerebrospinal fluid barrier as potentially important factors in the development of drug-resistant epilepsy [63]. An additional hypothesis to explain refractoriness mediated by MDR1 relates to reports linking an ABCB1 3435C–T single-nucleotide polymorphism or a three-single-nucleotide polymorphism haplotype containing 3435C–T to multidrug resistance in epilepsy in three retrospective case–control studies. A further meta-analysis failed to replicate the association [62].
An incomplete picture of transport mechanisms responsible for drug-resistance owing to accumulation defects has led to a wide array of opinions owing to inconsistent or conflicting data regarding the relative significance of a multitude of transport mechanisms. Cell lines, animal and clinical data indicate that MDR1, MRP1 and related transporters are clearly able to mediate drug accumulation defects in cultured malignant cells, but correlations with pathology, clinical resistance and outcomes in resistance to AEDs are less persuasive. A large number of small molecules, many of natural origin, are able to reverse multidrug resistance by inhibiting the functions of MDR1, MRP1 and sister proteins; their action has been considered a possible method to reverse multiple drug resistance. While a few compounds have actually reached clinical trials [101,102], a clear beneficial effect has not been shown in cancer patients after P-gp blockade [80]. An additional trial with the P-gp blocker verapamil is currently planned [103].
Expert commentary
Given the knowledge acquired by the combined clinical and preclinical, laboratory efforts, it appears that at present no magic bullet is available to treat multiple drug resistance in epileptic patients. Improved presurgical mapping and patient selection make surgical management more attractive than in the past. This is confounded by the intrinsic risk of surgical procedures and, in countries with free-market healthcare, significant costs to the patients.
Recent clinical trials [101–103] have enrolled (or plan to enroll) patients for add-on therapy with inhibitors of transporters involved in drug resistance to small molecules [59]. Preliminary data are not yet available, but the lessons learned from neuron–oncology suggest limited efficacy and side effects [80]. The whole field of pharmacokinetic drug resistance has been a focus of renewed attention with the discovery of relatively common polymorphisms for the MDR1 (or ABCB1) gene [61,81]. However, after a short lag-time, what appeared to be a likely and exploitable mechanism of multiple drug resistance to AED has become another false start, as underscored by the lack of support for these initial findings. These results do not rule out per se that these variants are indeed involved in the process leading to refractoriness, but rather highlight the infantile condition of pharmacogenomics.
Surprisingly, old drugs, such as steroids, are teaching us new tricks. Dexamethasone is known as a potent anti-inflammatory drug, but also exerts powerful ‘BBB repair’ potency. Recent laboratory findings suggest that a detriment to brain delivery of AEDs is a hostile neuronal environment (e.g., brain edema) resulting from a disrupted BBB [58]. Therapies aimed at improving CNS homeostasis (e.g., dexamethasone) have also been successfully used to treat a variety of seizure disorders refractory to traditional AEDs. Finally, there is growing evidence linking systemic inflammation to seizures [44,54]. This mechanism, if confirmed and further supported by clinical data, may further justify a broader use of NSAIDs or steroids even in epilepsies that are generally not assumed to be due to inflammatory or infectious events. The preliminary nature of the clinical findings strongly supports the development of multicenter clinical trials to test the best pharmacological and etiological approach [82].
Five-year view
The multifaceted nature of drug refractory epilepsy dictates the need for a multidisciplinary approach to therapeutic intervention. In the near future, the surgical approach is likely to remain the ‘drug of choice’. Under ideal circumstances, we will benefit from ground breaking surgical advancement, improvement in the capacity for early diagnosis and strengthening of the bridge between clinical experience and basic research. Ideally, one may foresee the ability to predict or at least identify the drug-resistant share of the disease. The development of markers of drug resistance will include specific imaging sequences, EEG signatures and serological markers associated with AED levels or metabolism. The disease as a whole may be revealed by brain signaling molecules (e.g., brain proteins or protein fragments, autoantibodies or nucleic acids). The use of markers of drug resistance will impact the pharmacological route to specific combination of AEDs and accelerate the development of unconventional drugs (e.g., steroids). Today, early prognosis of drug resistance has been shown to shorten time to surgery and to avoid the exacerbation of brain damage and decline of cognitive function induced by seizures and AEDs. Finally, a better transition from the bedside to bench is required. The clinical experience is rarely taken into account when designing the basic science experiments. We often rely on animal models that are obsolete and based on the use of an acute neuronal trigger, while epidemiology dictates modeling of a complex, ‘holistic’ disease. The dissociation between science and medicine has been furthered by the mechanistic reductionism adopted by many epilepsy researchers. This has led to findings that, although reproducible within a certain experimental model, are not expandable to the clinical reality.
Key issues.
Re-evaluation of previous knowledge together with analysis and interpretation of current results have suggested the following:
Multiple drug resistance can be seen either as a disease condition or as a comobidity separated from epilepsy.
The mechanisms underlying the phenomenon of drug resistance may not necessarily be the same as those responsible for seizure disorders.
Resective surgery treats both conditions by abrogating the seizure focus as well as the cells and molecules responsible for drug resistance.
If two distinct pathologies lead to multiple drug resistant epilepsy, one may wish to treat them as separate conditions.
Recently, the therapeutic use of steroids has been shown to reduce seizure burden in many epileptic pathologies, which may result from improved drug distribution into the brain.
Acknowledgments
The authors would like to acknowledge the contributions of Vincent Fazio for the extensive assistance he provided in editing and compiling of this manuscript for publication.
Financial & competing interests disclosure: This work was supported by NIH-RO1 NS43284, NIH-RO1 NS38195, NIH-R21 HD057256 to Damir Janigro. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Tiziana Granata, Email: granata.t@istituto-besta.it, Department of Neurology, Cleveland, OH, USA, Department of Child Neurology, Carlo Besta Neurological Institute, Milan, Italy, Tel.: +39 022 394 302, Fax: +39 027 063 8217.
Nicola Marchi, Email: marchin@ccf.org, Department of Cell Biology and Cerebrovascular Research Cleveland Clinic Foundation, Cleveland, OH, USA, Tel.: +1 216 445 0561, Fax: +1 216 445 1466.
Erin Carlton, Email: ecarlto2@utnet.utoledo.edu, Cerebrovascular Research Cleveland Clinic Foundation, Cleveland, OH, USA, Tel.: +1 216 445 0561, Fax: +1 216 445 1466.
Chaitali Ghosh, Email: ghoshc@ccf.org, Department of Cell Biology and Cerebrovascular Research Cleveland Clinic Foundation, Cleveland, OH, USA, Tel.: +1 216 445 0561, Fax: +1 216 445 1466.
Jorge Gonzalez-Martinez, Email: gonzalj1@ccf.org, Department of Neurological Surgery, Cleveland, OH, USA, Tel.: +1 216 445 0561, Fax: +1 216 445 1466.
Andreas V Alexopoulos, Email: alexopa@ccf.org, Cleveland Clinic Epilepsy Center, 9500 Euclid Ave, S-51, Cleveland, OH 44195, USA, Tel.: +1 216 444 3629, Fax: +1 216 445 4378.
Damir Janigro, Departments of Neurological Surgery, Molecular Medicine and Cell Biology and the Cerebrovascular Research Cleveland Clinic Foundation, Cleveland, OH, USA, Tel.: +1 216 445 0561, Fax: +1 216 445 1466.
References
- 1.Picot MC, Baldy-Moulinier M, Daures JP, Dujols P, Crespel A. The prevalence of epilepsy and pharmacoresistant epilepsy in adults: a population-based study in a Western European country. Epilepsia. 2008;49(7):1230–1238. doi: 10.1111/j.1528-1167.2008.01579.x. [DOI] [PubMed] [Google Scholar]
- 2.Kwan P, Brodie MJ. Epilepsy after the first drug fails: substitution or add-on? Seizure. 2000;9(7):464–468. doi: 10.1053/seiz.2000.0442. [DOI] [PubMed] [Google Scholar]
- 3.Arts WF, Brouwer OF, Peters AC, et al. Course and prognosis of childhood epilepsy: 5-year follow-up of the Dutch study of epilepsy in childhood. Brain. 2004;127:1774–1784. doi: 10.1093/brain/awh200. [DOI] [PubMed] [Google Scholar]
- 4.Sillanpaa M, Schmidt D. Natural history of treated childhood-onset epilepsy: prospective, long-term population-based study. Brain. 2006;129:617–624. doi: 10.1093/brain/awh726. [DOI] [PubMed] [Google Scholar]
- 5.Callaghan BC, Anand K, Hesdorffer D, Hauser WA, French JA. Likelihood of seizure remission in an adult population with refractory epilepsy. Ann Neurol. 2007;62(4):382–389. doi: 10.1002/ana.21166. [DOI] [PubMed] [Google Scholar]
- 6.Luciano AL, Shorvon SD. Results of treatment changes in patients with apparently drug-resistant chronic epilepsy. Ann Neurol. 2007;62(4):375–381. doi: 10.1002/ana.21064. [DOI] [PubMed] [Google Scholar]
- 7.Tetto A, Manzoni P, Millul A, et al. The costs of epilepsy in Italy – a prospective cost-of-illness study in referral patients with disease of different severity. Epilepsy Res. 2002;48(3):207–216. doi: 10.1016/s0920-1211(02)00013-x. [DOI] [PubMed] [Google Scholar]
- 8.Langfitt JT, Holloway RG, McDermott MP, et al. Health care costs decline after successful epilepsy surgery. Neurology. 2007;68(16):1290–1298. doi: 10.1212/01.wnl.0000259550.87773.3d. [DOI] [PubMed] [Google Scholar]
- 9.Forsgren I, Beghi E, Ekman M. Cost of epilepsy in Europe. Eur J Neurol. 2005;12:54–58. doi: 10.1111/j.1468-1331.2005.01191.x. [DOI] [PubMed] [Google Scholar]
- 10.Tellez-Zenteno JF, Dhar R, Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128:1188–1198. doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- 11.Engel J, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy – report of the quality standards subcommittee of the American Academy of Neurology, in association with the American Epilepsy Society and the American Association of Neurological Surgeons. Neurology. 2003;60(4):538–547. doi: 10.1212/01.wnl.0000055086.35806.2d. [DOI] [PubMed] [Google Scholar]
- 12.Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311–318. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 13.Wieser HG, Hane A. Antiepileptic drug treatment before and after selective amygdalohippocampectomy. Epilepsy Res. 2003;55(3):211–223. doi: 10.1016/s0920-1211(03)00116-5. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt D, Baumgartner T, Loscher L. Seizure recurrence after planned discontinuation of antiepileptic drugs in seizure-free patients after epilepsy surgery: A review of current clinical experience. Epilepsia. 2004;45(2):179–186. doi: 10.1111/j.0013-9580.2004.37803.x. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt D. Can epilepsy surgery cure epilepsy? Epilepsia. 2004;45:25–26. doi: 10.1111/j.0013-9580.2004.37803.x. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt D. Long-term seizure control of AEDs after epilepsy surgery. Ann Neurol. 2004;56:S24–S24. [Google Scholar]
- 17.Marchi N, Hallene KL, Kight KM, et al. Significance of MDR1 and multiple drug resistance in refractory human epileptic brain. BMC Med. 2004;2:37. doi: 10.1186/1741-7015-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Vliet E, Aronica E, Redeker S, et al. Selective and persistent upregulation of mdr1b mRNA and P-glycoprotein in the parahippocampal cortex of chronic epileptic rats. Epilepsy Res. 2004;60(2–3):203–213. doi: 10.1016/j.eplepsyres.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Rizzi M, Caccia S, Guiso G, et al. Limbic seizures induce P-glycoprotein in rodent brain: functional implications for pharmacoresistance. J Neurosci. 2002;22(14):5833–5839. doi: 10.1523/JNEUROSCI.22-14-05833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janigro D, Perju C, Fazio V, et al. Alternating current electrical stimulation enhanced chemotherapy: a novel strategy to bypass multidrug resistance in tumor cells. BMC Cancer. 2006;6:72. doi: 10.1186/1471-2407-6-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeGiorgio CM, Murray D, Markovic D, Whitehurst T. Trigeminal Nerve Stimulation for epilepsy: long-term feasibility and efficacy. Neurology. 2009;72(10):936–938. doi: 10.1212/01.wnl.0000344181.97126.b4. [DOI] [PubMed] [Google Scholar]
- 22.DeGiorgio CM, Shewmon A, Murray D, Whitehurst T. Pilot study of trigeminal nerve stimulation (TNS) for epilepsy: a proof-of-concept trial. Epilepsia. 2006;47(7):1213–1215. doi: 10.1111/j.1528-1167.2006.00594.x. [DOI] [PubMed] [Google Scholar]
- 23.Lega BC, Halpern CH, Jaggi JL, Baltuch GH. Deep brain stimulation in the treatment of refractory epilepsy: update on current data and future directions. Neurobiol Dis. 2009 doi: 10.1016/j.nbd.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Salanova V, Worth R. Neurostimulators in epilepsy. Curr Neurol Neurosci Rep. 2007;7(4):315–319. doi: 10.1007/s11910-007-0048-9. [DOI] [PubMed] [Google Scholar]
- 25.Alexopoulos AV, Gonugunta V, Yang J, Boulis NM. Electrical stimulation and gene-based neuromodulation for control of medically-refractory epilepsy. Acta Neurochir. 2007;(97)(Pt 2):293–309. doi: 10.1007/978-3-211-33081-4_33. [DOI] [PubMed] [Google Scholar]
- 26.Klitgaard H, Matagne A, Gobert J, Wulfert E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur J Pharmacol. 1998;353(2–3):191–206. doi: 10.1016/s0014-2999(98)00410-5. [DOI] [PubMed] [Google Scholar]
- 27.Kaminski RM, Matagne A, Leclercq K, et al. SV2A protein is a broad-spectrum anticonvulsant target: functional correlation between protein binding and seizure protection in models of both partial and generalized epilepsy. Neuropharmacology. 2008;54(4):715–720. doi: 10.1016/j.neuropharm.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 28.Glauser T, Kluger G, Sachdeo R, Krauss G, Perdomo C, Arroyo S. Rufinamide for generalized seizures associated with Lennox–Gastaut syndrome. Neurology. 2008;70(21):1950–1958. doi: 10.1212/01.wnl.0000303813.95800.0d. [DOI] [PubMed] [Google Scholar]
- 29.Hakimian S, Cheng-Hakimian A, Anderson GD, Miller JW. Rufinamide: a new anti-epileptic medication. Expert Opin Pharmacother. 2007;8(12):1931–1940. doi: 10.1517/14656566.8.12.1931. [DOI] [PubMed] [Google Scholar]
- 30.Beydoun A, D'Souza J, Hebert D, Doty P. Lacosamide: pharmacology, mechanisms of action and pooled efficacy and safety data in partial-onset seizures. Expert Rev Neurother. 2009;9(1):33–42. doi: 10.1586/14737175.9.1.33. [DOI] [PubMed] [Google Scholar]
- 31.Rostock A, Tober C, Rundfeldt C, et al. D-23129: a new anticonvulsant with a broad spectrum activity in animal models of epileptic seizures. Epilepsy Res. 1996;23(3):211–223. doi: 10.1016/0920-1211(95)00101-8. [DOI] [PubMed] [Google Scholar]
- 32.Reddy DS, Rogawski MA. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther. 2000;295(3):1241–1248. [PubMed] [Google Scholar]
- 33.Kerrigan JF, Shields WD, Nelson TY, et al. Ganaxolone for treating intractable infantile spasms: a multicenter, open-label, add-on trial. Epilepsy Res. 2000;42(2–3):133–139. doi: 10.1016/s0920-1211(00)00170-4. [DOI] [PubMed] [Google Scholar]
- 34.Levy R, Cooper P. Ketogenic diet for epilepsy. Cochrane Database Syst Rev. 2003;3:CD001903. doi: 10.1002/14651858.CD001903. [DOI] [PubMed] [Google Scholar]
- 35.Vining EP, Freeman JM, Ballaban-Gil K, et al. A multicenter study of the efficacy of the ketogenic diet. Arch Neurol. 1998;55(11):1433–1437. doi: 10.1001/archneur.55.11.1433. [DOI] [PubMed] [Google Scholar]
- 36.Keene DL. A systematic review of the use of the ketogenic diet in childhood epilepsy. Pediatr Neurol. 2006;35(1):1–5. doi: 10.1016/j.pediatrneurol.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Henderson CB, Filloux FM, Alder SC, Lyon JL, Caplin DA. Efficacy of the ketogenic diet as a treatment option for epilepsy: meta-analysis. J Child Neurol. 2006;21(3):193–198. doi: 10.2310/7010.2006.00044. [DOI] [PubMed] [Google Scholar]
- 38.Groesbeck DK, Bluml RM, Kossoff EH. Long-term use of the ketogenic diet: outcomes of 28 children with over 6 years diet duration. Neurology. 2006;66(5):A41–A41. doi: 10.1017/S0012162206002143. [DOI] [PubMed] [Google Scholar]
- 39.Groesbeck DK, Bluml RM, Kossoff EH. Long-term use of the ketogenic diet in the treatment of epilepsy. Dev Med Child Neurol. 2006;48(12):978–981. doi: 10.1017/S0012162206002143. [DOI] [PubMed] [Google Scholar]
- 40.Bough KJ, Rho JM. Anticonvulsant mechanisms of the ketogenic diet. Epilepsia. 2007;48(1):43–58. doi: 10.1111/j.1528-1167.2007.00915.x. [DOI] [PubMed] [Google Scholar]
- 41.Schachter SC. Botanicals and herbs: a traditional approach to treating epilepsy. Neurotherapeutics. 2009;6(2):415–420. doi: 10.1016/j.nurt.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gayatri NA, Ferrie CD, Cross H. Corticosteroids including ACTH for childhood epilepsy other than epileptic spasms. Cochrane Database Syst Rev. 2007;1:CD005222. doi: 10.1002/14651858.CD005222.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Nolan M, Bergazar M, Chu B, Cortez MA, Snead OC., 3rd Clinical and neurophysiologic spectrum associated with atypical absence seizures in children with intractable epilepsy. J Child Neurol. 2005;20(5):404–410. doi: 10.1177/08830738050200050201. [DOI] [PubMed] [Google Scholar]
- 44.Vezzani A, Granata T. Brain inflammation in epilepsy: experimental and clinical evidence. Epilepsia. 2005;46(11):1724–1743. doi: 10.1111/j.1528-1167.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 45.Verhelst H, Boon P, Buyse G, et al. Steroids in intractable childhood epilepsy: clinical experience and review of the literature. Seizure. 2005;14(6):412–421. doi: 10.1016/j.seizure.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Gupta R, Appleton R. Corticosteroids in the management of the paediatric epilepsies. Arch Dis Child. 2005;90(4):379–384. doi: 10.1136/adc.2004.051375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Araki T, Otsubo H, Makino Y, et al. Efficacy of dexamathasone on cerebral swelling and seizures during subdural grid EEG recording in children. Epilepsia. 2006;47(1):176–180. doi: 10.1111/j.1528-1167.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 48.Cucullo L, Hallene K, Dini G, Dal Toso R, Janigro D. Glycerophosphoinositol and dexamethasone improve transendothelial electrical resistance in an in vitro study of the blood–brain barrier. Brain Res. 2004;997(2):147–151. doi: 10.1016/j.brainres.2003.09.079. [DOI] [PubMed] [Google Scholar]
- 49.van Vliet EA, da Costa-Araujo S, Redeker S, van Schaik R, Aronica E, Gorter JA. Blood–brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130(Pt 2):521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- 50.Seiffert E, Dreier JP, Ivens S, et al. Lasting blood–brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 2004;24(36):7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomkins O, Kaufer D, Korn A, et al. Frequent blood–brain barrier disruption in the human cerebral cortex. Cell Mol Neurobiol. 2001;21(6):675–691. doi: 10.1023/A:1015147920283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marchi N, Angelov L, Masaryk T, et al. Seizure-promoting effect of blood–brain barrier disruption. Epilepsia. 2007;48(4):732–742. doi: 10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marchi N, Oby E, Fernandez N, et al. In vivo and in vitro effects of pilocarpine: relevance to epileptogenesis. Epilepsia. 2007;48(10):1934–1946. doi: 10.1111/j.1528-1167.2007.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fabene PF, Navarro MG, Martinello M, et al. A role for leukocyte–endothelial adhesion mechanisms in epilepsy. Nat Med. 2008;14(12):1377–1383. doi: 10.1038/nm.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vezzani A, Janigro D. Inflammation. In: Engel G, Pedley TA, editors. Epilepsy: a Comprehensive Textbook. Lippincott, Williams and Wilkins; PA, USA: 2008. pp. 267–276. [Google Scholar]
- 56.Marchi N, Batra A, Fan Q, et al. Antagonism of peripheral inflammation prevents status epilepticus. Neurobiol Dis. 2009;33(2):171–271. doi: 10.1016/j.nbd.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cavalheiro EA, Naffah-Mazzacoratti MG, Mello LE, Leite JP. The pilocarpine model of seizures. In: Pitkanen A, Mosh'e SL, Schwartzkroin PA, editors. Models of Seizures and Epilepsy. Elsevier; NY, USA: 2006. pp. 443–446. [Google Scholar]
- 58.Marchi N, Betto G, Fazio V, et al. Blood–brain barrier damage and brain penetration of antiepileptic drugs: role of serum proteins and brain edema. Epilepsia. 2009;50(4):664–677. doi: 10.1111/j.1528-1167.2008.01989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005;6(8):591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- 60.Hoffmeyer S, Burk O, von Richter O, et al. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA. 2000;97(7):3473–3478. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siddiqui A, Kerb R, Weale ME, et al. Association of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1. N Engl J Med. 2003;348(15):1442–1448. doi: 10.1056/NEJMoa021986. [DOI] [PubMed] [Google Scholar]
- 62.Bournissen FG, Moretti ME, Juurlink DN, Koren G, Walker M, Finkelstein Y. Polymorphism of the MDR1/ABCB1 C3435T drug-transporter and resistance to anticonvulsant drugs: a meta-analysis. Epilepsia. 2008;50(4):898–903. doi: 10.1111/j.1528-1167.2008.01858.x. [DOI] [PubMed] [Google Scholar]
- 63.Oby E, Janigro D. The blood–brain barrier and epilepsy. Epilepsia. 2006;47(11):1761–1774. doi: 10.1111/j.1528-1167.2006.00817.x. [DOI] [PubMed] [Google Scholar]
- 64.Loscher W, Potschka H. Role of multidrug transporters in pharmacoresistance to antiepileptic drugs. J Pharmacol Exp Ther. 2002;301(1):7–14. doi: 10.1124/jpet.301.1.7. [DOI] [PubMed] [Google Scholar]
- 65.Perucca E. Current trends in antiepileptic drug therapy. Epilepsia. 2003;44(Suppl. 4):41–47. doi: 10.1046/j.1528-1157.44.s4.1.x. [DOI] [PubMed] [Google Scholar]
- 66.Perucca E. An introduction to antiepileptic drugs. Epilepsia. 2005;46(Suppl. 4):31–37. doi: 10.1111/j.1528-1167.2005.463007.x. [DOI] [PubMed] [Google Scholar]
- 67.Sisodiya SM. Malformations of cortical development: burdens and insights from important causes of human epilepsy. Lancet Neurol. 2004;3(1):29–38. doi: 10.1016/s1474-4422(03)00620-3. [DOI] [PubMed] [Google Scholar]
- 68.Vreugdenhil M, Wadman WJ. Modulation of sodium currents in rat CA1 neurons by carbamazepine and valproate after kindling epileptogenesis. Epilepsia. 1999;40(11):1512–1522. doi: 10.1111/j.1528-1157.1999.tb02034.x. [DOI] [PubMed] [Google Scholar]
- 69.Remy S, Urban BW, Elger CE, Beck H. Anticonvulsant pharmacology of voltage-gated Na+ channels in hippocampal neurons of control and chronically epileptic rats. Eur J Neurosci. 2003;17(12):2648–2658. doi: 10.1046/j.1460-9568.2003.02710.x. [DOI] [PubMed] [Google Scholar]
- 70.Remy S, Gabriel S, Urban BW, et al. A novel mechanism underlying drug resistance in chronic epilepsy. Ann Neurol. 2003;53(4):469–479. doi: 10.1002/ana.10473. [DOI] [PubMed] [Google Scholar]
- 71.Ragozzino D, Palma E, Di Angelantonio S, et al. Rundown of GABA type A receptors is a dysfunction associated with human drug-resistant mesial temporal lobe epilepsy. Proc Natl Acad Sci USA. 2005;102(42):15219–15223. doi: 10.1073/pnas.0507339102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oby E, Caccia S, Vezzani A, et al. In vitro responsiveness of human-drug-resistant tissue to antiepileptic drugs: insights into the mechanisms of pharmacoresistance. Brain Res. 2006;1086(1):201–213. doi: 10.1016/j.brainres.2006.02.068. [DOI] [PubMed] [Google Scholar]
- 73.Loscher W, Klotz U, Zimprich F, Schmidt D. The clinical impact of pharmacogenetics on the treatment of epilepsy. Epilepsia. 2009;50(1):1–23. doi: 10.1111/j.1528-1167.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- 74.Schmidt D, Loscher W. New developments in antiepileptic drug resistance: an integrative view. Epilepsy Curr. 2009;9(2):47–52. doi: 10.1111/j.1535-7511.2008.01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dombrowski S, Desai S, Marroni M, et al. Overexpression of multiple drug resistance genes in endothelial cells from patients with refractory epilepsy. Epilepsia. 2001;42(12):1504–1507. doi: 10.1046/j.1528-1157.2001.12301.x. [DOI] [PubMed] [Google Scholar]
- 76.Aronica E, Gorter JA, Ramkema M, et al. Expression and cellular distribution of multidrug resistance-related proteins in the hippocampus of patients with mesial temporal lobe epilepsy. Epilepsia. 2004;45(5):441–451. doi: 10.1111/j.0013-9580.2004.57703.x. [DOI] [PubMed] [Google Scholar]
- 77.Aronica E, Gorter JA, Redeker S, et al. Localization of breast cancer resistance protein (BCRP) in microvessel endothelium of human control and epileptic brain. Epilepsia. 2005;46(6):849–857. doi: 10.1111/j.1528-1167.2005.66604.x. [DOI] [PubMed] [Google Scholar]
- 78.Sisodiya SM, Beck H, Loescher W, Vezzani A. Mechanisms of Drug Resistance. In: Engel G, Pedley TA, editors. Epilepsy: a Comprehensive Textbook. Lippincott Williams and Wilkins; PA, USA: 2008. pp. 1279–1290. [Google Scholar]
- 79.Marchi N, Guiso G, Caccia S, et al. Determinants of drug brain uptake in a rat model of seizure-associated malformations of cortical development. Neurobiol Dis. 2006;24(3):429–442. doi: 10.1016/j.nbd.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 80.Pusztai L, Wagner P, Ibrahim N, et al. Phase II study of tariquidar, a selective P-glycoprotein inhibitor, in patients with chemotherapy-resistant, advanced breast carcinoma. Cancer. 2005;104(4):682–691. doi: 10.1002/cncr.21227. [DOI] [PubMed] [Google Scholar]
- 81.Kerb R, Hoffmeyer S, Brinkmann U. ABC drug transporters: hereditary polymorphisms and pharmacological impact in MDR1, MRP1 and MRP2. Pharmacogenomics. 2001;2(1):51–64. doi: 10.1517/14622416.2.1.51. [DOI] [PubMed] [Google Scholar]
- 82.Granata T, Obino L, Ragona F, et al. Steroid treatment is effective in the treatement of status epilepticus in children. Program and Abstracts of the 62nd Annual Meeting of the American Epilepsy Society; Seattle, WA, USA. Dec, 2008. pp. 5–9. Abstract 8853. [Google Scholar]
- 83.Takano A, Kusuhara H, Suhara T, et al. Evaluation of in vivo P-glycoprotein function at the blood–brain barrier among MDR1 gene polymorphisms by using C-11-verapamil. J Nucl Med. 2006;47(9):1427–1433. [PubMed] [Google Scholar]
Websites
- 101.Evaluating the transporter protein inhibitor probenecid in patients with epilepsy. http://clinicaltrials.gov/ct2/show/NCT00610532?term=epilepsy&recr=Open&rank=59.
- 102.P-Glycoprotein Inhibition as Adjunct Treatment for Medically Refractory Epilepsy. http://clinicaltrials.gov/ct2/show/NCT00524134.
- 103.Verapamil and Catamenial Epilepsy. http://clinicaltrials.gov/ct2/show/NCT00559169?term=epilepsy&recr=Open&rank=21.