Abstract
Black cohosh (Actaea racemosa L. [syn. Cimifuga racemosa L.]) extracts (BCE) are marketed worldwide for the management of menopausal symptoms. However, recently more than 75 cases of hepatotoxicity associated with black cohosh ingestion have been reported. While these cases have not been fully substantiated for causality, the data suggest that herb-drug interactions may be involved rather than a direct hepatotoxic event. This work describes the in vitro inhibition of four CYP450 enzymes (1A2, 2D6, 2C9, 3A4) by black cohosh extracts and identifies the active inhibitory constituents. Ethanol extracts (75 and 80% ethanol) and a 40% isopropanol extract induced a concentration-dependent inhibition of all CYP450 isozyme activities, with median inhibitory concentrations (IC50) ranging from 21.9 μg/ml to 65.0 μg/ml. Isolation of the active chemical constituents, showed that the triterpene glycosides were weakly active (IC50 25-100 μM), while fukinolic acid and cimicifugic acids A and B strongly inhibited all CYP isozymes (IC50 1.8-12.6 μM). None of the extracts inhibited the growth of Hep-G2 cells in concentrations up to 50 μg/ml. These data suggest that BCEs are not directly hepatotoxic, but may have the potential to induce herb-drug interactions, which may in turn explain the rare cases of hepatotoxicity observed in women using multiple medications and dietary supplements, including black cohosh.
Keywords: Black cohosh, cytochrome P450, hepatotoxicity, herb-drug interactions, menopause
Introduction
Menopause is a universal female experience associated with disturbing symptoms such as hot flashes, night sweats, vaginal dryness, and irritability [1-5]. In addition to these acute symptoms, the incidence of chronic disease, such as cardiovascular disease, osteoporosis, and dementia are all significantly increased in the post-menopausal period [1-3]. Hormone therapy (HT; estrogen and/or progesterone) remains the gold standard for the symptomatic treatment of menopause, although since the publication of the Women's Health Initiative, many women are searching for alternative treatments for menopause, including herbal medicines [1, 3, 5]. One herbal therapy for the management of menopausal symptoms is black cohosh, known scientifically as Actaea racemosa L. [3, 5-9].
Black cohosh is a coarse perennial woodland herb with large compound leaves, and a thick, knotted rhizome (root) system [3, 5]. The plant is native to North America, with a distribution from southern Canada to Georgia. There are numerous vernacular (common) names for this plant, including black snakeroot, black root, bugbane, rattle root, rattle top, rattle squawroot, and rattle-weed. Historically, black cohosh rhizomes (roots) were used as a medicine by Native Americans (Penobscot, Winnebago and Dakota), for the treatment of coughs, colds, constipation, fatigue, and rheumatism, as well as to increase breast milk production [3, 6-8], The therapeutic use of black cohosh for these conditions has not been scientifically investigated. In 1832, a tincture of black cohosh rhizome was used for the treatment of pain and inflammation associated with endometriosis, rheumatism, neuralgia and dysmenorrhea, but again there are no clinical data to support these uses [3, 5]. More recently, extracts of black cohosh have been marketed worldwide for the management of menopausal symptoms [3-9]. The clinical efficacy of these products for the treatment of vasomotor symptoms associated with menopause has been repeatedly tested in over 15 clinical trials [reviewed in 10, 11], with positive results reported in most studies [10, 11], however the two most recent clinical trials were negative [12, 13].
Published clinical data for black cohosh shown few adverse events [3, 5-7, 10-13], including the newest trials [12, 13]. In fact, one of the most recent studies, reported no significant differences between the black cohosh treatments and placebo for any of the assessed safety parameters which included breast and endometrial safety, assessment of liver enzymes, complete blood count, or lipid profiles [12]. In particular, there was no evidence for hepatotoxicity of black cohosh during the 12-month intervention [12]. Thus, it is surprising that over the past seven years approximately 78 cases of hepatotoxicity associated with the ingestion of black cohosh containing products have been reported in the literature, as well as to regulatory agencies worldwide [8, 9]. These cases ranged in severity from minor elevations in liver enzymes to liver failure resulting in transplantation and a few deaths [8, 9]. However, as is often with dietary supplements all of the cases were poorly documented, and confounding variables included failure to identify the BC product; use of herbal mixtures with multiple ingredients in addition to BC; co-medication with synthetic drugs and dietary supplements including herbal ones; concomitant missing temporal association between BC use and development of liver disease; not specified modalities of BC treatment; failure of de-challenge after BC discontinuation; pre-existing liver diseases; insufficiently excluded other liver diseases and the possible presence of alternative liver diseases [8, 9].
Black cohosh preparations ranked eighth of all herbal supplements sold in mainstream retail outlets in 2004 [14]. However, total retail sales of black cohosh in all channels of trade are difficult to estimate, but may be as high as $76 million (data from 2003) [14]. Considering the millions of doses of black cohosh that are used by women worldwide, the cases of hepatotoxicity are a rare occurrence, but still of significant concern. While these cases have not been fully substantiated for causality, there is a possibility that an herb-drug interaction may be involved rather than direct hepatotoxic effects due to the fact that many of these women used multiple drug and dietary supplements product and alcohol concomitantly [8, 9].
Cytochrome P450 is a superfamily of hemoproteins (heme-containing monooxygenases) of which there are approximately 50 known CYP enzymes in the human body [15-16]. Cytochrome P450 (P450 or CYP) is involved in drug metabolism, and can be divided into many subfamilies, e.g. CYP1A2, 2C9, 2C19, 2D6, 2E1, 3A4, each of which is linked to a different set of drugs, and numerous reviews have been published on this topic [15-16]. Studies of the biochemical and enzymatic properties of these enzymes and activity have greatly enhanced the understanding of several aspects of clinical pharmacology such as pharmacokinetic variability, drug toxicity, and drug interactions. Three CYP families, namely CYP1, CYP2 and CYP3 are responsible for approximately 80% of hepatic drug metabolism, carcinogenesis and degradation of xenobiotics [16]. Thus, modulation of isozymes in all three of these subfamilies would indicate significant potential for herb-drug interactions and present the possibility for drug-induced hepatotoxicity. In vitro evidence has suggested that botanical dietary supplements, including black cohosh, may modulate the activity and expression of CYP isozymes [15, 17]. However, the in vivo findings in humans have shown weak or equivocal results [18-20].
This work reports the in vitro inhibition of the activities of CYP1A2, 2C9, 2D6, and 3A4 isozymes by three clinically relevant extracts of black cohosh. The active constituents responsible for these activities were identified using bioassay-guided fractionation. Furthermore, the work also assessed the effects of black cohosh extracts and pure compounds on the growth of human hepatocellular carcinoma (Hep-G2) cells in vitro.
Materials and Methods
Chemicals and Reagents
HPLC grade solvents acetonitrile and methanol (Fisher Scientific, Itasca, IL) were used for sample preparation and HPLC analysis. Reagents were obtained from Fisher Scientific and Sigma Chemical Co. (St. Louis, MO).
Plant Materials and Extracts
Black cohosh (Actaea racemosa L. syn. Cimicifuga racemosa) roots/rhizomes were obtained from PureWorld Botanicals, Inc. (South Hackensack, NJ) and Chromadex (Santa Ana, CA). A dried 40% isopropanol extract was also prepared and dissolved in DMSO. This extract is standardized to contain 1 mg of triterpene glycosides calculated as 23-epi-26-deoxyactein per 20 mg of extract. For the other two extracts, the dried, milled, rhizomes of A. racemosa (1 kg) were exhaustively extracted with 80% or 75% ethanol, and the triterpene glycoside concentrations were determined by HPLC based on the protocols described [21, 22]. Pure actein (compound 1) was isolated as colorless needles (MW 676, C37H56O11); 23-epi-26-deoxyactein (compound 2) was also isolated as colorless needles (MW 660, C37H56O10); and cimiracemoside A (MW 632; C36H56O9) was also isolated. On the basis of 1H NMR spectra and LC/MS/MS, three triterpene glycosides were confirmed as cimiracemoside A; 23-epi-26-deoxyactein, and actein. The fukinolic acid and the cimicifugic acids A and B were isolated and identified as previously described [23] using bioassay guided fractionation. Individual purified triterpene glycosides from black cohosh for HPLC standards were purchased from ChromaDex, Inc. (Santa Ana, CA).
CYP Isozyme Bioassays
The extracts and pure compounds were screened for their ability to inhibit the activities of CYP1A2, 2C9, 2D6, and 3A4 isozymes using a rapid high-throughput in vitro fluorometric microtiter plate assay as previously described [24, 25]. The enzymes were obtained from human recombinant insect Sf9 cells and assays for the activities of four principal human cytochromes P450 metabolizing enzymes, CYP1A2, CYP2C9, CYP2D6, and CYP3A4 were performed. Two direct fluorometric assays were used. For CYP1A2, CYP2C9, and CYP2D6, 3-cyano-7-ethoxycoumarin (CEC) (White) was used as the substrate. The CEC substrate concentration for CYP2C9 was 25 μM, for CYP1A2, 5 μM was used and for CYP2C9 the CRC concentration used was 50 μM. Resorufin benzyl ether [BzRes (Burke)] was used as the substrate for CYP3A4 at a concentration of 50 μM [24]. The assays were conducted in 96-well microtiter plates and the substrates were prepared as homogenous suspensions in pH 7.5 buffer (potassium phosphate 75 mM) by sonication. All extracts were dissolved in either methanol or DMSO. Final solvent was less than 1%. Control wells contained vehicle solvent or sterile distilled water and NADPH (×-nicotinamide adenine dinucleotide phosphate, reduced form; Sigma Chemical Co., St. Louis, MO) solution; blank wells contained distilled water or vehicle solvent and buffer solution (1:4.7 v/v mixture of 75 mM potassium phosphate in DW, pH 7.5); test wells consisted of extract or pure compound and NADPH solution. Test-blank wells consisted of the corresponding extract, pure compound and buffer solution. After substrate and inhibitor addition the plates were pre-warmed to 37°C. Incubations were initiated by the addition of 0.1 ml of pre-warmed enzyme and cofactors. Final incubation volume was 0.2 ml. The concentrations of enzymes (baculovirus/insect cell-expressed human cytochromes P450) and co-factors were as described [25]. Incubations were carried out for 30 min (CYP1A2 and CYP3A4) or 45 min (CYP2C9 and CYP2D6) and stopped by the addition of 0.1 ml of 60% acetonitrile, 40% 0.1 M Tris, pH 9. Metabolite formation was linear for these incubation times. Fluorescence per well was measured using a Synergy HT multi-detection microplate reader (Biotek, Winooski VT). The CEC metabolite, 3-cyano-7-hydroxycoumarin, was measured using an excitation wavelength of 420 nm (50-nm bandwidth) and emission wavelength of 485 nm (20-nm bandwidth). The BzRes metabolite, resorufin, was measured using an excitation wavelength of 530 nm (25-nm bandwidth) and emission wavelength of 590 nm (35-nm bandwidth). Detection of the products of either assay was linear over the range used for these assays. Data were exported and analyzed using an Excel spread sheet and IC50 values were calculated by linear interpolation. For CYP1A2 the reference inhibitor compound used was furafylline, for CYP2C9 with inhibitor control was sulfaphenazole, for CYP2D6, quinidine was used and for CYP3A4 ketoconazole was the control compound. Each assay was repeated in triplicate. Median inhibitory concentrations (IC50) were only determined for extracts showing >50% inhibition. For IC50 determinations, the concentration range of each extract used was 1-250 μg/ml [25]. Extracts inducing >50% inhibition at a concentration of 50 μg/ml were further assessed for IC50s. Only data sets yielding the highest readings without saturation were used to calculate the percent inhibition. The resultant percent inhibition calculations based on the mathematical combinations for the differences in fluorescence between the test/test-blank wells and the mean difference between each control and blank well. Control reference compounds were run with every assay and median inhibitory concentrations for the reference compounds were obtained.
Cell Cultures
Human hepatocellular liver carcinoma cells (Hep-G2 cells (ATCC, Manassus, VA) were maintained in RPMI 1640 (Gibco BRL) containing 10% FBS (Gibco BRL), 2 mg/ml sodium bicarbonate, 1% penicillin sodium salt and 1% streptomycin sulfate [26]. All extracts and compounds were dissolved in DMSO at a concentration range of 1-100 μg/ml, and diluted in tissue culture medium before use. Cells were plated in 96-well microassay culture plates and grown in RPMI 1640 medium with 10% FBS for 24 h prior to treatment. Tested compounds were then added to the wells to achieve final concentrations ranging from 1 to 100 μg/ml. After treatment, all the cultures were incubated at 37°C, 5% CO2 for 48 h followed by the MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] assay as described [26].
MTT Cytotoxicity Assay
Cells were harvested in the exponential phase from culture flask with 0.25% trypsin/0.1% EDTA (Sigma-Aldrich Chemical, St. Louis, MO). Cells were washed, centrifuged, and counted via hemocytometer and viability determined by 0.5% trypan blue vital dye exclusion. Only harvested aliquots showing greater than 90% viability were used in these studies. Cells were transferred to flat-bottom 96-well microtiter plates (Costar) at a concentration of 50,000 cells per well in 0.1 ml and cultured as described above. Preliminary studies demonstrated that this concentration of cells provided appropriate confluence at the end of the assay and provided an optimal MTT absorbance value. The cells were allowed to adhere for 24 hours in 5% CO2 at 37°C. Medium was then replaced and various concentrations of plant extracts were added and allowed to incubate for 72 hours. Cell viability was then determined for each concentration of extract tested by the colorimetric assay described [26] The yellow tetrazolium salt MTT (Sigma-Aldrich Chemical, St. Louis, MO) is converted into a purple formazan product by living cells in direct proportion to cell number and activity [26] Briefly, 5 μl of a 10 mg/ml solution of MTT in phosphate-buffered saline (0.01 mol/L of PO4, 0.85% NaCl, at pH of 7.4 was added to each well and incubated for 4 hours at 37°C. After the incubation period, 100 μL of acid isopropanol (0.04N HCl) was added to each well to dissolve the dark blue crystals. The absorbance of each well was measured by a multiple scanning spectrophotometer (Power Wave 200, Bio-Tek Instruments) at 570 nm, with a reference wavelength of 630 nm. Percent viability was determined by (absorbance of extract)/(control cells alone) × 100, where (absorbance of extract) = mean absorbance of extract wells at a given concentration and (control cells alone) = mean absorbance of control wells. Data are means from three replicate wells and three separate experiments. Microscopic inspection of wells revealed that decreased absorbance values from control wells correlated with decreased cell number and not with diminished metabolic rate [26].
Statistical Analysis
The values for the enzyme activities were compared by analysis of variance (ANOVA) followed by Dunnett's test. Statistically significant differences were determined at p ≤ 0.05.
Results
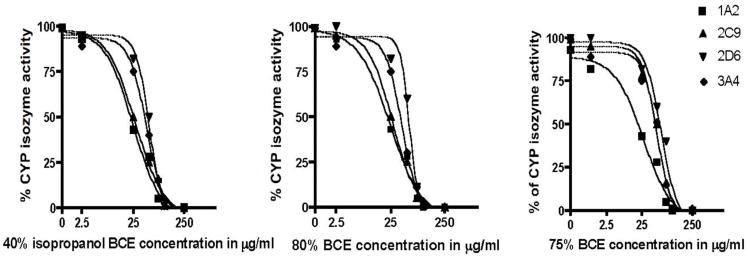
All three black cohosh extracts (BCEs) inhibited the activities of the four CYP isozymes in a concentration-dependent manner (Fig. 1). The median inhibitory concentrations (IC50s) of the three BCEs ranged from 21.9 to 75 μg/ml (Table 1). The 40% isopropanol extract was slightly more active than either the 75 or 80% ethanol extracts for all CYP isozymes tested, however these results were not statistically significant. Inhibitor control compounds were within normal concentration limits for each of the CYP assays (Table 2) [27-31]. The isolated triterpene glycosides cimiracemoside A, 23-epi-26-deoxyactein, and actein inhibited the activities of CYP2C9 and CYP3A4, with an IC50 range of 16.9-33.8 μM. None of the triterpene glycosides tested inhibited CYP1A2 at concentrations up to 100 μM. Jiang et al. [21] reported that the total percentage of the triterpene glycosides and the phenolic constituents in a 75% ethanol and 40% isopropanol extracts of black cohosh roots and rhizomes ranged from 6.9 to 10.3% for the triterpene glycosides and from 5.2 to 5.8% for the phenolic constituents [21]. Thus, the observation that the three BCEs inhibited all four CYP isozymes similarly, may be explained by the chemistry of the extracts.
Fig. 1.
Concentration-dependent inhibition of CYP1A2, 2D6, 2C9 and 3A4 by a 40% isopropanol, 75% and 80% ethanol extracts of black cohosh rhizomes.
Table 1. Median Inhibitory Concentrations (IC50) for Human Recombinant CYPs for Black Cohosh (BC) Extracts (μg/ml) and Pure Compounds (μM).
| Plant Extract | CYP1A2 | CYP3A4 | CYP2C9 | CYP2D6 |
|---|---|---|---|---|
| BC-80% ethanol | 31.4 | 45.8 | 35.5 | 75.0 |
| BC-75% ethanol | 38.8 | 48.1 | 50.0 | 76.7 |
| BC-40% isopropanol | 21.2 | 35.3 | 23.6 | 48.2 |
| CR-A1 | na | 51.3 | 40.5 | 32.2 |
| Actein | na | 35.2 | 25.0 | 51.1 |
| Deoyxactein2 | na | 28.7 | 25.9 | 100.0 |
| Fukinolic acid | 1.8 | 7.2 | 7.1 | 5.4 |
| CAA3 | 7.2 | 9.7 | 8.3 | 9.0 |
| CAB4 | 7.35 | 9.8 | 12.5 | 12.6 |
Cimiracemoside A.
Deoxyactein = 23-epi-26-deoxyactein.
CAA = Cimicifugic acid A.
CAB = Cimicifugic acid B.
na: not active at concentrations of 1-100 μg/ml; each IC50 concentration is the result of three experiments in triplicate.
Table 2. Median Inhibitory Concentrations of Reference Inhibitor Compounds for CYP 1A2, 2C9, 2D6, and 3A4 Isozyme Assays.
| CYP Isozyme | Reference Compound | IC50 μM | S.D. | Literature |
|---|---|---|---|---|
| 1A2 | Furafylline | 0.96 | ±0.06 | 0.5-6.0 μM [27] |
| 2C9 | Sulfaphenazole | 0.31 | ±0.04 | 0.14-0.7 μM [28] |
| 2D6 | Quindine | 0.04 | ±0.002 | 20-150 nM [29, 30] |
| 3A4 | Ketoconazole | 0.036 | ±0.004 | 0.07-8.5 μM [31] |
For CYP 1A2 the reference inhibitor compound used was furafylline, for CYP 2C9 with inhibitor control was sulfaphenazole, for CYP 2D6, quinidine was used and for CYP 3A4 ketoconazole was the control compound. The IC50 obtained for each of the control compounds (known inhibitors) were within the limits published in the scientific literature. Each assay was repeated in triplicate.
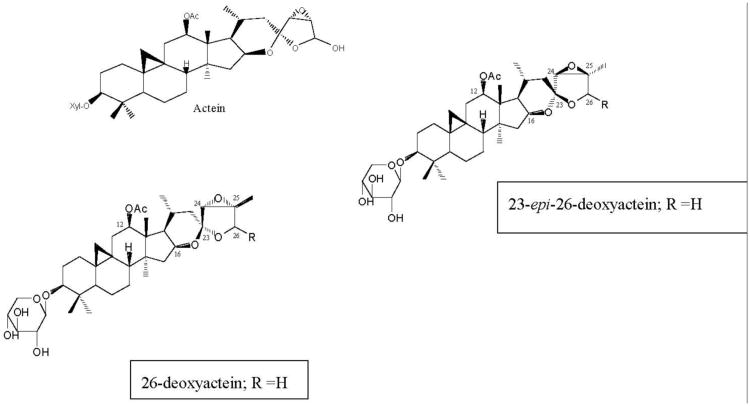
The triterpene glycosides (TGs; Fig. 2) had previously been isolated as the active constituents of black cohosh rhizomes [21, 22], and were thought to be the active constituents that inhibit the activity of CYP3A4 in vitro. However, the data presented here demonstrate that the TGs at best, weakly inhibit the activities of CYP isozymes, and only at higher concentrations. In addition, the TGs had no effect on CYP1A2 at concentrations up to 100 μM.
Fig. 2.
Chemical structures of the triterpene glycosides isolated from black cohosh root extracts with inhibitory activities in CYP 2D6, 2C9 and 3A4 bioassays.
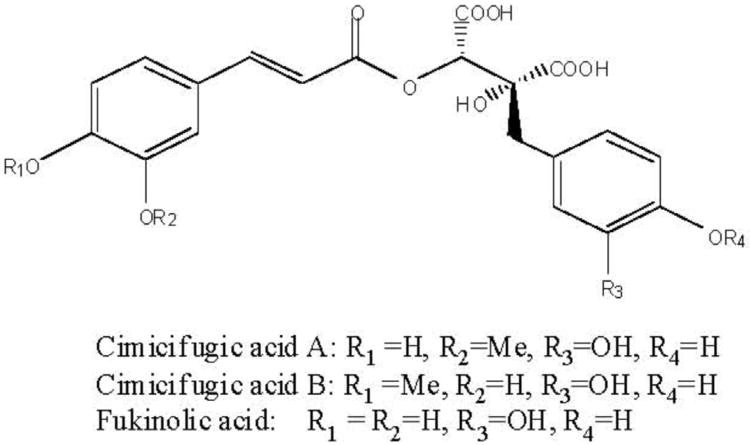
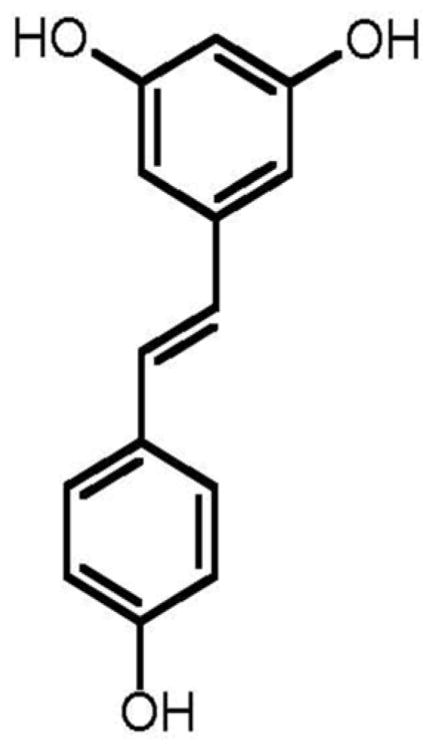
The three phenolic constituents fukinolic acid, and its derivatives cimicifugic acids A and B (Fig. 3), were significantly (p < 0.05) more active than any of the TGs tested, and inhibited all four CYP isozymes with an IC50 range of 1.8-12.6 μM. All three of these compounds inhibited the activity of CYP 1A2 in vitro at very low concentrations (IC501.8-7.2 μM), with fukinolic acid being the most active inhibitor of CYP1A2 (IC50 1.8 μM). Interestingly, resveratrol (Fig. 4), a naturally occurring stilbene with a chemical structure similar to fukinolic acid has demonstrated similar activity with an inhibitory IC50 1.89 μM in CYP 1A2 [27].
Fig. 3.
Chemical structures of fukinolic acid and cimicifugic acids A and B isolated from black cohosh root extracts with inhibitory activities in CYP 1A2, 2D6, 2C9 and 3A4 bioassays.
Fig. 4.
Resveratrol, a naturally occurring stilbene from red wine.
None of the extracts exhibited cytotoxicity in Hep-G2 cells up to concentrations of 50 μg/ml, with > 90% viability at this concentration. At higher concentrations (>100 μg/ml) all three extracts of black cohosh inhibited cell proliferation by 89-100%.
Discussion
In the past few years, there have been approximately 78 case reports suggesting that administration of black cohosh extracts may cause hepatoxicity, in the form of severe acute hepatitis and fulminant liver failure [8, 9]. These case reports of hepatotoxicity are disturbing and inexplicable, as black cohosh has been used in over 2800 patients in human studies and clinical trials with a low incidence of side effects, and previously had no history of hepatoxic effects [5-7, 8-9, 12-13]. Even the two most recent NIH-funded clinical trials report no serious adverse events [12, 13]. Previous in vitro testing of black cohosh extracts reported no effect on rat hepatocytes or HepG2 cells [32]. Lude et al. [33] demonstrated that BCEs only have a direct cytotoxic effects in HepG2 cells at concentrations of 75 mg/ml or higher. The results presented here confirm these data, as none of the extracts or pure compounds were cytotoxic in Hep-G2 cells at concentrations up to 50 μg/ml. Furthermore, Lude et al. [33] also assessed microvesicular steatosis of the liver in rats treated with black cohosh and found that a very high dose of 1000 mg extract per kg body weight was needed to observe any liver toxicity. Thus, the probability that BCEs, are directly hepatotoxic appears remote, when these products are used alone in normal therapeutic doses.
However, the other possible explanation for these case reports may be due to an interaction of black cohosh extracts with the drug metabolizing CYP isozymes, especially 3A4 and 2D6. In fact, in most of the rare cases of heptotoxicity associated with black cohosh were observed in menopausal women using black cohosh containing supplements in combination with other herbals, alcohol and/or prescription medications [8, 9]. In this work, three extracts of black cohosh inhibited the CYP isozymes 1A2, 2C9, 2D6 and 3A4 in vitro, in a concentration-dependent manner. Interestingly, the 40% isopropanol extract was slightly more active than the other two extracts, particularly against the 1A2 isozyme. However, these results were not statistically significant. Bioassay guided fractionation lead to the isolation of three triterpene glycosides (TGs), namely actein, 23-epi-26-deoxyactein and cimiracemoside A. All three compounds inhibited isozymes 2C9, 2D6 and 3A4 but did not inhibit CYP1A2 in concentrations up to 100 μM.
Previously, Tsukamoto et al., [32] had identified six TGs, including 26-deoxyactein, as inhibitors of CYP3A4. The reported IC50 for this compound was 100 μM, although the IC50 for their black cohosh extract was 27 μM, suggesting that 26-deoxyactein was not the active constituent [32]. The data from this work support the results of Tsukamoto et al. [32], as the IC50s for the three black cohosh extracts tested ranged from 35-48 μg/ml. Furthermore, we have identified 23-epi-26-deoxyactein (Fig. 2), an isomer of 26-deoxyactein [22], that differs in structure only in the steriochemistry at C 23 position (Fig. 2), as the most active TG, with an IC50 28.7 μM, which is similar to the activity of the extract. The average daily dose of a standardized black cohosh extract is 40-80 mg, and the total triterpene glycoside concentration of the extracts ranges between 2.5-10%. Thus, even at the highest concentration of 10%, a daily dose of 80 mg would deliver only 8 mg of total triterpene glycosides, and after absorption and distribution in the body, it would be difficult to achieve sufficiently high enough concentrations of TGs to inhibit the CYP isozymes. A daily dose of 25 to 50 times higher would be needed to achieve concentrations of TGs sufficient to inhibit the activities of the CYP isozymes, if this would even be possible.
Our results further demonstrate that the phenolic constituents fukinolic acid, cimicifugic acids A and B are significantly more active than the TGs against all CYP isozymes tested, and particularly inhibited CYP1A2 at very low concentrations indicating that they are more likely to be the CYP inhibitors of black cohosh extracts. Fukinolic acid and cimicifugic acids A and B were isolated from an n-butanol soluble fraction of a black cohosh extracts that exhibited significant binding to the serotonin receptor 7 [34], and it has been suggested that black cohosh extracts may have a serotonergic mechanism of action [35]. It is well known that modulators of the serotonin system such as some serotonin reuptake inhibitors (SSRIs), as well as serotonin and norepinephrine reuptake inhibitors (SNRIs) inhibit CYP isozymes, including CYP3A4, 1A2 and 2D6 [36]. Thus, it is possible that fukinolic acid and cimicifugic acids A and B may inhibit the CYP isozyme activities with a mechanism similar to these drugs.
While black cohosh extracts appear to inhibit the CYP isozymes in vitro, corroboration of the in vivo results have been equivocal in healthy young human subjects [18-20]. Gurley and co-workers have performed extensive in vivo studies addressing the effects of various dietary supplements on CYP activities in humans [18-20]. Oral administration of a black cohosh root extract (1090 mg twice daily standardized to 0.2% triterpene glycosides) to 12 young healthy volunteers for 28 days did not produce significant changes in phenotypic markers of CYP1A2, CYP2D6, and CYP3A4, however a small (∼7%) but significant inhibition of CYP2D6 was observed [18]. In the second study, involving 16 healthy young subjects (8 female), oral administration of 40 mg twice daily of a black cohosh extract (standardized to containing 0.2% triterpene glycosides) for 14 days exerted no significant effects on CYP2D6 activity [20]. No clinically relevant effect on CYP3A4 activity was observed in 19 healthy young subjects (9 females) administered an isopropanol extract of black cohosh standardized to 2.5% triterpene glycosides at a daily dose of 80 mg for 14 days [19]. Thus, in healthy young subjects of both sexes, the impact of 14-28 days of therapeutic doses of black cohosh extracts for 14-28 days on CYP isozymes appears to be minimal.
However, the adverse events reports associating the ingestion of black cohosh containing supplements with hepatotoxicity have been observed in women of peri- to post-menopausal age (39-72 years old) [8, 9]. Many of the affected women used the black cohosh containing products in therapeutic doses for longer than one month; many had multiple chronic diseases, and used multiple medications, as well as alcohol concomitantly [8, 9]. Drug-induced hepatotoxicity is most frequently observed in older women, with more than 80% of cases in women with an average age of 45-60 years [37]. The varied manifestations of liver injury include steatosis, acute and chronic hepatitis, hepatic fibrosis, zonal or diffuse hepatic necrosis, bile duct injury, veno-occlusive disease, and acute liver failure requiring liver transplantation [8, 9, 38]. Therefore, while the human data for black cohosh shows equivocal or no effect in healthy young subjects (including women), no studies have investigated the effects of black cohosh on CYP-450 isozymes in older menopausal women. It is recognized that the activities of human CYP-450 isozymes may be affected by gender, and hormonal factors such as the menopausal [39-41]. Thus, investigations using healthy subjects while informative do not reflect the true clinical situation in menopausal women with multiple pathologies using multiple prescription medications, supplements and alcohol. Thus, it would be beneficial to test black cohosh supplements in this specific population to determine effects on CYPP450.
Black cohosh is an herbal medicine that has been used in the United States for over 100 years and is currently being used by women worldwide for the management of menopausal symptoms. The ever-expanding case reports of hepatotoxicity associated with BCEs even when used in recommended doses are inexplicable, but need to be seriously addressed considering the number of women using these products. Our data suggest that compounds in BCEs may inhibit CYP450 isozymes, although the concentration of the compounds needed to inhibit CYP isozymes in vivo would be 25 to 50 times a normal daily dose. Extrapolation of in vitro data to determine the safety of an herbal product based is limited as the bioavailability and distribution of the isolated compounds in the body is currently not well understood. Data suggesting that BCEs inhibit the proliferation of Hep-G2 cells at higher concentrations may imply that users of BCEs exposed to common hepatotoxins such as alcohol and medications deleterious hepatotoxic effects may not be able to repair any hepatic injury, thereby exacerbating any limited hepatoxicity. Therefore, new human studies involving menopausal women using normal standards of care, clinically relevant BCEs, and perhaps in combination with other drugs or alcohol would be clinically significant.
As part of a previously published review [8], the United the United States Dietary Supplement Information Expert Committee has recommended that black cohosh products should be labeled to include a cautionary statement [8]. The cautionary statement: “Discontinue use and consult a healthcare practitioner if you have a liver disorder or develop symptoms of liver trouble, such as abdominal pain, dark urine, or jaundice”, was put in place to alert consumers and healthcare professionals to pay close attention to minimize potential risk [8].
Acknowledgments
We would like to specifically thank Dr. Guido Pauli, CBDSR-UIC for the kind provision of a purified sample of fukinolic acid. This work was made possible by a grant AT02381-02 from NCCAM/NIH to GBM. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM or NIH.
References
- 1.Guay MP, Dragomir A, Pilon D, Moride Y, Perreault S. Changes in pattern of use, clinical characteristics and persistence rate of hormone replacement therapy among postmenopausal women after the WHI publication. Pharmacoepidemiol Drug Saf. 2007;16:17–27. doi: 10.1002/pds.1273. [DOI] [PubMed] [Google Scholar]
- 2.Houmard BS, Seifer DB. Predicting the Onset of Menopause. In: Seifer DB, Kennard EA, editors. Menopause: Endocrinology and Management. Totwa, New Jersey: Humana Press; 1999. pp. 1–19. [Google Scholar]
- 3.Doyle BJ, Mahady GB. Phytotherapies for menopause. Drugs of the Future. 2007;32:897–905. [Google Scholar]
- 4.Locklear TD, Doyle BJ, Caceres A, Perez A, Mahady GB. Menopause a universal female experience: lessons from central america. Current Reviews in Women's Health. 2007;4:1–10. [Google Scholar]
- 5.Mahady GB. Black cohosh (Actaea racemosa): review of the clinical data for safety and efficacy in menopausal symptoms. Treat Endocrinol. 2005;4:177–84. doi: 10.2165/00024677-200504030-00006. [DOI] [PubMed] [Google Scholar]
- 6.Kronenberg F, Fugh-Berman A. Complementary and alternative medicine for menopausal symptoms: a review of randomized, controlled trials. Ann Intern Med. 2002;137:805–13. doi: 10.7326/0003-4819-137-10-200211190-00009. [DOI] [PubMed] [Google Scholar]
- 7.Low Dog T, Powell KL, Weisman SM. Critical evaluation of the safety of Cimicifuga racemosa in menopause symptom relief. Menopause. 2003;10:299–313. doi: 10.1097/01.GME.0000056039.51813.21. [DOI] [PubMed] [Google Scholar]
- 8.Mahady GB, Low Dog T, Barrett ML, et al. United States Pharmacopoeia review of the black cohosh case reports of hepatoxicity. Menopause. 2008;15:628–38. doi: 10.1097/gme.0b013e31816054bf. [DOI] [PubMed] [Google Scholar]
- 9.Teschke R, Bahre R, Genthner A, Fuchs J, Schmidt-Taenzer W, Wolff A. Suspected black cohosh hepatotoxicity--challenges and pitfalls of causality assessment. Maturitas. 2009;63:302–14. doi: 10.1016/j.maturitas.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Borrelli F, Ernst E. Black cohosh (Cimicifuga racemosa) for menopausal symptoms: a systematic review of its efficacy. Pharmacol Res. 2008;58:8–14. doi: 10.1016/j.phrs.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Palacio C, Masri G, Mooradian AD. Black cohosh for the management of menopausal symptoms: a systematic review of clinical trials. Drugs Aging. 2009;26:23–36. doi: 10.2165/0002512-200926010-00002. [DOI] [PubMed] [Google Scholar]
- 12.Geller SE, Shulman LP, van Breemen RB, et al. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: a randomized controlled trial. Menopause. 2009;16:1156–66. doi: 10.1097/gme.0b013e3181ace49b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newton KM, Reed SD, LaCroix AZ, Grothaus LC, Ehrlich K, Guiltinan J. Treatment of vasomotor symptoms of menopause with black cohosh, multibotanicals soy hormone therapy, or placebo: a randomized trial. Ann Intern Med. 2006;145:869–79. doi: 10.7326/0003-4819-145-12-200612190-00003. [DOI] [PubMed] [Google Scholar]
- 14.Prendy ML, De Angelis P, Chamberlain JL. Gen Tec Rep SRS–97. Asheville, NC: U.S. Department of Agriculture Forest Service Southern Research Station; 2006. Black cohosh Actaea racemosa: an annotated bibliography; pp. 97pp. 1–99. [Google Scholar]
- 15.Strandell J, Neil A, Carlin G. An approach to the in vitro evaluation of potential for cytochrome P450 enzyme inhibition from herbals and other natural remedies. Phytomed. 2004;11:98–104. doi: 10.1078/0944-7113-00379. [DOI] [PubMed] [Google Scholar]
- 16.Venkatakrishnan K, Von Moltke LL, Greenblatt DJ. Human drug metabolism and the cytochromes P450: application and relevance of in vitro models. J Clin Pharmacol. 2001;41:1149–79. doi: 10.1177/00912700122012724. [DOI] [PubMed] [Google Scholar]
- 17.Gaube F, Wolfl S, Pusch L, Kroll TC, Hamburger M. Gene expression profiling reveals effects of Cimicifuga racemosa (L.) NUTT (black cohosh) on the estrogen receptor positive human breast cancer cell line MCF-7. BMC Pharmacol. 2007;7:11–21. doi: 10.1186/1471-2210-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurley BJ, Gardner SF, Hubbard MA, et al. In vivo effects of goldenseal, kava kava, black cohosh, and valerian on human cytochrome P450 1A2, 2D6, 2E1, and 3A4/5 phenotypes. Clin Pharmacol Ther. 2005;77:415–26. doi: 10.1016/j.clpt.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurley B, Hubbard MA, Williams DK, et al. Assessing the clinical significance of botanical supplementation on human cytochrome P450 3A activity: comparison of a milk thistle and black cohosh product to rifampin and clarithromycin. J Clin Pharmaco. 2006;46:201–13. doi: 10.1177/0091270005284854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurley BJ, Swain A, Hubbard MA, et al. Clinical assessment of CYP2D6-mediated herb-drug interactions in humans: effects of milk thistle, black cohosh, goldenseal, kava kava, St John's wort, and Echinacea. Mol Nutr Food Res. 2008;52:755–63. doi: 10.1002/mnfr.200600300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang B, Kronenberg F, Nuntanakorn P, Qiu MH, Kennelly EJ. Evaluation of the botanical authenticity and phytochemical profile of black cohosh products by high-performance liquid chromatography with selected ion monitoring liquid chromatography-mass spectrometry. J Agric Food Chem. 2006;54:3242–53. doi: 10.1021/jf0606149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen SN, Li W, Fabricant DS, et al. Isolation, structure elucidation, and absolute configuration of 26-deoxyactein from Cimicifuga racemosa and clarification of nomenclature associated with 27-deoxyactein. J Nat Prod. 2002;65:601–5. doi: 10.1021/np010494t. [DOI] [PubMed] [Google Scholar]
- 23.Nuntanakorn P, Jiang B, Einbond LS, et al. Polyphenolic constituents of Actaea racemosa. J Nat Prod. 2006;69:314–8. doi: 10.1021/np0501031. [DOI] [PubMed] [Google Scholar]
- 24.Foster BC, Vandenhoek S, Hana J, et al. In vitro inhibition of human cytochrome P450-mediated metabolism of marker substrates by natural products. Phytomedicine. 2003;10:334–42. doi: 10.1078/094471103322004839. [DOI] [PubMed] [Google Scholar]
- 25.Crespi CL, Miller VP, Penman BW. Microtiter plate assays for inhibition of human drug metabolizing cytochromes P450. Anal Biochem. 1997;248:188–90. doi: 10.1006/abio.1997.2145. [DOI] [PubMed] [Google Scholar]
- 26.Mosmann T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 27.Yueh MF, Kawahara M, Raucy J. Cell-based high-throughput bioassays to assess induction and inhibition of CYP1A enzymes. Toxicol In Vitro. 2005;19:275–87. doi: 10.1016/j.tiv.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Baldwin SJ, Bloomer JC, Smith GJ, Ayrton AD, Clarke SE, Chenery RJ. Ketoconazole and sulphaphenazole as the respective selective inhibitors of P4503A and 2C9. Xenobiotica. 1995;25:261–7. doi: 10.3109/00498259509061850. [DOI] [PubMed] [Google Scholar]
- 29.Bourrié M, Meunier V, Berger Y, Fabre G. Cytochrome P450 isoform inhibitors as a tool for the investigation of metabolic reactions catalyzed by human liver microsomes. J Pharmacol Exp Ther. 1996;277:321–32. [PubMed] [Google Scholar]
- 30.Masubuchi: Masubuchi Y, Hosokawa S, et al. Cytochrome P450 isozymes involved in propranolol metabolism in human liver microsomes The role of CYP2D6 as ring-hydroxylase and CYP1A2 as N-desisopropylase. Drug Metab Dispos. 1994;22:909–15. [PubMed] [Google Scholar]
- 31.Yin H, Racha J, Li SY, Olejnik N, Satoh H, Moore D. Automated high throughput human CYP isoform activity assay using SPE-LC/MS method: application in CYP inhibition evaluation. Xenobiotica. 2000;30:141–54. doi: 10.1080/004982500237749. [DOI] [PubMed] [Google Scholar]
- 32.Tsukamoto S, Aburatani M, Ohta T. Isolation of CYP3A4 Inhibitors from the Black Cohosh (Cimicifuga racemosa) Evid Based Complement Alternat Med. 2005;2:223–6. doi: 10.1093/ecam/neh086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lüde S, Török M, Dieterle S, et al. Hepatic effects of Cimicifuga racemosa extract in vivo and in vitro. Cell Mol Life Sci. 2007;64:2848–57. doi: 10.1007/s00018-007-7368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fabricant DS, Nikolic D, Lankin DC, Chen SN, Jaki BU, Krunic A, van Breemen RB, Fong HH, Farnsworth NR, Pauli GF. Cimipronidine, a cyclic guanidine alkaloid from Cimicifuga racemosa. J Nat Prod. 2005;68:1266–7. doi: 10.1021/np050066d. [DOI] [PubMed] [Google Scholar]
- 35.Burdette JE, Liu J, Chen SN, et al. Black cohosh acts as a mixed competitive ligand and partial agonist of the serotonin receptor. J Agric Food Chem. 2003;51:5661–70. doi: 10.1021/jf034264r. [DOI] [PubMed] [Google Scholar]
- 36.Spina E, Santoro V, D'Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30:1206–27. doi: 10.1016/s0149-2918(08)80047-1. [DOI] [PubMed] [Google Scholar]
- 37.Stedman C. Herbal hepatotoxicity. Semin Liver Dis. 2002;22:195–206. doi: 10.1055/s-2002-30104. [DOI] [PubMed] [Google Scholar]
- 38.Chitturi S, Farrell GC. Herbal hepatotoxicity: an expanding but poorly defined problem. J Gastroenterol Hepatol. 2000;15:1093–99. doi: 10.1046/j.1440-1746.2000.02349.x. [DOI] [PubMed] [Google Scholar]
- 39.Harris RZ, Benet LZ, Schwartz JB. Gender effects in pharmacokinetics and pharmacodynamics. Drugs. 1995;50:222–39. doi: 10.2165/00003495-199550020-00003. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka E. Gender-related differences in pharmacokinetics and their clinical significance. J Clin Pharm Ther. 1999;24:339–46. doi: 10.1046/j.1365-2710.1999.00246.x. [DOI] [PubMed] [Google Scholar]
- 41.Scandlyn MJ, Stuart EC, Rosengren RJ. Sex-specific differences in CYP450 isoforms in humans. Expert Opin Drug Metab Toxicol. 2008;4:413–24. doi: 10.1517/17425255.4.4.413. [DOI] [PubMed] [Google Scholar]