Abstract
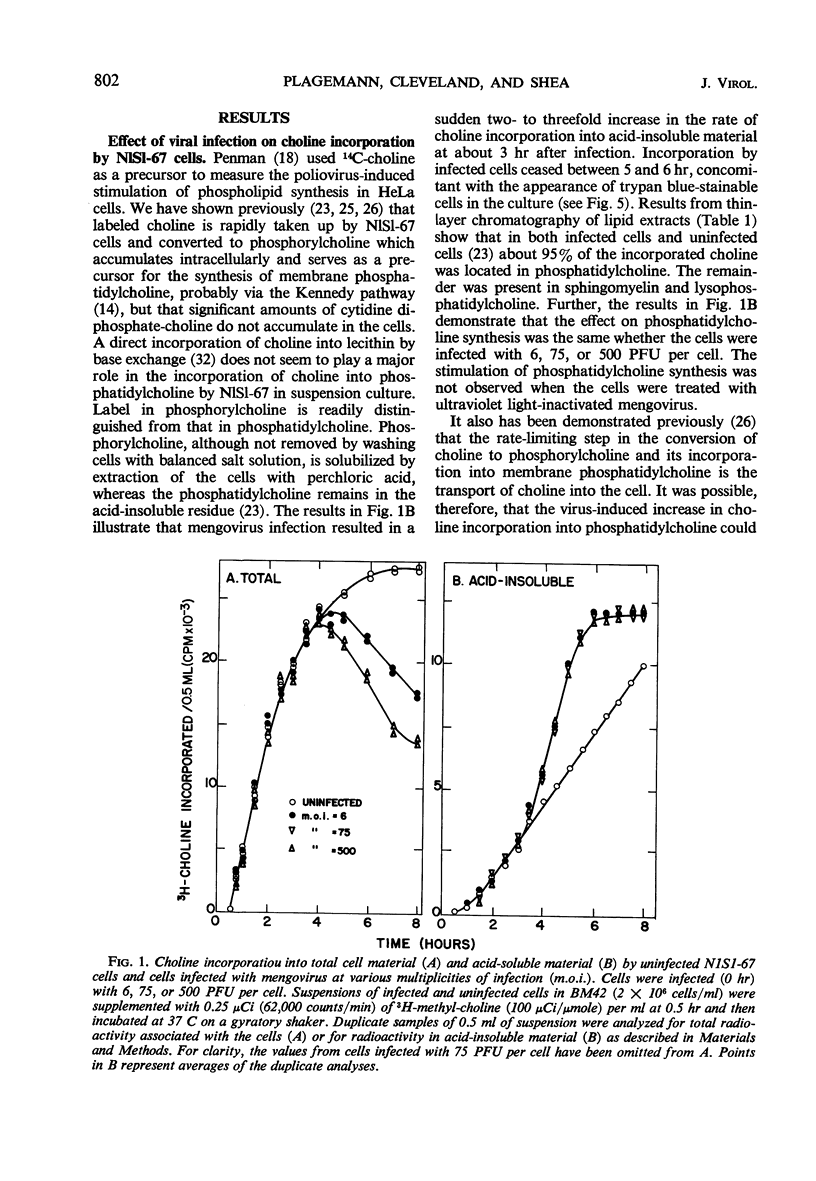
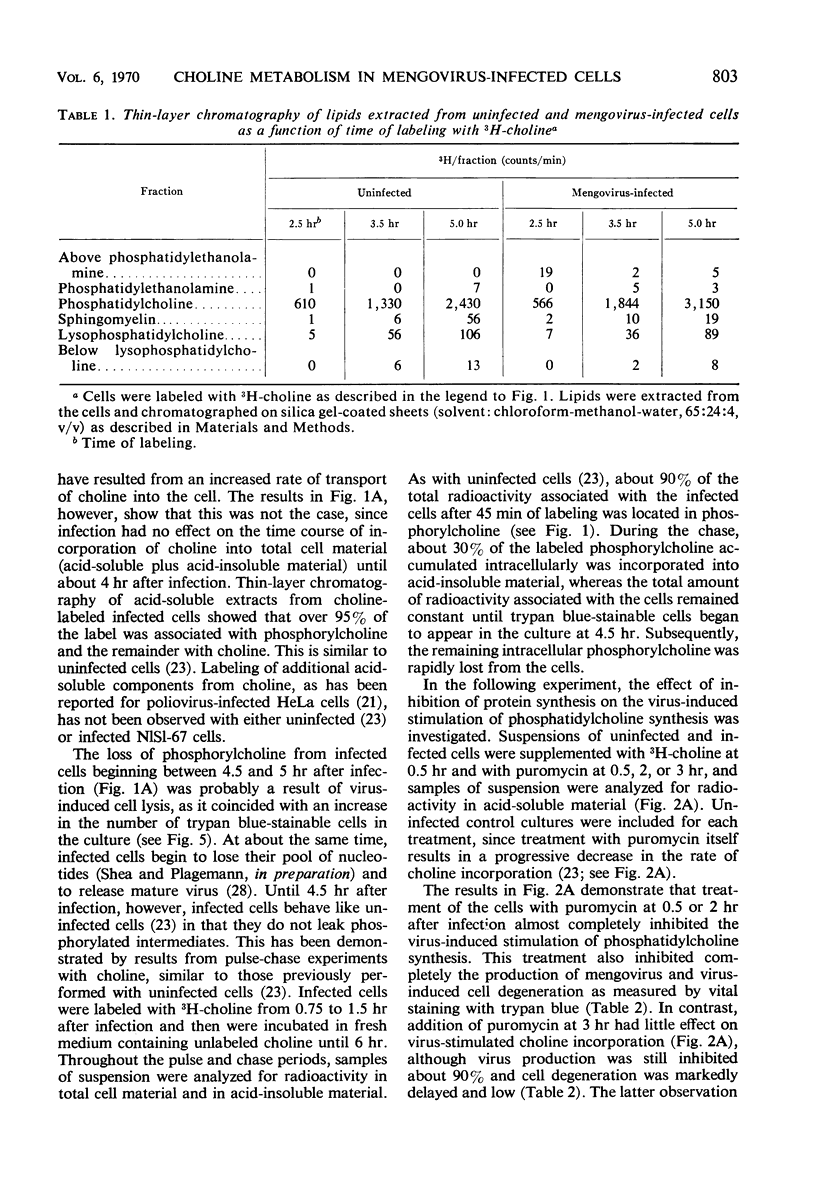
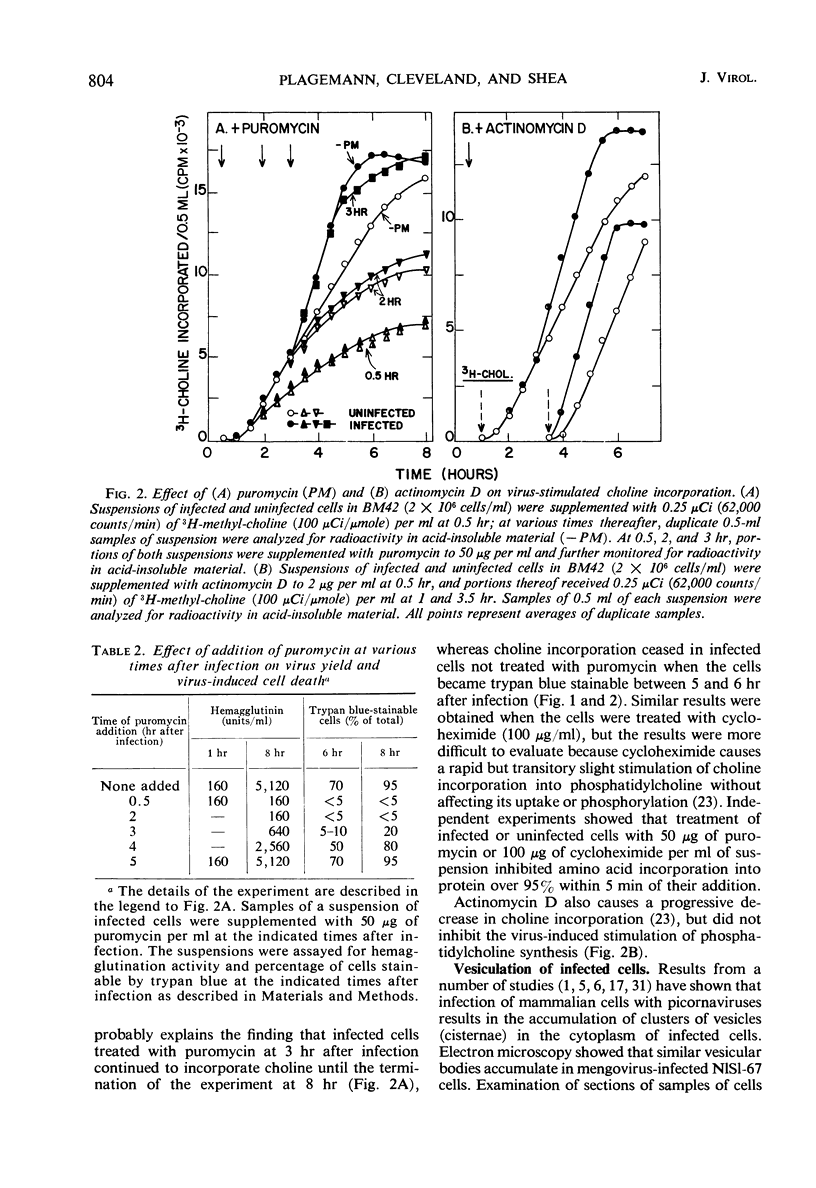
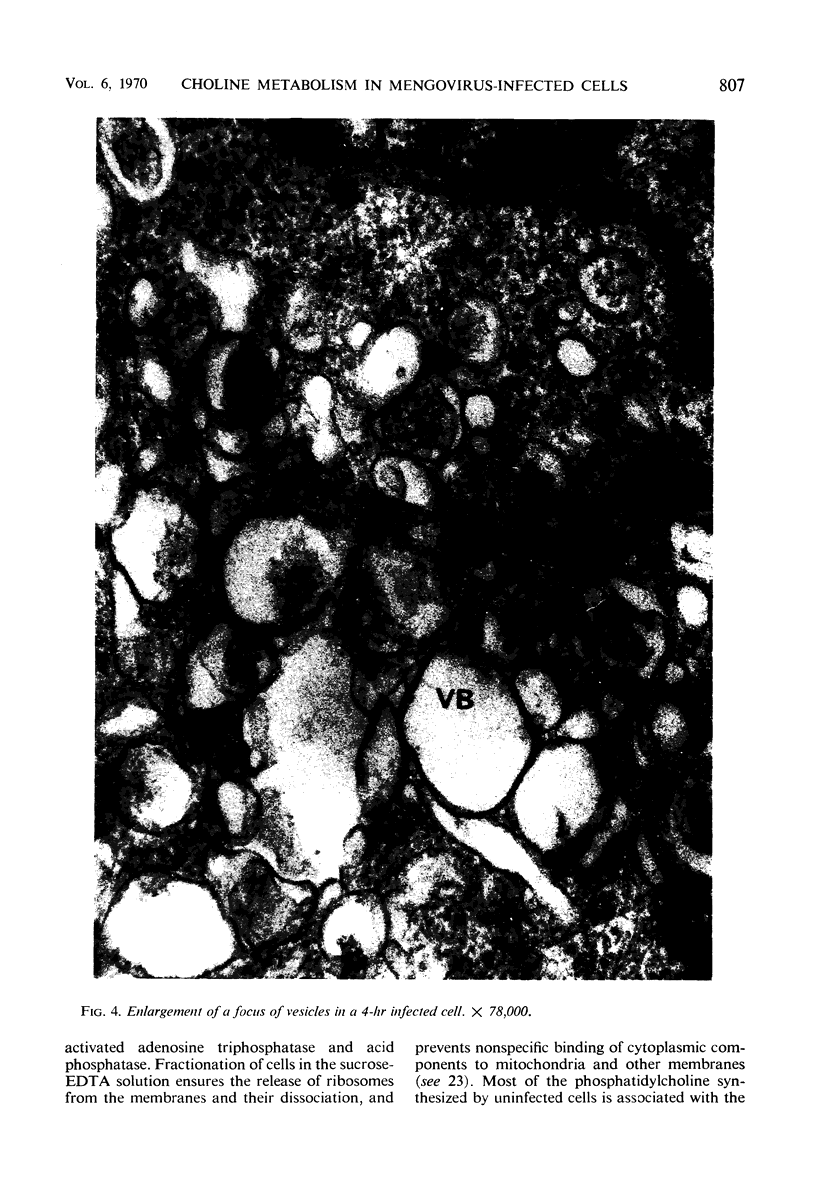
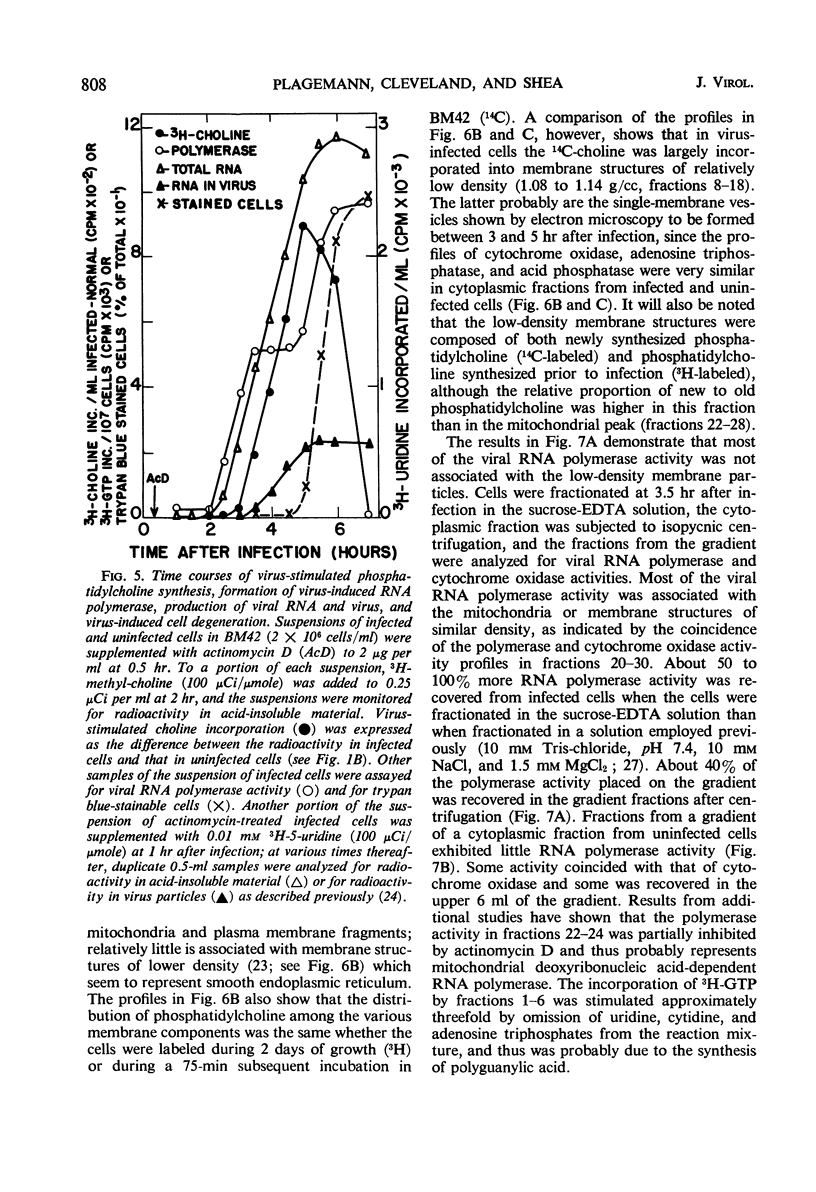
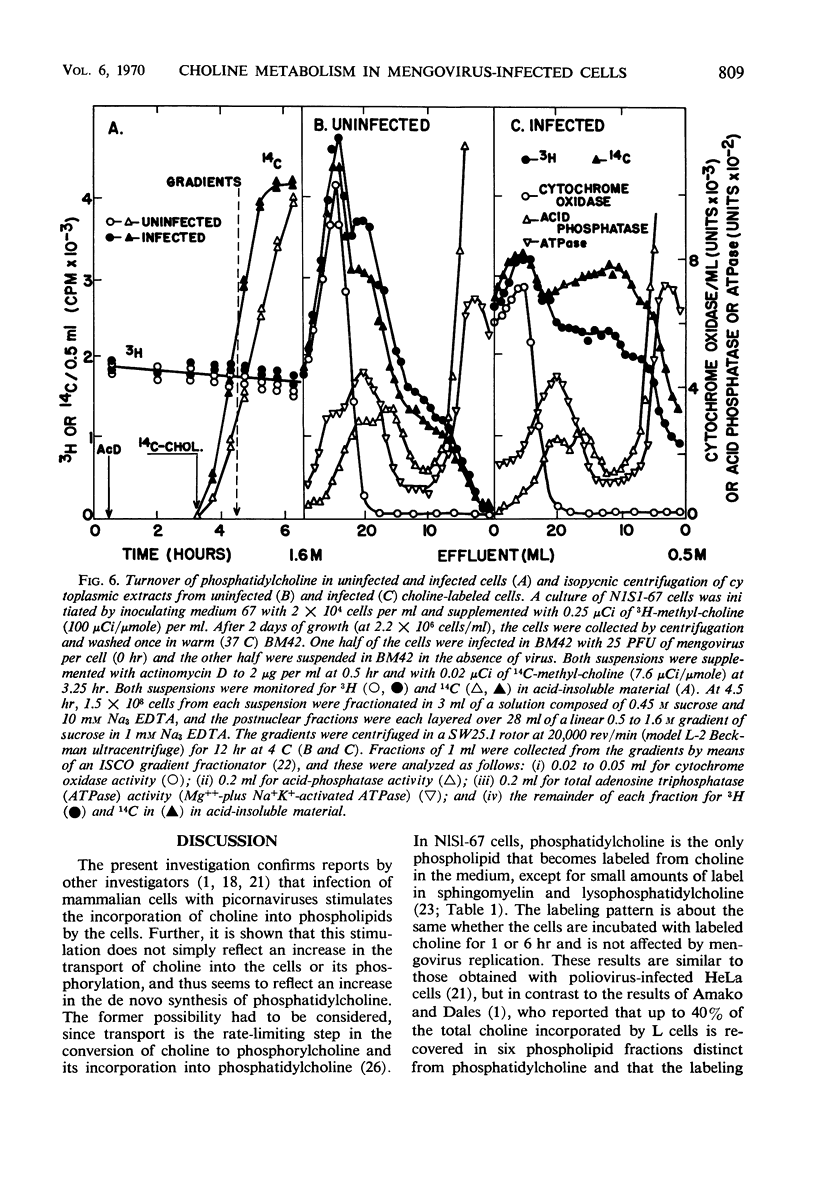
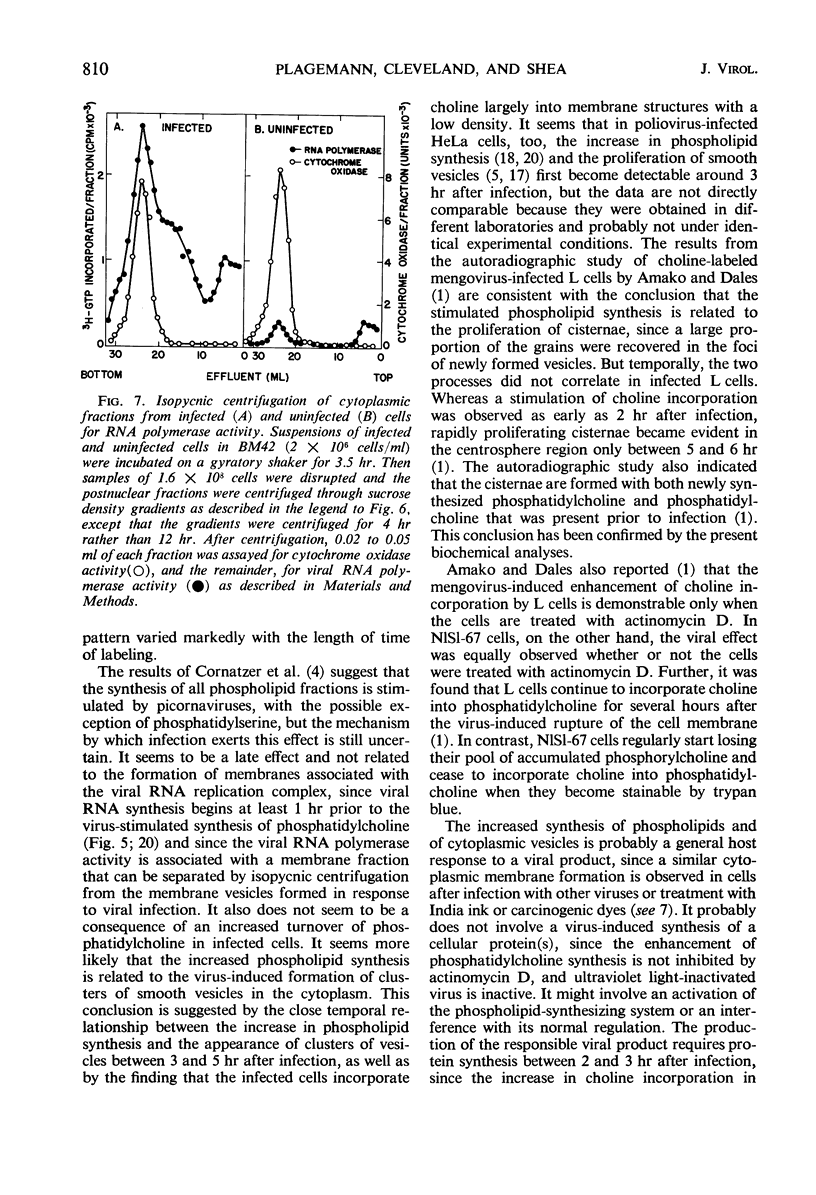
Infection of Novikoff rat hepatoma cells (subline NlSL-67) with mengovirus resulted in a two- to threefold increase in the rate of choline incorporation into membrane phosphatidylcholine at about 3 hr after infection, without affecting the rate of transport of choline into the cell or its phosphorylation. The time course of virus-stimulated phosphatidylcholine synthesis was compared with the time courses of other virus-induced processes during a single cycle of replication. The formation of viral ribonucleic acid (RNA) polymerase and of viral RNA commenced about 1 hr earlier than the virus-stimulated choline incorporation. Further, isopycnic centrifugation of cytoplasmic extracts indicated that the excess of phosphatidylcholine synthesized by infected cells is not located in the membrane structures associated with the viral RNA replication complex, but with structures of a lower density (1.08 to 1.14 g/cc). These membrane structures probably represent the smooth vesicles which accumulate in the cytoplasm of infected cells during the period of increased phosphatidylcholine synthesis between 3 and 5 hr after infection. They are formed with both newly synthesized phosphatidylcholine and phosphatidylcholine present prior to infection. However, concomitant protein synthesis is not required for the stimulated synthesis of membranes; the effect was not inhibited by treating the cells with inhibitors of protein synthesis at 3 hr after infection, although virus production was inhibited about 90% and virus-induced cell degeneration was markedly reduced and delayed. Production of mature virus began normally at about the same time as the stimulation of phosphatidylcholine synthesis. Treatment of infected cells with puromycin at 2 hr, on the other hand, completely inhibited the stimulation of phosphatidylcholine synthesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amako K., Dales S. Cytopathology of Mengovirus infection. II. Proliferation of membranous cisternae. Virology. 1967 Jun;32(2):201–215. doi: 10.1016/0042-6822(67)90270-x. [DOI] [PubMed] [Google Scholar]
- Arlinghaus R. B., Polatnick J. Detergent-solubilized RNA polymerase from cells infected with foot-and-mouth disease virus. Science. 1967 Dec 8;158(3806):1320–1322. doi: 10.1126/science.158.3806.1320. [DOI] [PubMed] [Google Scholar]
- BALTIMORE D. IN VITRO SYNTHESIS OF VIRAL RNA BY THE POLIOVIRUS RNA POLYMERASE. Proc Natl Acad Sci U S A. 1964 Mar;51:450–456. doi: 10.1073/pnas.51.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORNATZER W. E., SANDSTROM W., FISCHER R. G. The effect of poliomyelitis virus type I (Mahoney strain) on the phospholipid metabolism of the HeLa cell. Biochim Biophys Acta. 1961 May 13;49:414–415. doi: 10.1016/0006-3002(61)90151-2. [DOI] [PubMed] [Google Scholar]
- DALES S., EGGERS H. J., TAMM I., PALADE G. E. ELECTRON MICROSCOPIC STUDY OF THE FORMATION OF POLIOVIRUS. Virology. 1965 Jul;26:379–389. doi: 10.1016/0042-6822(65)90001-2. [DOI] [PubMed] [Google Scholar]
- DALES S., FRANKLIN R. M. A comparison of the changes in fine structure of L cells during single cycles of viral multiplication, following their infection with the viruses of Mengo and encephalomyocarditis. J Cell Biol. 1962 Aug;14:281–302. doi: 10.1083/jcb.14.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S., SIMINOVITCH L. The development of vaccinia virus in Earle's L strain cells as examined by electron microscopy. J Biophys Biochem Cytol. 1961 Aug;10:475–503. doi: 10.1083/jcb.10.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALGARNO L., MARTIN E. M. STUDIES ON EMC VIRAL RNA SYNTHESIS AND ITS LOCALIZATION IN INFECTED KREBS ASCITES CELLS. Virology. 1965 Jul;26:450–465. doi: 10.1016/0042-6822(65)90008-5. [DOI] [PubMed] [Google Scholar]
- DARNELL J. E., Jr, LEVINTOW L. Poliovirus protein: source of amino acids and time course of synthesis. J Biol Chem. 1960 Jan;235:74–77. [PubMed] [Google Scholar]
- Ehrenfeld E., Maizel J. V., Summers D. F. Soluble RNA polymerase complex from poliovirus-infected HeLa cells. Virology. 1970 Apr;40(4):840–846. doi: 10.1016/0042-6822(70)90129-7. [DOI] [PubMed] [Google Scholar]
- FRANKLIN R. M., BALTIMORE D. Patterns of macromolecular synthesis in normal and virus-infected mammalian cells. Cold Spring Harb Symp Quant Biol. 1962;27:175–198. doi: 10.1101/sqb.1962.027.001.019. [DOI] [PubMed] [Google Scholar]
- HAUSEN P., VERWOERD D. W. STUDIES ON THE MULTIPLICATION OF A MEMBER OF THE COLUMBIA SK GROUP (ME VIRUS) IN L CELLS. III. ALTERATION OF RNA AND PROTEIN SYNTHETIC PATTERNS IN VIRUS-INFECTED CELLS. Virology. 1963 Dec;21:617–627. doi: 10.1016/0042-6822(63)90235-6. [DOI] [PubMed] [Google Scholar]
- KENNEDY E. P. Metabolism of lipides. Annu Rev Biochem. 1957;26:119–148. doi: 10.1146/annurev.bi.26.070157.001003. [DOI] [PubMed] [Google Scholar]
- MARTIN E. M., WORK T. S. Studies on protein and nucleic acid metabolism in virus-infected mammalian cells. 5. The kinetics of synthesis of virus protein and of virus ribonucleic acid in Krebs II mouse-ascites-tumour cells infected with encephalomyocarditis virus. Biochem J. 1962 Jun;83:574–582. doi: 10.1042/bj0830574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattern C. F., Daniel W. A. Replication of poliovirus in HeLa cells: electron microscopic observations. Virology. 1965 Aug;26(4):646–663. doi: 10.1016/0042-6822(65)90328-4. [DOI] [PubMed] [Google Scholar]
- PENMAN S., BECKER Y., DARNELL J. E. A CYTOPLASMIC STRUCTURE INVOLVED IN THE SYNTHESIS AND ASSEMBLY OF POLIOVIRUS COMPONENTS. J Mol Biol. 1964 Apr;8:541–555. doi: 10.1016/s0022-2836(64)80010-3. [DOI] [PubMed] [Google Scholar]
- PENMAN S. STIMULATION OF THE INCORPORATION OF CHOLINE IN POLIOVIRUS-INFECTED CELLS. Virology. 1965 Jan;25:149–152. doi: 10.1016/0042-6822(65)90263-1. [DOI] [PubMed] [Google Scholar]
- Penman S., Summers D. Effects on host cell metabolism following synchronous infection with poliovirus. Virology. 1965 Dec;27(4):614–620. doi: 10.1016/0042-6822(65)90187-x. [DOI] [PubMed] [Google Scholar]
- Philipson L., Bengtsson S., Dinter Z. The reversion of guanidine inhibition of poliovirus synthesis. Virology. 1966 Jun;29(2):317–329. doi: 10.1016/0042-6822(66)90039-0. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G. Choline metabolism and membrane formation in rat hepatoma cells grown in suspension culture. I. Incorporation of choline into phosphatidylcholine of mitochondria and other membranous structures and effect of metabolic inhibitors. Arch Biochem Biophys. 1968 Oct;128(1):70–87. doi: 10.1016/0003-9861(68)90009-x. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G. Choline metabolism and membrane formation in rat hepatoma cells grown in suspension culture. II. Phosphatidylcholine synthesis during growth cycle and fluctuation of mitochondrial density. J Cell Biol. 1969 Sep;42(3):766–781. doi: 10.1083/jcb.42.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P. G. Mengovirus replication in Novikoff rat hepatoma and mouse L cells: effects on synthesis of host-cell macromolecules and virus-specific synthesis of ribonucleic acid. J Virol. 1968 May;2(5):461–473. doi: 10.1128/jvi.2.5.461-473.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P. G. Reversible inhibition of induction of mengovirus RNA polymerase and of virus maturation in Novikoff rat hepatoma cells by phenethyl alcohol. Virology. 1968 Feb;34(2):319–330. doi: 10.1016/0042-6822(68)90242-0. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Roth M. F. Permeation as the rate-limiting step in the phosphorylation of uridine and choline and their incorporation into macromolecules by Novikoff hepatoma cells. Competitive inhibition by phenethyl alcohol, persantin, and adenosine. Biochemistry. 1969 Dec;8(12):4782–4789. doi: 10.1021/bi00840a020. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Swim H. E. Replication of mengovirus. I. Effect on synthesis of macromolecules by host cell. J Bacteriol. 1966 Jun;91(6):2317–2326. doi: 10.1128/jb.91.6.2317-2326.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P. G., Swim H. E. Symposium on replication of viral nucleic acids. 3. Replication of mengovirus ribonucleic acid. Bacteriol Rev. 1966 Jun;30(2):288–308. doi: 10.1128/br.30.2.288-308.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P. G., Swim H. E. Synthesis of ribonucleic acid by mengovirus-induced RNA polymerase in vitro: nature of products and of RNase-resistant intermediate. J Mol Biol. 1968 Jul 14;35(1):13–25. doi: 10.1016/s0022-2836(68)80034-8. [DOI] [PubMed] [Google Scholar]
- SCHARFF M. D., MAIZEL J. V., Jr, LEVINTOW L. PHYSICAL AND IMMUNOLOGICAL PROPERTIES OF A SOLUBLE PRECURSOR OF THE POLIOVIRUS CAPSID. Proc Natl Acad Sci U S A. 1964 Feb;51:329–337. doi: 10.1073/pnas.51.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M. S., Halperen S., Harkin J. C. Cytoplasmic membrane-bound vesicles in echovirus 12-infected cells. Virology. 1968 Oct;36(2):241–253. doi: 10.1016/0042-6822(68)90141-4. [DOI] [PubMed] [Google Scholar]
- Treble D. H., Frumkin S., Balint J. A., Beeler D. A. The entry of choline into lecithin, in vivo, by base exchange. Biochim Biophys Acta. 1970 Feb 10;202(1):163–171. doi: 10.1016/0005-2760(70)90227-4. [DOI] [PubMed] [Google Scholar]
- Ward G. A., Plagemann P. G. Fluctuations of DNA-dependent RNA polymerase and synthesis of macromolecules during the growth cycle of Novikoff rat hepatoma cells in suspension culture. J Cell Physiol. 1969 Jun;73(3):213–231. doi: 10.1002/jcp.1040730307. [DOI] [PubMed] [Google Scholar]