STUDY QUESTION
Could early CPAP be an alternative to routine intubation and surfactant administration in extremely preterm infants?
METHODS
Randomized multicenter controlled trial
Population
Extremely low birth weight infants (1316 infants) 24+0 to 27+6 weeks gestational age
Intervention
CPAP Group
In the delivery room, CPAP was administered by means of a T-piece resuscitator, or an equivalent device. CPAP or ventilation with positive end-expiratory pressure (PEEP) (at a recommended pressure of 5 cm of water) was used if the infant received positive pressure ventilation during resuscitation. CPAP was continued until the infant's admission to the NICU. Intubation was performed based on specific criteria.
Surfactant group
All infants in the surfactant group were to be intubated in the delivery room and were to receive surfactant within 1 hour after birth with continued ventilation thereafter. The infants were to be extubated within 24 hours after meeting specific criteria.
Outcomes
The primary outcome was death or bronchopulmonary dysplasia (physiological definition), prespecified secondary outcomes included bronchopulmonary dysplasia as defined by the receipt of any supplemental oxygen at 36 week's postmenstrual age, pneumothorax, intraventricular, hemorrhage, and the need for chest compressions or epinephrine during resuscitation.
Randomization
Randomization method details were not mentioned, and it was performed before delivery.
Allocation concealment
Performed using specially prepared double-sealed envelopes.
Blinding
Not blinded.
Completeness of follow up
Complete.
Main results
1316 infants were enrolled.
Mean gestational age 26.2 and mean birth weight 830 g in both groups.
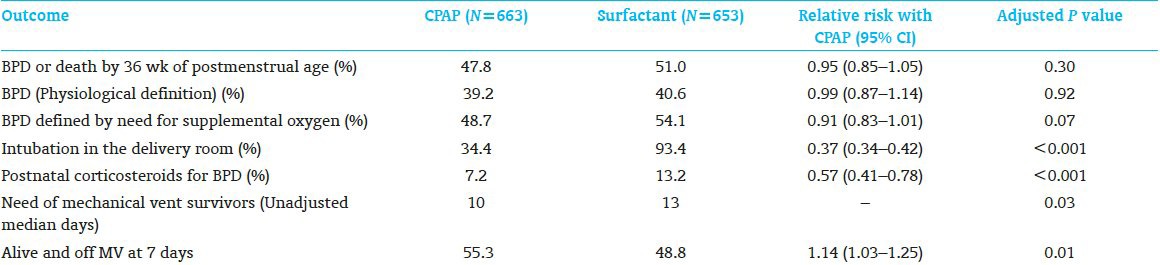
The main results are listed in the Table 1.
Table 1.
Main results
CONCLUSION
Early CPAP for the extremely low birth weight infant is associated with decreased exposure to intubation and mechanical ventilation, without any increase in measured morbidities or apparent complications.
COMMENTARY
In this study, CPAP has been shown as an acceptable alternative to intubation and surfactant therapy in extremely low weight infants. The study showed no significant difference between the two groups in the rate of death or bronchopulmonary dysplasia. Infants who received CPAP less frequently required intubation in the delivery room or postnatal corticosteroids for bronchopulmonary dysplasia compared with those receiving surfactant treatment. In addition, infants on CPAP group required less ventilation days and were more likely to be alive and free from mechanical ventilation by day 7. Interestingly, there was a high rate of intubation and surfactant treatment among infants assigned to CPAP, as well as high rate of antenatal steroids use 96%. This trial enrolment was antenatal so might not represent the populations intended to study.[1] Eligible infants were assigned to treatment irrespective of whether they were breathing spontaneously or in distress, so some infant may be intubated while they do not need to be. Compared with COIN trial,[2] this study showed no increase in the rate of air leaks by using CPAP possibility due to the use of lower CPAP 5, high rate of antenatal steroids utilization, and large proportion of infants receiving surfactant in the CPAP group (67%).
Extremely low weight infants are surfactant deficient, and neonatologists and practitioners may reasonably choose to use CPAP as an alternative to routine intubations in selected cases, i.e., centers with high rate of prenatal care, antenatal steroid use, presence of trained staff, and delivery room evaluations to identify who may need early surfactant.
Abstracted from
Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Early CPAP versus surfactant in extremely preterm infants. New Engl J Med 2010;362:1970-9.
ClinicalTrials.gov, NCT00233324
REFERENCES
- 1.Rich WD, Auten KJ, Gantz MG, Hale EC, Hensman AM, Newman NS, et al. Antenatal consent in the SUPPORT trial: Challenges, costs, and representative enrollment. Pediatrics. 2010;126:e215–21. doi: 10.1542/peds.2009-3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2008;358:700–8. doi: 10.1056/NEJMoa072788. [DOI] [PubMed] [Google Scholar]


