Abstract
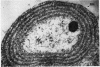
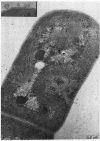
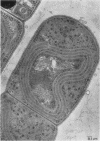
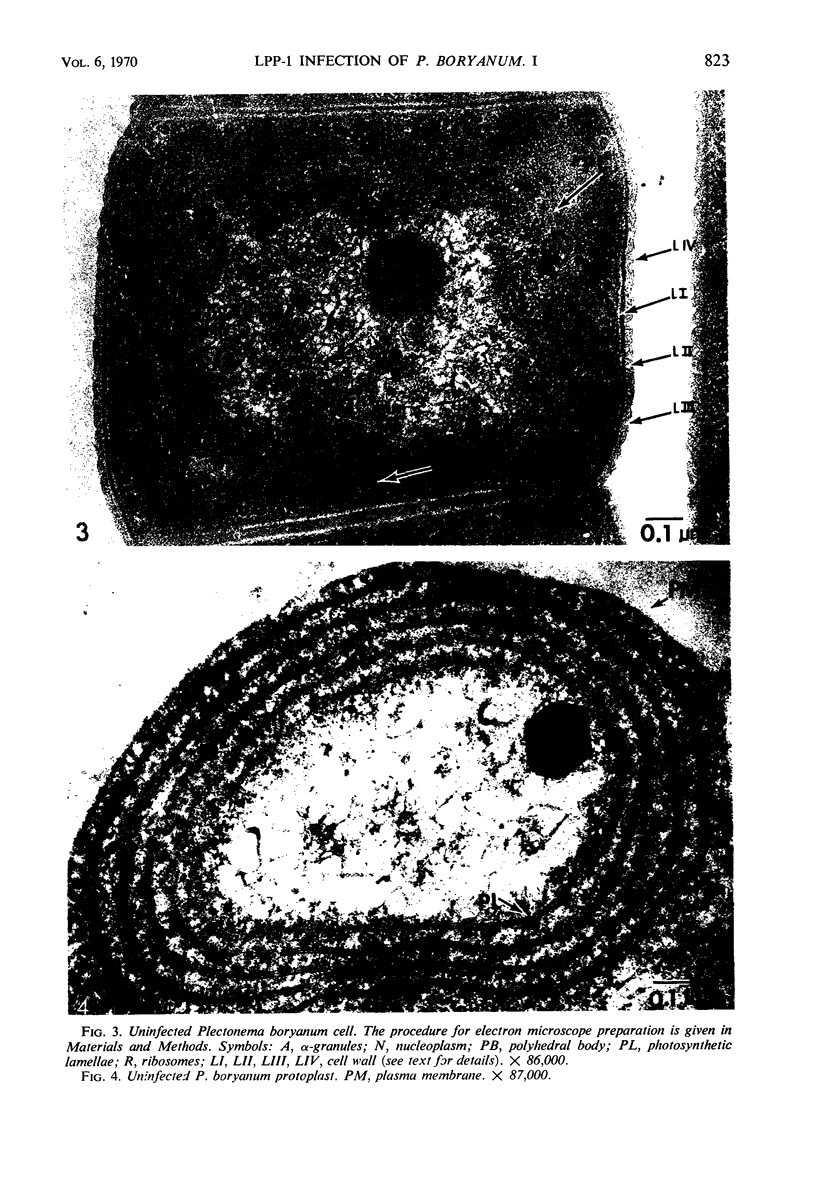
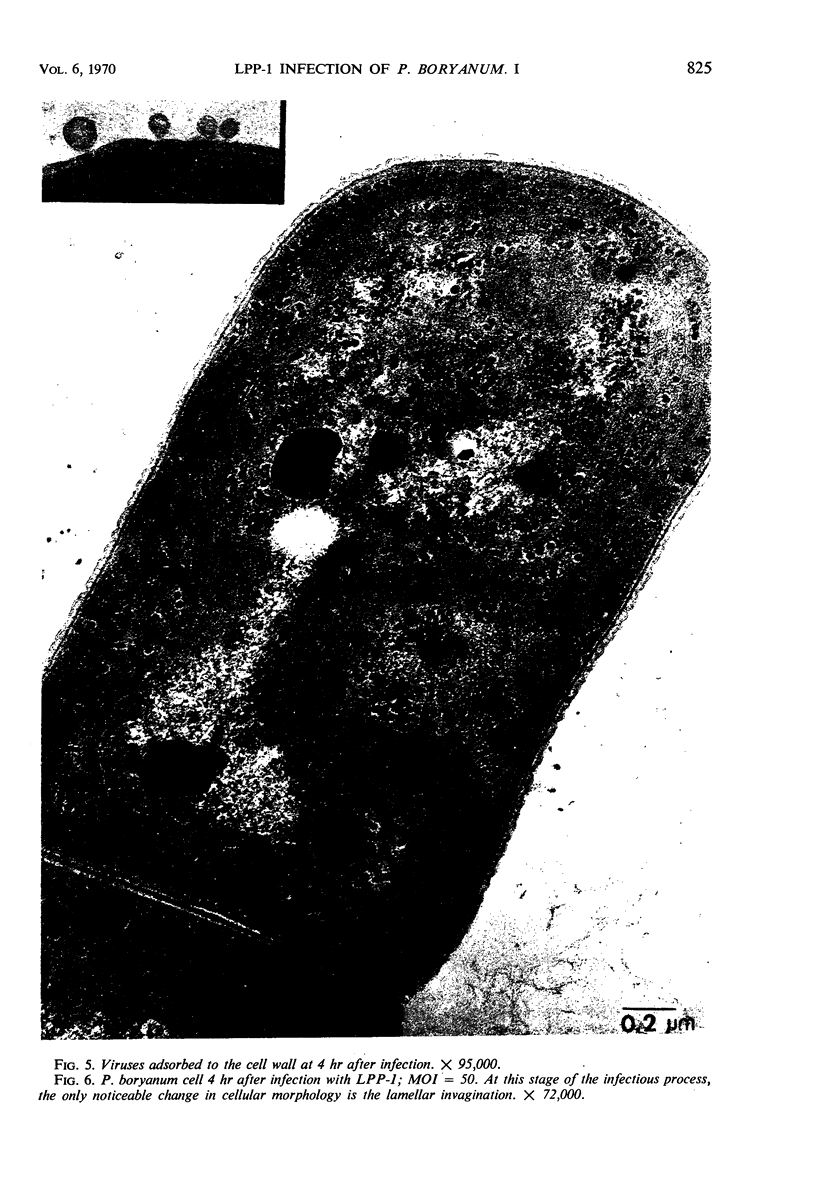
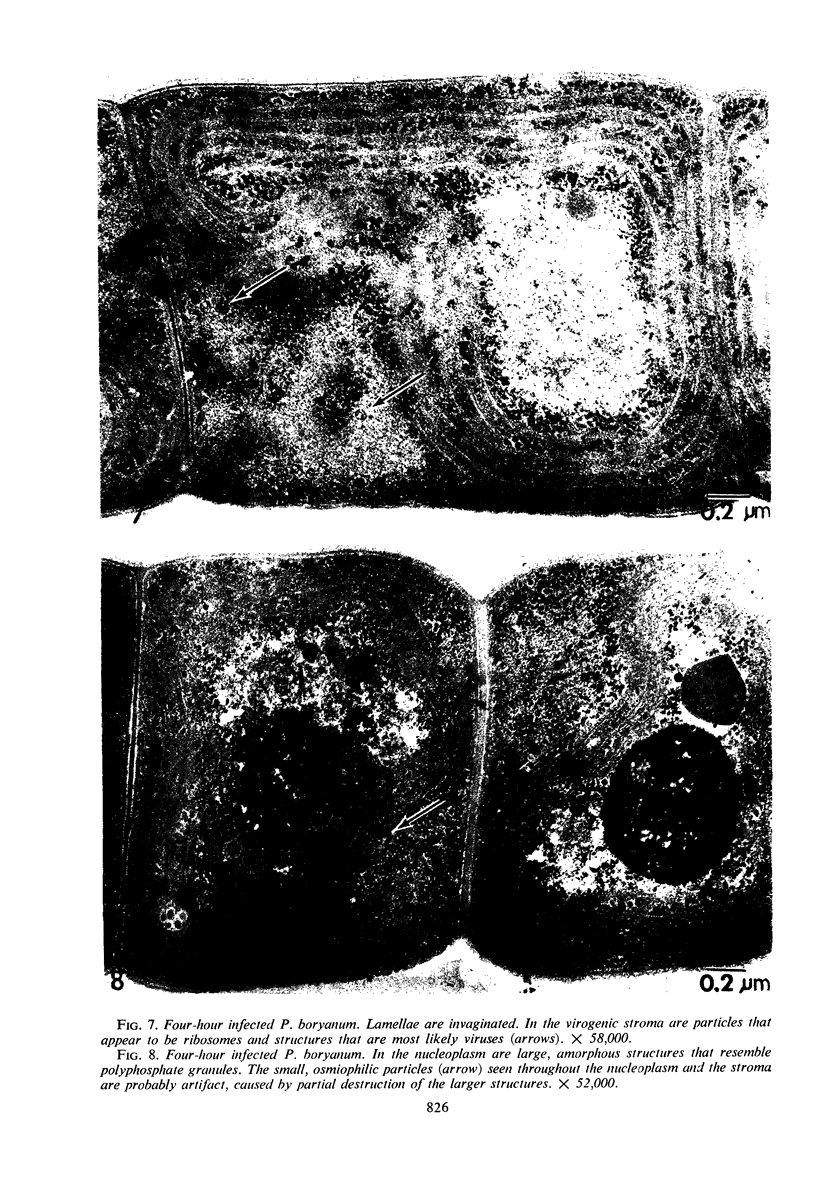
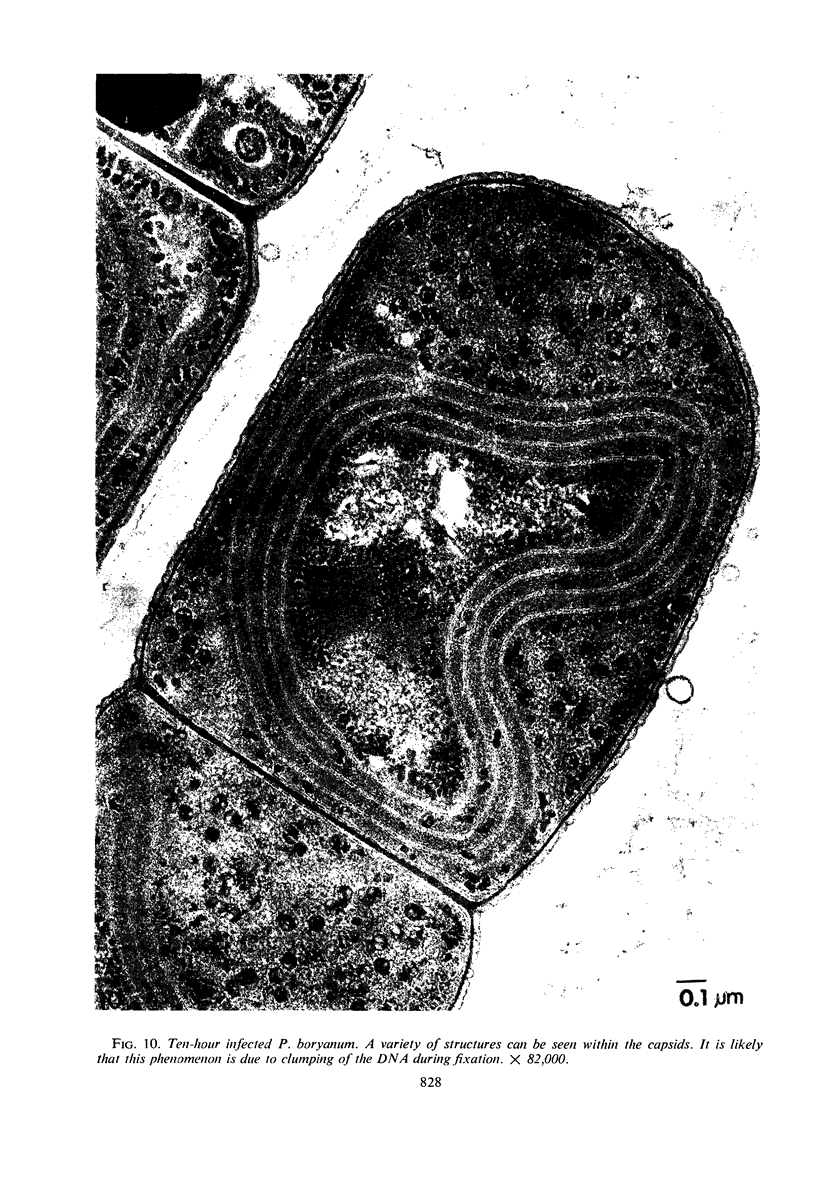
One-step growth and intracellular growth experiments were performed at high multiplicities of the virus LPP-1 during the infection of the blue-green alga Plectonema boryanum. The eclipse period lasts until 4 hr after infection, the latent period terminates at 6 hr, and the rise period continues until 14 to 16 hr after infection. The burst size was independent of multiplicity of infection over the ranges from 1 to 50. The burst size was 3,000 to 5,000 plaque-forming units (PFU) per infectious center or about 200 PFU per cell. Samples for electron microscopy were taken at characteristic times during the lytic cycle. The first sign of viral infection was the invagination of the photosynthetic lamellae at 3 hr after infection. Mature virions were visible at 4 hr. By 6 to 7 hr, many mature intracellular viral particles could be seen, with lysis beginning at 7 hr. By 10 hr after infection, all infected cells contained mature virions. No evidence for mass migration of performed viral precursors was obtained. The invagination of the lamellae could be prevented by the early addition of chloramphenicol, which implies that this process requires protein synthesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. M. Ultrastructure of the cell wall and cell division of unicellular blue-green algae. J Bacteriol. 1968 Sep;96(3):842–852. doi: 10.1128/jb.96.3.842-852.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins J. Preparation of Metabolically Active Protoplasts and Particle Preparations from the Blue-Green Alga, Phormidium luridum. Plant Physiol. 1967 Oct;42(10):1442–1446. doi: 10.1104/pp.42.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDRICKS W. W., JAGENDORF A. T. A SOLUBLE COMPONENT OF THE HILL REACTION IN ANACYSTIS NIDULANS. Arch Biochem Biophys. 1964 Jan;104:39–49. doi: 10.1016/s0003-9861(64)80032-1. [DOI] [PubMed] [Google Scholar]
- Ginzburg D., Padan E., Shilo M. Effect of cyanophage infection on CO2 photoassimilation in Plectonema boryanum. J Virol. 1968 Jul;2(7):695–701. doi: 10.1128/jvi.2.7.695-701.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E. Vegetative bacteriophage and the maturation of the virus particles. Adv Virus Res. 1961;8:1–61. doi: 10.1016/s0065-3527(08)60682-x. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R., Haselkorn R. Morphology of a virus of blue-green algae and properties of its deoxyribonucleic acid. J Virol. 1967 Apr;1(2):344–361. doi: 10.1128/jvi.1.2.344-361.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R., Haselkorn R. Studies on the structure of blue-green algae virus LPP-1. Virology. 1968 Apr;34(4):664–674. doi: 10.1016/0042-6822(68)90087-1. [DOI] [PubMed] [Google Scholar]
- Padan E., Ginzburg D., Shilo M. The reproductive cycle of cyanophage LPP1-G in Plectonema boryanum and its dependence on photosynthetic and respiratory systems. Virology. 1970 Mar;40(3):514–521. doi: 10.1016/0042-6822(70)90194-7. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIS H., SINGH R. N. Electron microscope studies on blue-green algae. J Biophys Biochem Cytol. 1961 Jan;9:63–80. doi: 10.1083/jcb.9.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SECHAUD J., KELLENBERGER E. Lyse précoce, provoquée par le chloroforme, chez les bactéries infectées par du bactériophage. Ann Inst Pasteur (Paris) 1956 Jan;90(1):102–106. [PubMed] [Google Scholar]
- SHATKIN A. J. A chlorophyll-containing cell fraction from the blue-green alga, Anabaena variabilis. J Biophys Biochem Cytol. 1960 Jun;7:583–584. doi: 10.1083/jcb.7.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUSOR W. A., KROGMANN D. W. HILL ACTIVITY IN CELL-FREE PREPARATIONS OF A BLUE-GREEN ALGA. Biochim Biophys Acta. 1964 Jul 29;88:11–19. doi: 10.1016/0926-6577(64)90150-0. [DOI] [PubMed] [Google Scholar]
- Sherman L. A., Haselkorn R. LPP-1 infection of the blue-green alga Plectonema boryanum. 3. Protein synthesis. J Virol. 1970 Dec;6(6):841–846. doi: 10.1128/jvi.6.6.841-846.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. A., Haselkorn R. LPP-1 infection of the blue-green alga Plectonema boryanum. II. Viral deoxyribonucleic acid synthesis and host deoxyribonucleic acid breakdown. J Virol. 1970 Dec;6(6):834–840. doi: 10.1128/jvi.6.6.834-840.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. M., Brown R. M., Jr, Goldstein D. A., Walne P. L. Culture methods for the blue-green alga Plectonema boryanum and its virus, with an electron microscope study of virus-infected cells. Virology. 1966 Apr;28(4):580–591. doi: 10.1016/0042-6822(66)90243-1. [DOI] [PubMed] [Google Scholar]
- Smith K. M., Brown R. M., Jr, Walne P. L., Goldstein D. A. Electron microscopy of the infection process of the blue-green alga virus. Virology. 1966 Oct;30(2):182–192. doi: 10.1016/0042-6822(66)90094-8. [DOI] [PubMed] [Google Scholar]
- Smith K. M., Brown R. M., Jr, Walne P. L. Ultrastructural and time-lapse studies on the replication cycle of the blue-green algal virus LPP-1. Virology. 1967 Feb;31(2):329–337. doi: 10.1016/0042-6822(67)90178-x. [DOI] [PubMed] [Google Scholar]
- Zagury D., Model P., Pappas G. D. A simplified technique for preparing thin sections of bacterial and viral suspensions: application to Escherichia coli and bacteriophage T2. Virology. 1967 Oct;33(2):347–352. doi: 10.1016/0042-6822(67)90154-7. [DOI] [PubMed] [Google Scholar]