Abstract
Background:
Bacterial sepsis is one of the major causes of mortality in newborn infants. Mortality increases when sepsis is associated with neutropenia.
Materials and Methods:
We conducted a prospective, randomized, double-blind, placebo-controlled trial of recombinant human granulocyte colony-stimulating factor on preterm neonates (gestational age (GA) <34 weeks) with sepsis and absolute neutrophil count (ANC) of <1500 cells/mm3. Mortality, duration of Neonatal Intensive Care Unit (NICU) stay, hematological parameters (ANC, platelet count, and total leukocyte count) were compared between the two groups. The GCSF group (n=39) received GCSF intravenously in a single daily dose of 10 μg/kg/day in a 5% dextrose solution over 20-40 min for three consecutive days, while the control group (n=39) received placebo of an equivalent volume of 5% dextrose.
Results:
Baseline demographic profile among the two groups was comparable. Mortality rate in the GCSF group was significantly lower than in the control group (10% vs. 35%; P<0.05). By day 3 of treatment, ANC in the GCSF group was significantly higher (3521±327) compared to 2094±460 in the control group, with P value being <0.05. Duration of NICU stay also decreased significantly in the GCSF group.
Conclusion:
The administration of GCSF in preterms with septicemia and neutropenia resulted in lower mortality rates. Further studies are required to confirm our results and establish this adjunctive therapy in neonatal sepsis.
Keywords: Granulocyte colony-stimulating factor, neonates, neutropenia, preterm, septicemia
INTRODUCTION
Bacterial sepsis is one of the major causes of mortality in newborns. The mortality rate of neonates with bacterial sepsis varies between 15% and 75%, depending on the organism and other associated complications.[1,2] Neutropenia is a common association of neonates with sepsis and is associated with increased risk of death.[3,4] Compared to adults, the unique susceptibility of neonates to sepsis associated neutropenia is due to a smaller neutrophil storage pool, reduced capacity of neutrophils to be mobilized from bone marrow, and a slower regeneration of neutrophils from bone marrow.[5] Also, neutrophil functioning abnormalities may co-exist in neonates and these neonates fail to mount an adequate immune response during overwhelming bacterial sepsis.[6] As a result, in addition to the conventional therapy for neonatal sepsis with antibiotic medications and supportive care, several new modes of immunotherapy such as granulocyte transfusions and intravenous immunoglobulin administration have been used to reduce mortality without any proven positive result.[7,8] Intravenous immunoglobulin have failed to create a major impact in reducing sepsis-related mortality and now the attention is more on the potential enhancement of phagocytic immunity using the hemopoietic colony-stimulating factors.[9,10] Studies have shown that granulocyte colony-stimulating factor (GCSF) can prime neutrophils for increased respiratory burst and chemotactic responses.[1] In addition, in newborns with sepsis, short-term therapy with GCSF increased the neutrophil count[11] and improved survival.[12] GCSF therapy in very low birth weight (VLBW) infants was demonstrated to be safe and tolerance is good.[13] Clinical trials in neonates have been preceded by extensive in vitro and animal studies because of the concern about acute and long-term toxicities of such agents in neonates.[10] There is a dearth of studies and clinical trials in this field and there is a pressing need of such studies to establish or to reject the use of GCSF to improve survival in neonatal sepsis.[14,15] The aim of this study was to determine whether the administration of GCSF is effective in reversing neutropenia and lowering mortality rates in preterms with sepsis.
MATERIALS AND METHODS
Study design and patient selection
A prospective, randomized, controlled double-blind trial was conducted in the Neonatal Intensive Care unit (NICU) of Medical College, Kolkata, India, from September 2011 to January 2012, with the approval of the institutional ethics committee, registered with the Clinical Trials Registry of India (CTRI) (2011).
An informed consent had been taken from each of the participating neonate's parents. Sepsis was diagnosed when a neonate had new-onset symptoms (e.g., respiratory distress, new apnea, temperature instability, inability to suck, lethargy, or other accepted clinical signs of sepsis),[12] a positive sepsis screen based on total leukocyte count, absolute neutrophil count (ANC), immature to total neutrophils ratio, micro-ESR, C-reactive protein (CRP),[16] and at least one positive blood culture in 1st 28 days of life. Blood cultures were repeated in all patients 72 h after randomization. Neutropenia was defined as a total neutrophil count <1500 cells/mm3 using a stringent adaptation of the criteria of Manroe et al.[17]
Inclusion criteria
-
a)
All premature infants of gestational age (GA) <34 weeks were eligible for the study if they had a peripheral blood neutrophil count <1500 cells/mm3 for at least 24 h during the 1st 3 weeks of life
-
b)
Positive clinical signs of sepsis
-
c)
A positive sepsis screen and
-
d)
At least a single positive blood culture
-
e)
Weighing between 1100 and 2500 g
-
f)
Adequate renal and liver function.
Exclusion criteria
-
a)
Major congenital anomaly,
-
b)
Stigma of congenital infection, and
-
c)
Severe lesions diagnosed by cranial ultrasound (e.g., intraventricular hemorrhage grade 3 and 4 and major ischemic lesions)
-
d)
Newborns with altered liver and renal functions were excluded.
The included infants were randomized to receive either recombinant GCSF (rGCSF) (Filgastrim (Grafeel) batch no. GFAV00511 mfg date March 2011) or placebo using a randomly generated (computer-generated) predetermined schedule. Investigators were divided into two teams – blinded and unblinded. Those collecting the data and following babies during the study period were blinded to the study assignment. A computer-generated random number table was followed and an allocation number was assigned to each random number. Corresponding allocation numbers were also present concealed in the cover of each medication/placebo, which were not accessible to the blinded team. The unblinded team administered the medications following randomization. We followed the schedule specified by Kocherlakota and La Gamma.[18,19] The product was infused intravenously and slowly in a single daily dose of 10 μg/kg/day in a 5% dextrose solution over 20-40 min for 3 consecutive days, starting no longer than 48 h after neutropenia was diagnosed. Placebo consisted of an equivalent volume of 5% dextrose. The total volume injected in the both cases was 0.66 ml/kg.[11]
Outcome
Primary
GCSF significantly increased the ANC in preterm babies with neutropenia and sepsis.
Secondary
Mortality and duration of NICU stay was also significantly reduced in those babies with sepsis and neutropenia treated with GCSF.
The included infants showed no signs of disturbance in respiration, heart rate, or blood pressure during administration of the study medicine. Routine examination was performed daily, and vitals and all systems were closely monitored until discharge. ANC was monitored before the third dose and it was held if ANC exceeded 20,000.[19]
Prior to the study, maternal characteristics, approximate gestational age, anthropometry, neonatal vitals, serum biochemistry, CRP, blood sugar, and electrolytes were recorded. Gestational age was confirmed by New Ballard scoring system.[20] Both groups (n=39) each were treated with appropriate conventional therapeutic interventions. Antibiotic regimens were modified subsequently according to blood culture reports and sensitivity patterns, and those with negative blood cultures on all occasions were excluded from the study. Complete blood counts were obtained by counter autoanalyzer machine at study entry, and after treatment at 48 h, 72 h, and 7 days. White blood cells (WBCs) were also counted on a hemocytometer, and differential count was obtained by manual counting from Wright stained blood films. The ANC was determined by the percentage of polymorphonuclear leukocytes (PMLs) and band forms identified manually. There was no significant difference in the two forms of determination. Metamyelocytes and myelocytes were not included as they contribute minimally to the total count, being functionally inactive.[21,22] All WBCs were corrected for the presence of nucleated red blood cells (RBCs). Hemoglobin and platelet counts were measured pre-and post-treatment. All infants received antibiotics for at least 5 days.
Statistical analysis
As we are taking placebo as control, we are doing superiority trial, parallel design with equal group allocation. Sample size was determined by convenience.
Groups were made for weight on admission, demographic characteristics, hematological responses, NICU stay, and mortality. Data were expressed as numbers (%), median range, and mean±SD. P value <0.05 was taken as significant. For the continuous data, normal distribution was used for comparison of mean values and Fisher's exact t test was used; for binary data, binomial distribution was presumed and t test was used. Statistical analysis was done using the software STATA version 12.
RESULTS
Baseline demographic profile and study flowchart
A total of 1748 preterm babies weighing <2500 g were admitted at the institute during the study period, of which 653 were admitted in NICU with features of sepsis, hence assessed for eligibility. Figure 1 shows the flow of subjects through the study. A total of 78 babies were enrolled and randomized to receive either GCSF (n=39) plus conventional care or placebo with 5% dextrose solution plus conventional care. All babies completed the study. Table 1 shows the baseline demographic profile of the babies in the two treatment groups.
Figure 1.
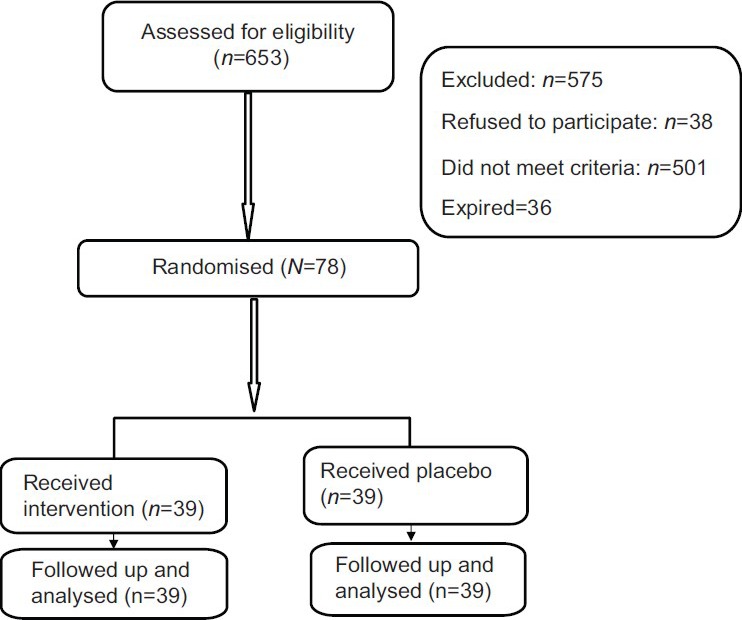
Flow of patients through the trial
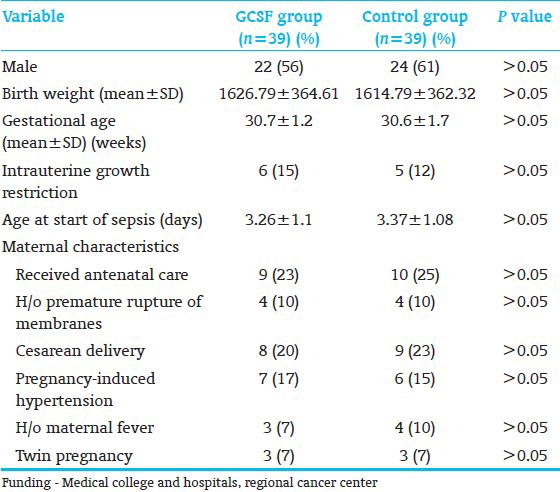
Table 1.
Baseline demographic profile of participants in two groups
Microorganisms isolated at study entry in the blood culture of GCSF group were Klebsiella pneumoniae (n=18), Pseudomonas aeruginosa (n=12), Enterobacter sp. (n=4), Coagulase-negative Staphylococcus (CONS) (n=4), and Acinetobacter sp. (n=1). Microorganisms at study entry in the other group were K. pneumoniae (n=16), P. aeruginosa (n=13), Enterobacter sp. (n=2), CONS (n=5), Candida sp. (n=1), and Acinetobacter sp. (n=2).
Hematological indices
At the study entry, the ANC in the GCSF group was 1349±226 compared to 1499±258 in the other group. The total leukocyte count in the GCSF group was 6358±1266 compared to 6548±1187 in the other group.
By Day 3 (72 h) of starting the intervention, the GCSF group had significantly higher ANC compared to the other group. ANC in the GCSF group was 3521±327 compared to 2094±460 in the other group, with the P value being <0.05.
By Day 5, the GCSF group had an ANC of 4872±913 compared to 4052±1011 in the other group, with a P value <0.05.
At Day 7, the GCSF group's ANC was 5917±1047 compared to 5034±936 in the other group.
Mortality
There were 4 (10%) deaths in the GCSF group compared to 14 (35%) in the other group, which was significantly low (P<0.05).
Treatment group
P value: The probability of survival for the treatment group
(GCSF)=0.9
Mean survival=35.1
Variance: Variance around this P=0.09
Variance around this mean=1.8735
95% Confidence Interval: (35.1±[1.96×1.8735])=(31.43 38.77) Control group
P value: The probability of survival for the control
group=0.65
Mean survival=25.35
Variance: Variance around this P=0.2275
Variance around this mean=2.9787
95% Confidence Interval: (25.35±[1.96×2.9787])=(19.51 31.19)
Improvement=90%-65%=25% increase in survival probability
Relative Risk Reduction: ([0.9-0.65]/0.65)×100=38.46%
The causes of deaths among those in the GCSF group were septic shock (n=2), (IVH) Intaventricular Hemorrhage Grade 3 and Grade 4 (n=1), and (DIC) Disseminated Intravascular Coagulation (n=1). In the control group, the causes of death were septic shock (n=7), DIC (n=5), and respiratory failure due to pulmonary hemorrhage (n=2).
The duration of stay in neonatal intensive care unit would go down for granulocyte colony-stimulating factor
To verify this claim, a regression of the duration of stay had been carried out on birth weight, gestational age, and a treatment dummy, and the sign of the dummy variable turned out to be negative (−12.16) and statistically significant at less than 1% level. This result has been obtained by regression analysis using dummy variable.
DISCUSSION
Our study demonstrated that preterm babies with sepsis and neutropenia who were treated with GCSF for 3 days along with conventional care had a significantly lower mortality than the control group. GCSF increased ANC, and ANC showed a significant increase over 72 h. Overall, favorable prognosis in neonatal septicemia depends on effective host mechanism which again depends on normal hematological indices.[23] Neonates born to pregnant women with preeclampsia have 50% chance of having neutropenia. Neutropenia has variable course typically lasting from days to weeks in the affected infants.[24] Studies have shown that neutropenic neonates born to mothers with preeclampsia have increased risk of sepsis. In our study, 17% in the study group and 15% in the control group had mothers with preeclampsia. These neonates were neutropenic and developed sepsis subsequently. Newborns with neonatal alloimmune neutropenia develop transient neutropenia that recovers spontaneously after an average of 11 weeks. In general, infectious complications are minor, and most series report no septic deaths.[24] When necessary, patients respond well to GCSF.
Neutropenia, when associated with neonatal sepsis, worsens the prognosis.[22] An immaturity in the quantitative and qualitative aspects of phagocytic immunity contributes to a state of relative immunodeficiency in newborn infants.[24] GCSF is a physiological regulator of myelopoiesis and an activator of mature effective neutrophil function.[25] It supports the clonal growth of neutrophil progenitors, primes neutrophils to increased expression of chemotactic receptors, and enhances antibody-dependent cellular cytotoxicity.[11] Compared to adults, newborns do not seem to generate GCSF effectively.[26] Estimates suggest that when sepsis is associated with severe neutropenia, mortality exceeds 50%.[27] Relative neutropenia, though, is a low-risk group in developed countries; in developing countries with resource-limited settings, sepsis-related neonatal neutropenia is a significant cause of neonatal mortality and morbidity.[28] In addition, in developing countries, the microbiological organisms causing septicemia are different from those in developed nations; in developing countries organisms are mostly gram negative such as Klebsiella and Pseudomonas.[29]
There have been studies on the use of GCSF both as an adjunctive to treatment in neutropenic septicemic neonates and also its prophylactic use in preterm neonates, but all these studies are heterogeneous with regard to patient selection, duration of intervention, dosage and route of intervention, and outcome criteria. Duration of intervention varies widely in studies, mostly between 5 and 7 days. Our study utilized 3 days which has been shown to be effective in increasing ANC and reducing mortality with minimum possible intervention, thereby reducing the cost of treatment and any possibility of side effects.
Among the different studies, most are with positive outcomes; but those with negative outcomes are a recently published multicenter randomized control trial exploring the use of prophylactic GCSF, and there was no significant difference between two groups in terms of mortality, short-term morbidity, or sepsis-free interval,[30] and another study on prophylactic GCSF by Cairo et al., which did not show any significant difference.[31]
There have been debates on whether there are subgroups of infants who are better candidates for the study, as less mature infants benefit more than older infants.[11] Time dependence of response is very important as in a vast majority of cases where neutropenia occurs early, a second course of treatment is not indicated as infants who are neutropenic by 2nd week of life do not have increased risk of infection.[32] A Cochrane review[11,32] of this effect found no evidence of adding GCSF or (GMCSF) Granulocyte Macrophage Colony Stimulating Factor to antibiotic therapyas adjuvant therapy reduced immediate cause of mortality.[33]
Our study showed remarkable results both in terms of mortality and duration of NICU stay with the use of GCSF, and since conflicting results have been obtained in different studies, we suggest that there is an urgent need of such studies to include or discard totally the use of GCSF as an adjunctive to conventional treatment of septicemia in preterm neonates.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Siegel JD, McCracken GH., Jr Sepsis neonatorum. N Engl J Med. 1981;304:642–7. doi: 10.1056/NEJM198103123041105. [DOI] [PubMed] [Google Scholar]
- 2.Polin RA, St Geme JW., 3rd Neonatal sepsis. Adv Pediatr Infect Dis. 1992;7:25–61. [PubMed] [Google Scholar]
- 3.Philip AG, Hewitt JR. Early diagnosis of neonatal sepsis. Pediatrics. 1980;65:1036–41. [PubMed] [Google Scholar]
- 4.Mathur NB, Singh A, Sharma VK, Satyanarayana L. Evaluation of risk factors for neonatal sepsis. Indian Pediatr. 1996;33:817–22. [PubMed] [Google Scholar]
- 5.Banerjee MC, Seer CP. The current role of colony stimulating factors in prevention and treatment of neonatal sepsis. Semin Neonatol. 2002;7:335–49. doi: 10.1016/s1084-2756(02)90116-8. [DOI] [PubMed] [Google Scholar]
- 6.Cairo MS. Cytokines: A new immunotherapy. Clin Perinatol. 1991;81:343–55. [PubMed] [Google Scholar]
- 7.Fanaroff AA, Korones SB, Wright LL, Wright EC, Poland RL, Bauer CB, et al. A controlled trial of intravenous immune globulin to reduce nosocomial infections in very low birth weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med. 1994;330:1107–13. doi: 10.1056/NEJM199404213301602. [DOI] [PubMed] [Google Scholar]
- 8.Souza LM, Boone TC, Gabrilove J, Lai PH, Zsebo KM, Murdock DC, et al. Recombinant human granulocyte colony-stimulating factor: Effects on normal and leukemic myeloid cells. Science. 1986;232:61–5. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- 9.Lacy JB, Ohlsson A. Administration of intravenous immunoglobulins for prophylaxis or treatment of infection in preterm neonates: Metaanalyses. Arch Dis Child Fetal Neonatal Ed. 1995;72:151–5. doi: 10.1136/fn.72.3.f151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carr R, Modi N. Haemopoietic colony stimulating factors for preterm neonates. Arch Dis Child Fetal Neonatal Ed. 1997;76:F128–33. doi: 10.1136/fn.76.2.f128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillian ER, Christensen RD, Suen Y, Ellis R, van de Ven C, Cairo MS. A randomised placebo controlled trial of recombinant human granulocyte macrophage colony stimulating factor administration in newborn infants with presumed sepsis: Significant induction of peripheral and bone marrow neutrophilia. Blood. 1994;84:1427–33. [PubMed] [Google Scholar]
- 12.Kocherlakota P, La Gamma EF. Human granulocyte colony-stimulating factor may improve outcome attributable to neonatal sepsis complicated by neutropenia. Pediatrics. 1997;100:E6. doi: 10.1542/peds.100.1.e6. [DOI] [PubMed] [Google Scholar]
- 13.Sreenan C, Osiovich H. Myeloid colony stimulating factors: Use in the newborn. Arch Pediatr Adolesc Med. 1999;153:984–8. doi: 10.1001/archpedi.153.9.984. [DOI] [PubMed] [Google Scholar]
- 14.Carr R, Brocklehurst P, Dore CJ, Modi N. Granulocyte-macrophage colony stimulating factor administered as prophylaxis for reduction of sepsis in extremely preterm, small for gestational age neonates (the PROGRAMS trial): A single-blind, multicentre, randomised controlled trial. Lancet. 2009;373:226–33. doi: 10.1016/S0140-6736(09)60071-4. [DOI] [PubMed] [Google Scholar]
- 15.Ozdemir E, Kakehi Y, Nakamura E, Kinoshita H, Terachi T, Okada Y, et al. HLA-DRB1 *0101 and *0405 as protective alleles in Japanese patients with renal cell carcinoma. Cancer Res. 1997;57:742–6. [PubMed] [Google Scholar]
- 16.Meherban S. 7th ed. New Delhi, India: Sagar Publications; 2010. Care of the Newborn; pp. 225–6. [Google Scholar]
- 17.Manroe BL, Weinberg AG, Rosenfeld Cr, Browne R. The neonatal blood count in health and disease: Reference values for neutrophilic cells. J Pediatr. 1979;95:89–98. doi: 10.1016/s0022-3476(79)80096-7. [DOI] [PubMed] [Google Scholar]
- 18.Kocherlakota P, La Gamma Ef. Preliminary report: RhGMCSF may reduce the incidence of neonatal sepsis in prolonged preeclampsia – associated- neutropenia. Pediatrics. 1998;102:1107–11. doi: 10.1542/peds.102.5.1107. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn P, Messer J, Paupe A, Espagne S, Kacet N, Mouchnino G, et al. A multicenter, randomised placebo–controlled trial of prophylactic recombinant granulocyte-colony stimulating factor in preterm neonates with neutropenia. J Pediatr. 2009;155:324–30. doi: 10.1016/j.jpeds.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard Score expanded to include extremely premature infants. J Pediatr. 1991;119:417–23. doi: 10.1016/s0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- 21.Babior BM, Stossel TP. New York, NY: Churchill Livingstone; 1984. Hematology: A pathophysiological approach; p. 167. [Google Scholar]
- 22.Bilgin K, Yaramis A, Haspolat K, Taş MA, Günbey S, Derman O. A randomised trial of granulocyte-macrophage colony–stimulating factor in neonates with sepsis and neutropenia. Pediatrics. 2001;107:36–41. doi: 10.1542/peds.107.1.36. [DOI] [PubMed] [Google Scholar]
- 23.Gathwala G, Walia M, Bala H, Singh S. Recombinant human granulocyte colony stimulating factor in preterm neonates with sepsis and relative neutropenia: A randomised, single blind, non placebo–controlled trial. J Trop Pediatr. 2012;58:12–8. doi: 10.1093/tropej/fmr012. [DOI] [PubMed] [Google Scholar]
- 24.Mouzenho A, Rosenfeld CR, Sanchez PJ, Risser R. Effect of maternal hypertension on neonatal neutropenia and risk of nosocomial infection. Pediatrics. 1992;90:430–5. [PubMed] [Google Scholar]
- 25.Devriendt K, Kim AS, Mathijs G, Frints SG, Schwartz M, Van Den Oord JJ, et al. Constitutively activating mutation in WASP causes X-linked severe congenital neutropenia. Nat Genet. 2001;27:313–7. doi: 10.1038/85886. [DOI] [PubMed] [Google Scholar]
- 26.Cairo MS. Neonatal neutrophil host defense. Prospects for immunologic enhancement during neonatal sepsis. Am J Dis Child. 1989;143:40–6. doi: 10.1001/archpedi.1989.02150130050014. [DOI] [PubMed] [Google Scholar]
- 27.Demetri G, Griffin J. Granulocyte colony stimulating factor and its receptor. Blood. 1992;78:2791. [PubMed] [Google Scholar]
- 28.Cairo M, Suen Y, Knoppel E, Dana R, Park L, Clark S, et al. Decreased GCSF and IL-3 production and gene expression from mononuclear cells of newborn infants. Pediatr Res. 1992;31:574–8. doi: 10.1203/00006450-199206000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Omar SA, Salhadar A, Wooliever DE, Alsgaard PK. Late onset neutropenia in very low birth weight infants. Pediatrics. 2000;106:E55. doi: 10.1542/peds.106.4.e55. [DOI] [PubMed] [Google Scholar]
- 30.Carr R, Brocklehusrst P, Dore CJ, Modi N. Granulocyte macrophage colony stimulating factor administered as prophylaxis for reduction of sepsis in extremely preterm, small for gestational neonates: A single blind, multicentre, randomized controlled trial. Lancet. 2009;373:226–33. doi: 10.1016/S0140-6736(09)60071-4. [DOI] [PubMed] [Google Scholar]
- 31.Cairo MS, Agosti J, Ellis R, Laver JJ, Puppala B, deLemos R, et al. A randomized double blind, placebo controlled trial of prophylactic recombinant human granulocyte macrophage colony stimulating factor to reduce nosocomial infections in very low birth weight neonates. J Pediatr. 1999;134:64–70. doi: 10.1016/s0022-3476(99)70373-2. [DOI] [PubMed] [Google Scholar]
- 32.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. Trends in neonatal morbidity and mortality for very low birth weight infants. Am J Obstet Gynecol. 2007;196:147.e1–8. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Jawson MS, Jones MH, Linch DC. The effects of recombinant human granulocyte macrophage colony stimulating factor on the neutrophil respiratory burst in the term and preterm infant when studied in whole blood. Pediatr Res. 1994;36:623–7. doi: 10.1203/00006450-199411000-00016. [DOI] [PubMed] [Google Scholar]


