Abstract
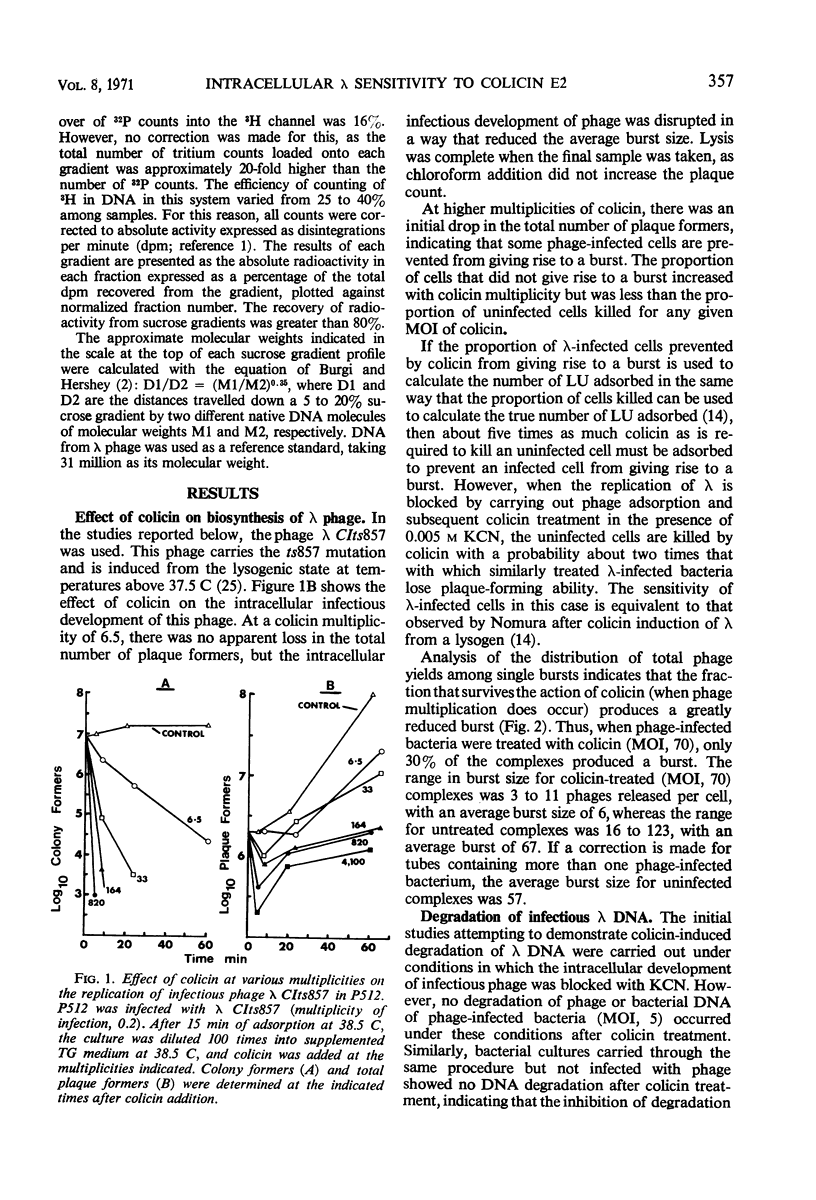
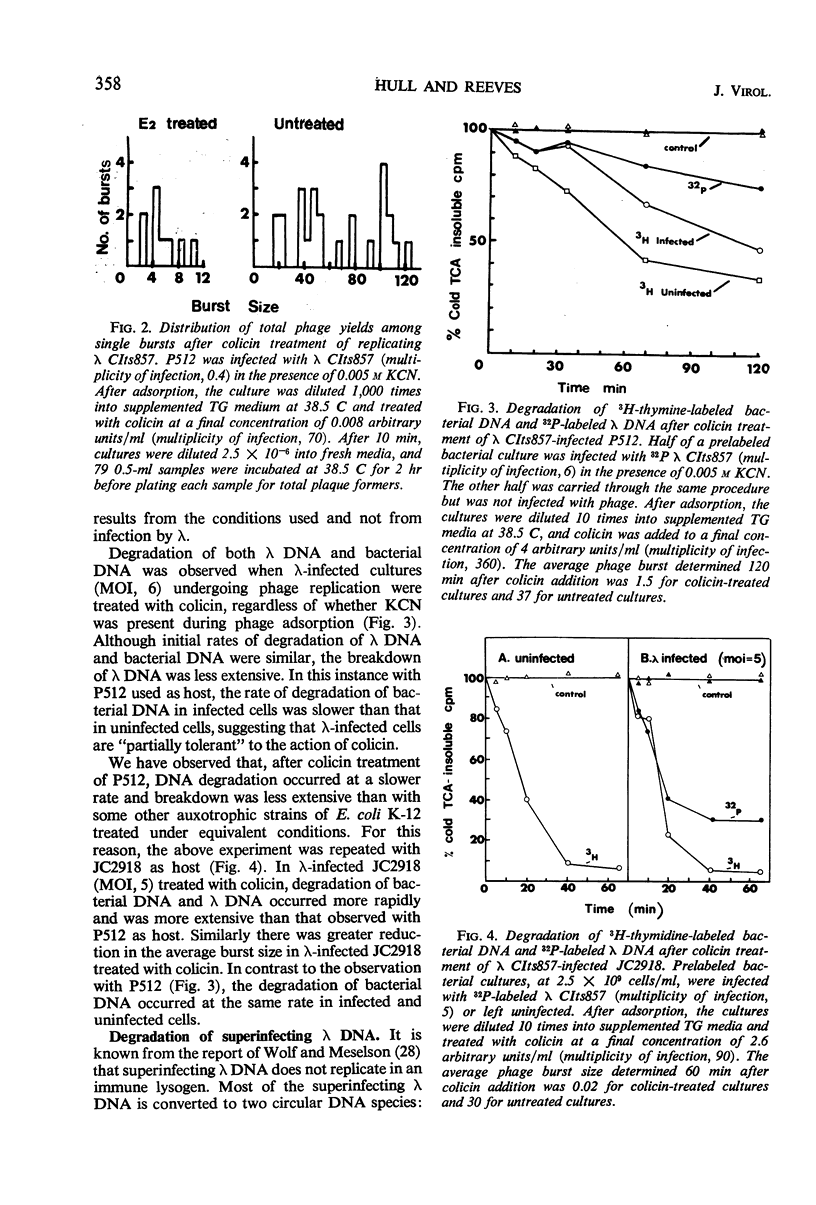
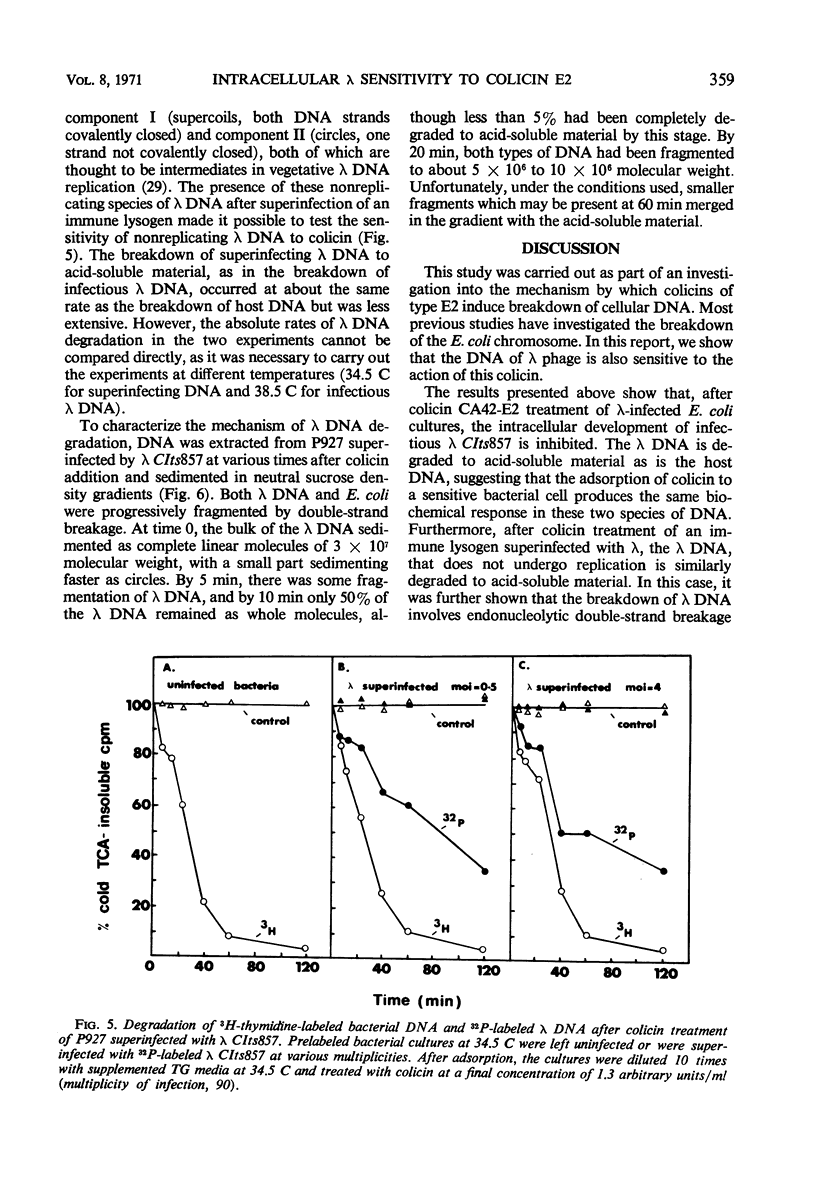
Treatment of Escherichia coli K-12 infected by λ CIts857 with colicin CA42-E2 resulted in partial inhibition of the infectious process. Uninfected bacteria were killed by colicin with a probability of about five times that with which similarly treated λ-infected bacteria lose plaque-forming ability. The λ deoxyribonucleic acid (DNA), when present in a bacterial cell either as the replicating DNA of infectious phage or as the nonreplicating DNA of superinfecting phage, was degraded to acid-soluble material after colicin treatment. Analysis of the intermediates of DNA breakdown has revealed that degradation of the DNA to acid-soluble material is preceded by endonucleolytic fragmentation of the chromosome at a limited number of sites. This is the same mechanism of degradation previously observed for E. coli DNA after colicin treatment.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C., Goldthwait D. A. Endonuclease II of E. coli. I. Isolation and purification. Proc Natl Acad Sci U S A. 1969 Mar;62(3):934–940. doi: 10.1073/pnas.62.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C., Hadi S. M., Goldthwait D. A. Endonuclease II of Escherichia coli. II. Enzyme properties and studies on the degradation of alkylated and native deoxyribonucleic acid. J Biol Chem. 1969 Nov 10;244(21):5879–5889. [PubMed] [Google Scholar]
- GOEBEL W. F., BARRY G. T., SHEDLOVSKY T. Colicine K. I. The production of colicine K in media maintained at constant pH. J Exp Med. 1956 May 1;103(5):577–588. doi: 10.1084/jem.103.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOTTA Y., BASSEL A. MOLECULAR SIZE AND CIRCULARITY OF DNA IN CELLS OF MAMMALS AND HIGHER PLANTS. Proc Natl Acad Sci U S A. 1965 Feb;53:356–362. doi: 10.1073/pnas.53.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell E. H., Daverin C. I. Pre-fork synthesis: a model for DNA replication. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1065–1071. doi: 10.1073/pnas.64.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland E. M., Holland I. B. Induction of DNA breakdown and inhibition of cell division by colicin E2. Nature of some early steps in the process and properties of the E-2-specific nuclease system. J Gen Microbiol. 1970 Dec;64(2):223–239. doi: 10.1099/00221287-64-2-223. [DOI] [PubMed] [Google Scholar]
- Kutter E. M., Wiberg J. S. Degradation of cytosin-containing bacterial and bacteriophage DNA after infection of Escherichia coli B with bacteriophage T4D wild type and with mutants defective in genes 46, 47 and 56. J Mol Biol. 1968 Dec;38(3):395–411. doi: 10.1016/0022-2836(68)90394-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nomura M. Colicins and related bacteriocins. Annu Rev Microbiol. 1967;21:257–284. doi: 10.1146/annurev.mi.21.100167.001353. [DOI] [PubMed] [Google Scholar]
- Obinata M., Mizuno D. Mechanism of colicin E2-induced DNA degradation in Escherichia coli. Biochim Biophys Acta. 1970 Feb 18;199(2):330–339. doi: 10.1016/0005-2787(70)90076-6. [DOI] [PubMed] [Google Scholar]
- REEVES P. THE BACTERIOCINS. Bacteriol Rev. 1965 Mar;29:24–45. doi: 10.1128/br.29.1.24-45.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves P. Mode of action of colicins of types E1, E2, E3, and K. J Bacteriol. 1968 Nov;96(5):1700–1703. doi: 10.1128/jb.96.5.1700-1703.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves P. Mutants resistant to colicin CA42-E2: cross resistance and genetic mapping of a special class of mutants. Aust J Exp Biol Med Sci. 1966 Jun;44(3):301–315. doi: 10.1038/icb.1966.29. [DOI] [PubMed] [Google Scholar]
- Ringrose P. Sedimentation analysis of DNA degradation products resulting from the action of colicin E2 on Escherichia coli. Biochim Biophys Acta. 1970 Aug 8;213(2):320–334. doi: 10.1016/0005-2787(70)90040-7. [DOI] [PubMed] [Google Scholar]
- SMITH H. O., LEVINE M. THE SYNTHESIS OF PHAGE AND HOST DNA IN THE ESTABLISHMENT OF LYSOGENY. Virology. 1965 Apr;25:585–590. doi: 10.1016/0042-6822(65)90086-3. [DOI] [PubMed] [Google Scholar]
- SUSSMAN R., JACOB F. [On a thermosensitive repression system in the Escherichia coli lambda bacteriophage]. C R Hebd Seances Acad Sci. 1962 Feb 19;254:1517–1519. [PubMed] [Google Scholar]
- Szybalski W., Kubinski H., Sheldrick P. Pyrimidine clusters on the transcribing strand of DNA and their possible role in the initiation of RNA synthesis. Cold Spring Harb Symp Quant Biol. 1966;31:123–127. doi: 10.1101/sqb.1966.031.01.019. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLF B., MESELSON M. REPRESSION OF THE REPLICATION OF SUPERINFECTING BACTERIOPHAGE DNA IN IMMUNE CELLS. J Mol Biol. 1963 Dec;7:636–644. doi: 10.1016/s0022-2836(63)80110-2. [DOI] [PubMed] [Google Scholar]
- Young E. T., 2nd, Sinsheimer R. L. Vegetative lambda DNA. 3. Pulse-labeled components. J Mol Biol. 1968 Apr 14;33(1):49–59. doi: 10.1016/0022-2836(68)90280-5. [DOI] [PubMed] [Google Scholar]



