Abstract
mTOR integrates signals from nutrients and growth factors to control protein synthesis, cell growth, and survival. Although mTOR has been established as a therapeutic target in hematologic malignancies, its physiological role in regulating hematopoiesis remains unclear. Here we show that conditional gene targeting of mTOR causes bone marrow failure and defects in multi-lineage hematopoiesis including myelopoiesis, erythropoiesis, thrombopoiesis, and lymphopoiesis. mTOR deficiency results in loss of quiescence of hematopoietic stem cells, leading to a transient increase but long-term exhaustion and defective engraftment of hematopoietic stem cells in lethally irradiated recipient mice. Furthermore, ablation of mTOR causes increased apoptosis in lineage-committed blood cells but not hematopoietic stem cells, indicating a differentiation stage-specific function. These results demonstrate that mTOR is essential for hematopoietic stem cell engraftment and multi-lineage hematopoiesis.
Introduction
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase.1 In response to nutrients, growth factors, and intracellular energy status, mTOR is activated by signaling through phosphatidylinositol-3-OH (PI 3) kinase, PDK1 and Akt.2 mTOR activation leads to phosphorylation of the translational regulators S6K1 and 4E-BP to regulate protein synthesis, cell growth, and metabolism, and to cell survival via phosphorylating Akt on Ser473.2,3
In the hematopoietic system, studies using mTOR inhibitor rapamycin or its analogs have suggested a role for mTOR in megakaryocyte and dendritic cell proliferation and differentiation.4,5 Hyper-activation of mTOR by deletion of phosphatase and tensin homolog (PTEN) or tuberous sclerosis complex (TSC), negative regulators of mTOR, results in long-term hematopoietic stem cell (HSC) exhaustion.6–8 Nonetheless, such a gain-of-function approach is not sufficient to reveal the physiological role of mTOR. Because gene targeting of mTOR in embryonic stem cells results in early embryonic lethality,9 tissue-specific gene knockout mouse model of mTOR has recently been generated.10 In the present studies, we have examined the physiological roles of mTOR in hematopoiesis and hematopoietic stem cell (HSC) function by using a hematopoietic-specific inducible mouse knockout model. We show that mTOR deficiency causes bone marrow (BM) failure and a markedly decreased production of all blood lineage cells, as well as impaired HSC engraftment.
Methods
Mice
Conditional gene-targeted mTORloxp/loxp mice were generated as described previously.9 To delete mTOR in vivo in hematopoietic stem cells, mTORloxp/loxp;Mx-Cre+ mice were generated by breeding mTORloxp/loxp mice with Mx-Cre+ transgenic mice carrying a bacteriophage Cre recombinase driven by an interferon-α-inducible Mx1 promoter. The expression of Cre was induced by 6–8 intraperitoneal (i.p.) injections of 10 μg/g of body weight polyinosine-polycytidine (pIpC) (Amersham Pharmacia Biotech, Piscataway, NJ, USA) into the Mx-Cre+ mice at 2-day intervals.
Blood lineage analysis
Single cell suspensions were incubated for 20 min at room temperature with various combinations of the following cell-surface marker antibodies: PE-Gr1 (clone: RB6-8C5), FITC-Mac1 (clone: M1/70), FITC-Ter119 (clone: TER-119), PE-CD71 (clone: C2), FITC-B220 (clone: RA3-6B2), Percp-Cy5.5-IgM (clone: G155-228), Percp-Cy5.5-CD4 (clone: RM4-5), FITC-CD8 (clone: 53-6.7), PE-Cy7-CD150 (clone: TC15-12F12.2), FITC-CD41 (clone: MWReg30), FITC-CD48 (clone: HM48-1), FITC-CD34 (clone: RAM34), PE-Sca1 (clone: D7), APC-c-Kit (clone: ACK2), PE-Cy7- CD16/CD32 (clone: 93), APC-Cy7-IL7R (clone: A7R34), PE-H2Kb (clone: AF6-88.5), PE-CD45.1 (clone: A20), and FITC-CD45.2 (clone: 104). All the antibodies were purchased from BD Biosciences except FITC-CD34, APC-c-Kit, PE-Cy7-CD16/CD32, and APC-Cy7-IL7R (eBiosciences) and PE-Cy7-CD150 (Biolegend). Immunolabeled cells were analyzed by flow cytometry.
Colony formation assay
Bone marrow cells (5 × 104 cells) were cultured in 1 mL methyl-cellulose medium (1% methylcellulose, 30% fetal bovine serum (FBS), 2% penicillin and streptomycin, 1% bovine serum albumin (BSA), and 10−4 M β-mercaptoethanol) containing 4 U/mL erythropoietin (EPO), 100 ng/mL rrSCF, 100 ng/mL granulocyte-colony stimulating factor (G-CSF), and 100 ng/mL IL-3 for seven days and colony-forming unit of multiple myeloid progenitors (CFU-C) and erythroid burst-forming unit (BFU-E) were counted. For erythroid CFU (CFU-E) assays, 2 × 105 BM cells were cultured in 1 mL methylcellulose medium supplemented with 100 ng/mL rrSCF and 4 U/mL EPO for two days.
Cell cycle and survival analysis
For assessment of cell cycle status of HSCs, mice were given a single i.p. injection of BrdU (250 mg/kg of body weight). Two hours later, BM cells were harvested and stained for surface markers and then fixed and stained with anti-BrdU antibody and 7-AAD using the Cytofix/Cytoperm Kit (BD Biosciences), according to the manufacturer’s instructions. For survival assays, the apoptotic cell population was determined by annexin V staining. Cells were analyzed by flow cytometry.
Quantitative real-time polymerase chain reaction
Total RNA was isolated using RNeasy Micro Kit (Qiagen). First-strand complementary DNA synthesis was primed with random hexamers (PE Applied Biosystems) from sample RNA by using the Sensiscript RT Kit (Qiagen). Real-time quantitative polymerase chain reaction (PCR) was carried out in an ABI Prism 7700 Sequence Detector using SYBR Green PCR Master Mix reagent (Applied Biosystems). Primer sequences were: mouse Rb1, forward 5′-atctacctcccttgccctgt-3′, reverse 5′-gaaggcgtgcaca gagtgta-3′; mouse E2f5, forward 5′-actcagggcctatccatgtg-3′, reverse 5′-ggggaacaggaaaaaccact-3′; and mouse Mcl1, forward 5′-tgggtttgtg-gagttcttcc-3′, reverse 5′-aaagccagcagcacatttct-3′.
Transplantation and engraftment assays
Donor BM cells were injected into the tail veins of recipient mice. For competitive repopulation analysis, donor BM cells were mixed with recipient BM cells at a 1:1 or 7:3 ratio and injected into the recipient mice. Chimerism (donor-derived cells) was determined by FACS analysis of peripheral blood or BM of the recipient mice after transplantation.
Results
mTOR deficiency causes pancytopenia
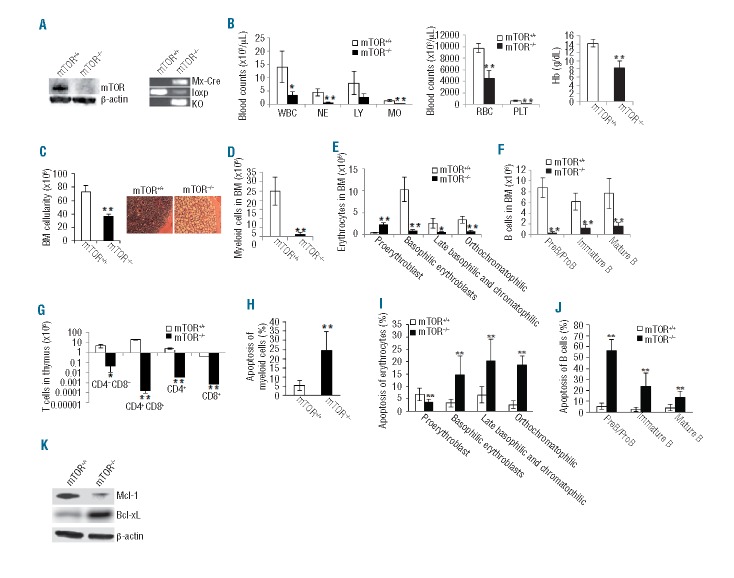
To define the physiological function of mTOR in hematopoiesis, we deleted mTOR gene in adult mTORloxp/loxp;Mx1-Cre+ mice by 6–8 i.p. injections of polyinosine-polycytidine (pIpC). Immunoblotting and genotyping confirmed mTOR deletion in BM cells of the pIpC-treated mTORloxp/loxp;Mx1-Cre+ (mTOR−/−) mice (Figure 1A). mTOR−/− mice died 7–17 days after pIpC injection (data not shown) and the mice were sacrificed for analysis in the time window. mTOR−/− mice displayed a marked decline in white blood cell counts (WBC) in peripheral blood, apparently due to reduced number of neutrophils (NE) and monocytes (MO) (Figure 1B). In addition, the mice showed a marked reduction in platelets (PLT) and severe anemia with significantly decreased red blood cells (RBC) and hemoglobin (Hb) compared to mTOR+/+ controls (Figure 1B). mTOR deficiency resulted in a 50% loss of BM cellularity, which was further verified by hematoxylin and eosin (H&E) staining (Figure 1C). Within the BM, total myeloid (Gr-1+Mac-1+) cell numbers were drastically reduced (Figure 1D and Online Supplementary Figure S1A), and erythroid progenitor differentiation was blocked at the proerythroblast stage (Figure 1E and Online Supplementary Figure S1B). B-cell progenitors were also reduced at all developmental stages (Figure 1F and Online Supplementary Figure S1C). Within the thymus, mTOR−/− mice exhibited a defect in T-cell progenitor differentiation as manifested by a marked reduction in CD4−CD8−, CD4+CD8+, CD4+, and CD8+ thymocytes (Figure 1G and Online Supplementary Figure S1D). The defects of mTOR−/− lineage-committed cells are associated with increased apoptosis in these cells including myeloid (Figure 1H and Online Supplementary Figure S1E), erythroid (Figure 1I and Online Supplementary Figure S1F) and B lineages (Figure 1J and Online Supplementary Figure S1G). To begin to understand the molecular mechanisms of increased apoptosis in mTOR−/− lineage-committed cells, we detected the expression of Mcl-1 and Bcl-xL, two critical pro-survival factors. mTOR−/− lineage-positive (Lin+) cells displayed a diminished expression of Mcl-1 but increased level of Bcl-xL (Figure 1K). It seems that the increased apoptosis in mTOR−/− lineage-committed cells is due, at least partially, to decreased Mcl-1, whereas increased Bcl-xL might be a compensatory effect of increased apoptosis. Taken together, mTOR is essential for all hematopoietic lineage development.
Figure 1.
mTOR deficiency causes impaired hematopoiesis. (A) Western blotting of mTOR protein (left) and PCR genotyping mTOR loxP and knockout (KO) alleles (right) in bone marrow (BM) cells. The Mx1-Cre allele was also genotyped. (B) Blood count parameters. WBC: white blood cell; NE: neutrophil; LY: lymphocyte; MO: monocyte; RBC: red blood cell; PLT: platelet; Hb: hemoglobin. (C) Total number of BM cells (left) and hematoxylin and eosin staining of bone sections (right). (D) The number of myeloid cells (Gr1+Mac1+) in BM. (E) The numbers of erythroid lineages in BM were quantified by FACS. Erythroid cells at different developmental stages include proerythroblasts (Ter119med CD71hi), basophilic erythroblasts (Ter119hiCD71hi), late basophilic and chromatophilic erythroblasts (Ter119hiCD71med), and orthochromatophilic erythroblasts (Ter119hiCD71lo). (F) The numbers of B-cell lineage in BM were analyzed by FACS. B cells at different developmental stages include preB/proB (B220loIgM−), immature B (B220loIgM+), and mature B(B220hiIgM+). (G) The numbers of T-cell lineage in thymus were analyzed by FACS. (H–J) The percentages of apoptotic myeloid cells (H), erythrocytes (I) and B cells (J) were analyzed by Annexin V staining. (K) Western blotting of Mcl-1 and Bcl-xL in lineage positive (Lin+) BM cells. Mouse number for each group n=5. *P<0.05; **P<0.01. Error bars represent mean ± s.d.
mTOR regulates HSC and hematopoietic progenitor cell homeostasis
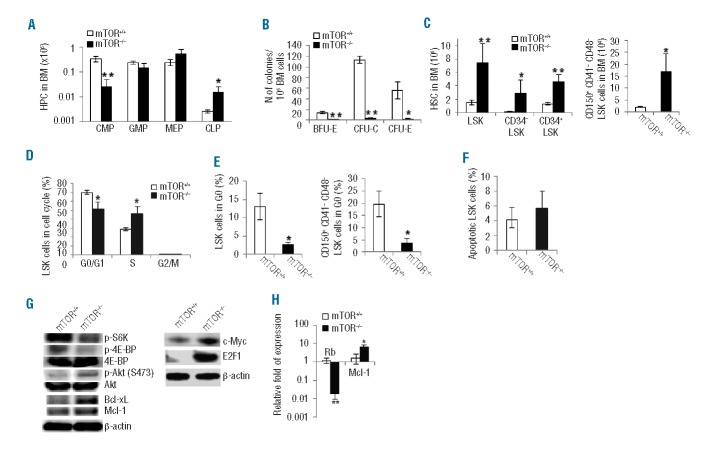
The widespread effect of mTOR deficiency on blood lineages prompted us to examine the effect of mTOR knockout on HSCs and hematopoietic progenitor cells (HPCs). In the HPC compartments, we detected a decrease in common myeloid progenitors (CMPs) but an increase in common lymphoid progenitors (CLPs) in mTOR−/− mice compared to mTOR+/+ controls (Figure 2A and Online Supplementary Figure S2A). Further analysis revealed that mTOR deficiency impaired colony-forming ability of HPCs as shown by markedly reduced activities of BFU-E, CFU-E, and CFU-C (Figure 2B). In the HSC compartments, mTOR−/− BM contained approximately 4-fold more primitive LSK (Lin− scal+c-kit+) cells than mTOR+/+ controls (Figure 2C and Online Supplementary Figure S2A). The increased LSK cells in mTOR−/− BM appeared to stem from an increase in both short-term (CD34+LSK) and long-term (CD34−LSK or CD150+CD41−CD48−LSK) HSCs (Figure 2C and Online Supplementary Figure S2A and B). A BrdU labeling assay revealed a 60% increase in mTOR−/− LSK cells at the S phase (BrdU+) of the cell cycle compared to mTOR+/+ controls (Figure 2D and Online Supplementary Figure S2C) indicating that mTOR deficiency leads to a loss of HSC quiescence. Indeed, we found that mTOR deficiency caused a significant reduction in LSK cells or more primitive CD150+CD41−CD48−LSK cells at the G0 phase (Figure 2E and Online Supplementary Figure S2D). In contrast to the cell cycle alterations, no difference in apoptosis was detected between mTOR−/− and mTOR+/+ LSK cells (Figure 2F and Online Supplementary Figure S2E); this is unexpected given the recognized role of mTOR in regulating cell survival.2,3 Interestingly, although mTOR deficiency does not affect survival status of LSK cells, it increases apoptosis of more mature blood cell lineages including myeloid and erythroid cells (Figure 1H and I and Online Supplementary Figure S1E and F), suggesting a differentiation stage-specific role for mTOR.
Figure 2.
Deletion of mTOR affects HSC and HPC homeostasis. (A) The numbers of common myeloid progenitors (CMPs) (Lin−Sca1−c-Kit+CD34+CD16/CD32mid), granulocyte-macrophage progenitors (GMPs) (Lin−Sca1−c-Kit+CD34+ CD16/CD32hi), megakaryocyte-erythroid progenitors (MEPs) (Lin−Sca1−c-Kit+CD34−CD16/CD32lo), and common lymphoid progenitors (CLP) (Lin−IL7R+Sca1medc-Kitmed-hi) in bone marrow (BM) were analyzed by FACS. (B) The colony-forming activities of CFU-E, CFU-C and BFU-E were examined using the total BM cells. (C) The numbers ofLSK (Lin− scal+c-kit+), CD34−LSK, CD34+LSK, and CD150+CD41−CD48−LSK cells in BM were analyzed by FACS. (D) Cell cycle profile of LSK cells. LSK cells were labeled with BrdU in vivo, followed by BrdU and 7-AAD staining and FACS analysis (G0/G1:BrdU−7-AADlo; S:BrdU+; G2/M:BrdU−7-AADhi). (E) The percentages of LSK progenitor cells and CD150+CD41-CD48-LSK stem cells in G0 phase (pyronin Y-Hoechst 33342lo) of cell cycle were determined. (F) The percentage of apoptotic LSK cells was analyzed by Annexin V staining. (G) Western blot analysis of the signaling activities in Lin- cells. (H) Quantitative RT-PCR analysis of LSK cells for the expression of indicated genes. n=3–5 in each test group. *P<0.05; **P<0.01. Error bars represent mean ± s.d.
To examine the molecular mechanisms related to the accelerated cell cycle status and the apparently unaltered survival activity in mTOR−/− HSCs and HPCs, we first analyzed the activities (phosphorylation) of S6K and 4E-BP that act downstream of mTOR to promote ribosome biogenesis essential for cell growth and survival.2,3mTOR−/− Lin- cells had attenuated activities of S6K and 4E-BP in vivo (Figure 2G) or in response to stem cell factor (SCF) stimulation in vitro (Online Supplementary Figure S3). mTOR deficiency in Lin− or LSK cells resulted in a marked increase in cell cycle-promoting Myc and E2F1 and a downregulation of the cell cycle suppressor Rb (Figure 2G and H). In addition, increased basal activity of Akt and higher levels of Bcl-xL and Mcl-1 were evident in mTOR−/− Lin− or LSK cells (Figure 2G and H). The increased Akt phosphorylation in these cells may be ascribable to the absence of the well-documented S6K-mediated inhibitory feedback.11,12 These results indicate that mTOR is involved in balanced signaling of stem and progenitor cell survival and proliferation, and is required for maintaining HSC quiescence.
Because Mx1-cre expression is not restricted in hematopoietic cells, the observed HSC phenotypes in mTOR−/− mice could be due to a perturbed BM microenvironment. To overcome this caveat, we transplanted mTORloxp/loxp;Mx1−Cre+ or control mTORloxp/loxp;Mx1-Cre- BM cells into lethally irradiated BoyJ congenic mice and induced mTOR deletion in blood lineages two months after engraftment (Online Supplementary Figure S4A). The mTOR−/− recipient mice phenocopied primary mice showing an increase in total, CD34− and CD34+ LSK cell numbers and a decrease in myeloid cells, erythrocytes, and B cells in the BM and T cells in thymus compared to mTOR+/+ recipient mice (Online Supplementary Figure S4B–F), indicating that mTOR plays a cell autologous role in maintaining HSC and early progenitor homeostasis and hematopoiesis.
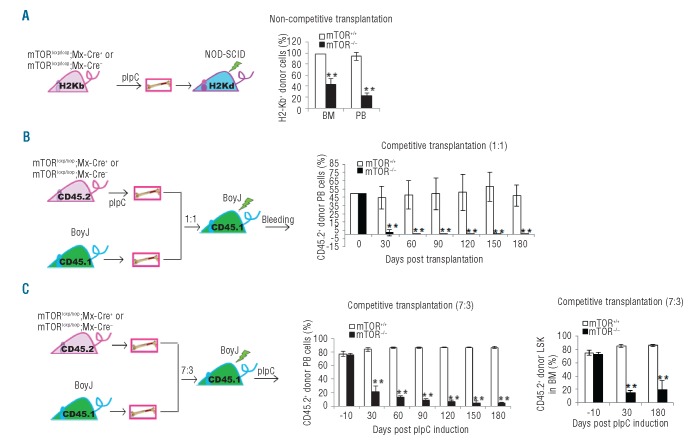
mTOR regulates HSC and HPC repopulating potential
To further examine the role of mTOR in stem cell regulation, we next determined the impact of mTOR deficiency on HSC repopulating capability. In a non-competitive setting, mTOR deletion was induced before BM transplantation into immunodeficient NOD/SCID mice (Figure 3A). The mTOR loss caused a decrease in H2Kb+ donor-derived cells in the BM and peripheral blood 30 days after transplantation (Figure 3A and Online Supplementary Figure S5A). In a competitive setting, mTOR deletion was induced either before (Figure 3B) or after (Figure 3C) transplantation of donor cells with recipient competitor cells into congenic mice. A progressive decrease in CD45.2+ donor-derived cells was detected in the peripheral blood in mTOR−/− recipient mice ((Figure 3B and C and Online Supplementary Figure S5B). Similarly, the mTOR−/− CD45.2+ LSK cells were significantly reduced 30 days after pIpC induction compared to mTOR+/+ controls (Figure 3C and Online Supplementary Figure S5C). Therefore, mTOR deficiency causes defective engraftment and hematopoietic repopulation of stem/progenitor cells in transplantation models.
Figure 3.
mTOR deficiency causes an impaired repopulating potential of HSCs. (A) Left: schematics of donor transplantation into NOD-SCID mice. Right: bone marrow (BM) cells from pIpC-treated donor mice were transplanted into sub-lethally irradiated NOD/SCID mice. The donor-derived cells in BM and peripheral blood (PB) of recipient mice were analyzed identified by H2-Kb+ staining. (B) Left hand side: schematics of competitive donor transplantation into syngeneic BoyJ mice. Right: BM cells from pIpC-treated donor mice (C57Bl/6) were mixed at 1:1 ratio with BM cells from BoyJ recipient mice. The cell mixtures were transplanted into lethally irradiated BoyJ rmice. The donor-derived cells were identified by CD45.2+ staining. (C) Left: schematics of competitive donor transplantation into syngeneic BoyJ mice. Middle and right: BM cells from donor mice were mixed at 7:3 ratio with BM cells from BoyJ recipient mice. The cell mixtures were transplanted into lethally irradiated BoyJ mice. The recipient mice were injected with pIpC 2 months post transplantation. The percentage of overall donor-derived cells in PB of the recipient mice were analyzed at various days post pIpC induction by CD45.2 staining (middle). The percentage of donor-derived LSK cells in BM of the recipient mice were determined at 30 days and 180 days post pIpC induction (right). N=3–5. **P<0.01. Error bars represent mean ± s.d.
Discussion
In this study, we have found that mTOR knockout mice develop BM failure due to impaired HSC homeostasis, engraftment, and differentiation. mTOR deficiency drives HSCs from quiescence into rapid cycling and transiently increases HSC number. Such a transient increase in HSCs associated with a loss of quiescence may lead to their long-term exhaustion.6–8 Indeed, mTOR deficiency results in a depletion of HSCs over time in competitively transplanted mice. Whether increased cycling of the stem cells causes a decline and exhaustion of the HSCs in primary mTOR−/− mice is difficult to determine due to the rapid death of the animal. Interestingly, mTOR deficiency causes certain hematopoietic phenotypes similar to that of TSC1 or PTEN deletion that causes mTOR activation, suggesting that a tightly regulated mTOR activity is required for HSC maintenance and hematopoiesis. mTOR deficiency causes reduced engraftment, which differs from a recent report that addition of the mTOR inhibitor, rapamycin, to a cytokine cocktail of SCF, TPO and FL enhances the engraftment of human CD34+ cord blood cells into NSG mice.13 Such a discrepancy highlights the need for careful examinations of genetic models that may be helpful in revealing differences in effects between drug targets such as mTOR and chemical inhibitors that may not be specific to a target or may affect only a part of the target path-ways. mTOR signals through two parallel molecular complexes: mTOR complex 1 ( mTORC1) and mTORC2.1 Most of the hematopoietic phenotypes in mTOR-deficient mice recapitulate that of Raptor, an essential component of mTORC1, deficient mice. For instance, both mTOR deficiency and Raptor deficiency cause loss of quiescence and increased cell cycling of HSCs, without affecting their survival status.14,15 In line with recent reports that ablation of mTORC2 component Rictor has negligible effects on the majority of hematopoietic cells,16–18 mTOR appears to function in HSCs and hematopoietic lineage development dependent of mTORC1, but not of mTORC2. One exception is that mTOR regulation of T-lymphocyte development requires both mTORC1 and mTORC2, because deletion of either Raptor (data not shown) or Rictor impairs thymocyte differentiation.16,17 Interestingly, while mTOR deficiency causes a reduction in myeloid cells, these cells are increased in Raptor−/− or Raptor−/−; Rictor−/− mice.14 Thus, mTOR may act in a way that is not strictly dependent on mTORC1 and mTORC2 in myeloid cells. In support of this, we have found that mTOR regulates FANCD2, a key component of Fanconi anemia DNA repair pathway, and genomic stability independent of mTORC1 or mTORC2 in HSCs.19 Similarly, mTOR deficiency in muscle cells more severely affects muscle contractile properties and reduces dystrophin expression compared to Raptor−/−, Rictor−/−, or Raptor−/−; Rictor−/− mice.11 Furthermore, mTOR knockdown by RNAi represses translation efficiency of terminal oligopyrimidine mRNA, whereas Raptor or Rictor deficiency has only a marginal effect on terminal oligopyrimidine mRNA translation.20 Thus, it is likely that mTOR functions through mTORC1 and/or mTORC2 is contingent upon cell type and physiological context.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
YZ is supported by an NIH grant (NIH P30 DK090971).
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Gan B, DePinho RA. mTORC1 signaling governs hematopoietic stem cell quiescence. Cell Cycle. 2009;8(7):1003–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster KG, Fingar DC. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem. 2010;285(19):14071–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Proud CG. The multifaceted role of mTOR in cellular stress responses. DNA Repair (Amst). 2004;3(8–9):927–34 [DOI] [PubMed] [Google Scholar]
- 4.Raslova H, Baccini V, Loussaief L, Comba B, Larghero J, Debili N, et al. Mammalian target of rapamycin (mTOR) regulates both proliferation of megakaryocyte progenitors and late stages of megakaryocyte differentiation. Blood. 2006;107(6):2303–10 [DOI] [PubMed] [Google Scholar]
- 5.Sathaliyawala T, O’Gorman WE, Greter M, Bogunovic M, Konjufca V, Hou ZE, et al. Mammalian target of rapamycin controls dendritic cell development downstream of Flt3 ligand signaling. Immunity. 2010;33(4):597–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martelli AM, Evangelisti C, Chiarini F, Grimaldi C, Cappellini A, Ognibene A, et al. The emerging role of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling network in normal myelopoiesis and leukemogensis. Biochim Biophys Acta. 2010;1803(9):991–1002 [DOI] [PubMed] [Google Scholar]
- 7.Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441(7092):518–22 [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Liu Y, et al. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205(10):2397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, et al. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol Cell Biol. 2004;24(21):9508–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30(6):832–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Risson V, Mazelin L, Roceri M, Sanchez H, Moncollin V, Corneloup C, et al. Muscle inactivation of mTOR causes metabolic and dystrophin defects leading to severe myopathy. J Cell Biol. 2009;187(6):859–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Um SH, D’Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 2006;3(6):393–402 [DOI] [PubMed] [Google Scholar]
- 13.Rohrabaugh SL, Campbell TB, Hangoc G, Broxmeyer HE. Ex vivo rapamycin treatment of human cord blood CD34+ cells enhances their engraftment of NSG mice. Blood Cell Mol Dis. 2011;46(4):318–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalaitzidis D, Sykes SM, Wang Z, Punt N, Tang Y, Ragu C, et al. mTOR complex 1 plays critical roles in hematopoiesis and Pten-loss-evoked leukemogenesis. Cell Stem Cell. 2012;11(3):429–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoshii T, Tadokoro Y, Naka K, Ooshio T, Muraguchi T, Sugiyama N, et al. mTORC1 is essential for leukemia propagation but not stem cell self-renewal. J Clin Invest. 2012;122(6): 2114–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang F, Wu Q, Ikenoue T, Guan KL, Liu Y, Zheng P. A critical role for rictor in T lymphopoiesis. J Immunol. 2012;189(4):1850–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee K, Nam KT, Cho SH, Gudapati P, Hwang Y, Park DS, et al. Vital roles of mTOR complex 2 in Notch-driven thymocyte differentiation and leukemia. J Exp Med. 2012;209(4):713–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magee JA, Ikenoue T, Nakada D, Lee JY, Guan KL, Morrison SJ. Temporal Changes in PTEN and mTORC2 Regulation of Hematopoietic Stem Cell Self-Renewal and Leukemia Suppression. Cell Stem Cell. 2012;11(3):415–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo F, Li J, Du W, Zhang S, O’Connor M, Thomas G, Kozma S, et al. mTOR regulates DNA damage response through NF-κB-mediated FANCD2 pathway in hematopoietic cells. Leukemia. 2013. March 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patursky-Polischuk I, Stolovich-Rain M, Hausner-Hanochi M, Kasir J, Cybulski N, Avruch J, et al. The TSC-mTOR pathway mediates translational activation of TOP mRNAs by insulin largely in a raptor- or rictor-independent manner. Mol Cell Biol. 2009;29(3):640–9 [DOI] [PMC free article] [PubMed] [Google Scholar]