Abstract
Accumulation of myocardial iron is the cause of heart failure and early death in most transfused thalassemia major patients. T2* cardiovascular magnetic resonance provides calibrated, reproducible measurements of myocardial iron. However, there are few data regarding myocardial iron loading and its relation to outcome across the world. A survey is reported of 3,095 patients in 27 worldwide centers using T2* cardiovascular magnetic resonance. Data on baseline T2* and numbers of patients with symptoms of heart failure at first scan (defined as symptoms and signs of heart failure with objective evidence of left ventricular dysfunction) were requested together with more detailed information about patients who subsequently developed heart failure or died. At first scan, 20.6% had severe myocardial iron (T2*≤10ms), 22.8% had moderate myocardial iron (T2* 10–20ms) and 56.6% of patients had no iron loading (T2*>20ms). There was significant geographical variation in myocardial iron loading (24.8–52.6%; P<0.001). At first scan, 85 (2.9%) of 2,915 patients were reported to have heart failure (81.2% had T2* <10ms; 98.8% had T2* <20ms). During follow up, 108 (3.8%) of 2,830 patients developed new heart failure. Of these, T2* at first scan had been less than 10ms in 96.3% and less than 20ms in 100%. There were 35 (1.1%) cardiac deaths. Of these patients, myocardial T2* at first scan had been less than 10ms in 85.7% and less than 20ms in 97.1%. Therefore, in this worldwide cohort of thalassemia major patients, over 43% had moderate/severe myocardial iron loading with significant geographical differences, and myocardial T2* values less than 10ms were strongly associated with heart failure and death.
Introduction
Over 300,000 children are born each year with a hemoglobinopathy, of whom more than 25,000 have thalassemia major (TM) and need regular transfusions to survive beyond infancy. Of the TM patients who receive transfusion, less than half obtain adequate iron chelation therapy and approximately 3,000 die each year due to uncontrolled iron overload in their mid-20s.1 These figures almost certainly underestimate the true frequency of TM, but still indicate that TM is a substantial health issue worldwide. Myocardial iron overload leading to heart failure remains the leading cause of death. The onset of ventricular dysfunction can be rapid and results from iron toxicity including oxidative damage to membrane lipids and enzymes in the mitochondrial respiratory chain,2 which occurs as a late phenomenon after a prolonged period of iron loading.3 Cardiovascular magnetic resonance (CMR) using T2* is routinely used in many countries for identifying patients with myocardial iron loading and guiding chelation therapy tailored to the heart. Myocardial T2* is calibrated to the myocardial iron concentration,4 and has been shown to improve with intensive iron chelation therapy in parallel with the ejection fraction.5 Myocardial T2* is the only validated non-invasive measure of myocardial iron loading and is superior for this purpose to surrogates such as serum ferritin, liver iron, ventricular ejection fraction and tissue Doppler parameters.3,6–10 Although worldwide survival is still poor,11 life expectancy is increasing in some countries with regular blood transfusion and well-managed iron chelation therapy.12–14 In most cases, chronic myocardial siderosis is both preventable and reversible with modern chelation regimes.15–17 Progress has also been made in treatment of acute heart failure and LV systolic dysfunction.18,19 A substantial 71% decrease in deaths has been observed in the UK thalassemia cohort since the introduction of T2* CMR and improved iron chelation.20,21 Other countries have also reported improved management of cardiac iron using T2* CMR.22–25 Whilst myocardial T2* has shown strong prognostic value in the UK cohort for prediction of cardiac complications,26 the data are limited on the burden of cardiac iron loading or the application of CMR T2* in different parts of the world. We therefore performed an international survey of centers that use T2* to assess its wider clinical application and the degree of myocardial iron loading in a “real-life” setting.
Methods
CMR parameters
Myocardial T2* was derived from the decay curve generated from a multi-echo gradient echo sequence with a range of echo times (TE), as previously described.27 Whilst the exact sequence parameters varied between scanners, it has been shown that T2* measurement is robust to these minor differences and transferable between scanners across multiple different vendor platforms.28–31 All T2* measurements were performed at 1.5T by experienced operators at each site using a single mid-ventricular short axis slice with a region of interest (ROI) in the septum. Centers used Thalassemia-tools (a plugin of CMRtools, Cardiovascular Imaging Solutions, London, UK) or other commercial software for the calculation of T2*. Left ventricular ejection fraction was calculated using a standardized protocol from a stack of equally spaced short-axis cine slices.32
Recruitment of sites
The lead clinician in 65 TM centers was approached by email and invited to participate. Forty-seven centers expressed an initial interest and 35 completed surveys were returned with full information (a response rate of 74% with a total of 3,445 patients). The majority of centers (27 of 35) involved in the initial data collection for this study had undergone rigorous quality control with site visits and training in both T2* acquisition and image analysis. Only data from these validated sites were included in the final analysis for this study (27 sites with a total of 3095 patients). All data were fully anonymized.
Baseline data collection
Each center was asked for baseline data on TM patients who had undergone T2* CMR scans between 2001 and 2008. For the patients’ first scan, we requested the numbers of patients in three strata of severity of myocardial iron loading. These were: T2* less than 10ms (severe); 10–20ms (moderate), and more than 20ms (normal). At the time of this first scan, we also requested: a) the proportion of patients taking iron chelation; b) the proportion who had been on iron chelation for more than five years; and c) the total number of patients with heart failure defined as symptoms (such as breathlessness) or signs of cardiac failure associated with objective evidence of ventricular dysfunction at rest (reduced left ventricular ejection fraction <56% measured by CMR). This cutoff value was derived from the lower limit of the normal range for LV ejection fraction.32
Cardiovascular outcomes
To assess the usefulness of T2* in predicting serious adverse outcomes, we asked for the number of patients who had either died from cardiac causes or had developed heart failure after the first CMR scan and for the prior myocardial T2* value to be confirmed for each case.
Ethics and statistics
The study protocol was reviewed by the institutional research ethics committee. A waiver of informed consent was approved for the anonymous data collection associated with this project. All centers were required to obtain local permission for the use of anonymized data and, where necessary, full local institutional review board approval was granted. All data were analyzed using STATA version 10.1 (StataCorp, Texas, USA). Geographical differences were assessed using a χ2 test. Linear regression analysis was performed to assess association between variables. P<0.05 was considered significant.
Results
Worldwide use of T2*
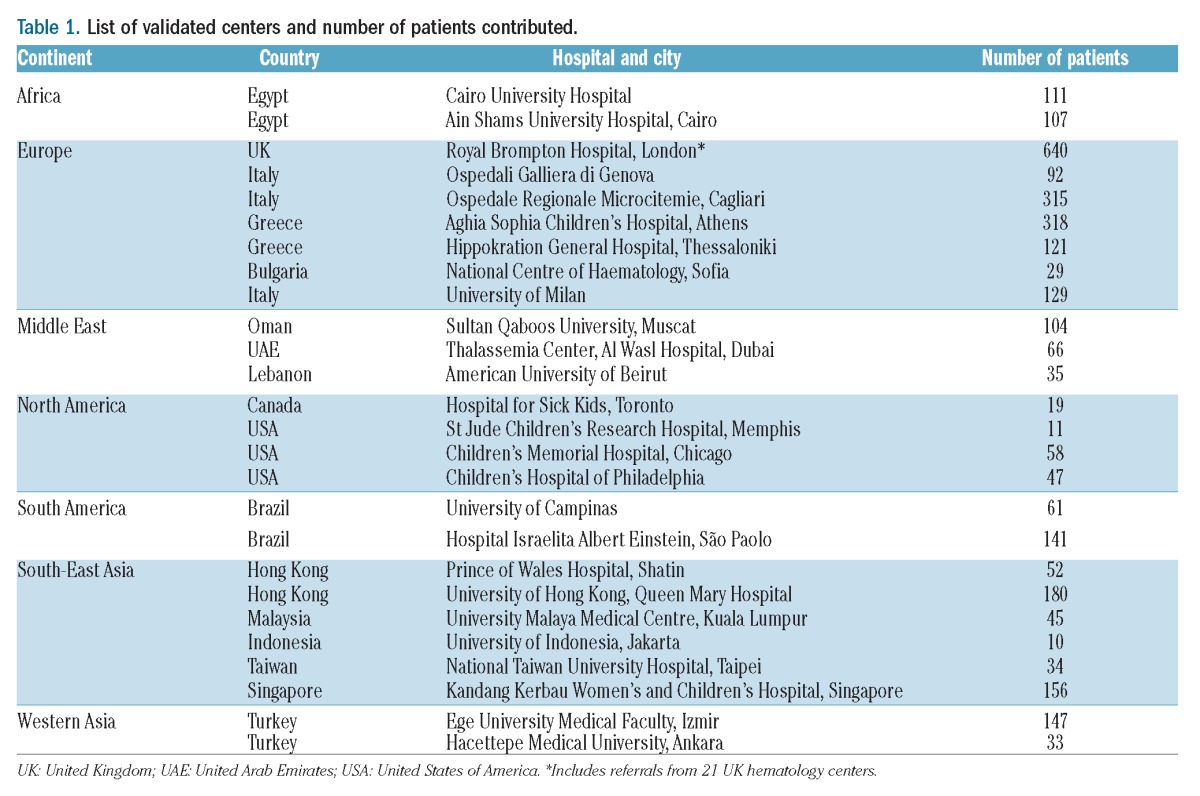
Data were analyzed from 27 fully validated centers that returned completed survey results, representing a total of 3,095 TM patients from Europe, the Middle East, North America, South America, North Africa and Asia. A full list of the sites and patient numbers reported in this study is given in Table 1.
Table 1.
List of validated centers and number of patients contributed.
Baseline findings at first CMR scan
The baseline T2* values revealed that 56.6% of patients had no significant cardiac iron loading (T2* >20ms), 22.8% had moderate loading (T2* 10–20ms) and 20.6% had severe loading (T2* ≤10ms). Overall, 43.4% of patients had myocardial iron loading with a T2* less than 20ms. The prevalence of myocardial iron loading (T2* <20ms) varied significantly between regions with the lowest level being found in patients from Egypt (24.8%) and the highest in South-East Asia (52.7%; P<0.001). A similar distribution was found for the prevalence of severe iron loading (T2* <10ms) with significant geographical differences (P<0.001). A full breakdown of geographical variation in terms of percentage of patients with T2* less than 20ms and less than 10ms, together with odds ratios of having T2* less than 20ms and less than 10ms (with Europe as the reference group), is shown in Table 2 and presented graphically in Figures 1 and 2. A very high proportion (96.7%) of patients undergoing their first T2* scan were taking regular iron chelation and 92.9% had been taking iron chelation therapy for more than five years. In 11 of the 27 centers, all patients had been on chelation therapy for more than five years at the time of their first scan.
Table 2.
Geographical difference in T2*.
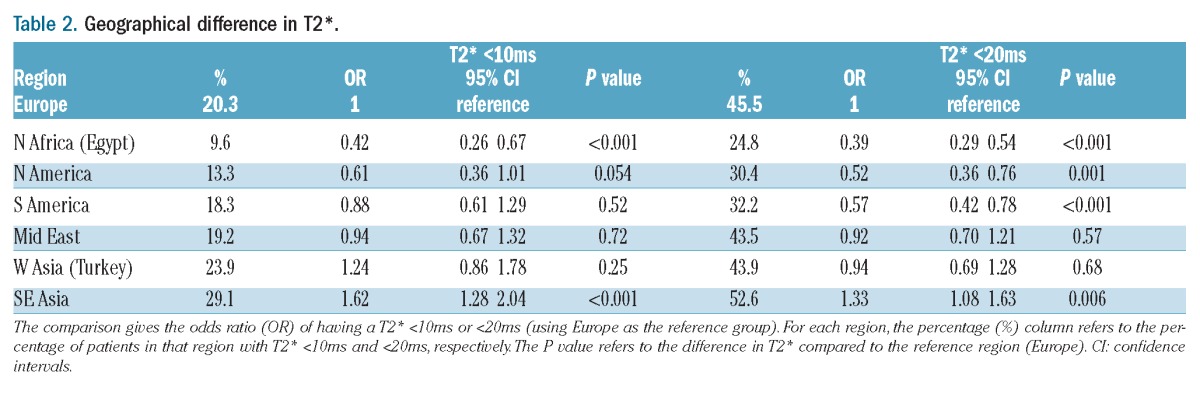
Figure 1.
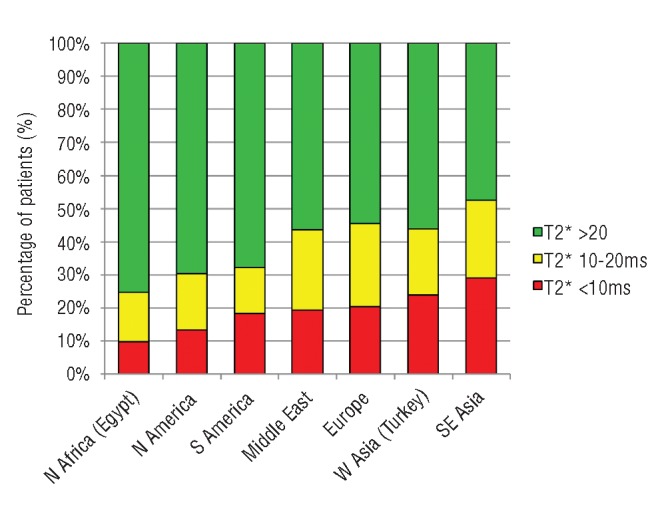
Myocardial iron loading by region. Percentage of patients with myocardial T2* values <10ms (red), between 10 and 20ms (yellow) and >20ms (green) at baseline scan. Data are presented in Table 2 with regions in order of increasing percentage of iron loading (T2* <20ms).
Figure 2.
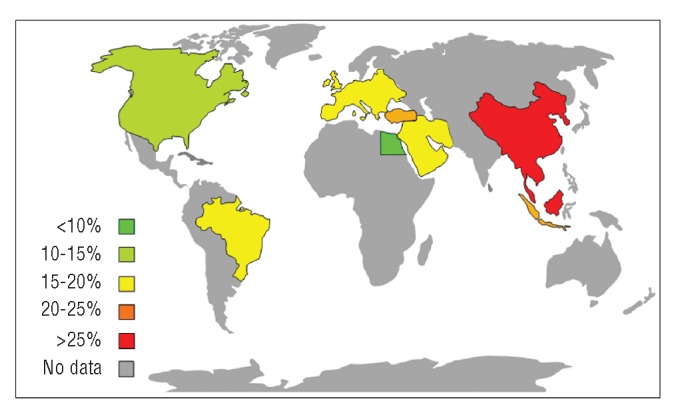
World map showing the distribution of the prevalence of severe myocardial iron loading at first CMR scan. The color scale represents the percentage of patients in each region who have severe myocardial iron loading with T2* values <10ms.
Prevalence of heart failure at first scan and T2* distribution
For 180 of the 3,095 patients, there was not enough information available from the clinical records to allow the centers to make an accurate diagnosis of heart failure. Therefore, these patients were excluded from the heart failure analysis. For the remaining 2,915 patients, 85 (2.9%) had confirmed heart failure at the time of the first scan with NYHA class II or above symptoms and ventricular dysfunction at rest. The majority of these patients (n=69, 81.2%) had T2* values less than 10ms with almost all patients (n=84, 98.8%) having T2* values less than 20ms (Figure 3A).
Figure 3.
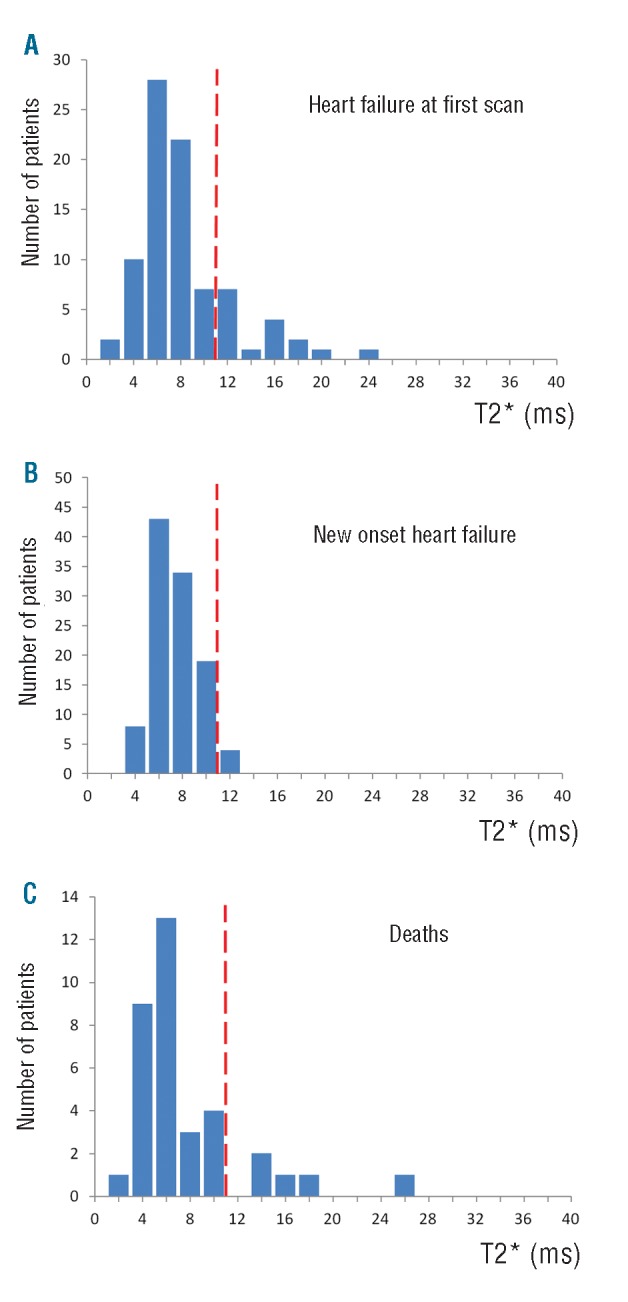
Histograms of the distribution of T2* values. The broken red line represents a T2* value of 10ms. A. Myocardial T2* values for patients with heart failure at first scan. B. Myocardial T2* values for patients who developed new onset heart failure subsequent to their initial CMR scan. C. Myocardial T2* values for patients who died of cardiovascular causes subsequent to their initial CMR scan.
T2* predicting the development of new onset heart failure
In the cohort of patients who did not have heart failure at the first scan (n=2,830), there were 108 (3.8%) who subsequently developed heart failure during follow up. The overwhelming majority of these patients (n=104, 96.3%) had T2* values less than 10ms. All patients (100%) who went on to develop heart failure had a myocardial T2* of less than 20ms with no patient with a T2* value of more than 12ms (Figure 3B). The median time (Q1, Q3) from scan to onset of symptoms was 140 (7, 259) days but there was no relationship between myocardial T2* and time to onset of heart failure (R2 <0.001; P=0.86). Of the 637 patients with T2* less than 10ms at first scan, 104 (16.3%) developed heart failure in comparison to 4 (0.6%) of the 705 patients with T2* between 10 and 20ms (P<0.0001), giving an odds ratio of 32.3 for developing new-onset heart failure if T2* was less than 10ms versus T2* between 10 and 20ms.
Deaths
There were 35 cardiac deaths reported. The distribution of T2* values (Figure 3C) showed that the majority (n=30, 85.7%) were less than 10ms, with 34 patients (97.1%) having a myocardial T2* less than 20ms. There was one outlier value with a high T2* (24.1ms). It was confirmed that the primary cause of death was cardiac failure and the scan images were reviewed to check the T2* measurement. No problem with the initial T2* measurement was identified. The median time (Q1, Q3) from scan date to death was 226 [65, 353] days, and again there was no relationship between T2* and time to death (R2=0.05; P=0.24). Of patients with T2* less than 10ms at first scan, 4.7% died in comparison to 0.6% of those who initially had T2* between 10 and 20ms (P<0.0001), giving an odds ratio of 8.6 for death if T2* was less than 10ms versus T2* between 10 and 20ms.
Discussion
We have presented summary results for myocardial T2* from 3,095 TM patients, which represents approximately 8% of the most recently published WHO estimate of 37,866 patients worldwide who receive chelation therapy (although these numbers are considered an underestimate).1 These data from 27 validated centers demonstrate 4 major findings: 1) the primary dissemination of T2* CMR technology has been successfully achieved with robust independent operation of individual sites in a wide geographical distribution; 2) myocardial T2* values of less than 10ms have important and generalized prognostic value across the world to predict future heart failure and death, which agrees with findings from the UK,26 and suggests that T2* CMR has the potential to play a significant global role in the identification of patients with myocardial iron load who are at increased risk of events, and in the prevention of events through early tailored cardiac iron chelation treatment; 3) there is a high international prevalence of myocardial iron overload with 43.4% having a myocardial T2* less than 20ms, and 20.6% having a T2* less than 10ms; and 4) there is considerable geographical variation in myocardial iron overload.
The observed association of myocardial T2* less than 10ms with the future development of heart failure proved generally robust in this survey, but some cases were seen with higher myocardial T2* values. As a result, the strength of lower T2* values for associating patients with risk of heart failure in this “real-life” study is less than in previous reports.26 There are several possible explanations for this finding. First, we cannot exclude technical issues associated with the T2* CMR acquisition and image analysis that might have resulted in incorrectly high T2* values as overestimation of T2* can occur when the minimum echo time (TE) is too long.33 However, most modern scanners are capable of running a minimum TE of ~2ms, which makes this potential source of error unlikely. All centers in this survey had undergone a validation and teaching process at the time of T2* scanning initiation, with a visit by a clinical/physics team and scanning of phantoms of known T2* values for verification of accuracy. We believe this process is important for clinical governance. In general, it is a more common error to overestimate T2* rather than to underestimate it at the data analysis stage if the data noise in the signal intensity values at long TE is not handled appropriately.34 Second, the diagnosis of heart failure in TM patients may not be clear-cut. This is because of the chronic anemia, high cardiac index and general symptomatology of TM patients. We required an objective measure of depressed ejection fraction for diagnosis of heart failure in order to minimize false positive diagnosis, but analysis error and false positive identification of cases cannot be excluded. Third, it is likely that some cases of heart failure result from causes other than myocardial iron overload, with the leading alternative cause being myocarditis. Other than myocarditis, there is also potential for a tachycardia-related cardiomyopathy or high cardiac output heart failure in these chronically anemic patients.35 Expert opinion generally holds that occasional cases of fulminant heart failure have been seen with normal or only mildly abnormal myocardial T2*. There have been reports in the past of a more common occurrence of myocarditis from Greece,36 but only one case report which showed myocarditis with a normal myocardial T2* but no heart failure.37 Our survey data support the possible occurrence of myocarditis as a cause of heart failure, although it appears to be rare and, in general, mild to moderate myocardial iron loading may be an important cofactor in its development, as new-onset heart failure with myocardial T2* values more than 20ms was not seen, and only one of the 85 patients (1.2%) with heart failure at the time of first CMR scan had myocardial T2* more than 20ms (with an absolute T2* value of 23.6ms). It is known that these T2* values correspond to a myocardial iron concentration of approximately 1.0mg/g dry weight,4 which is still above the upper limit of normal for the heart of 0.5 mg/g dry weight.38 Only one cardiac death was recorded in association with a myocardial T2* more than 20ms.
The variation in myocardial iron loading around the world is of interest (Figure 2). This could be due to several possible factors. One major factor is the relative utilization of transfusions and iron chelation treatment. All patients were transfused in this survey and over 96% were receiving regular iron chelation, with almost 93% on chelation for at least five years. We do not have data on the number of transfusions per year, the pre-transfusion hemoglobin threshold, or the type and dose of iron chelator, so no conclusions can be drawn on this issue. With modern migration, patients within each geographical region are somewhat mixed, which is likely to reduce any real observable differences between regions. Although we believe the patients from each center to be representative of the center and the region, we cannot exclude selection bias. This is of particular interest in relation to the very low levels of iron loading in the Egyptian population. Whether this represents the fact that only those patients with adequate chelation therapy have access to T2* or that this is a younger population remains unclear. We did not collect data on patient age. It is also possible that access to “tailored” chelation therapy is higher in the Egyptian than in the south-east Asian cohort and where the availability of chelation is limited, it is possible that there would be a greater degree of cardiac iron loading. Of note, there are also differences in patient cohorts between different regions with a higher number of transfusion-dependent hemoglobin E/beta thalassaemia patients in South-East Asia; however, the current study only looked at patients with beta thalassemia major.
One intriguing possibility that arises, however, is that the data are also compatible with differing pre-disposition to cardiac iron loading. This might arise from variations in genetic modifiers of cardiac iron loading. While for the moment there are little data to support this hypothesis, it would be interesting to compare any genetic modifiers that might be found in the future between the Egyptian and the south-east Asian populations, the two groups that showed widest difference in prevalence.
Limitations
Our survey was of a highly selected population of TM patients and represents just the tip of the iceberg of the worldwide burden of disease. The majority of patients with TM live in countries with limited access to transfusion and regular chelation therapy, and for these patients advanced imaging modalities such as CMR are not affordable or available. CMR becomes important in the larger scheme of healthcare provision only when the basics of care for TM patients have been satisfied. Limitation of access to CMR could lead to systematic bias in disease prevalence, although it is not certain whether this would cause under or overestimation of cardiac iron loading. However, in regions where transfusion is available but access to CMR is either limited or unavailable, it is likely that there would be more patients with excess cardiac iron. We also need to consider how long centers have had both CMR T2* and appropriate chelation therapy available with which to tailor patient care.
A further limitation of this study was the clinical confirmation of heart failure. In some centers, there was not enough clinical data available to allow accurate diagnosis of heart failure and, therefore, although the LV ejection fraction was known, it was not possible to analyze the T2* values with respect to heart failure in these patients. This resulted in the exclusion of 180 patients from this part of the analysis. Each center used its own approved software to determine LV ejection fraction and it is acknowledged that this could give rise to variations in LVEF. In turn, this could lead to variation in the local diagnosis of heart failure according to the specified criteria. Each center performed its own T2* analysis independently and there are possible small differences in T2* which could result between centers, although the effects are unlikely to be clinically significant. A central ‘core-lab’ approach for measurement of both T2* and EF was not a practical option given the numbers involved. However, this could be considered for future studies. In centers where detailed site visits for validation and setup were not performed, data were not included in the final analysis. This limited the total number of patients. However, the 8 centers which did not have this level of validation returned data on 300 patients (8.8% of the total number) and had similar event rates to all remaining sites with 3 deaths (8.1% of the total number of deaths) and 8 new-onset heart failure (6.9% of the total number of patients with new-onset heart failure).
Due to the intentionally simple nature of the survey and the large numbers involved, we did not request detailed demographic information and we only asked for specific T2* results and scan dates for those patients with new-onset heart failure or who had died. As we did not have access to the complete dataset of all T2* scan results and dates, we were unable to perform a full survival analysis (including Kaplan-Meier curves). We only requested basic information regarding chelation therapy and were, therefore, unable to capture data on transfusion and chelation practice at each center which was sufficiently meaningful to comment on the likely influence of these factors in the observed geographical distribution of cardiac T2*. However, the clinical outcomes related to low cardiac T2* in this “real-life” study were clear.
Conclusions
These data show that there is a substantial prevalence of myocardial iron loading in a large international sample of TM patients who are regularly transfused and taking iron chelation therapy. The use of the threshold of T2* of 10ms below which the risk of cardiac complications rises significantly in previous reports has been confirmed and alternative explanations for heart failure other than myocardial iron loading appear to be rare. The consistency of findings from independent centers worldwide indicates that myocardial T2* is a robust clinical technique with potential for further expansion to guide chelation regimes which are tailored to the heart to prevent heart failure and prolong survival. These findings provide an invaluable insight into the burden of cardiac disease and the effects of cardiac iron overload worldwide. A major co-ordinated international effort is needed to continue to improve access to T2* CMR, although the provision of affordable and adequate transfusions and iron chelation therapy are primary issues.
Footnotes
Funding
This work was supported by the National Institute for Health Research Cardiovascular Biomedical Research Unit of Royal Brompton and Harefield NHS Foundation Trust and Imperial College London.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org
References
- 1.Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008;86(6):480–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Link G, Pinson A, Hershko C. Iron loading of cultured cardiac myocytes modifies sarcolemmal structure and increases lysosomal fragility. J Lab Clin Med. 1993; 121 (1):127–34 [PubMed] [Google Scholar]
- 3.Nienhuis AW, Griffith P, Strawczynski H, Henry W, Borer J, Leon M, et al. Evaluation of cardiac function in patients with thalassemia major. Ann N Y Acad Sci. 1980;344:384–96 [DOI] [PubMed] [Google Scholar]
- 4.Carpenter JP, He T, Kirk P, Roughton M, Anderson LJ, de Noronha SV, et al. On T2* magnetic resonance and cardiac iron. Circulation. 2011;123(14):1519–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson LJ, Holden S, Davis B, Prescott E, Charrier CC, Bunce NH, et al. Cardiovascular T2-star (T2*) magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001; 22(23):2171–9 [DOI] [PubMed] [Google Scholar]
- 6.Telfer PT, Prestcott E, Holden S, Walker M, Hoffbrand AV, Wonke B. Hepatic iron concentration combined with long-term monitoring of serum ferritin to predict complications of iron overload in thalassaemia major. Br J Haematol. 2000;110(4):971–7 [DOI] [PubMed] [Google Scholar]
- 7.Leonardi B, Margossian R, Colan SD, Powell AJ. Relationship of magnetic resonance imaging estimation of myocardial iron to left ventricular systolic and diastolic function in thalassemia. JACC Cardiovasc Imaging. 2008;1(5):572–8 [DOI] [PubMed] [Google Scholar]
- 8.Fitchett DH, Coltart DJ, Littler WA, Leyland MJ, Trueman T, Gozzard DI, et al. Cardiac involvement in secondary haemochromatosis: a catheter biopsy study and analysis of myocardium. Cardiovascular Res. 1980;14(12):719–24 [DOI] [PubMed] [Google Scholar]
- 9.Olson LJ, Edwards WD, McCall JT, Ilstrup DM, Gersh BJ. Cardiac iron deposition in idiopathic hemochromatosis: histologic and analytic assessment of 14 hearts from autopsy. J Am Coll Cardiol. 1987; 10(6): 1239–43 [DOI] [PubMed] [Google Scholar]
- 10.Olson LJ, Edwards WD, Holmes DR, Jr, Miller FA, Jr, Nordstrom LA, Baldus WP. Endomyocardial biopsy in hemochromatosis: clinicopathologic correlates in six cases. J Am Coll Cardiol. 1989;13(1):116–20 [DOI] [PubMed] [Google Scholar]
- 11.Telfer P. Update on survival in thalassemia major. Hemoglobin. 2009;33 Suppl 1:S76–80 [DOI] [PubMed] [Google Scholar]
- 12.Olivieri N, Nathan DG, MacMillan JH, Wayne AS, Liu PP, McGee A, et al. Survival in medically treated patients with homozygous beta-thalassemia. N Engl J Med. 1994; 331(9):574–8 [DOI] [PubMed] [Google Scholar]
- 13.Borgna-Pignatti C, Rugolotto S, De Stefano P, Zhao H, Cappellini MD, Del Vecchio GC, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89(10):1187–93 [PubMed] [Google Scholar]
- 14.Telfer P, Coen PG, Christou S, Hadjigavriel M, Kolnakou A, Pangalou E, et al. Survival of medically treated thalassemia patients in Cyprus. Trends and risk factors over the period 1980–2004. Haematologica. 2006;91 (9):1187–92 [PubMed] [Google Scholar]
- 15.Pennell DJ, Berdoukas V, Karagiorga M, Ladis V, Piga A, Aessopos A, et al. Randomized controlled trial of deferiprone or deferoxamine in beta-thalassemia major patients with asymptomatic myocardial siderosis. Blood. 2006;107(9):3738–44 [DOI] [PubMed] [Google Scholar]
- 16.Tanner MA, Galanello R, Dessi C, Smith GC, Westwood MA, Agus A, et al. A randomized, placebo controlled, double blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation. 2007;115(14):1876–84 [DOI] [PubMed] [Google Scholar]
- 17.Pennell DJ, Porter JB, Cappellini MD, El-Beshlawy, Chan LL, Aydinok Y, et al. Efficacy of deferasirox in reducing and preventing cardiac iron overload in beta-thalassemia. Blood. 2010;115(12):2364–71 [DOI] [PubMed] [Google Scholar]
- 18.Anderson LJ, Westwood MA, Holden S, Davis B, Prescott E, Wonke B, et al. Myocardial iron clearance during reversal of siderotic cardiomyopathy with intravenous desferrioxamine: a prospective study using T2* cardiovascular magnetic resonance. Br J Haematol. 2004;127(3):348–55 [DOI] [PubMed] [Google Scholar]
- 19.Tanner MA, Galanello R, Dessi C, Smith GC, Westwood MA, Agus A, et al. Combined chelation therapy in thalassemia major for the treatment of severe myocardial siderosis with left ventricular dysfunction. J Cardiovasc Magn Reson. 2008;10: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Modell B, Khan M, Darlison M. Survival in beta thalassaemia major in the UK: data from the UK Thalassaemia Register. Lancet. 2000;355(9220):2051–2 [DOI] [PubMed] [Google Scholar]
- 21.Modell B, Khan M, Darlison M, Westwood MA, Ingram D, Pennell DJ. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008; 10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolnagou A, Economides C, Eracleous E, Kontoghiorghes GJ. Long term comparative studies in thalassemia patients treated with deferoxamine or a deferoxamine/deferiprone combination. Identification of effective chelation therapy protocols. Hemoglobin. 2008;32(1–2):41–7 [DOI] [PubMed] [Google Scholar]
- 23.Telfer PT, Warburton F, Christou S, Hadjigavriel M, Sitarou M, Kolnagou A, et al. Improved survival in thalassemia major patients on switching from desferrioxamine to combined chelation therapy with desferrioxamine and deferiprone. Haematologica. 2009;94(12):1777–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pathare A, Taher A, Daar S. Deferasirox (Exjade) significantly improves cardiac T2* in heavily iron-overloaded patients with beta-thalassemia major. Ann Hematol. 2010;89(4):405–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ladis V, Chouliaras G, Berdoukas V, Chatziliami A, Fragodimitri C, Karabatsos F, et al. Survival in a large cohort of Greek patients with transfusion-dependent beta thalassaemia and mortality ratios compared to the general population. Eur J Haematol. 2011;86(4):332–8 [DOI] [PubMed] [Google Scholar]
- 26.Kirk P, Roughton M, Porter JB, Walker JM, Tanner MA, Patel J, et al. Cardiac T2* magnetic resonance for prediction of cardiac complications in thalassemia major. Circulation. 2009;120(20):1961–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westwood M, Anderson LJ, Firmin DN, Gatehouse PD, Charrier CC, Wonke B, et al. A single breath-hold multiecho T2* cardiovascular magnetic resonance technique for diagnosis of myocardial iron overload. J Magn Reson Imaging. 2003;18(1):33–9 [DOI] [PubMed] [Google Scholar]
- 28.Westwood MA, Anderson LJ, Firmin DN, Gatehouse PD, Lorenz CH, Wonke B, et al. Interscanner reproducibility of cardiovascular magnetic resonance in the early diagnosis of myocardial iron overload. J Magn Reson Imaging. 2003;18(5):616–20 [DOI] [PubMed] [Google Scholar]
- 29.Westwood MA, Firmin DN, Gildo M, Renzo G, Stathis G, Markissia K, et al. Intercentre reproducibility of magnetic resonance T2* measurements of myocardial iron in thalassaemia. Int J Cardiovasc Imaging. 2005;21(5):531–8 [DOI] [PubMed] [Google Scholar]
- 30.Tanner MA, He T, Westwood MA, Firmin DN, Pennell DJ. Thalassemia International Federation Heart T2* Investigators Multi-center validation of the transferability of the magnetic resonance T2* technique for the quantification of tissue iron. Haematologica. 2006;91(10):1388–91 [PubMed] [Google Scholar]
- 31.Kirk P, He T, Anderson LJ, Roughton M, Tanner MA, Lam WW, et al. International reproducibility of single breathhold T2* MR for cardiac and liver iron assessment among five thalassemia centers. J Magn Reson Imaging. 2010;32(2):315–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maceira AM, Prasad SK, Khan M, Pennell DJ. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2006;8(3):417–26 [DOI] [PubMed] [Google Scholar]
- 33.Ghugre NR, Enriquez CM, Coates TD, Nelson MD, Jr, Wood JC. Improved R2* measurements in myocardial iron overload. J Magn Reson Imaging 2006;23(1):9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He T, Gatehouse PD, Kirk P, Mohiaddin RH, Pennell DJ, Firmin DN. Myocardial T*2 measurement in iron-overloaded thalassemia: an ex vivo study to investigate optimal methods of quantification. Magn Reson Med. 2008;60(2):350–6 [DOI] [PubMed] [Google Scholar]
- 35.Marsella M, Borgna-Pignatti C, Meloni A, Caldarelli V, Dell’Amico MC, Spasiano A, et al. Cardiac iron and cardiac disease in males and females with transfusion-dependent thalassemia major: a T2* magnetic resonance imaging study. Haematologica. 2011;96(4):515–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kremastinos DT, Tiniakos G, Theodorakis GN, Katritsis DG, Toutouzas PK. Myocarditis in beta-thalassemia major. A cause of heart failure. Circulation. 1995; 91(1):66–71 [DOI] [PubMed] [Google Scholar]
- 37.Roghi A, Dellegrottaglie S, Pedrotti P, Pedretti S, Cassinerio E, Cappellini MD. Unexpected myocarditis in thalassaemia major patient screened for iron load cardiomyopathy. BMJ Case Rep. 2009; pii: bcr08.2008.0811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins W, Taylor WH. Determination of iron in cardiac and liver tissues by plasma emission spectroscopy. Ann Clin Biochem. 1987;24(5):483–7 [DOI] [PubMed] [Google Scholar]


