Abstract
Painful episodes of vaso-occlusion are the leading cause of hospitalizations and emergency department visits in sickle cell disease, and are associated with increased mortality. Low nitric oxide bioavailability contributes to vasculopathy in sickle cell disease. Since arginine is the obligate substrate for nitric oxide production, and an acute deficiency is associated with pain, we hypothesized that arginine may be a beneficial treatment for pain related to sickle cell disease. Thirty-eight children with sickle cell disease hospitalized for 56 episodes of pain were randomized into this double-blinded placebo-controlled trial. Patients received L-arginine (100 mg/kg tid) or placebo for 5 days or until discharge. A significant reduction in total parenteral opioid use by 54% (1.9±2.0 mg/kg versus 4.1±4.1 mg/kg, P=0.02) and lower pain scores at discharge (1.9±2.4 versus 3.9±2.9, P=0.01) were observed in the treatment arm compared to the placebo one. There was no significant difference in hospital length of stay (4.1±01.8 versus 4.8±2.5 days, P=0.34), although a trend favored the arginine arm, and total opioid use was strongly correlated with the duration of the admission (r=0.86, P<0.0001). No drug-related adverse events were observed. Arginine therapy represents a novel intervention for painful vaso-occlusive episodes. A reduction of narcotic use by >50% is remarkable. Arginine is a safe and inexpensive intervention with narcotic-sparing effects that may be a beneficial adjunct to standard therapy for sickle cell-related pain in children. A large multi-center trial is warranted in order to confirm these observations.
Introduction
Sickle cell disease (SCD) is an autosomal recessive disorder of the β-globin gene, in which hemoglobin-S polymerizes in erythrocytes under deoxygenated conditions, causing occlusion of the small blood vessels. Pain is the clinical hallmark of SCD with painful vaso-occlusive episodes (VOE) being common, debilitating, and a medical emergency.1 VOE are the leading cause of admission to hospital, emergency room visits, missed school, and are associated with an increased mortality rate.2 Nationally, 78% of the nearly 200,000 annual emergency department visits for SCD are for a complaint of pain,3 yet there is no effective therapy that targets the underlying mechanisms of VOE. Symptomatic relief with analgesics and hydration is the only currently available treatment, and this has not changed in decades. Episodic periods of severe pain lead to high use of health care resources,4 and high readmission rates within 72 hours despite initial hospitalization for pain management.2 Hospital admission rates are particularly high for children with SCD5 presenting to an emergency department with VOE, with pediatric hospitalization rates commonly over 60%.6 Although the reasons for high pediatric admission rates are unknown, many children and adults with SCD live with some degree of daily pain that they or their families try to control at home through various methods. It is when the pain becomes acutely worse, and unbearable, that they present to the emergency department, often in acute distress. Novel approaches to the treatment of acute pain in SCD, which could be utilized in the emergency department as well as on the hospital ward, are urgently needed.
Vaso-occlusion is believed to be the root cause of sickle cell pain. Nitric oxide (NO) is a potent vasodilator7 that plays a role in the vaso-occlusive complications of SCD.8–11 NO is produced in the endothelium from its obligate substrate L-arginine, which is converted to citrulline by a family of enzymes, the NO synthases (NOS).7 Although NOS expression and activity are increased, SCD is characterized by a state of NO resistance, NO inactivation, and impaired NO bioavailability.10,12,13 Under conditions of increased inflammation or oxidative stress, the compensatory up-regulation of NO likely becomes overwhelmed and ineffective resulting in vascular dysfunction.11 Normal arginine metabolism is also impaired in SCD,14–19 and differs in children compared to adults.16 We found that adults with SCD are arginine-deficient at steady-state,11,16 whereas children have plasma levels that are similar to those of normal controls in steady-state.16 However, plasma arginine concentration decreases significantly in both adults and children with VOE and is associated with low NO metabolite (NOx) levels.16 The lowest arginine levels were found in children requiring admission for VOE,16 with arginine levels returning to baseline during convalescence in the hospital. Of interest, low plasma arginine alone was a sensitive predictor for admission,16 while NOx levels were not, suggesting a function for arginine bioavailability in VOE severity that goes beyond NO. Although supplemental arginine increases plasma NOx in normal controls, when given to SCD patients at steady-state, a paradoxical decrease in NOx occurs that is not overcome by higher doses,20 indicating that arginine is metabolized differently in patients with SCD than in healthy volunteers. However, when a single dose of arginine is given to patients with SCD during VOE, there is a robust dose-dependent increase in plasma NOx.20 Based on these promising data, we designed a randomized, placebo-controlled trial to determine the safety and efficacy of arginine therapy in children with SCD requiring hospitalization for severe pain necessitating parenteral narcotics.
Methods
Study design
This study was a single-center, prospective, randomized, double-blind, placebo-controlled, phase 2 trial designed to explore the effectiveness of the arginine intervention in participants with SCD requiring hospitalization for VOE. Data outcome measures included length of stay (LOS) in hospital (days), total narcotic use over the course of the emergency department visit and hospitalization (mg/kg), and pain scores (10-cm linear visual analog scale and Faces Pain Scale). Further details are provided in the Online Supplementary Methods.
Standard intravenous (IV) opioid analgesic equivalents were used: 10 mg morphine sulfate = 100 mg meperidine = 2 mg hydromorphone hydrochloride. A hospital admission for a pain episode was considered a distinct event if it occurred more than 2 weeks after a previous pain episode requiring parenteral opioid therapy.
The study protocol was approved by the Institutional Review Board at the Children’s Hospital & Research Center at Oakland (CHRCO): informed consent was obtained for all patients enrolled, and assent was obtained from all children age 7 years and older. Children with an established diagnosis of SCD age 3–19 years with VOE requiring parenteral opioids and admission to hospital were recruited from emergency departments, hematology clinics, day hospitals and wards. Patients were recruited as a convenience sample during times when the principal investigator or study nurse was on-site and available to consent, a legal guardian was present, and the research pharmacist was available to perform the randomization.
Patients were consented within 24 hours of admission to the hospital and randomized to receive IV or oral (PO) study drug, L-arginine hydrochloride (100 mg/kg/dose three times/day with a maximum dose of 10 g for 15 doses or until discharge, whichever occurred first) or placebo. Placebo capsules appeared identical to the study drug (700 mg capsules) and were matched to the study drug by Tyson Pharmaceuticals for color and size, while normal saline was used for the IV placebo. Block randomization was performed by the hospital pharmacist at CHRCO. Exclusion criteria included known hepatic or renal insufficiency, presenting hemoglobin (Hb) <5 g/dL or immediate need for red blood cell transfusion, pregnancy, mental status changes or concern for stroke, >10 hospital admissions per year or a history of dependence on opioids, or a known allergy to arginine. A standardized treatment and monitoring program for VOE, utilized by CHRCO, was followed.
Safety and efficacy
Safety assessments included reports of adverse events (AE) and serious adverse events (SAE), clinical laboratory assessments, physical examination, vital signs, development of acute chest syndrome (ACS), and need for red blood cell transfusion. Safety and efficacy assessments were conducted at baseline, at discharge, and during routine clinical blood sampling. It was standard clinical practice to analyze the complete blood count with hemoglobin concentration and reticulocyte count during an emergency department visit for pain, while a complete metabolic panel and repeat complete blood count were performed when clinically indicated. Efficacy was judged by a statistically significant decrease in LOS in hospital, total narcotic use or pain score in the arginine treatment arm compared to in the placebo one.
Statistical analysis
The statistical analysis of the data is described in the Online Supplementary Material.
Results
Patients’ characteristics
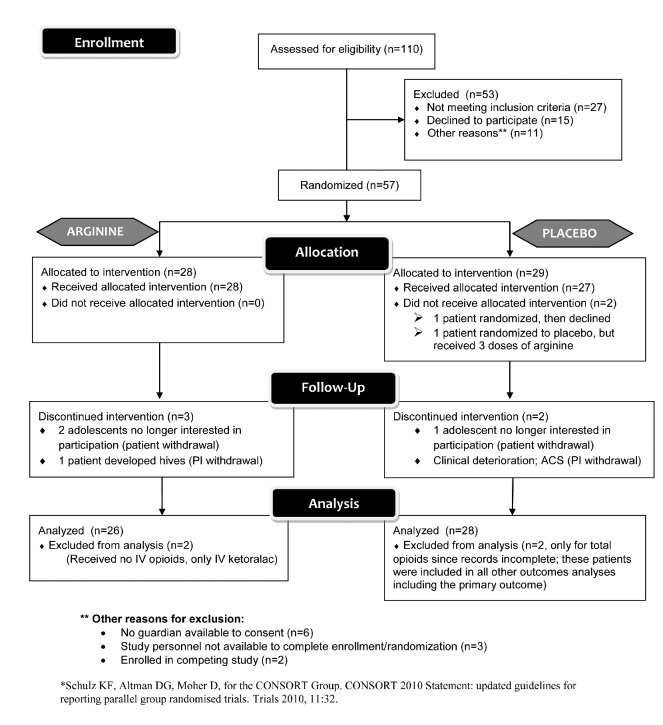
Figure 1 summarizes the CONSORT flow diagram for enrollment and analysis. A total of 38 patients with 56 distinct VOE completed randomization and received the study drug or placebo in this prospective, double-blind, placebo-controlled trial, 28 in each arm (arginine versus placebo). Two patients randomized to the arginine arm received IV ketorolac only for pain and no parenteral narcotics throughout their hospital stay, a requirement for eligibility. These patients were excluded from analyses after the study was unblinded and total opioid use was calculated. Therefore, 54 VOE events were analyzed in total. Table 1 summarizes the patients’ demographics and clinical parameters while Table 2 includes laboratory parameters collected as part of clinical care. Forty-seven episodes (85.5%) were evaluated in the emergency department, while seven patients were admitted to the ward directly from a hematology clinic or day hospital. The average age of the patients was 13.9 ± 4 years (range, 3.6–19 years), and 53% were female. Seventy-three percent of the pain episodes involved children with Hb-SS, 18% had Hb-SC, and 9% carried S-β thalassemia. Age and gender were equally distributed between groups, and there were no significant differences in sickle-hemoglobin genotype, oxygen saturation, respiratory rate, fever, severity of anemia, reticulocyte count, white blood cell count, pain scores, time to first parenteral opioid from presentation to triage, or therapeutic interventions (ketoralac, benadryl, antibiotics, intravenous fluid use) between the study arms. At presentation (day 1), systolic blood pressure was higher in the arginine arm than in the placebo arm.
Figure 1.
CONSORT flow diagram. Of 110 pain events assessed for study participation during years 2000–2007, 57 pain events were randomized into this placebo-controlled trial and 56 received either arginine therapy or placebo per protocol, with 28 events in each arm. Two patients were excluded after randomization into the arginine arm when it was determined that they had received no parenteral opioids throughout their admission, an eligibility criterion. Narcotic records were incomplete for two patients randomized to the placebo arm; these two patients were excluded from the total opioid use analysis only. One patient randomized to the placebo arm received three doses of arginine due to a pharmacy error. A total of five patients were withdrawn from the study and intervention discontinued, however their data were included in the intent-to-treat analysis. The Children’s Hospital & Research Center Oakland admits ~ 160 children with SCD and painful vaso-occlusive events year. Patients enrolled were among a convenience sample recruited weekdays when the study principal investigator (PI) or research nurse was on-site and available to consent for the study, a legal guardian was available to provide consent, and the pharmacy could perform the randomization.
Table 1.
Patients’ demographics and clinical parameters.
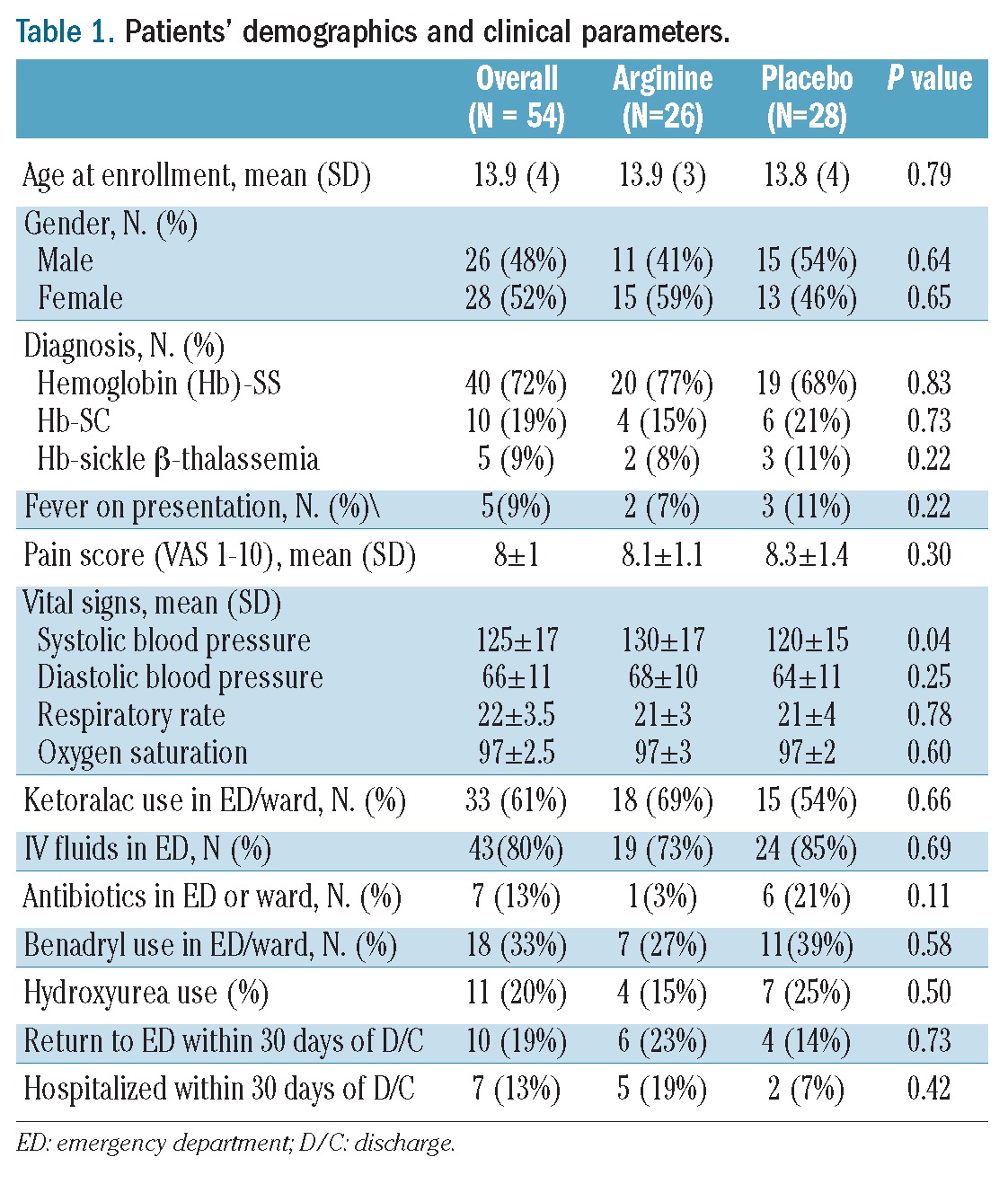
Table 2.
Impact of study drug on laboratory parameters, vital signs and pain scores.
The overall time between triage in the emergency department or presentation to the clinic and delivery of first randomized study drug dose was 20.4±11 hours, with no difference between the placebo and treatment arms.
Five patients (9%) withdrew from the study after initiation of the study drug (3 in the arginine arm and 2 in the placebo arm). Of these patients, three adolescents were no longer interested in participating (2 in the arginine arm, and 1 in the placebo arm), and asked to withdraw from the study without providing a particular reason. Two patients were withdrawn by the principal investigator for adverse events although the study drug code was not unblinded until after completion of the study. One patient in the arginine arm developed hives, considered possibly related to the study drug, and a second patient experienced clinical deterioration during the evolution of ACS (placebo arm, see adverse events). In a protocol deviation, one patient randomized to the placebo arm was erroneously crossed over for three doses of arginine due to a pharmacy medication error.
Effects of arginine on length of stay in hospital
The mean LOS was 4.5±2.2 days. There was not a significant difference between the two groups (mean±SD: 4.1±1.8 versus 4.8±2.5 days, P=0.34; arginine versus placebo arm), although the LOS tended to be shorter in the patients treated with arginine.
When patients receiving oral therapy only were excluded (n=6), the mean LOS for the IV arginine arm decreased by 2.4 hours while that for the IV placebo arm was unchanged (4.0±1.8 versus 4.8±2.5 days, P=0.20). Interestingly, LOS decreased over time for unclear reasons from 6±3 days for all admissions in 1998 at CHRCO (data used for sample size calculation) to 4.5±2.2 days in our study cohort from 2000–2008, a trend that has continued at this institution with a 2012 mean LOS of 3.3±2.2 days (n=124 VOE admissions).21
Effects of arginine on total opioid use
The majority of episodes (50/54; 93%) were treated with IV morphine sulfate as the parenteral opioid to treat pain, while three events were treated with IV hydromorphone and one event was treated with IV meperidine. A 54% significant reduction in total opioid use (mg/kg) over the course of the hospital stay was observed in patients in the treatment arm receiving arginine compared to those in the placebo arm (mean±SD: 1.9±2.0 mg/kg; n=26 versus 4.1±4.1 mg/kg; n=26, P=0.02; Figure 2A). Total opioid use correlated strongly with LOS in hospital (r=0.86, P<0.0001; Figure 2B).
Figure 2.
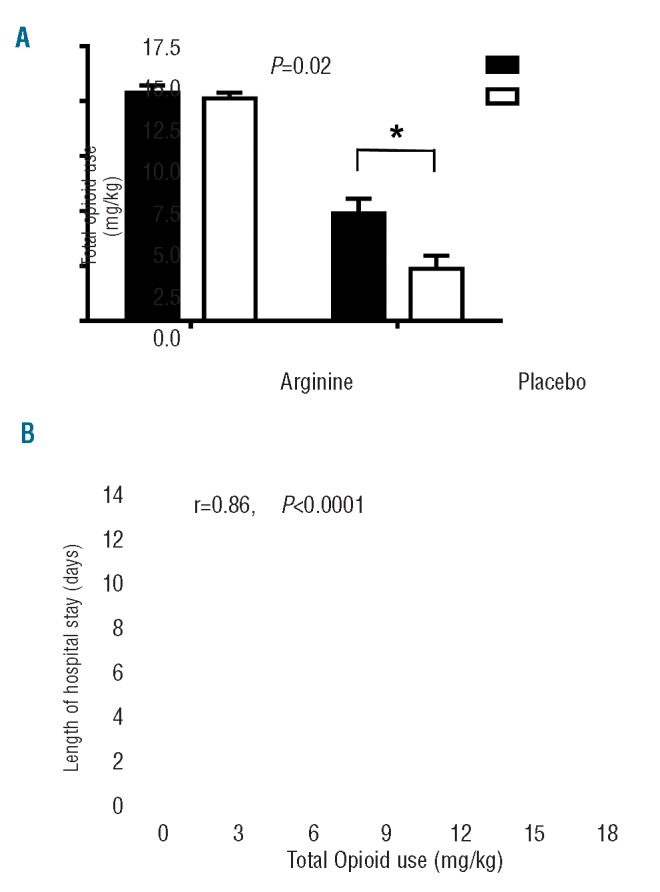
Impact of arginine therapy on total opioid use (mg/kg) and Pearson’s correlation between total opioid use (mg/kg) and total length of hospital stay (days). (A) Arginine supplementation (unfilled circles) led to a significant and clinically relevant reduction in total opioid use by 54% over the course of the hospital stay compared to total opioid use in the placebo group (filled circles). The difference remains significant even when the two outliers with the largest total opioid use in the placebo arm are excluded from the analysis (P=0.04). (B) Total opioid use (mg/kg) is directly correlated to length of hospital stay (r=0.86, P<0.0001).
When patients receiving oral therapy only were excluded (n=6), the total opioid use (mg/kg) was similarly reduced (mean±SD: 2.0±2.0 mg/kg; n=23 versus 4.2 ± 4.1 mg/kg; n=23, P=0.03) in the IV arginine versus placebo groups.
When the two outliers in the placebo arm receiving the highest total opioid treatment were excluded from analysis, the difference between the arginine and placebo arms remained statistically significant (1.9±2.0 mg/kg; n=26 versus 3.1±2.4 mg/kg; n=24, P=0.04).
Effects of arginine on pain scores
Pain scores, based on a 10-cm visual analog scale from 0 to 10 and recorded in the emergency department or in the clinic during the initial presentation, were similar in both the arginine and placebo groups (Figure 3). As expected, pain scores had decreased significantly in both the arginine and placebo arms at the time of discharge compared to the initial evaluation. However pain scores were significantly lower at discharge in the arginine arm than in the placebo arm (mean±SD: 1.9±2.4 versus 3.9±2.9, P=0.01). Comparing the differences in the change from presentation (day 1) to day of discharge adjusting for day 1 values between the placebo and arginine arms, the decrease in pain scores was larger in the arginine arm than in the placebo arm (P=0.01).
Figure 3.
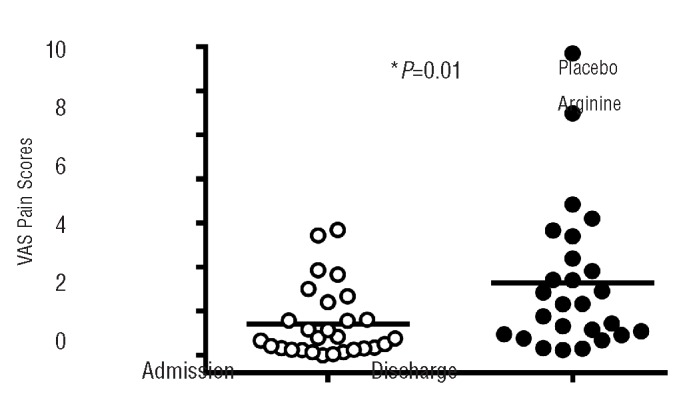
Impact of arginine therapy on pain scores. 10-cm visual analog scale (VAS) pain scores were similar at the time of admission in both groups, but were 2 cm lower at discharge in the arginine group compared to the placebo group (P=0.01).
The impact of arginine therapy on pain scores at discharge was similar when patients receiving oral therapy only (n=6) were excluded (1.7±2.0 versus 3.9±3.0, P=0.03, arginine versus placebo).
Effects of arginine on clinical and laboratory parameters
Arginine had no impact on blood pressure, heart rate or laboratory parameters compared to placebo, although systolic blood pressure and white blood cell count decreased similarly in both groups at discharge compared to the values at admission for VOE. In patients for whom data were available, no significant differences were observed between subjects in the arginine treatment group or placebo group with regards to pre- and post-therapy liver enzymes (alanine and aspartate transaminases), total/indirect bilirubin, renal function (blood-urea nitrogen or creatinine), or hemoglobin concentration. At discharge there was a trend towards lower reticulocyte count in the arginine arm (mean±SD: 10.3±6 to 8.4±5%, P=0.08, n=25) compared to the placebo arm (9.7±7 to 9.4±7%, P=0.99, n=23) and a trend towards higher oxygen saturation in the arginine arm (P=0.06). As expected during VOE admissions, mean hemoglobin dropped significantly from presentation within 24 hours (9.4±1.7 versus 8.6±1.7 g/dL, P<0.0001, n=38 with day 2 measurements) in the group as a whole, when day 2 measurements were available; however, there was no difference in the hemoglobin concentration at discharge in the arginine arm compared to the placebo arm (Table 2). Although only a limited number of patients had blood chemistry analyses performed on admission, 85% (46/54) of patients had a comprehensive metabolic panel performed prior to discharge. There were no statistically or clinically significant differences between liver function tests or creatinine levels at discharge, although blood urea nitrogen tended to increase in the arginine arm, with a statistically significant, but not clinically relevant difference between the two groups (10±4 versus 7±3, P=0.02). When using an analysis of covariance model comparing the differences in the change from initial presentation (day 1) to day of discharge adjusting for day 1 values between the placebo and arginine arms, it was noted that the increase in oxygen saturation was larger in the arginine group than in the placebo group (P=0.05), while there was a greater decrease in platelet count in the placebo group after treatment than in the arginine group. No other significant changes in clinical or laboratory variables between presentation and discharge, including blood-urea nitrogen, were noted in the two groups in this analysis.
Four episodes of ACS, defined as a fever and new infiltrates on a chest radiograph, developed in the study participants, three in the treatment arm and one in the placebo arm. None had fever during their initial presentation in the emergency department. One patient (in the placebo arm) experienced clinical deterioration associated with ACS during hospitalization requiring emergency transfusion and transfer from the ward to the pediatric intensive care unit. No clinical deterioration or transfers to the pediatric intensive care unit occurred in the arginine arm. Nine patients received red blood cell transfusions during the hospitalization, five in the treatment arm and four in the placebo arm. All patients who developed ACS (n=4) received a blood transfusion.
Repeat enrollment
After excluding two patients from the arginine group who did not receive any IV opioids for their pain, 36 unique patients were evaluated for 54 distinct, independent pain episodes requiring hospitalization. Over an 8-year period, nine patients were enrolled twice, two patients enrolled three times, and two patients enrolled four times. The mean time between re-enrollment for all participants was 13.7±11 months. Mean±SD total opioid use was 1.98±1.5 versus 3.05±1.9 mg/kg (P=0.11) for arginine versus placebo recipients, respectively, in a paired analysis of patients randomized to both arms (n=5 paired events for 10 VOE admissions). When only unique patients were analyzed (n=36; 18 in the placebo arm and 18 receiving arginine), using their first presentation for pain in the emergency department for analysis, total opioid use (1.9±2.0 versus 3.7±4.0 mg/kg, P=0.11, arginine versus placebo), LOS in hospital (4.2±1.6 versus 5.0±3 days, P=0.22, arginine versus placebo) and pain scores at discharge (2.0±3 versus 4.0±3, P=0.14, arginine versus placebo) were similar to analyses that included all events, although statistical significance was lost due to the inadequate sample size.
Adverse events
Three adverse or severe adverse events were reported. No serious drug-related adverse events were observed, although one AE and one SAE occurred that led to discontinuation of the study drug, and a decision by the principal investigator to withdraw the participant from the study without breaking the blinded study drug code until the completion of the trial. One patient (randomized to the arginine arm) developed hives during infusion of the study drug. Although the patient had a history of allergy to paper tape, and paper tape had been inadvertently used, the study drug was discontinued and the patient was withdrawn from the trial due to an AE that was possibly related to the study drug. A second patient (randomized to the placebo group) was withdrawn from the study after experiencing an acute clinical deterioration during the evolution of ACS, requiring an emergency red blood cell transfusion, and transfer from the ward to the intensive care unit. A second SAE was reported in a patient randomized to the placebo group: this patient had clinically relevant increases in liver function enzymes (alanine transaminase rose from 44 U/L on admission to 197 U/L on day 4 while aspartate transaminase increased from 64 to 200 U/L). The clinical team felt that this was likely related to SCD, but could possibly be related to the study drug, so a SAE was reported to the Institutional Review Board and Data Safety Monitoring Board, but the patient continued participation in the study and was closely monitored. No other patients experienced clinical deterioration, and no other adverse events occurred. Arginine and placebo were well tolerated.
Discussion
This is the first randomized placebo-controlled study to demonstrate benefits of arginine therapy in children with SCD hospitalized for severe pain. Total opioid use decreased by 54% and pain scores were significantly lower at discharge in the group treated with arginine compared to the group given placebo. Although the primary outcome was LOS, and overall mean LOS decreased over time,21 ultimately the study was not sufficiently powered to detect a change in LOS. However, the trend to a decrease by 17 hours in the arginine group was a clinically relevant improvement for patients and their families. Given that LOS correlated strongly with total opioid use (r=0.86, P<0.0001), a larger-scale, multi-center trial is needed to confirm our observations. Delivering the study drug as early as possible after presentation to the emergency department or clinic may have a greater impact on LOS, especially since many patients received their first dose of medication more than 24 hours into their hospitalized pain event. Anecdotally it is interesting to note that two patients with pain severe enough to require admission randomized to the arginine arm received no parenteral opioids throughout their hospital stay, although these patients were excluded from the analysis,.
We have previously demonstrated that a dysregulation of the arginine metabolome contributes to complications in SCD, including VOE and early mortality.14,16,22,23 The mechanistic impact of arginine supplementation cannot be elucidated by this trial, and is a limitation of this study. However an impressive back-bone of basic science and already published pre-clinical efficacy data are now further supported by our efficacy findings with regards to important clinical outcomes such as pain severity and opioid use. In transgenic mouse models of SCD, L-arginine supplementation inhibits the red cell Gardos channels,24 reduces red cell density,24 improves perfusion, and reduces lung injury, microvascular vaso-occlusion and mortality.25–27 Arginine also increases erythrocyte glutathione levels in both mice25 and humans,28 and may down-regulate inflammatory pathways.29 In addition, arginine is a key substrate in creatine synthesis, an important metabolic pathway not yet sufficiently studied in SCD that may be affected by an arginine-deficient state. Although the role of NO in SCD has become controversial,30,31 these studies demonstrate that the mechanistic impact of arginine goes beyond NO. Clinically, rapid healing of leg ulcers was anecdotally reported with oral L-arginine hydrochloride22 and IV arginine-butyrate in both SCD and thalassemia.32 Short-term arginine therapy improved pulmonary hypertension in SCD,22 and acutely increased both plasma NOx and exhaled NO when administered to ethnically matched normal controls and SCD patients with VOE.20,33 However when given to SCD patients at steady-state, a paradoxical decrease in plasma NOx; this decrease is not overcome by higher doses,20 indicating that arginine is metabolized differently in SCD than in healthy volunteers, and differently in SCD at steady-state than during periods of acute illness including painful VOE and ACS.16,20,33 These early observations may explain the negative outcome of the Comprehensive Sickle Cell Centers’ (CSCC) arginine trial,34 particularly since the primary outcome measure of that study was an increase in plasma NOx concentration, a biomarker which actually decreases with arginine supplementation in SCD patients at steady-state.20 Furthermore, low-dose arginine therapy is likely to be sub-therapeutic in SCD, and may represent an additional flaw in the design of the CSCC prophylactic arginine trial, as doses used were close to placebo based on the cardiovascular literature.35 Previous studies have shown that low-dose arginine is unlikely to affect NO synthesis,35 an observation confirmed in SCD by the CSCC study.34 Since the capacity of arginine to increase NOx production in SCD and VOE is dose-dependent,20 higher concentrations of plasma arginine are likely needed29 to overcome multi-factorial effects including the impact of arginase and asymmetric dimethylarginine (ADMA) on global arginine bioavailability.11
Independently of SCD, low global arginine bioavailability is associated with major adverse cardiovascular events including mortality in patients screened for cardiovascular disease,36 coronary artery disease,37 and malaria,38 and is associated with the risk of pulmonary hypertension.14,23,39
Increased arginase activity from both inflammatory triggers and more significantly from erythrocyte release of arginase during hemolysis,14 intracellular arginine transport inhibition, renal dysfunction (which impairs the major route of endogenous arginine biosynthesis),11,23 the presence of elevated endogenous NOS inhibitors such as ADMA40–42 (a competitive inhibitor of arginine transport and NOS43), uncoupled NOS27 and other consequences of oxidative stress25,26,44,45 lead to low global arginine bioavailability in SCD. Although mechanisms of arginine dysregulation are complex and multifactorial,11,23 they can be overcome through arginine supplementation, a phenomenon known as the “arginine paradox”.46 Arginine may have greater therapeutic potential than NO gas in SCD47 because of its multi-faceted nature that extends beyond its function as the obligate NO substrate. Hemolysis will drive arginine consumption, which will ultimately exacerbate NO sequestration and decreased NO synthesis.11 Under conditions of hypoxia, high ADMA, low arginine, or low levels of essential NOS cofactors,48 NOS will uncouple, producing reactive oxygen species instead of NO, further reducing NO bioavailability and adding to the milieu of oxidative stress. An imbalance between endothelial NOS-derived NO and superoxide generation exists in SCD.49 Up-regulation of NOS would, therefore, enhance oxidative stress when the local milieu favors NOS uncoupling. Indeed, studies in transgenic sickle cell mice demonstrate that NOS activity is paradoxically increased and uncoupled while NO bioavailability is low.27 Inhaled NO gas may be rapidly sequestered by superoxide, forming peroxynitrite, which is known to cause lung damage and cell death. It is plausible that NO and possibly even sildenafil therapy in SCD may lack therapeutic benefit47,50 in an environment of oxidative stress or in the absence of sufficient L-arginine bioavailability.
Although all pain episodes were independent events often separated by years, repeat patient participation was an unavoidable limitation of this study for feasibility reasons linked to enrollment of children with an orphan disease in a single center. Nevertheless, the promising results justify planning a multi-center trial involving a larger cohort of patients and provided pilot data to enable more accurate power calculations to determine the required sample size for desired outcome measures.
Experience with arginine therapy in SCD is growing,20,22,25,28,33,34 and no safety issues have emerged. We learned through this study that intravenous administration is preferred by patients and improves feasibility of study drug delivery compared to oral administration in the acute setting. Arginine therapy is a novel intervention for VOE. A reduction of total opioid use by more than 50% with arginine therapy is remarkable and was combined with improved pain scores at discharge. Arginine is a safe and efficacious intervention with narcotic-sparing effects that may be a beneficial adjunct to standard therapy for VOE. A large multi-center trial is warranted in order to confirm these observations.
Acknowledgments
The authors would like to thank the attending staff and nurses of the emergency department and hematology clinic for their assistance in this study, as well as the staff from the Children’s Hospital & Research Center Oakland (CHRCO) Pediatric Clinical Research Center. We also thank Jane van Warmerdam CNP for her assistance with enrollment of patients. Finally, we thank our patients with sickle cell disease and their families for their participation.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
This study was supported in part by NIH-NHLBI grant K23 HL 04386-05, FDA grant 1R01FD003531-04 and CTSA grant UL1 RR024131 (to CRM).
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997; 337(11):762–9 [DOI] [PubMed] [Google Scholar]
- 2.Brousseau DC, Owens PL, Mosso AL, Panepinto JA, Steiner CA. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303(13):1288–94 [DOI] [PubMed] [Google Scholar]
- 3.Yusuf HR, Atrash HK, Grosse SD, Parker CS, Grant AM. Emergency department visits made by patients with sickle cell disease: a descriptive study, 1999–2007. Am J Prev Med. 2011;38(4 Suppl):S536–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanzkron S, Carroll CP, Haywood C., Jr The burden of emergency department use for sickle-cell disease: an analysis of the national emergency department sample database. Am J Hematol. 2010;85(10):797–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bundy DG, Strouse JJ, Casella JF, Miller MR. Urgency of emergency department visits by children with sickle cell disease: a comparison of 3 chronic conditions. Acad Pediatr. 2011;11(4):333–41 [DOI] [PubMed] [Google Scholar]
- 6.Frei-Jones MJ, Baxter AL, Rogers ZR, Buchanan GR. Vaso-occlusive episodes in older children with sickle cell disease: emergency department management and pain assessment. J Pediatr. 2008;152(2):281–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329 (27):2002–12 [DOI] [PubMed] [Google Scholar]
- 8.Kaul DK, Liu XD, Fabry ME, Nagel RL. Impaired nitric oxide-mediated vasodilation in transgenic sickle mouse. Am J Physiol Heart Circ Physiol. 2000;278(6): H1799–806 [DOI] [PubMed] [Google Scholar]
- 9.Aslan M, Ryan T, Adler B, Townes T, Parks D, Thompson J, et al. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci. 2001;98(26):15215–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood KC, Hsu LL, Gladwin MT. Sickle cell disease vasculopathy: a state of nitric oxide resistance. Free Radic Biol Med. 2008;44(8): 1506–28 [DOI] [PubMed] [Google Scholar]
- 11.Morris CR. Mechanisms of vasculopathy in sickle cell disease and thalassemia. Hematology Am Soc Hematol Educ Program. 2008;2008:177–85 [DOI] [PubMed] [Google Scholar]
- 12.Reiter C, Wang X, Tanus-Santos J, Hogg N, Cannon R, Schechter A, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle cell disease. Nat Med. 2002;8(12):1383–9 [DOI] [PubMed] [Google Scholar]
- 13.Aslan M, Freeman BA. Oxidant-mediated impairment of nitric oxide signaling in sickle cell disease--mechanisms and consequences. Cell Mol Biol (Noisy-le-grand). 2004;50(1):95–105 [PubMed] [Google Scholar]
- 14.Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, VS, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension and mortality in sickle cell disease. JAMA. 2005;294(1):81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enwonwu CO. Increased metabolic demand for arginine in sickle cell anaemia. Med Sci Res. 1989;17:997–8 [Google Scholar]
- 16.Morris CR, Kuypers FA, Larkin S, Vichinsky E, Styles L. Patterns of arginine and nitric oxide in sickle cell disease patients with vaso-occlusive crisis and acute chest syndrome. J Pediatr Hematol Oncol. 2000; 22(6):515–20 [DOI] [PubMed] [Google Scholar]
- 17.Waugh W, Daeschner C, Files B, Gordon D. Evidence that L-arginine is a key amino acid in sickle cell anemia - a preliminary report. Nutritional Res. 1999;19:501–18 [Google Scholar]
- 18.Lopez B, Kreshak A, Morris CR, Davis-Moon L, Ballas S, Ma X. L-arginine levels are diminished in adult acute vaso-occlusive sickle cell crisis in the emergency department. Br J Haematol. 2003;120(3): 532–4 [DOI] [PubMed] [Google Scholar]
- 19.Schnog JB, Jager EH, van der Dijs FP, Duits AJ, Moshage H, Muskiet FD, et al. Evidence for a metabolic shift of arginine metabolism in sickle cell disease. Ann Hematol. 2004;83:371–5 [DOI] [PubMed] [Google Scholar]
- 20.Morris CR, Kuypers FA, Larkin S, Sweeter N, Simon J, Vichinsky EP, et al. Arginine therapy: A novel strategy to increase nitric oxide production in sickle cell disease. Brit J Haematol. 2000;111(12):498–500 [DOI] [PubMed] [Google Scholar]
- 21.Morris CR, Barreda F, Leibovich SA, Rutherford M, Saulys A, Stewart M, et al. Quality improvement goals for sickle cell disease pain management in an urban pediatric emergency department: we can do better! 54th American Society of Hematology Annual Meeting, Atlanta, GA, December 2012 abstract 2101. [Google Scholar]
- 22.Morris CR, Morris SM, Jr, Hagar W, van Warmerdam J, Claster S, Kepka-Lenhart K, et al. Arginine therapy: a new treatment for pulmonary hypertension in sickle cell disease? Am J Respir Crit Care Med. 2003;168(1):63–9 [DOI] [PubMed] [Google Scholar]
- 23.Morris CR, Gladwin MT, Kato G. Nitric oxide and arginine dysregulation: a novel pathway to pulmonary hypertension in hemolytic disorders. Curr Mol Med 2008;8 (7):81–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romero J, Suzuka S, Nagel R, Fabry M. Arginine supplementation of sickle transgenic mice reduces red cell density and Gardos channel activity. Blood. 2002;99 (4):1103–8 [DOI] [PubMed] [Google Scholar]
- 25.Dasgupta T, Hebbel RP, Kaul DK. Protective effect of arginine on oxidative stress in transgenic sickle mouse models. Free Radic Biol Med. 2006;41(12):1771–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaul DK, Zhang X, Dasgupta T, Fabry ME. Arginine therapy of transgenic-knockout sickle mice improves microvascular function by reducing non-nitric oxide vasodilators, hemolysis, and oxidative stress. Am J Physiol Heart Circ Physiol. 2008;295(1): H39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, et al. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109(7):3088–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Little JA, Hauser KP, Martyr SE, Harris A, Maric I, Morris CR, et al. Hematologic, biochemical, and cardiopulmonary effects of L-arginine supplementation or phosphodiesterase 5 inhibition in patients with sickle cell disease who are on hydroxyurea therapy. Eur J Haematol. 2009;82(4):315–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Archer DR, Stiles JK, Newman GW, Quarshie A, Hsu LL, Sayavongsa P, et al. C-reactive protein and interleukin-6 are decreased in transgenic sickle cell mice fed a high protein diet. J Nutr. 2008;138(6): 1148–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gladwin MT, Barst RJ, Castro OL, Gordeuk VR, Hillery CA, Kato GJ, et al. Pulmonary hypertension and NO in sickle cell. Blood 2010;116(5):852–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bunn HF, Nathan DG, Dover GJ, Hebbel RP, Platt OS, Rosse WF, et al. Pulmonary hypertension and nitric oxide depletion in sickle cell disease. Blood. 2010;116(5):687–92 [DOI] [PubMed] [Google Scholar]
- 32.Sher GD, Olivieri NG. Rapid healing of leg ulcers during arginine butyrate therapy in patients with sickle cell disease and thalassemia. Blood. 1994;84(7):2378–80 [PubMed] [Google Scholar]
- 33.Morris CR, Vichinsky EP, van Warmerdam J, Machado L, Kepka-Lenhart D, Morris SM, Jr, et al. Hydroxyurea and arginine therapy: impact on nitric oxide production in sickle cell disease. J Pediatr Hematol Oncol. 2003;25(8):629–34 [DOI] [PubMed] [Google Scholar]
- 34.Styles L, Kuypers F, Kesler K, Riess U, Lebeau P, Nagel R, et al. Arginine therapy does not benefit children with sickle cell anemia: results of the comprehensive sickle cell center multi-center study. 35th Convension of the National Sickle Cell Disease Program and the Sickle Cell Disease Association of America; 2007; Washington, DC; 2007. abstract no. 114. [Google Scholar]
- 35.Maxwell AJ, Cooke JP. Cardiovascular effects of L-arginine. Curr Opin Nephrol Hypertens. 1998;7(1):63–70 [DOI] [PubMed] [Google Scholar]
- 36.Tang WH, Wang Z, Cho L, Brennan DM, Hazen SL. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J Am Coll Cardiol. 2009;53(22):2061–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sourij H, Meinitzer A, Pilz S, Grammer TB, Winkelmann BR, Boehm BO, et al. Arginine bioavailability ratios are associated with cardiovascular mortality in patients referred to coronary angiography. Atherosclerosis. 2011;218(1):220–5 [DOI] [PubMed] [Google Scholar]
- 38.Omodeo-Sale F, Cortelezzi L, Vommaro Z, Scaccabarozzi D, Dondorp AM. Dysregulation of L-arginine metabolism and bioavailability associated to free plasma heme. Am J Physiol Cell Physiol. 2010;299(1):C148–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris CR, Teehankee C, Kato G, Gardner J, McGlothlin D, Malloy M, et al. Decreased arginine bioavailability contributes to the pathogenesis of pulmonary artery hypertension. American College of Cardiology Annual Meeting; 2005 March 6–9; Orlando, Florida; 2005 [Google Scholar]
- 40.Schnog JB, Teerlink T, van der Dijs FP, Duits AJ, Muskiet FA. Plasma levels of asymmetric dimethylarginine (ADMA), an endogenous nitric oxide synthase inhibitor, are elevated in sickle cell disease. Ann Hematol. 2005;84(5):282–6 [DOI] [PubMed] [Google Scholar]
- 41.Landburg PP, Teerlink T, Muskiet FA, Duits AJ, Schnog JJ. Plasma concentrations of asymmetric dimethylarginine, an endogenous nitric oxide synthase inhibitor, are elevated in sickle cell patients but do not increase further during painful crisis. Am J Hematol. 2008;83(7):577–9 [DOI] [PubMed] [Google Scholar]
- 42.Kato GJ, Wang Z, Machado RF, Blackwelder WC, Taylor JG, Hazen SL. Endogenous nitric oxide synthase inhibitors in sickle cell disease: abnormal levels and correlations with pulmonary hypertension, desaturation, haemolysis, organ dysfunction and death. Br J Haematol. 2009;145(4):506–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vallance P. The asymmetrical dimethylarginine/dimethylarginine dimethylaminohy-drolase pathway in the regulation of nitric oxide generation. Clin Sci. 2001;100(2):159–60 [PubMed] [Google Scholar]
- 44.Hebbel RP. Auto-oxidation and a membrane-associated ‘Fenton reagent’: a possible explanation for development of membrane lesions in sickle erythrocytes. Clin Haematol. 1985;14(1):129–40 [PubMed] [Google Scholar]
- 45.Morris CR, Suh JH, Hagar W, Larkin S, Bland DA, Steinberg MH, et al. Erythrocyte glutamine depletion, altered redox environment, and pulmonary hypertension in sickle cell disease. Blood. 2008;140:104–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gornik HL, Creager MA. Arginine and endothelial and vascular health. J Nutr. 2004;134(10 Suppl):2880S–7S; discussion 95S [DOI] [PubMed] [Google Scholar]
- 47.Gladwin MT, Kato GJ, Weiner D, Onyekwere OC, Dampier C, Hsu L, et al. Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: a randomized controlled trial. JAMA. 2011;305 (9):893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berka V, Yeh HC, Gao D, Kiran F, Tsai AL. Redox function of tetrahydrobiopterin and effect of L-arginine on oxygen binding in endothelial nitric oxide synthase. Biochemistry. 2004;43(41):13137–48 [DOI] [PubMed] [Google Scholar]
- 49.Wood KC, Hebbel RP, Lefer DJ, Granger DN. Critical role of endothelial cell-derived nitric oxide synthase in sickle cell disease-induced microvascular dysfunction. Free Radic Biol Med. 2006;40(8):1443–53 [DOI] [PubMed] [Google Scholar]
- 50.Machado RF, Barst RJ, Yovetich NA, Hassell KL, Kato GJ, Gordeuk VR, et al. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood. 2011;118(4):855–64 [DOI] [PMC free article] [PubMed] [Google Scholar]