Abstract
Achievement of complete molecular response in patients with chronic phase chronic myeloid leukemia has been recognized as an important milestone in therapy cessation and treatment-free remission; the identification of predictors of complete molecular response in these patients is, therefore, important. This study evaluated complete molecular response rates in imatinib-treated chronic phase chronic myeloid leukemia patients with major molecular response by using the international standardization for quantitative polymerase chain reaction analysis of the breakpoint cluster region-Abelson1 gene. The correlation of complete molecular response with various clinical, pharmacokinetic, and immunological parameters was determined. Complete molecular response was observed in 75/152 patients (49.3%). In the univariate analysis, Sokal score, median time to major molecular response, ABCG2 421C>A, and regulatory T cells were significantly lower in chronic phase chronic myeloid leukemia patients with complete molecular response than in those without complete molecular response. In the multivariate analysis, duration of imatinib treatment (odds ratio: 1.0287, P=0.0003), time to major molecular response from imatinib therapy (odds ratio: 0.9652, P=0.0020), and ABCG2 421C/C genotype (odds ratio: 0.3953, P=0.0284) were independent predictors of complete molecular response. In contrast, number of natural killer cells, BIM deletion polymorphisms, and plasma trough imatinib concentration were not significantly associated with achieving a complete molecular response. Several predictive markers for achieving complete molecular response were identified in this study. According to our findings, some chronic myeloid leukemia patients treated with imatinib may benefit from a switch to second-generation tyrosine kinase inhibitors (ClinicalTrials.gov, UMIN000004935).
Introduction
The introduction of imatinib mesylate, a specific breakpoint cluster region-Abelson1 (BCR-ABL1) tyrosine kinase inhibitor, has improved the outcome of patients with chronic myeloid leukemia (CML). A multinational, randomized phase III study (IRIS) demonstrated that imatinib is significantly superior to interferon-α plus low-dose cytarabine as first-line therapy in terms of hematologic and cytogenetic responses, tolerability, and progression-free survival in patients with newly diagnosed chronic phase CML (CML-CP).1 Continuous treatment with imatinib as initial therapy induced durable responses in a high proportion of CML-CP patients at a 5-year follow-up. Cumulative complete hematologic response and complete cytogenetic response rates at 60 months were 98% and 87%, respectively.2 Based on the results of these and other studies, imatinib has become the standard therapy for CML-CP.
Molecular response assessment is valuable in evaluating the therapeutic efficacy of tyrosine kinase inhibitors in CML. A major molecular response (MMR) is an important surrogate biomarker for the prediction of long-term outcome of imatinib treatment in CML. CML-CP patients who achieved MMR at 18 months had a significantly lower risk of disease progression than patients who did not achieve MMR.3
Recently, complete molecular response (CMR) has been evaluated in clinical studies as a surrogate biomarker for the prediction of long-term outcome of imatinib therapy in CML-CP. CMR is defined as at least a 4.5-log reduction of BCR-ABL1 transcripts or undetectable transcripts by real-time quantitative polymerase chain reaction (qRT-PCR). In a landmark analysis by Press et al., CML patients who achieved CMR by 18 months had a significantly longer relapse-free survival than patients who achieved MMR.4 In an intention-to-treat analysis, the cumulative incidence of CMR on imatinib therapy was 0.5% at 1 year and 8.3% at 5 years in newly diagnosed CML-CP patients.5 CMR may also represent a new surrogate biomarker in the era of second-generation tyrosine kinase inhibitors, which have produced significantly superior molecular responses compared with imatinib. In the Evaluating Nilotinib Efficacy and Safety in Clinical Trials-newly diagnosed patients (ENESTnd) phase III study, significantly more patients achieved CMR at 12, 24, and 36 months in the nilotinib groups than in the imatinib group (11%, 25%, and 32% with 300 mg nilotinib twice daily, respectively; 7%, 19%, and 28% with 400 mg nilotinib twice daily, respectively; and 1%, 9%, and 15% with imatinib, respectively).6,7 The Dasatinib versus Imatinib Study In Treatment-Naive CML patients (DASISION) a phase III trial, also showed that significantly more patients achieved CMR at 24 months in the dasatinib group than in the imatinib group (17% versus 8%).8 Moreover, CMR may be a first milestone toward the development of a successful strategy for discontinuation therapy. In the Stop Imatinib (STIM) study, 41% of CML patients with a stable CMR for at least 2 years were able to sustain the CMR 12 months after cessation of imatinib.9 In an update of the STIM trial at 48 months of follow-up, the overall probability of maintenance of CMR at 24 and 36 months was 39% (95% CI 29–48).10
qRT-PCR-based molecular monitoring of BCR-ABL transcripts is used to assess treatment response in CML. qRT-PCR is only clinically useful if it is conducted under a rigorous quality control regimen so that fluctuations in the BCR-ABL1 level can be confidently attributed to a biological cause rather than assay variation. An international scale has been proposed to further improve the value of qRT-PCR results in the setting of clinical practice.11 The European Leukemia Net (ELN) defined MMR as BCR-ABL1 transcripts ≤0.1% compared with ABL (or other house-keeping gene) transcripts in peripheral blood by the standardized international scale of qRT-PCR (IS-PCR).9 CMR is defined as undetectable transcripts at different times in peripheral blood by nested PCR (sensitivity >104).12 Because the potential for patients’ eligibility for tyrosine kinase inhibitor cessation studies is becoming a more widely discussed topic and area for research, Cross et al. recently reviewed the standardized definitions of molecular response in CML based on BCR-ABL1 transcript levels.13 According to the latest definitions of molecular response, MR3 is defined as BCR-ABL1 transcripts ≤0.1%, MR4 as BCR-ABL1 transcripts ≤0.01%, and MR4.5 as BCR-ABL1 transcripts ≤0.0032%.13
The purpose of this multicenter clinical study was to evaluate CMR rates in imatinib-treated CML-CP patients with MMR using the IS-PCR. The correlation of CMR with clinical, immunological, and pharmacokinetic parameters in CML-CP patients was also evaluated.
Methods
Patients and treatment
Twenty-one institutions in Japan participated in this multicenter trial. Patients who were ≥16 years of age, with a confirmed diagnosis of Philadelphia chromosome-positive CML-CP, treated with ongoing imatinib at any dose, and with an estimated MMR within 3 months before study entry were eligible for inclusion. All CML patients were in the chronic phase with no history of progression to accelerated phase or blast crisis. Molecular assessments for minimal residual disease quantification according to ELN recommendations were performed before the participants’ inclusion in the study. Patients who had previously been treated with hydroxyurea, low-dose cytarabine, or interferon-α before imatinib administration were allowed to participate in the study. Patients who had undergone allogeneic stem cell transplantation or received chemotherapy combined with imatinib or other tyrosine kinase inhibitors were excluded from the study. Patients with severe complications or uncontrollable organ dysfunction were also excluded from the study. Imatinib treatment was continued for the duration of the study.
Study conduct
The study was conducted in accordance with the principles of the Declaration of Helsinki. Informed consent was obtained from all patients according to institutional guidelines. The study was approved by the Akita University Research Ethics Board and all institutional review boards of the participating centers.
Molecular response
IS-PCR was performed in a central laboratory using a MolecularMD One-Step qRT-PCR BCR-ABL kit (BML Inc., Kawagoe, Japan). MMR was defined as a 3-log reduction in BCR-ABL1 transcripts as determined by IS-PCR (BCR-ABLIS 0.0032%–0.1%), and at least 10,000 control genes (ABL) were required for a sample to be classified as adequate. CMR was defined as ≥4.5-log reduction in BCR-ABL1 transcripts (BCR-ABLIS ≤0.0032%) in peripheral blood samples collected twice on different days, and at least 32,000 control genes were required for a sample to be classified as adequate. To confirm the initial CMR (BCR-ABLIS ≤0.0032%), the IS-PCR assay was repeated at 6 months.
Imatinib concentration, genomics, and flow cytometry
Imatinib trough concentrations were determined using high-performance liquid chromatography as described previously.14 Genotyping of SLC22A1 (156T>C, 480G>C, 1022C>T, and 1222A>G),15ABCB1 3435T>C,16ABCG2 421C>A,17 and a BIM deletion polymorphism18 was performed as described previously. Peripheral blood T-cell subsets were analyzed by flow cytometry using a whole blood lysis technique. The monoclonal antibodies used in this study are described in the Online Supplementary Methods.
Statistical analyses
Statistical analyses were performed using SPSS statistical software (version 17.0, SPSS Japan Inc., Tokyo, Japan). Data are presented as median (range) or median (quartile 1–quartile 3). Differences in the various parameters between groups were evaluated using the Mann-Whitney U test or χ2 test. Time to MMR was measured from the date of diagnosis of CML or date of imatinib administration to the date of MMR achievement. The cumulative incidence of MMR was estimated using the Kaplan-Meier method. Time to MMR was compared between groups using a stratified log-rank test. Stepwise forward selection multiple regression analysis was performed to determine the effect of the variables examined in univariate analysis on CMR. A P value <0.05 was considered statistically significant.
Results
Patients and treatment
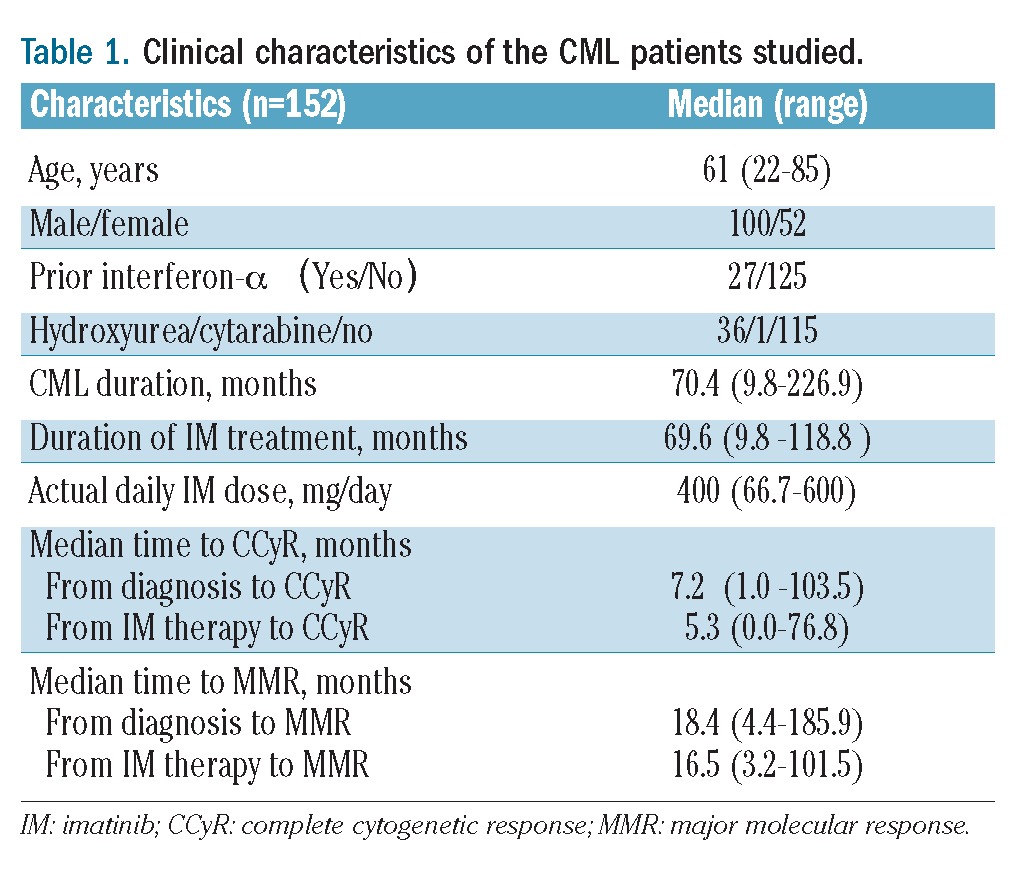
Between December 1, 2010 and July 30, 2011, 157 patients were recruited for this study, of whom 152 were included in the study. Five patients were excluded because a MMR was not detectable at the first IS-PCR testing. The cutoff date for this analysis was January 31, 2012 (6 months after the last patient had been enrolled). Adverse events including loss of cytogenetic response, progression to acute phase/blast crisis, and death from any cause were not reported in any patients during the course of this study. The demographic characteristics, baseline disease characteristics, and risk stratification of the patients are presented in Table 1. The median age of the patients was 61 years (range, 22–85 years). The male-to-female ratio was 100:52. Twenty-seven and 36 patients had received prior interferon-α and hydroxyurea treatment, respectively. Only one patient had received prior low-dose cytarabine. The median duration after CML diagnosis was 70.4 months (range, 9.8–226.9 months). The median duration of imatinib treatment was 69.6 months (range, 9.8–118.8 months), and the median actual daily dose of imatinib was 400 mg (range, 66.7–600 mg). Only one of the 152 patients received 600 mg/day imatinib from the onset of CML-CP; the other 151 patients were treated with ≤400 mg/day imatinib. The median time to complete cytogenetic response from CML diagnosis and imatinib administration was 7.2 months (range, 1.0–103.5 months) and 5.3 months (range, 0–76.8 months), respectively. The median time to MMR from CML diagnosis and imatinib administration was 18.4 months (range, 4.4–185.9 months) and 16.5 months (range, 3.2–101.5 months), respectively.
Table 1.
Clinical characteristics of the CML patients studied.
Evaluation of molecular response
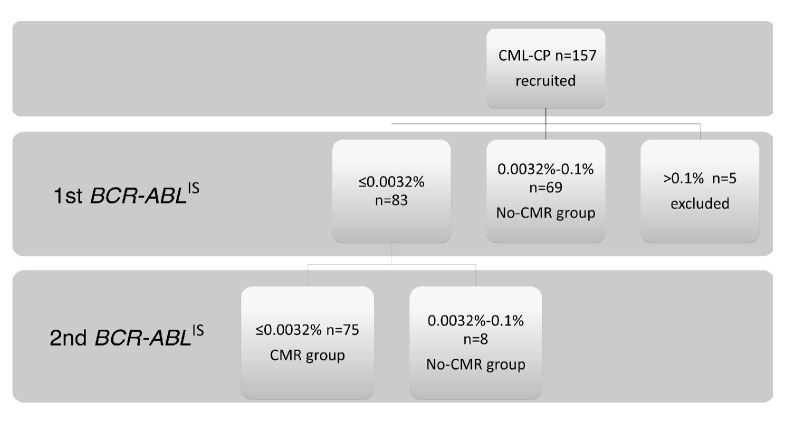
BCR-ABLIS 0.0032%–0.1% was detected in 69 patients (45.4%) at the first testing, and these patients were classified into the No-CMR group. Of the 152 patients analyzed, 75 (49.3%) were classified into the CMR group. BCR-ABLIS ≤0.0032% was detected at the first testing in eight patients but was not confirmed at the second testing. These eight patients were, therefore, classified into the No-CMR group. The study flow chart is shown in Figure 1.
Figure 1.
Study flow. One hundred fifty-seven patients were recruited for the study. Five patients were excluded because MMR was not detect ed at the first testing. BCR-ABLIS 0.0032%–0.1% was detected in 69 of the 152 patients at the first testing, and these patients were classified into the No-CMR group. Seventy-five patients with BCR-ABLIS&lE0.0032% confirmed at the first and the second testing were classified into the CMR group. BCR-ABLIS&lE0.0032% was detected at the first testing but not confirmed in the second testing in eight patients, who were classified into the No-CMR group.
Comparison of patients with and without complete molecular remission
Comparison of the clinical characteristics of patients who did and did not achieve CMR revealed several significant differences (Table 2). In the univariate analysis, the frequency of low-risk patients was significantly higher (P=0.0367) and the time to MMR from CML diagnosis and imatinib administration was significantly shorter in the CMR group than in the No-CMR group. The median time to MMR from CML diagnosis and imatinib administration was 13.8 and 13.3 months, respectively, in the CMR group and 22.2 and 18.6 months, respectively, in the No-CMR group (P=0.0224, P=0.0239). The cumulative incidence of MMR was significantly higher in the CMR group than in the No-CMR group (P=0.0126; Figure 2). Age, sex, prior CML therapy, and time to achieve a complete cytogenetic response were not significant predictive factors for achieving CMR.
Table 2.
Comparison of clinical characteristics of patients in the CMR and No-CMR groups.
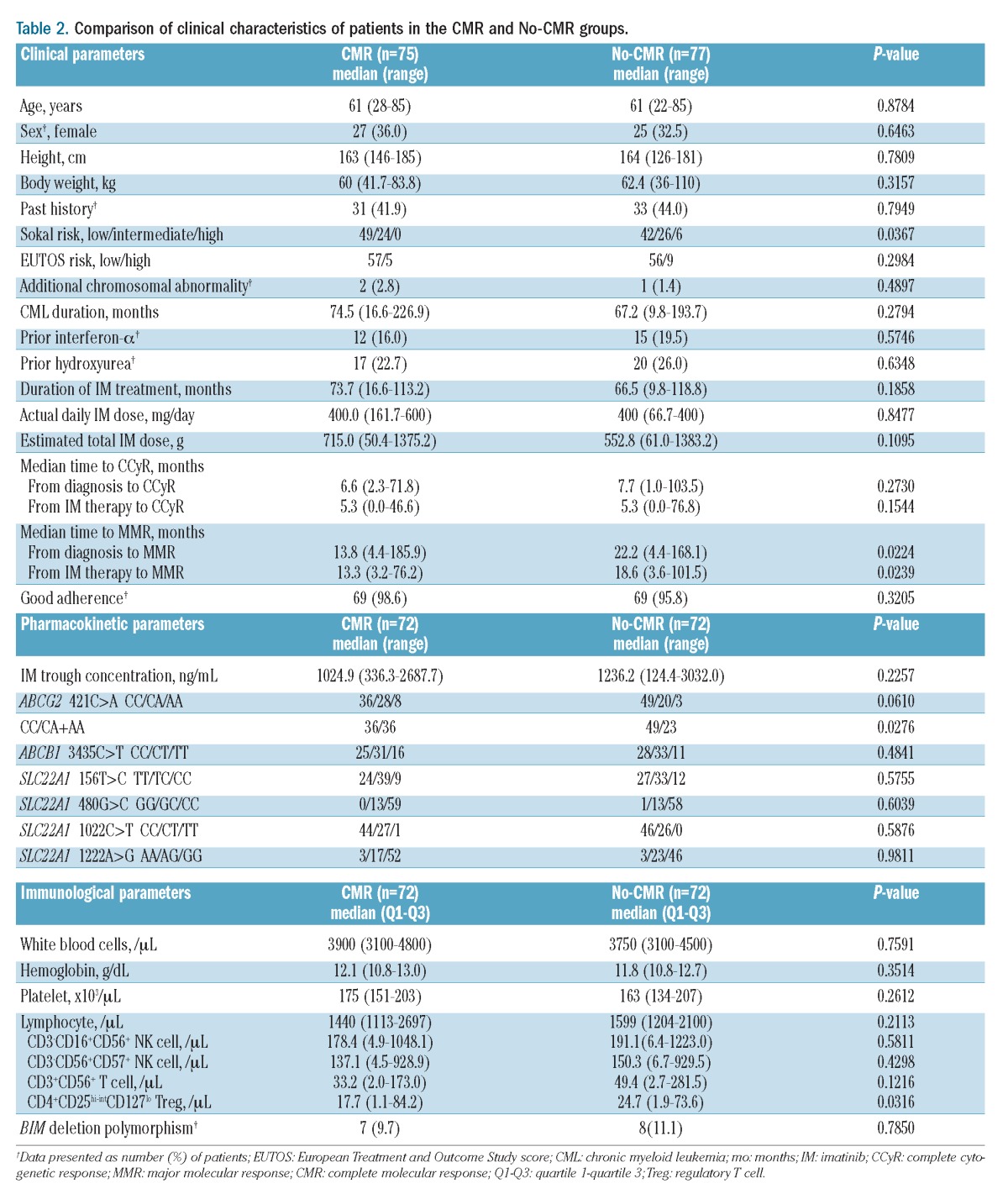
Figure 2.
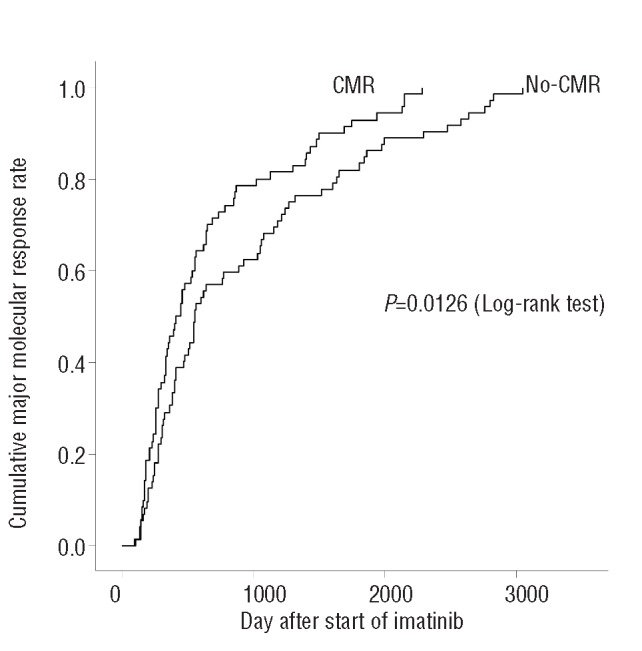
Cumulative incidence of MMR. Relationship between CMR and time to MMR after imatinib administration in CML-CP patients. Cumulative incidence of MMR was estimated using the Kaplan-Meier method, and the time to MMR was compared using the log-rank test. A significant difference in time to MMR between the CMR and No-CMR groups was observed (P=0.0126).
Comparisons of the pharmacokinetic, pharmacogenomic, and immunological parameters in the CMR and No-CMR groups are shown in Table 2. Because of insufficiently sized samples from eight patients, these parameters were analyzed in 144 of the 152 patients. Good adherence was confirmed in both the CMR and No-CMR groups, and the mean imatinib plasma trough concentration was above 1000 ng/mL in both groups. The frequency of patients with ABCG2 421A allele was significantly higher in the CMR group than in the No-CMR group (P=0.0276).
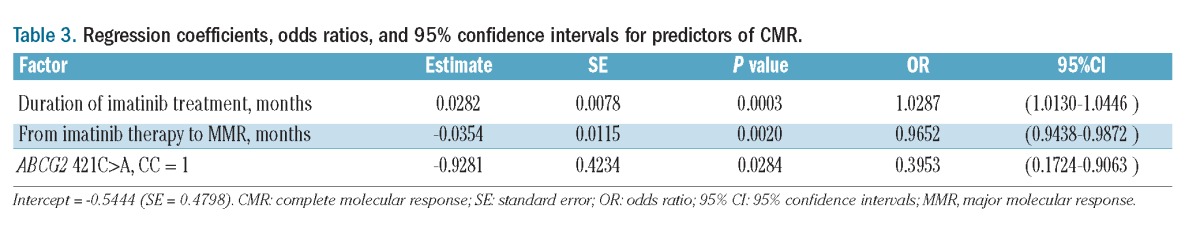
The absolute number of lymphocytes and the number of CD3−CD16+CD56+ natural killer (NK) cells, CD3−CD56+CD57+ NK cells, CD3+CD56+ T cells, and CD4+/CD25high-int/CD127low regulatory T (Treg) cells in the peripheral blood are shown in Table 2. The absolute number of lymphocytes and the number of NK cells and CD3+CD56+ T cells were not significantly different between the two groups, whereas the number of Treg cells was significantly lower in the CMR group than in the No-CMR group. The frequency of BIM deletion polymorphism was not significantly different between the two groups. In a multivariate regression analysis, duration of imatinib treatment, time to MMR from imatinib therapy, and the polymorphism of ABCG2 421C>A were independent predictors of CMR (Table 3).
Table 3.
Regression coefficients, odds ratios, and 95% confidence intervals for predictors of CMR.
Discussion
The purpose of CML treatment is to avoid disease progression from chronic phase to blast phase. Because CML patients who had an MMR have a significantly lower risk of disease progression, MMR at 18 months has been an important surrogate biomarker for the prediction of long-term outcome of imatinib treatment according to the ELN.12 The idea of treatment-free remission, which is defined as the absence of molecular recurrence after discontinuation of tyrosine kinase inhibitor therapy, was proposed after the STIM trial.9 CMR has been recognized as an important therapeutic milestone to treatment-free remission. Thus, it is necessary to identify new treatment strategies to increase the CMR rate in CML patients and to clarify the predictive factors for CMR achievement in these patients.
The 8-year cumulative incidence of CMR with imatinib therapy was 13.5% in study in Hammersmith Hospital (London, UK).19 However, the percentage of CML patients who achieve CMR in daily clinical practice in Japan is unknown, because IS-PCR is only used in clinical trials. In the current study, we confirmed that approximately 50% of CML-CP patients treated with imatinib (median duration of imatinib treatment: 69.9 months) achieved CMR in daily clinical practice in Japan. The CMR was confirmed twice at different time-points by IS-PCR, because it is known that BCR-ABL1 levels sometimes fluctuate between MR3 and MR4.5.20 Among 152 patients, eight (5.3%) had BCR-ABL1 levels that fluctuated, and they were classified into the No-CMR group.
The STIM study demonstrated that approximately 40% of CML patients with a stable CMR were able to sustain the CMR for more than 18 months after discontinuation of imatinib therapy.9,10 In the STIM study, the risk factors for molecular relapse were high Sokal risk score at the time of CML diagnosis and treatment with imatinib for <50 months.9 Although the median duration of imatinib treatment was long (69.9 months) and more than 70% of patients had a low Sokal score in the current study, low Sokal risk score and duration of imatinib treatment were significant predictive factors for CMR in the univariate and multivariate regression analyses, respectively. Age, sex, European Treatment and Outcome Study score (EUTOS),21 prior therapy for CML, and time to achieve complete cytogenetic response were not significant predictors of achieving CMR. In the multivariate regression analysis, time to MMR from imatinib therapy was an independent predictive factor for achieving CMR. BCR-ABLIS ≤0.0032% rates were significantly higher in newly diagnosed CML patients treated with nilotinib and dasatinib than in those treated with imatinib in the ENESTnd and DASISION trials.6,8 Furthermore, differences in the depth of the molecular response between nilotinib-and imatinib-treated patients increased over time (1% in the imatinib arm versus 11% in the nilotinib arm by 12 months; 23% in the imatinib arm versus 40% in the nilotinib arm by 48 months).22 Moreover, the landmark analysis of ENESTnd estimated that 53% and 65% of imatinib-treated CML patients with BCR-ABLIS ≤1% at 3 months would achieve BCR-ABLIS ≤0.0032% by 3 years and 4 years, respectively. However, patients with BCR-ABLIS 1–10% and >10% at 3 months were estimated to achieve BCR-ABLIS ≤0.0032% rates of 14% and 1% by 3 years and 24% and 5% by 4 years, respectively.23 The landmark analysis of the DASISION trial also estimated that early molecular response at 3 months correlated with 3-year outcomes and risk of transformation.24 Although more patients treated with second-generation tyrosine kinase inhibitors achieved an early molecular response at 3 months than did patients treated with imatinib, BCR-ABLIS >10% at 3 months was a warning sign of poor response in both landmark analyses.23,24 Taken together, these findings suggested that an early molecular response was associated with a deeper molecular response to CML treatment.
We expected that pharmacokinetic factors, such as drug adherence, plasma trough concentration, and polymorphism of imatinib transporters, would be predictors of CMR in CML. Previously, we reported that imatinib trough concentration was significantly higher in patients who achieved MMR than in those who did not achieve MMR.25 Furthermore, patients with imatinib trough concentration values >1002 ng/mL had a higher probability of achieving MMR in our large cohort.25 In the current study, the mean imatinib plasma trough concentration was above 1000 ng/mL in patients with and without CMR. However, the percentage of patients with CMR was significantly lower among those with the ABCG2 421C/C genotype than among those with the A allele. This finding suggests that the ABCG2 421A allele is associated with greater intracellular retention and, therefore, higher exposure to imatinib than the wild-type genotype. This finding is in agreement with the in vitro study by Imai et al., who demonstrated that the levels of expression of the protein BCRP, which is encoded by ABCG2, were markedly decreased by the ABCG2 421A allele compared with the 421C/C genotype.26 Although we previously reported that the ABCG2 421A allele was an independent predictor of a higher dose-adjusted imatinib trough concentration,27 this polymorphism is also a predictive factor for CMR in CML patients treated with imatinib. Polyspecific organic cation transporter-1, which is encoded by SLC22A1, has been reported to be a key determinant in the molecular response to imatinib by increasing intracellular uptake.28,29 However, a relationship between SLC22A1 genotypes and CMR rate was not observed in the present study. Further investigation of SLC22A1 genotypes as predictive factors for achieving CMR in CML is necessary to confirm our findings.
BIM, a pro-apoptotic member of the BCL2 family of proteins, is required for imatinib to induce apoptosis in kinase-driven leukemia. We previously reported that a common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to imatinib in CML.18 This polymorphism switches BIM splicing from exon 4 to exon 3 resulting in the expression of BIM isoforms lacking the pro-apoptotic BCL2 homology domain 3. In the current study, the frequency of BIM deletion was not significantly different between CML-CP patients with CMR and those without CMR. Clinical, pharmacokinetic, and immunological parameters were not significantly different between patients with and without the BIM deletion in the CMR group (Online Supplementary Table S1). However, patients with the BIM deletion had slightly higher trough levels than patients without BIM deletion (1482 ng/mL versus 996 ng/mL, P=0.091). Although not statistically significant, pharmacokinetic factors may overcome the negative effect of BIM deletion on achieving CMR. In contrast, Katagiri et al. reported that the BIM deletion polymorphism is a possible criterion for discontinuation of imatinib in CML patients.30 Although BIM deletion polymorphism was not associated with achieving CMR in our study, it may be a risk factor for relapse after discontinuation of imatinib.
Immunological surveillance, including cytotoxic T cells and/or NK cells, may be associated with CMR rate in response to imatinib in CML. Mustjoki et al. reported that clonal expansion of NK or T cells was associated with a favorable outcome in patients with Philadelphia chromosome-positive leukemia treated with dasatinib.31 In the current study, there was no significant difference in absolute number of lymphocytes, NK cells, or CD3+CD56+ T cells between CML-CP patients with CMR and those without CMR; however, the number of Treg cells was significantly lower in patients with CMR than in those without CMR. Balachandran et al. reported that T cells are crucial to the antitumor effects of imatinib in gastrointestinal stromal tumor (GIST).32 Imatinib therapy activates CD8+ T cells and induces Treg cell apoptosis in GIST.32 The significant difference in the absolute number of Treg cells between patients with CMR and those without CMR in the current study may be due to the induction of Treg cell apoptosis in the bone marrow. Thus, further investigation of the potential antitumor T-cell responses induced by imatinib in CML is warranted.
In summary, we confirmed a CMR rate of 50% in CML-CP patients with MMR treated with imatinib for more than 5 years. Duration of imatinib treatment, time to MMR from imatinib therapy, and frequency of ABCG2 421C/A were factors predicting the achievement of a CMR in CML-CP patients treated with imatinib. Further validation of these predictive factors in a large prospective study is warranted and will be important in identifying CML patients who can sustain CMR and treatment-free remission after discontinuation of imatinib therapy.
Acknowledgments
The authors would like to thank the Shimousa Hematology study group, the Akita CML study group, Osaka City University Hematology consortium, and the Nagasaki CML study group for their participation in this study, and Ms. Saori Takahashi and Ms. Yukiko Abe for secretarial and technical assistance.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.O’Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348(11):994–1004 [DOI] [PubMed] [Google Scholar]
- 2.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–17 [DOI] [PubMed] [Google Scholar]
- 3.Hughes TP, Hochhaus A, Branford S, Muller MC, Kaeda JS, Foroni L, et al. Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS). Blood. 2010;116(19):3758–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Press RD, Galderisi C, Yang R, Rempfer C, Willis SG, Mauro MJ, et al. A half-log increase in BCR-ABL RNA predicts a higher risk of relapse in patients with chronic myeloid leukemia with an imatinib-induced complete cytogenetic response. Clin Cancer Res. 2007;13(20):6136–43 [DOI] [PubMed] [Google Scholar]
- 5.de Lavallade H, Apperley JF, Khorashad JS, Milojkovic D, Reid AG, Bua M, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26(20):3358–63 [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian HM, Hochhaus A, Saglio G, De Souza C, Flinn IW, Stenke L, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12(9): 841–51 [DOI] [PubMed] [Google Scholar]
- 7.Saglio G, le Coutre PD, Pasquini R, Jootar S, Nakamae H, Flinn IW, et al. Nilotinib versus imatinib in patients (pts) with newly diagnosed philadelphia chromosome-positive (Ph+) chronic myeloid leukemia in chronic phase (CML-CP): ENESTnd 36-Month (mo) Follow-up. Blood (ASH Annual Meeting Abstracts). 2011;118 [Google Scholar]
- 8.Kantarjian HM, Shah NP, Cortes JE, Baccarani M, Agarwal MB, Undurraga MS, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2012;119(5):1123–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029–35 [DOI] [PubMed] [Google Scholar]
- 10.Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini FE, et al. Discontinuation of imatinib in patients with chronic myeloid leukemia who have maintained complete molecular response: update results of the STIM study. Blood (ASH Annual Meeting Abstracts). 2011;118:603 [Google Scholar]
- 11.Hughes T. ABL kinase inhibitor therapy for CML: baseline assessments and response monitoring. Hematology Am Soc Hematol Educ Program. 2006:211–8 [DOI] [PubMed] [Google Scholar]
- 12.Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cross NC, White HE, Muller MC, Saglio G, Hochhaus A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia. 2012;26(10):2172–5 [DOI] [PubMed] [Google Scholar]
- 14.Miura M, Takahashi N, Sawada K. Quantitative determination of imatinib in human plasma with high-performance liquid chromatography and ultraviolet detection. J Chromatogr Sci. 2011;49(5):412–5 [DOI] [PubMed] [Google Scholar]
- 15.Itoda M, Saito Y, Maekawa K, Hichiya H, Komamura K, Kamakura S, et al. Seven novel single nucleotide polymorphisms in the human SLC22A1 gene encoding organic cation transporter 1 (OCT1). Drug Metab Pharmacokinet. 2004;19(4):308–12 [DOI] [PubMed] [Google Scholar]
- 16.Cascorbi I, Gerloff T, Johne A, Meisel C, Hoffmeyer S, Schwab M, et al. Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin Pharmacol Ther. 2001;69(3):169–74 [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi D, Ieiri I, Hirota T, Takane H, Maegawa S, Kigawa J, et al. Functional assessment of ABCG2 (BCRP) gene polymorphisms to protein expression in human placenta. Drug Metab Dispos. 2005;33(1): 94–101 [DOI] [PubMed] [Google Scholar]
- 18.Ng KP, Hillmer AM, Chuah CT, Juan WC, Ko TK, Teo AS, et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med. 2012;18(4):521–8 [DOI] [PubMed] [Google Scholar]
- 19.Marin D, Bazeos A, Mahon FX, Eliasson L, Milojkovic D, Bua M, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Branford S, Ross D, Prime J, Field C, Altamura H, Yeoman A, et al. Early molecular response and female sex strongly predict achievement of stable undetectable BCR-ABL1, a criterion for imatinib discontinuation in patients with CML. Blood (ASH Annual Meeting Abstracts). 2012; 120:165 [Google Scholar]
- 21.Hasford J, Baccarani M, Hoffmann V, Guilhot J, Saussele S, Rosti G, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood. 2011;118 (3):686–92 [DOI] [PubMed] [Google Scholar]
- 22.Kantarjian HM, Kim DW, Issaragrisil S, Clark RE, Reiffers J, Nicolini FE, et al. ENESTnd 4-year (y) update: continued superiority of nilotinib vs imatinib in patients (pts) with newly diagnosed philadelphia chromosome–positive (Ph+) chronic myeloid leukemia in chronic phase (CML-CP). Blood (ASH Annual Meeting Abstracts). 2012;120:1676 [Google Scholar]
- 23.Hochhaus A, Hughes TP, Saglio G, Guilhot F, Al-Ali HK, Rosti G, et al. Outcome of patients with chronic myeloid leukemia in chronic phase (CML-CP) based on early molecular response and factors associated with early response: 4-year follow-up data from ENESTnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials Newly Diagnosed Patients). Blood (ASH Annual Meeting Abstracts). 2012; 120:167 [Google Scholar]
- 24.Saglio G, Kantarjian HM, Shah N, Jabbour EJ, Quintas-Cardama A, Steegmann JL, et al. Early response (molecular and cytogenetic) and long-term outcomes in newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): exploratory analysis of DASISION 3-year data. Blood (ASH Annual Meeting Abstracts). 2012;120:1675 [Google Scholar]
- 25.Takahashi N, Wakita H, Miura M, Scott SA, Nishii K, Masuko M, et al. Correlation between imatinib pharmacokinetics and clinical response in Japanese patients with chronic-phase chronic myeloid leukemia. Clin Pharmacol Ther. 2010;88(6):809–13 [DOI] [PubMed] [Google Scholar]
- 26.Imai Y, Nakane M, Kage K, Tsukahara S, Ishikawa E, Tsuruo T, et al. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol Cancer Ther. 2002;1(8):611–6 [PubMed] [Google Scholar]
- 27.Takahashi N, Miura M, Scott SA, Kagaya H, Kameoka Y, Tagawa H, et al. Influence of CYP3A5 and drug transporter polymorphisms on imatinib trough concentration and clinical response among patients with chronic phase chronic myeloid leukemia. J Hum Genet. 2010;55(11):731–7 [DOI] [PubMed] [Google Scholar]
- 28.White DL, Saunders VA, Dang P, Engler J, Venables A, Zrim S, et al. Most CML patients who have a suboptimal response to imatinib have low OCT-1 activity: higher doses of imatinib may overcome the negative impact of low OCT-1 activity. Blood. 2007;110(12):4064–72 [DOI] [PubMed] [Google Scholar]
- 29.White DL, Dang P, Engler J, Frede A, Zrim S, Osborn M, et al. Functional activity of the OCT-1 protein is predictive of long-term outcome in patients with chronic-phase chronic myeloid leukemia treated with imatinib. J Clin Oncol. 2010;28(16): 2761–7 [DOI] [PubMed] [Google Scholar]
- 30.Katagiri S, Umezu T, Ohyashiki JH, Ohyashiki K. The BCL2L11 (BIM) deletion polymorphism is a possible criterion for discontinuation of imatinib in chronic myeloid leukaemia patients. Br J Haematol. 2013;160(2):269–71 [DOI] [PubMed] [Google Scholar]
- 31.Mustjoki S, Ekblom M, Arstila TP, Dybedal I, Epling-Burnette PK, Guilhot F, et al. Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia. 2009;23(8):1398–405 [DOI] [PubMed] [Google Scholar]
- 32.Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med. 2011;17(9):1094–100 [DOI] [PMC free article] [PubMed] [Google Scholar]