Abstract
Clinical trials of immunotherapy in mantle cell lymphoma have not yet delivered desirable results, partly because of the inhibitory machinery of the tumor and its microenvironment. Here we investigated the role of B7-H1, a member of the B7 family of co-stimulatory/co-inhibitory ligands, in mantle cell lymphoma-mediated immunosuppression. Allogeneic CD3+, CD4+ and CD8+ T cells were purified and co-cultured with irradiated mantle cell lymphoma cells. Mantle cell lymphoma-reactive T-cell lines from HLA-A*0201+ healthy blood donors were generated after in vitro restimulation, and were subjected to functional tests. We found that B7-H1 expressed on mantle cell lymphoma cells was able to inhibit T-cell proliferation induced by the tumor cells, impair the generation of antigen-specific T-cell responses, and render mantle cell lymphoma cells resistant to T-cell-mediated cytolysis. Blocking or knocking down B7-H1 on mantle cell lymphoma cells enhanced T-cell responses and restored tumor-cell sensitivity to T-cell-mediated killing in vitro and in vivo. Knocking down B7-H1 on mantle cell lymphoma cells primed more CD4+ or CD8+ memory effector T cells. Our study demonstrates for the first time that lymphoma cell-expressed B7-H1 may lead to the suppression of host anti-tumor immune responses in mantle cell lymphoma and targeting tumor cell B7-H1 may represent a novel approach to improve the efficacy of immunotherapy in patients with mantle cell lymphoma.
Introduction
Mantle cell lymphoma (MCL) is a unique subtype of incurable B-cell neoplasm that accounts for about 6% of non-Hodgkin’s lymphoma.1 It is characterized by a specific t(11;14) (q13;q32) translocation, causing overexpression of cyclin D1.2 MCL is typically disseminated at presentation, containing a leukemic component in 20%–30% of patients.1 Most patients have advanced stage disease at initial diagnosis, and the prognosis is poorest among B-cell lymphoma patients, with a median survival of approximately 3–5 years.3 Although progress has been made in the management of MCL in the past decade, persistent remissions are usually not achieved and the management of patients with relapsed or refractory MCL is still challenging. Novel treatment options are, therefore, needed to improve the clinical outcome in MCL patients.
Immunotherapy may emerge as an effective treatment and may offer a potential for cure in several lymphoma subtypes in combination with other treatments. Studies of autologous idiotype-specific T cells and evidence of clinical response to allogeneic donor lymphocyte infusions show that anti-lymphoma responses can be generated.4 Idiotype protein has been proven to stimulate both humoral and cellular responses in lymphoma patients.5 Autologous idiotype protein can be formulated into an immunogenic antigen in follicular lymphoma patients with minimal residual disease,6 and a randomized, phase III clinical trial showed that vaccination with patient-specific hybridoma-derived idiotype vaccine in first remission prolonged disease-free survival in patients with follicular lymphoma.7 However, two other similar randomized, phase III trials failed to demonstrate clinical benefits in vaccinated lymphoma patients.8 The lack of clinical response may be related to the inhibitory mechanisms of tumor cells and/or their microenvironment, which may include impairment of co-stimulation and expression by tumor cells of co-inhibitory molecules that suppress T-cell responses.9 The tumor-cell inhibitory machinery must, therefore, be targeted to improve the efficacy of immunotherapy in lymphoma patients.
Previous studies have shown that mRNA of B7-H1 (Pdcd-1L1, PD-L1, CD274), a member of the B7 family of co-stimulatory/co-inhibitory ligands,10 is found in almost all normal tissues and organs in humans, while B7-H1 protein is rarely expressed in normal tissues and cells.11 B7-H1 induces an inhibitory signal to T-cell-mediated immunity, plays an important role in T-cell regulation of various immune responses, and is involved in peripheral tolerance, autoimmunity and infection.12 Recent studies demonstrated that the expression of B7-H1 protein is aberrantly up-regulated in various cancers such as gastric cancer, glioma, pancreatic cancer, renal cell carcinoma and urothelial cancer, and correlates with poor prognosis of the patients with these malignancies.11 B7-H1 over-expression appears as a possible mechanism for tumors to escape from host immune surveillance. The growth of a tumor-cell line was significantly increased after transfection with B7-H1.13 In a murine model of tumor dormancy, long-term persistent leukemic cells over-expressed B7-H1 and B7.1 and were more resistant to cytotoxic T lymphocyte (CTL)-mediated killing.14 In the present study, we investigated the role of B7-H1 in MCL-mediated immunosuppression in human MCL.
Methods
Patients and cell lines
The sources of primary MCL cells included bone marrow aspirates and peripheral blood from newly diagnosed and relapsed MCL patients, collected after obtaining informed consent. The study was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center. Twelve bone marrow aspirates were derived from seven newly diagnosed and five relapsed MCL patients who had bone marrow involvement. Peripheral blood containing leukemic MCL cells was collected from five patients with relapsed MCL who also had bone marrow involvement. Mononuclear cells were separated by Ficoll-Hypaque density centrifugation, and MCL cells were isolated using anti-CD19 magnetic microbeads (Miltenyi Biotec, Auburn, CA, USA). Purified MCL cells were cryopreserved in liquid nitrogen until use. K562 and four human MCL lines, SP53, Mino, Granta 519 and Jeko-1 (ATCC, Manassas, VA, USA), were maintained in RPMI-1640 medium (Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA, USA), penicillin (10,000 units/mL, Sigma), and streptomycin (10 mg/mL, Sigma).
Flow cytometry analysis
The expression of various surface MCL molecules, such as CD5, CD19, and CD20, was determined by direct immunofluorescence using FITC-conjugated antibodies against these molecules. After staining, cells were resuspended in phosphate-buffered saline and analyzed by a FACScan flow cytometer (Becton Dickinson). To examine the expression of B7-H1, we gated on CD19+/CD5+ cells to identify MCL tumor cells.
B7-H1 small hairpin RNA transfection of tumor cells by lentivirus
SP53 and Granta 519 cells were transfected using human Pdcd-1L1 or control small hairpin (sh)RNA lentiviral particles (Santa Cruz Biotechnology, Santa Cruz, CA, USA) according to the manufacturer’s protocol to knockdown B7-H1 expression in the cells. The untreated SP53 or Granta 519 cells are referred to as SP53-wt or Granta 519-wt, the control shRNA-transfected SP53 or Granta 519 cells as SP53-ctl or Granta 519-ctl, and the B7-H1 specific shRNA-transfected SP53 or Granta 519 cells as SP53-kd or Granta 519-kd, respectively.
Generation of tumor-reactive, alloantigen-specific cytotoxic T lymphocyte lines
Allogeneic CD3+ T cells were co-cultured in T-cell medium with irradiated SP53-wt, SP53-ctl, SP53-kd, Granta 519-wt, Granta 519-ctl, or Granta 519-kd. After at least four repeated cycles of in vitro restimulation, T-cell lines were generated, and named CTL-SP53-wt, CTL-SP53-ctl, CTL-SP53-kd, CTL-Granta 519-wt, CTL-Granta 519-ctl and CTL-Granta 519-kd, based on their stimulatory MCL cells. T-cell lines were expanded in T-cell medium containing recombinant interleukin (IL)-2, IL-7, and IL-15 for 2 weeks and subjected to functional tests.
Cytotoxicity assay
The standard 4-h 51Cr-release assay was performed to measure the cytolytic activity of the T-cell lines with target cells including SP53-wt, SP53-ctl, SP53-kd, Granta 519-wt, Granta 519-ctl, Granta 519-kd, primary tumor cells isolated from MCL patients, peripheral blood mononuclear cells (PBMC), B cells and K562 cells, as described previously.15
Statistical analysis
The Student’s t test was used to compare various experimental groups. A P value less than 0.05 was considered statistically significant. Unless otherwise indicated, means and standard deviations (SD) are shown.
Other methods
Details on the reverse transcriptase polymerase chain reaction analyses, western blot studies, proliferation assays, cytokine enzyme-linked immunosorbent assays (ELISA), generation of tumor-reactive, alloantigen-specific CTL lines, cytotoxic assays, and adoptive therapy in SCID mice are provided in the Online Supplementary Material.
Results
B7-H1 is constitutively expressed by mantle cell lymphoma cell lines and primary mantle cell lymphoma cells
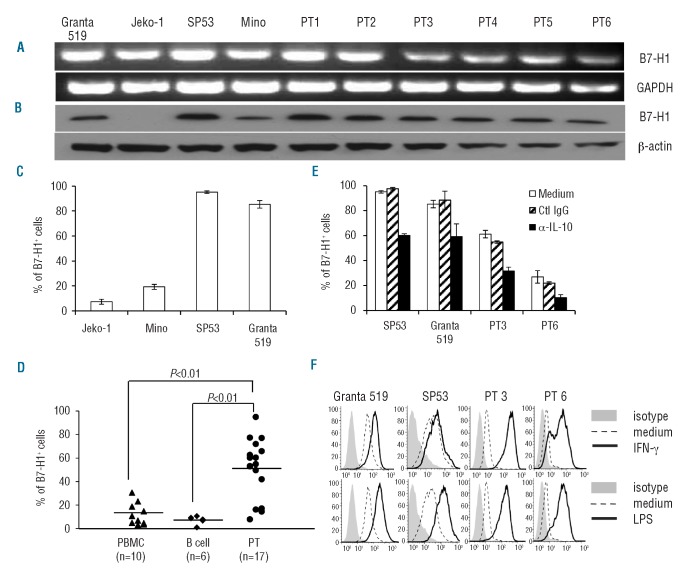
We found that B7-H1 mRNA (Figure 1A) and total protein (Figure 1B) were expressed in most MCL cell lines and all primary MCL cells from six patients examined. Flow cytometry analysis showed that surface B7-H1 was expressed in most MCL cell lines (Figure 1C) and primary MCL cells of 17 patients (Figure 1D), and the percentages of B7-H1+ cells were significantly higher in primary MCL cells than in normal PBMC (P<0.01) or B cells (P<0.01).
Figure 1.
Expression of B7-H1 by MCL cells. Expression of B7-H1 (A) mRNA by reverse transcriptase polymerase chain reaction, (B) total protein by western blots, and surface protein by flow cytometry analysis in (C) MCL cell lines and (D) primary tumor cells from 17 MCL patients, normal PBMC from ten blood donors, and B cells from six blood donors. Surface expression of B7-H1 on SP53 and Granta 519, and on primary MCL cells from two patients (PT3 and PT6) after 24 h incubation with (E) 20 μg/mL IL-10-neutralizing mAb (α-IL-10) or control IgG (Ctl IgG), or (F) with addition of 500 IU/mL IFN-γ or 1 μg/mL LPS. To examine the expression of B7-H1, we gated on CD19+/CD5+ cells to identify MCL tumor cells. Representative experiments of B7-H1 expression on MCL cells are shown.
As B7-H1 is usually an inducible molecule in normal cells, we investigated whether the tumor microenvironment or inflammatory factors may have contributed to MCL B7-H1 expression. Since our preliminary studies showed that MCL secreted IL-10 and tumor necrosis factor-α (TNF-α) (Online Supplementary Figure S1A), we used neutralizing monoclonal antibodies (mAb) to neutralize MCL-secreted IL-10 or TNF-α and found that B7-H1 expression was decreased by neutralizing IL-10 (Figure 1E) but not by TNF-α neutralization (data not shown). Moreover, interferon-γ (IFN-γ), which is the main inducer of B7-H1 expression in normal cells,16,17 significantly up-regulated B7-H1 expression in primary MCL cells and SP53 and Granta 519 cells (Figure 1F). As we detected expression of TLR4 in MCL cell lines and primary MCL cells from patients (Online Supplementary Figure S1B), we used lipopolysaccharide (LPS) to incubate MCL cells and found that B7-H1 surface expression was upregulated in MCL cells (Figure 1F). LPS also induced B7-H1 mRNA expression in dose- and time-dependent ways (Online Supplementary Figure S1C,D).
Blocking or knocking down B7-H1 on mantle cell lymphoma cells significantly increases their capacity to induce proliferation of alloreactive T cells
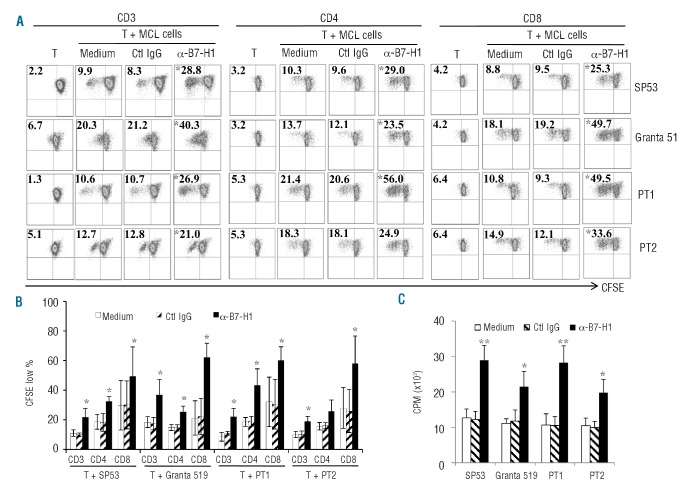
To investigate the functional significance of B7-H1 expressed on MCL cells, we co-cultured allogeneic CD3+, CD4+ and CD8+ T cells isolated from healthy donors with irradiated SP53, Granta 519, or primary MCL cells from two patients. B7-H1 blocking antibody or control IgG was used to pre-incubate MCL cells before adding the cells to allogeneic T cells. As shown in Figure 2A–C, the presence of B7-H1-blocking antibody, but not control IgG, significantly increased the proliferation of allogeneic CD3+, CD4+ and CD8+ T cells (P<0.05, compared with medium or IgG control). Similar results were also obtained when PD-1, which is a ligand for B7-H1, blocking antibody was used (Online Supplementary Figure S2A; P<0.05, compared with medium or control IgG). Indeed, alloreactive T cells expressed PD-1 on their surface (Online Supplementary Figure S2B). These findings indicate that B7-H1/PD-1 signaling inhibited T-cell proliferation in response to alloantigens.
Figure 2.
MCL-expressed B7-H1 inhibits allogeneic T-cell proliferation. (A) Representative results of proliferation of CD3+, CD4+ and CD8+ T cells, measured by CFSE dilution assay, in 7–10 day co-cultures with irradiated SP53, Granta 519 or primary MCL cells from two patients (PT1 and PT2) in the presence or absence of 20 μg/mL anti-B7-H1 mAb (α-B7-H1) or control IgG (Ctl IgG). Figures within dot plots represent the percentages of proliferating T cells; (B) summarized results of three independent experiments using the cell lines and primary MCL cells; and (C) proliferation of CD3+ T cells, measured by 3H-thymidine uptake assay, in 5-day co-cultures with irradiated SP53, Granta 519 or primary MCL cells from two patients (PT1 and PT2) in the presence or absence of 20 μg/mL anti-B7-H1 mAb (α-B7-H1) or control IgG (Ctl IgG). *P<0.05, **P<0.01, compared with medium or control IgG.
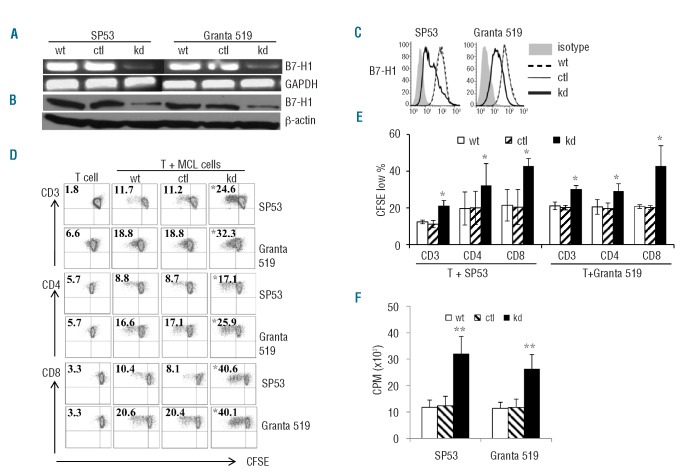
To confirm the role of B7-H1 in the suppression of T-cell proliferation, we knocked down B7-H1 gene expression in SP53 and Granta 519 cells by using B7-H1-specific shRNA lentiviral particles. Upon transfection, B7-H1-specific shRNA reduced B7-H1 gene (Figure 3A), total protein (Figure 3B) and surface protein (Figure 3C) expression, while the control shRNA did not. Consistent with antibody-blocking studies, knockdown of B7-H1 in MCL cells led to enhanced proliferation of alloreactive CD3+, CD4+ and CD8+ T cells (Figure 3D,E,F; P<0.05 or P<0.01, compared with control cells). These findings confirmed that MCL-associated B7-H1 inhibited T-cell proliferation.
Figure 3.
Knockdown of B7-H1 in MCL cells promotes allogeneic T-cell proliferation. Expression of B7-H1 (A) mRNA by reverse transcriptase polymerase chain reaction (B) total protein by western blots, and (C) surface protein by flow cytometry analysis of wild-type (wt) SP53 or Granta 519 and cells transfected with B7-H1-specific (kd) or control (ctl) shRNA. (D) Proliferation of CD3+, CD4+ and CD8+ T cells, measured by CFSE dilution assay, in 7–10 day co-cultures with irradiated SP53-wt, SP53-ctl or SP53-kd or Granta 519-wt, Granta 519-ctl or Granta 519-kd cells. Figures within dot plots represent the percentages of proliferating T cells. Representative results of three independent experiments are shown. (E) Summarized results of three independent experiments using the cell lines and primary MCL cells. (F) Proliferation of CD3+ T cells, measured by 3H-thymidine uptake assay, in 5-day co-cultures with irradiated SP53-wt, SP53-ctl or SP53-kd or Granta 519-wt, Granta 519-ctl or Granta 519-kd cells. *P<0.05, **P<0.01, compared with shRNA controls.
Generation of mantle cell lymphoma tumor-reactive T-cell lines
Although previous studies indicated that expression of B7-H1 in solid tumor cells may inhibit tumor-specific T cells, little is known about its potential contribution to the suppression of host antitumor immunity in MCL. To investigate this issue, we generated MCL-reactive T-cell lines from HLA-A*0201+ healthy blood donors. Irradiated SP53-wt, SP53-ctl, SP53-kd, Granta 519-wt, Granta 519-ctl and Granta 519-kd were used as stimulatory cells and co-cultured with allogeneic CD3+ T cells (both SP53 and Granta 519 are HLA-A*0201+). As shown in Figure 4, the percentages of CD8+ T cells in T-cell lines increased during in vitro stimulation (Figure 4A). Furthermore, MCL cells with B7-H1 knockdown induced better CD8+ T-cell proliferation (Figure 4B,C, P<0.05, compared with wild-type cells). These results indicated that expression of surface B7-H1 on MCL cells impaired the generation of a MCL-reactive CD8+ T-cell response.
Figure 4.
Generation of MCL-reactive T-cell lines. Flow cytometry analysis showing: (A) the frequencies of MCL-reactive CD3+CD8+ T cells in cultures with irradiated MCL cells during in vitro stimulation; and (B) proliferation of CD8+ T cells, measured by CFSE dilution assay, in T-cell lines generated in response to irradiated MCL cells for 5 days. Representative results from one of three blood donors are shown. (C) Summarized results of proliferation of CD8+ T cells of three blood donors are shown. (D) Cytotoxicity of CTL-Granta 519-wt and CTL-SP53-wt against target cells, including Granta 519, SP53, Jeko-1 and Mino, primary MCL cells from four patients, and PBMC and normal B cells from three of the four patients (patients 1–3; PBMC1-PBMC3 and B1–B3). Patients 1, 2, and 3 were HLA-A*0201+ and patient 4 was HLA-A*0201-. MDA231 cells were used as an HLA-mismatched control tumor target, K562 was used as a control for NK-cell activity. (E) Inhibition of T-cell-mediated cytotoxicity against Granta 519 or SP53 by mAb against MHC class I (α-MHC-I) or MHC class II (α-MHC-II). Isotypic IgG was used as a control (Ctl IgG). An effector:target (E:T) ratio of 20:1 was used. Representative results of T-cell lines generated from one healthy donor are shown in panels (A) and (B). Similar results were obtained with T-cell lines generated from three healthy donors. *P<0.05; **P<0.01, compared with control T cells.
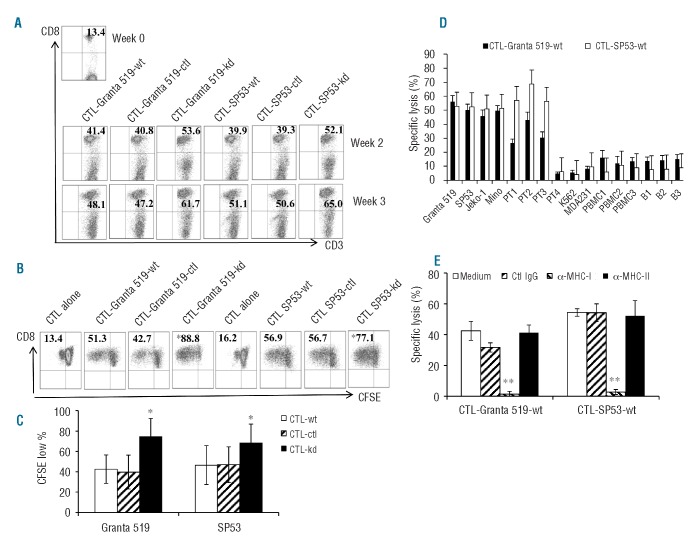
Next we examined the cytolytic activity of these T-cell lines. We used T-cell lines generated from wild-type Granta 519 or SP53 and showed that the T cells killed not only the stimulatory MCL cell lines, but also HLA-A*0201+ primary MCL cells (patients 1–3). No killing was observed on HLA-A*0201− primary MCL cells (patient 4) or K562 cells (Figure 4D), indicating that natural killer cells were not responsible for the killing. To determine MHC restriction of the T-cell–mediated cytotoxicity, we evaluated the inhibitory effects of anti-MHC mAb. As shown in Figure 4E, mAb against MHC class I (HLA-ABC) significantly inhibited killing of the target cells by the T-cell lines (P<0.01, compared with medium control). No inhibitory effect was observed with mAb against MHC class II (HLA-DR) and control IgG. The results indicate that the cytotoxicity of the T cells was attributed to MHC class I-restricted, CD8+ T-cell-mediated killing.
We also examined whether the T cells were cytolytic to normal hematopoietic cells and HLA-mismatched control tumor target MDA231. In these experiments, purified PBMC and B cells from MCL patients #1–3 as well as MDA231 were used as target cells to demonstrate whether the T cells were cytolytic to normal cells and HLA-mismatched control tumor target. As shown in Figure 4D, although the T cells were alloantigen-specific, less killing was observed against normal B cells or PBMC from the same patients or MDA231. These findings may suggest that the T cells recognized tumor-derived peptides in the context of allogeneic MHC molecules, which were not present on the surface of normal blood cells and HLA-mismatched control tumor target.
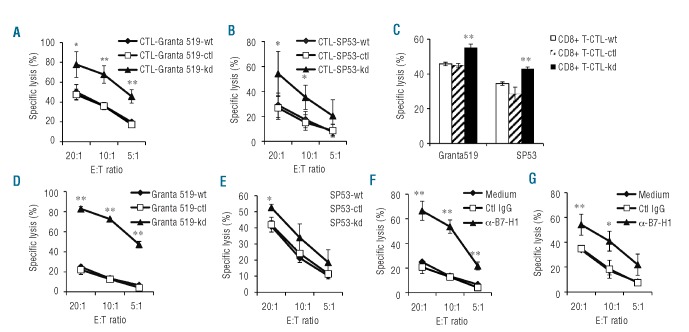
B7-H1 mediates a direct inhibitory effect on T-cell lysis of their targets
We examined the cytolytic activity of T-cell lines against MCL cells. As shown in Figure 5, the CTL-SP53-kd (Figure 5A) and CTL-Granta 519-kd (Figure 5B) lines showed better killing of their target cells as compared with control T-cell lines (P<0.05 to P<0.01). To exclude the possibility that the better killing was caused by more CD8+ T cells in the CTL-SP53-kd or CTL-Granta 519-kd line than in other cell lines, we isolated CD8+ T cells from the T-cell lines and compared their cytolytic activity. We found that CD8+ T cells isolated from CTL-SP53-kd or CTL-Granta 519-kd lines again showed better killing of their target cells as compared with the same numbers of CD8+ T cells from control T-cell lines (Figure 5C; P<0.01). More importantly, MCL cells with B7-H1 knockdown were significantly more sensitive than the wild-type or control cells to T-cell-mediated cytotoxicity (Figure 5D,E; P<0.05 to P<0.01). Moreover, when wild-type SP53 or Granta 519 cells were pre-incubated with B7-H1 blocking mAb, these cells became equally sensitive, as compared with B7-H1-knocked down cells, to the killing (Figure 5F,G; P<0.05 to P<0.01). Similar results were also obtained using PD-1 blocking mAb (Online Supplementary Figure S2C; P<0.01). These results strongly suggest that B7-H1/PD-1 signaling mediates a direct inhibitory effect on T-cell-mediated killing of their target MCL cells.
Figure 5.
Cytotoxicity of MCL-reactive T-cell lines. Cytotoxicity of (A) CTL-Granta 519-wt, CTL-Granta 519-ctl and CTL-Granta 519-kd against wild-type Granta 519; and (B) CTL-SP53-wt, CTL-SP53-ctl and CTL-SP53-kd against wild-type SP53 cells; (C) Purified CD8+ T cells from CTL-Granta 519-wt, CTL-Granta 519-ctl or CTL-Granta 519-kd or CTL-SP53-wt, CTL-SP53-ctl or CTL-SP53-kd T-cell lines against their respective target cells Grant 519 or SP53. An effector:target (E:T) ratio of 10:1 was used. (D) CTL-Granta 519-wt against three different target cells Granta 519-wt, Granta 519-ctl or Granta 519-kd tumor cells, or (E) CTL-SP53-wt against three different target cells SP53-wt, SP53-ctl or SP53-kd tumor cells; or (F) CTL-Granta 519-wt against Granta 519 pre-treated with 20 μg/mL anti-B7-H1 mAb (α-B7-H1) or control IgG (Ctl IgG); and (G) CTL-SP53-wt against SP53 pre-treated with 20 μg/mL anti-B7-H1 mAb (α-B7-H1) or control IgG (Ctl IgG). Panels (A), (B), (D) and (F) show results of T-cell lines generated from three healthy donors. Panels (C), (E) and (G) show representative results of T-cell lines generated from one healthy donor. *P<0.05; **P<0.01, compared with shRNA control T cells.
Mantle cell lymphoma-reactive T cells are memory effector T cells and express high levels of perforin and granzyme B
Flow cytometry analysis was used to examine the expression of granzyme, perforin and Fas ligand (FasL) by the T-cell lines. As shown in Online Supplementary Figure S3A,B, it seems that the T cells killed their target cells via the perforin/granzyme pathways, because they expressed high levels of perforin and granzyme B but not FasL. Either CD4+ or CD8+ T cells in the lines displayed CD45RAlowCD45ROhighCD44highCD62Llow phenotype (Online Supplementary Figure S3A,B), indicating that they were memory effector T cells.18,19 The expression of extracellular markers CD27 and CD28 has been used to define subsets of effector and memory T cells,20,21 and CD27 and CD28 are major co-stimulatory receptors for T-cell growth and cytokine production. As shown in Online Supplementary Figure S3A,B, CD27 expression was low in CD4+ and CD8+ T cells but nearly absent from CD4+ and CD8+ T cells in CTL-SP53-kd lines as compared to CTL-SP53-wt or CTL-SP53-ctl lines. However, CD28 was abundantly expressed in CD4+ and CD8+ T cells and was upregulated in effector CD8+ T cells in CTL-SP53-kd cells as compared to CTL-SP53-wt or CTL-SP53-ctl.
Mantle cell lymphoma-reactive T cells secrete interferon-γ and express CD107a
Two independent methods were used to examine the cytokine expression profiles of the T-cell lines. To determine cytokine secretion, an ELISA was used to detect IFN-γ levels in T-cell culture supernatants. After restimulation with SP53-wt, SP53-ctl or SP53-kd, all the T-cell lines secreted high levels of IFN-γ, however, CTL-SP53-kd cells secreted significantly higher levels of IFN-γ than the other two cell lines (Online Supplementary Figure S3C; P<0.01). We also examined secretion of IL-4, IL-6 and IL-10 by the T cells, which was undetectable (data not shown). Similarly, intracellular cytokine staining showed that the proportion of CD4+ or CD8+ T cells expressing IFN-γ was higher in the CTL-SP53-kd line than in the CTL-SP53-wt or CTL-SP53-ctl lines (Online Supplementary Figure S3D; P<0.05), while there were only few IL-4- or IL-17-expressing T cells. As CD107a has been described to be a marker for cytotoxic potential of effector CD8+ T cells,22,23 we examined its expression on our T cells. CD107a was expressed at a higher level on CD8+ T cells in the CTL-SP53-kd line than on CTL-SP53-wt or CTL-SP53-ctl lines (P<0.05). Collectively, this indicates that the T cells expressed IFN-γ, but not IL-4 or IL-17, and were thus type-1 T cells.24,25
Mantle cell lymphoma cells with down-regulated B7-H1 are more sensitive to killing by mantle cell lymhoma-specific cytotoxic T lymphocytes in vivo
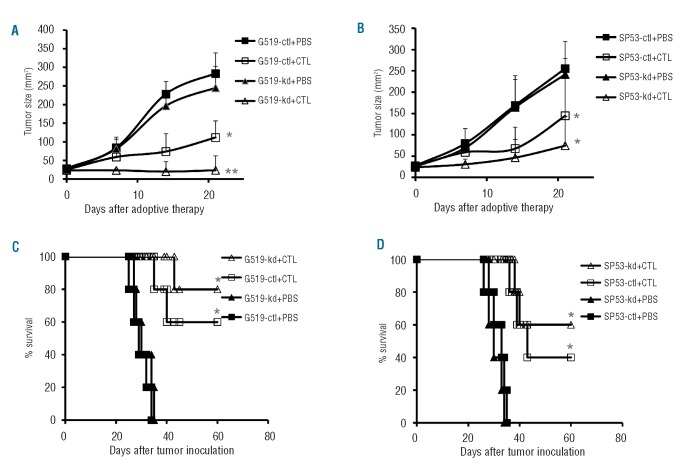
To confirm the effect of B7-H1 expression on MCL cells on T-cell-mediated cytolysis in vivo, we conducted an in vivo adoptive therapy study of MCL-specific CTL in MCL-established SCID mice. As shown in Figure 6, the wild-type (data not shown) and control (Granta-519-ctl and SP53-ctl) MCL tumors grew quickly in PBS-treated mice and the animals died within 40 days after tumor injection. Knockdown of B7-H1 (Granta-519-kd and SP53-kd) did not affect their growth in vivo. Adoptive transfer of MCL-specific CTL was therapeutic and eradicated tumors in 60% and 40% of mice injected with Granta-519-ctl and SP53-ctl, respectively, while the same treatment led to the survival of 80% and 60% of mice injected with Granta-519-kd and SP53-kd, respectively (P<0.05 and P<0.01, compared with controls). These results showed that down-regulation of B7-H1 on MCL cells improved the cells’ sensitivity to CTL-mediated killing in vivo.
Figure 6.
Down-regulation of B7-H1 improves the sensitivity of MCL cells to the killing of specific CTL. SCID mice (5 for each group) were subcutaneously injected with wild-type (data not shown), control Granta 519 or SP53 (G519-ctl or SP53-ctl), or B7-H1 knockdown Granta 519 or SP53 (G519-kd or SP53-kd). After the tumor burden had reached 25 mm2, mice were treated with CTL-Granta 519-wt or CTL-Sp53-wt, respectively, followed by injection of a high dose of recombinant human IL-2 (for details, see Design and Methods section). Mice treated with PBS served as controls. Tumor sizes were recorded twice a week. Mice were sacrificed once tumors reached 225 mm2. Tumor burden in mice injected with (A) Granta 519 and (B) SP53 MCL cells. The survival of mice injected with (C) Granta 519 and (D) SP53 MCL cells is also shown. *P<0.05; **P<0.01, compared with control MCL cells.
Discussion
There is accumulating evidence that tumor progression can be attributed to a variety of immune evasion strategies. The mechanisms include: (i) inhibition of the expression of certain cell surface antigen-presenting proteins, conferring them resistance to CTL;26 (ii) secretion of immunosuppressive factors, such as IL-6, IL-10, VEGF and TGF-β, that inhibit effector T-cell responses;27 (iii) expression of apoptosis-inducing cell surface proteins;9 and (iv) insufficient T-cell co-stimulation.9 However, the mechanism of tumor-mediated evasion of the immune system in MCL is largely unexplored. In this study, we report a new observation that B7-H1 gene and protein were expressed in most MCL cell lines and primary MCL cells from all patients examined, and expression of B7-H1 on the tumor cells led to inhibition of T-cell responses to tumor cells, which could occur during both priming and generation of an immune response and at the effector phase of T cells. Recognition of this new mechanism of MCL immune evasion will provide a new approach to the design of T-cell-based immunotherapy.
The use of different experimental systems to explore the role of B7-H1 expression may yield different results. Early studies showed that soluble B7-H1 immunoglobulin or cell-associated B7-H1 induced the proliferation of T cells in the presence of anti-CD3 mAb in vitro.28 However, recent studies demonstrated that the interaction of B7-H1 with PD-1, a member of the CD28 family up-regulated on activated T, B and myeloid cells, inhibited both CD4+ and CD8+ T-cell responses and was overcome by IL-2.29 In a murine acute myeloid leukemia model, B7-H1/PD-1 signaling inhibited antitumor immune responses and provided a rationale for clinical trials targeting this pathway in leukemia patients.30 Other studies showed that cancer cell-associated B7-H1 increased apoptosis of activated T cells in vitro, and this effect was mediated largely by one or more receptors other than PD-1.13 Recently, B7.1 has been described as a second binding receptor for B7-H1 and the B7-H1/B7.1 signaling pathway is functional in inhibiting alloimmune responses in vitro and in vivo.31 However, it is not known whether these negative pathways are important for immune evasion in MCL. In the present study, we aimed at investigating the significance of B7-H1 expressed by MCL cell lines and primary MCL cells in immune responses against the tumor cells. Under in vitro alloreactive co-culture conditions, we found that the proliferation of CD3+, CD4+ and CD8+ T cells in response to MCL cells significantly increased in the presence of B7-H1 or PD-1 blocking antibodies or when MCL-expressed B7-H1 was knocked down, implying that B7-H1/PD-1 signaling is directly involved in inhibition of T-cell responses to MCL cells. In line with our results, a recent study by Andorsky et al. showed that B7-H1 was also expressed by anaplastic large cell lymphomas and a subset of diffuse large B-cell lymphomas and inhibited the activity of tumor-associated T cells.32 In this study we used shRNA to knockdown B7-H1 expression on MCL cells. Although the knockdown was partial and incomplete, which is a limitation of the technology, T-cell responses to partially B7-H1-knocked down MCL cells were significantly increased as compared with the responses of control cells. This result indicates that decreased expression of B7-H1 on MCL cells triggered less negative signaling in T cells.
In this study we also investigated the contribution of B7-H1 on tumor cells to the suppression of host antitumor immunity in MCL. We generated T-cell lines from healthy donors using different, B7-H1-manipulated MCL cell lines as allogeneic antigen-presenting cells. We found that, compared with the wild-type or control cells, MCL cells with B7-H1 knockdown had a better capacity to prime tumor-reactive CD8+ T cells in vitro. These T cells also showed stronger killing of target tumor cells as compared with other T cells. Moreover, T-cell lines generated in response to B7-H1-knocked down MCL cells secreted more IFN-γ than other T-cell lines and these T cells did not secrete IL-4, IL-6, IL-10 or IL-17. As we started with CD3+ T cells, our T-cell lines contained both CD4+ and CD8+ T cells. However, it is evident that CD8+ T cells in the T-cell lines played the most important role because the cytolytic activity of the T cells was only blocked by mAb against MHC class I molecules. Flow cytometry analysis showed that the CD8+ T cells expressed high levels of perforin and granzyme B but not FasL, thus these cells may lyse their target cells via the perforin-granzyme pathways.33,34 On the other hand, the expression of B7-H1 on MCL cells rendered them more resistant to T-cell-mediated killing, as we showed that the T cells killed significantly more MCL cells with B7-H1 knockdown as compared to the wild-type or control cells. Similarly, when B7-H1 or PD-1 blocking mAb were added, wild-type MCL cells were as sensitive as B7-H1-knocked down cells to T-cell-mediated cytotoxicity. These findings indicate that B7-H1 expression on MCL cells not only impaired their ability to prime CD8+ T-cell responses but also rendered them more resistant to T-cell killing. These observations are in line with the concept of an overall suppressive regulatory function of B7-H1 for T-cell responses and suggest MCL-expressed B7-H1 as a possible mechanism of immune escape in the disease.
In conclusion, our study demonstrated that MCL cell lines and primary tumor cells expressed B7-H1, and cytokines and inflammatory factors such as IL-10, IFN-γ and LPS increased B7-H1 expression on MCL cells. MCL-expressed B7-H1 inhibited T-cell responses and protected MCL cells from direct immune attack by tumor-reactive T cells including CTL. Thus, our study identified tumor-expressed B7-H1 as an important negative regulator of immune responses in MCL and suggests that targeting B7-H1 may be a promising strategy to improve the efficacy of immunotherapy in patients, by enhancing the priming of tumor-specific immune responses and by sensitizing MCL cells to the killing mediated by tumor-specific effector cells.
Acknowledgments
We would like to thank the University of Texas MD Anderson Cancer Center Lymphoma Tissue Bank for providing patients’ samples. This facility is supported by the National Institutes of Health Lymphoma SPORE grant P50CA136411 and Fredrick B. Hagemeister Research Fund.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
This work was supported by Special Funds for International Cooperation of the National Natural Science Foundation of China (81120108018), Major Research Plan of the Chinese National Natural Science Foundation (91029740), Key Program of the Natural Science Foundation of Zhejiang, China (2009C03012-2) and the Cultivation Program for Distinguished Talented Persons of Health of Zhejiang, China. This work was also supported by National Cancer Institute R01 CA138402, R01 CA138398, and P50 CA142509, Leukemia and Lymphoma Society Translational Research Grants, Multiple Myeloma Research Foundation, the Commonwealth Foundation for Cancer Research and the Center for Targeted Therapy of The University of Texas MD Anderson Cancer Center.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117(1):26–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dreyling M, Hiddemann W. Current treatment standards and emerging strategies in mantle cell lymphoma. Hematology Am Soc Hematol Educ Program. 2009:542–51 [DOI] [PubMed] [Google Scholar]
- 3.Ghielmini M, Zucca E. How I treat mantle cell lymphoma. Blood. 2009;114(8):1469–76 [DOI] [PubMed] [Google Scholar]
- 4.Neelapu SS, Kwak LW, Kobrin CB, Reynolds CW, Janik JE, Dunleavy K, et al. Vaccine-induced tumor-specific immunity despite severe B-cell depletion in mantle cell lymphoma. Nat Med. 2005;11(9):986–91 [DOI] [PubMed] [Google Scholar]
- 5.Weng J, Neelapu SS, Woo AF, Kwak LW. Identification of human idiotype-specific T cells in lymphoma and myeloma. Curr Top Microbiol Immunol. 2011;344:193–210 [DOI] [PubMed] [Google Scholar]
- 6.Qin H, Cha SC, Neelapu SS, Lou Y, Wei J, Liu YJ, Kwak LW. Vaccine site inflammation potentiates idiotype DNA vaccine-induced therapeutic T cell-, and not B cell-, dependent antilymphoma immunity. Blood. 2009;114 (19):4142–9 [DOI] [PubMed] [Google Scholar]
- 7.Schuster SJ, Neelapu SS, Gause BL, Janik JE, Muggia FM, Gockerman JP, et al. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J Clin Oncol. 2011;29(20):2787–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freedman A, Neelapu SS, Nichols C, Robertson MJ, Djulbegovic B, Winter JN, et al. Placebo-controlled phase III trial of patient-specific immunotherapy with mitumprotimut-T and granulocyte-macrophage colony-stimulating factor after rituximab in patients with follicular lymphoma. J Clin Oncol. 2009;27(18):3036–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol. 2001;2(4): 293–9 [DOI] [PubMed] [Google Scholar]
- 10.Dong H, Strome SE, Matteson EL, Moder KG, Flies DB, Zhu G, et al. Costimulating aberrant T cell responses by B7-H1 autoanti-bodies in rheumatoid arthritis. J Clin Invest. 2003;111(3):363–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467–77 [DOI] [PubMed] [Google Scholar]
- 12.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19(7):813–24 [DOI] [PubMed] [Google Scholar]
- 13.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800 [DOI] [PubMed] [Google Scholar]
- 14.Saudemont A, Quesnel B. In a model of tumor dormancy, long-term persistent leukemic cells have increased B7-H1 and B7.1 expression and resist CTL-mediated lysis. Blood. 2004;104(7):2124–33 [DOI] [PubMed] [Google Scholar]
- 15.Qian J, Xie J, Hong S, Yang J, Zhang L, Han X, et al. Dickkopf-1 (DKK1) is a widely expressed and potent tumor-associated antigen in multiple myeloma. Blood. 2007;110 (5):1587–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170(3):1257–66 [DOI] [PubMed] [Google Scholar]
- 17.Mazanet MM, Hughes CC. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J Immunol. 2002;169(7):3581–8 [DOI] [PubMed] [Google Scholar]
- 18.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–23 [DOI] [PubMed] [Google Scholar]
- 19.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63 [DOI] [PubMed] [Google Scholar]
- 20.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8(4):379–85 [DOI] [PubMed] [Google Scholar]
- 21.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186(9):1407–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281(1–2):65–78 [DOI] [PubMed] [Google Scholar]
- 23.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294(1–2):15–22 [DOI] [PubMed] [Google Scholar]
- 24.Romagnani S. Human TH1 and TH2 subsets: doubt no more. Immunol Today. 1991;12(8): 256–7 [DOI] [PubMed] [Google Scholar]
- 25.Romagnani S. The Th1/Th2 paradigm and allergic disorders. Allergy. 1998;53(46 Suppl): 12–5 [DOI] [PubMed] [Google Scholar]
- 26.Zindl CL, Chaplin DD. Immunology. Tumor immune evasion. Science. 2010;328(5979): 697–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S, Yang J, Qian J, Wezeman M, Kwak LW, Yi Q. Tumor evasion of the immune system: inhibiting p38 MAPK signaling restores the function of dendritic cells in multiple myeloma. Blood. 2006;107(6): 2432–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–9 [DOI] [PubMed] [Google Scholar]
- 29.Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, et al. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32(3):634–43 [DOI] [PubMed] [Google Scholar]
- 30.Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 2009;114(8):1545–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang J, Riella LV, Chock S, Liu T, Zhao X, Yuan X, et al. The novel costimulatory programmed death ligand 1/B7.1 pathway is functional in inhibiting alloimmune responses in vivo. J Immunol. 2011;187 (3):1113–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andorsky DJ, Yamada RE, Said J, Pinkus GS, Betting DJ, Timmerman JM. Programmed death ligand 1 is expressed by non-Hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res. 2011;17(13):4232–44 [DOI] [PubMed] [Google Scholar]
- 33.Kägi D, Vignaux F, Ledermann B, Bürki K, Depraetere V, Nagata S, et al. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265 (5171):528–30 [DOI] [PubMed] [Google Scholar]
- 34.Kojima H, Shinohara N, Hanaoka S, Someya-Shirota Y, Takagaki Y, Ohno H, et al. Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity. 1994;1(5):357–64 [DOI] [PubMed] [Google Scholar]