Abstract
The role of reduced intensity allogeneic stem cell transplantation for the treatment of relapsed/refractory Hodgkin’s lymphoma remains controversial. We retrospectively analyzed 191 patients who underwent reduced intensity allogeneic stem cell transplantation between 1998 and 2008 for relapsed or refractory Hodgkin’s lymphoma and whose data were reported to the French registry. The median follow-up was 36 months. The estimated 3-year overall survival rate, progression-free survival rate, cumulative incidence of relapse and cumulative incidence of non-relapse mortality were 63%, 39%, 46%, and 16%, respectively. There was no difference in outcome between patients in complete response and in partial response at the time of transplantation with regards to overall survival (70% versus 74%, no significant difference) and progression-free survival (51% versus 42%, no significant difference). Patients with chemoresistant disease had a shorter overall survival (39% at 3 years; P=0.0003) and progression-free survival (18% at 3 years; P=0.001) than patients in complete remission. The use of umbilical cord blood as the source of stem cells was associated with a poor outcome with an increased risk of death with a hazard ratio of 3.49 (95% confidence interval: 1.26 to 9.63; P=0.016). The use of peripheral blood was associated with a better outcome for patients who where alive 1 year after transplantation with a hazard ratio of 0.38 (95% confidence interval: 0.17 to 0.83; P=0.016). Disease status at transplantation remains the most important risk factor for outcome. Our data suggest that the use of peripheral blood should be preferred whereas umbilical cord blood should be used with caution.
Introduction
In most cases, Hodgkin’s lymphoma is a curable disease with the first line of treatment. However, 15% to 25% of patients relapse or do not respond to this first-line therapy. For these patients, the standard of care is based on salvage courses of chemotherapy followed by autologous stem cell transplantation (SCT).1 Patients who relapse after autologous SCT or who are primary refractory to chemotherapy have a very poor prognosis and salvage treatment is less well codified.2,3 Allogeneic SCT is a treatment option for such patients, but the results are controversial and its role is still debated. Myeloablative conditioning regimens are associated with transplant-related mortality (rate 48 to 61%) and no obvious advantage when compared to autologous SCT.4–8 A graft-versus-lymphoma effect has been suggested.4,7 This concept is supported by the role of donor lymphocyte infusions, although data are conflicting.9 In contrast, reduced intensity conditioning is associated with an acceptable rate of non-relapse mortality in the most recently published studies and has, therefore, improved the prognosis of patients with refractory/relapsed Hodgkin’s lymphoma.10–18 Nevertheless the major cause of failure remains relapse with a rate of relapse of about 45%.15 A retrospective study using data from the European Group for Blood and Marrow Transplantation (EBMT) Registry found that reduced intensity conditioning allogeneic SCT was superior to standard conditioning SCT mainly because of the lower rate of non-relapse mortality in the reduced intensity conditioning group.11 More recently the EBMT reported the outcome of 285 patients who underwent reduced intensity conditioning allogeneic SCT for refractory/relapsed Hodgkin’s lymphoma and identified chemoresistant disease and poor performance status as factors predicting the outcome.15
In order to determine the role of reduced intensity conditioning allogeneic SCT in Hodgkin’s lymphoma, we conducted a multicenter retrospective study of 191 patients who underwent reduced intensity conditioning allogeneic SCT over a 10-year period (April 1998 - December 2008) in 28 centers and whose data were reported to the French Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC) registry. The aim of the study was to analyze the outcome of patients and to identify prognostic factors predicting the outcome.
Methods
This study was approved by the scientific board of the SFGM-TC and was performed according to institutional guidelines in agreement with the principles of the Declaration of Helsinki. Reduced intensity conditioning allogeneic SCT was defined as follows: busulfan ≤8 mg/kg with or without a purine analog, cyclophosphamide ≤60 mg/kg with or without a purine analog, total body irradiation ≤6 cGy (fractionated) with or without a purine analog, melphalan 140 mg/m2 with a purine analog.
The histological diagnosis was based on local review and the disease was staged according to the Ann Arbor system. Status at transplantation was defined according to Cheson’s criteria.19 Complete response was defined as the complete disappearance of all detectable clinical, pathological (i.e. bone marrow), and radiographic evidence of disease, all disease-related symptoms as well as the normalization of all biochemical abnormalities. Partial response was defined as a ≥ 50% decrease of all measurable lesions. Stable disease was defined as no response or a response < 50%. Progressive disease was defined as at least a 50% increase of any measurable lesion or appearance of any new lesion during or at the end of therapy. Relapse was defined as the appearance of any new lesion in patients who had achieved a complete response. Patients in complete response and in partial response at the time of allogeneic SCT were considered to have chemosensitive disease, whereas patients with stable or progressive disease were considered to have chemoresistant disease. All data were checked thoroughly by the local investigator and AM in order to be sure that the data were accurate.
End-points and statistical analysis
Data are presented as frequency (percent) or median (range). The end-points studied were overall survival, progression-free survival, non-relapse mortality, disease progression (relapse) as well as acute and chronic graft-versus-host disease (GVHD). Progression was defined as either relapse or disease progression for patients who did not achieve complete or partial response. Non-relapse mortality was defined as death without progression. Non-relapse mortality and disease progression were analyzed in a competing risks’ setting, in which each of these events was considered as a competing event for the other one. Acute and chronic GVHD were also analyzed in a competing risks’ setting with death and relapse as competing events. Patients who developed GVHD after donor lymphocyte infusion were considered as not having GVHD in these analyses. End-points were analyzed at the reference date of August 31st, 2009. Follow-up durations were thus computed as the time interval between the date of transplantation and the date of event, date of last follow-up or reference date, whichever occurred first. Cumulative incidence curves of acute and chronic GVHD, relapse and non-relapse mortality were estimated using the usual methodology.20 Overall and progression-free survival curves were estimated using the Kaplan-Meier product-limit estimator. Factors associated with overall survival and progression-free survival were analyzed using Cox proportional hazards models. Factors associated with non-relapse mortality and relapse were analyzed using Cox models for the cause-specific hazard.21 Univariable analyses were carried out for all four end-points. All factors were time-fixed covariates, except chronic GVHD which was considered as a time-dependent covariate in the Cox models. A multivariable analysis was then carried out for overall survival and relapse, where all factors with a P-value <0.20 in the univariable analyses were considered. No variable deletion procedure was used in the multivariable model, as the aim was to perform an adjusted analysis and not to develop a prognostic score. In each case, the proportional hazards assumption was checked by examination of Schoenfeld residuals and Grambsch and Therneau’s lack-of-fit test.22 In case of non-proportional hazards, models with time-dependent effects were used.
Survival and cumulative incidence results are presented as estimates and 95% confidence intervals (95% CI), and association of factors with end-points as hazard ratios (HR) (95% CI).
All tests were two-sided and P-values <0.05 were considered as indicating significant associations. Analyses were performed using R 2.10.1 statistical software [The R Foundation for Statistical Computing, Vienna, Austria (R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria 2009. URL http://www.R-project.org. ISBN 3-900051-07-0.)].
Results
Patients’ characteristics (Table 1)
Table 1.
Patient and transplant characteristics.
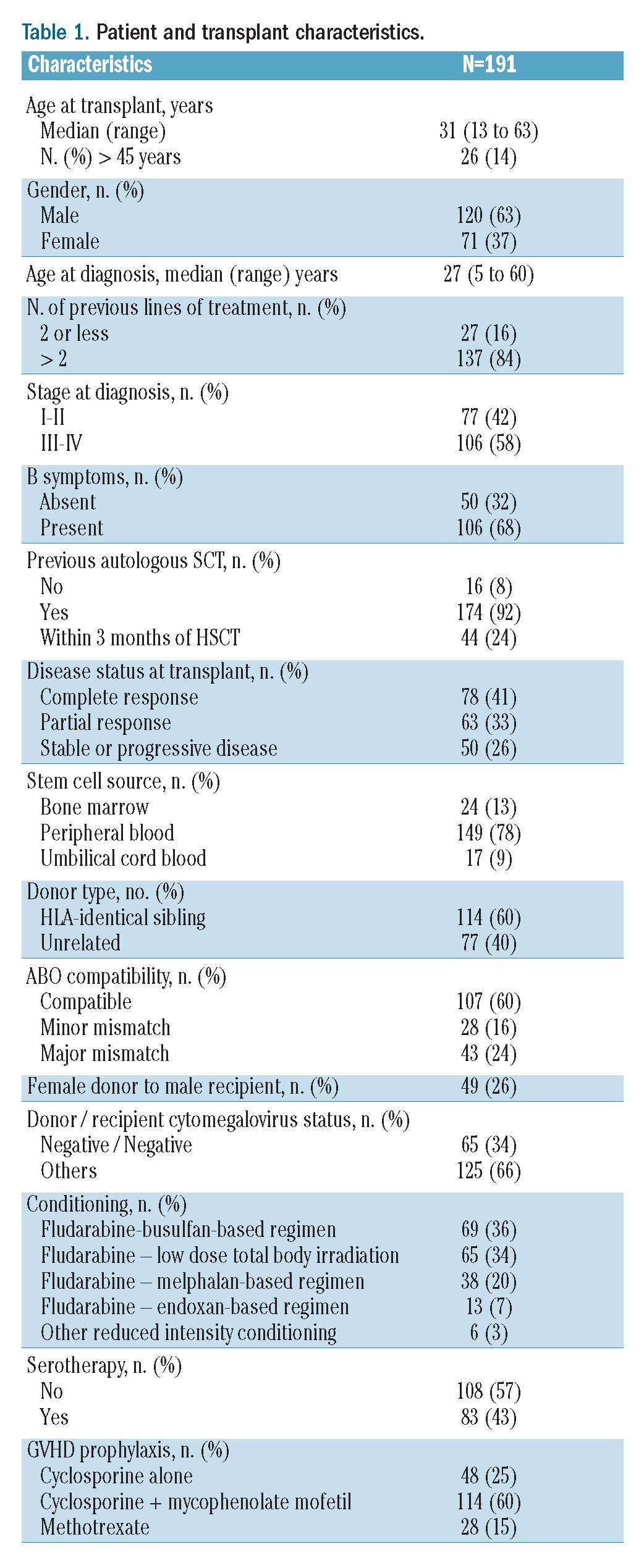
One hundred and ninety-one patients were enrolled in this study [median age at time of transplantation, 31 years (range, 13–63 years)]. The median number of previous lines of treatment before SCT was four (range, 2 – 8) and 174 patients (92%) had received a previous autologous SCT. Forty-four patients (24%) underwent allogeneic SCT within 3 months of autologous SCT. At the time of allogeneic SCT, 78 patients were in complete response (41%), 63 (33%), in partial response and 50 had stable or progressive disease (26%).
Transplant modalities and donors’ characteristics
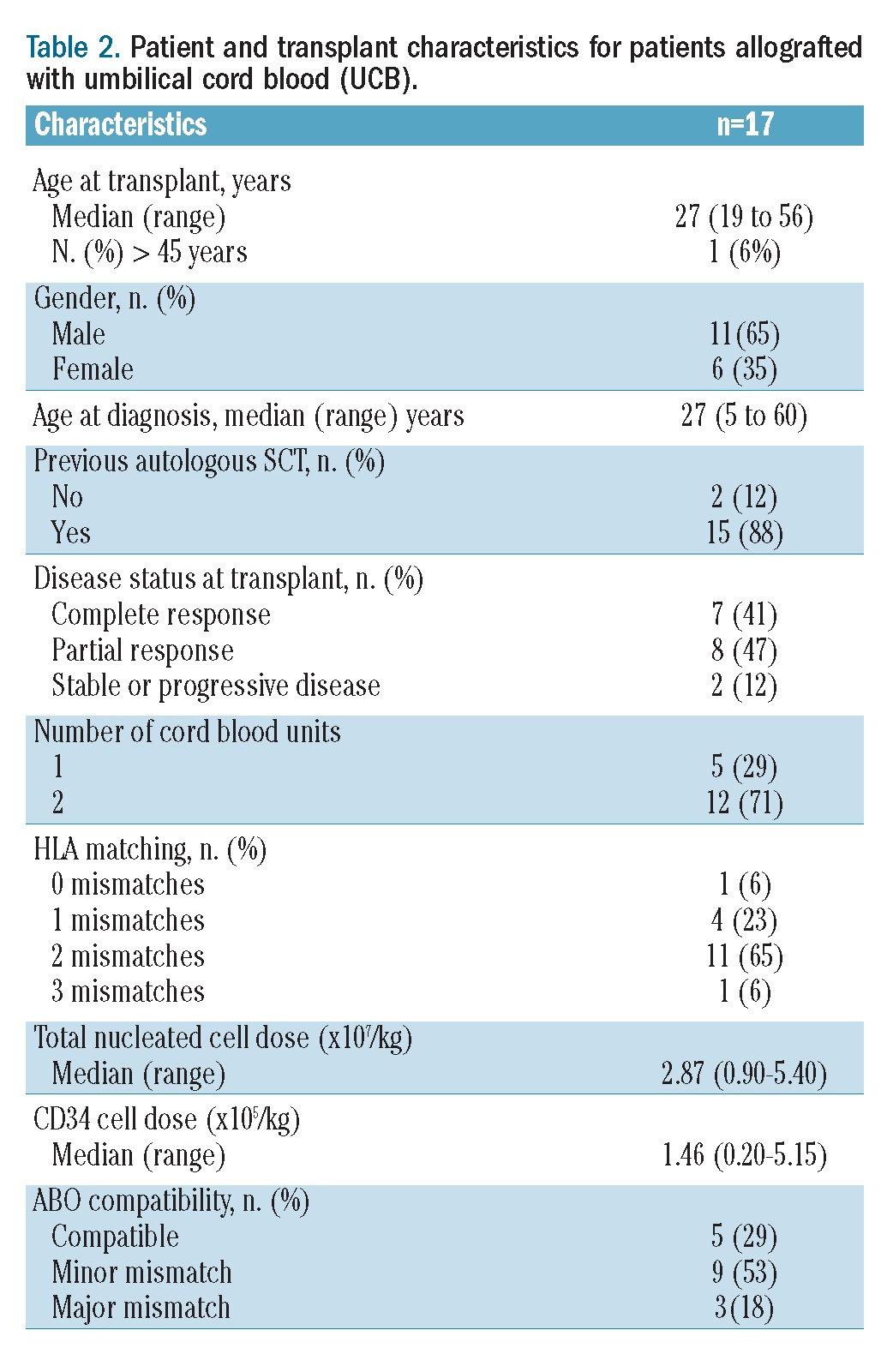
All patients received a reduced intensity regimen. The characteristics of the conditioning regimens are summarized in Table 1. The three most widely used conditioning regimens were a fludarabine + busulfan-based regimen (n=69, 36%), a fludarabine + low-dose total body irradiation-based regimen (n=65, 34%) and a fludarabine + melphalan-based regimen (n=38, 20%). Antithymocyte globulin was used in the conditioning regimen in 83 patients (43%). Post-transplantation GVHD prophylaxis was achieved using cyclosporine alone, cyclosporine and methotrexate, and cyclosporine and mycophenolate mofetil in 48 (25%), 28 (15%) and 114 (60%) cases, respectively. Donors were HLA identical siblings for 114 (60%) recipients and matched or mismatched unrelated donors for 77 (40%) recipients. The hematopoietic stem cell source was peripheral blood for 149 patients (78%), bone marrow for 24 patients (13%) and umbilical cord blood for the other 17 patients (9%). The disease status at time of allogeneic SCT was not statistically different among the three groups of patients divided according to stem cell source. The transplant characteristics for patients who were allografted with umbilical cord blood are summarized in Table 2.
Table 2.
Patient and transplant characteristics for patients allografted with umbilical cord blood (UCB).
Engraftment
Of 191 patients, five patients died before day 30 leaving 186 evaluable for engraftment. All patients engrafted except one who had a late rejection and underwent a second allogeneic SCT. The median time to neutrophil recovery (neutrophils >0.5×109/L) and platelet recovery (platelets >50×109/L) was 15 days and 13 days, respectively.
Graft-versus-host disease
The incidence rate of grade II–IV acute GVHD was 28%. The cumulative incidence of acute GVHD was 30% at 100 days. Chronic GVHD was evaluated in patients who survived and remained free of progression/relapse at day 100. The 1-year cumulative incidence rate of chronic and extensive chronic GVHD was 24% (95% CI: 18% to 31%) and 14% (95% CI: 9% to 9%), respectively. The impact of chronic GVHD and extensive chronic GVHD on the risk of progression was evaluated considering GVHD as a time-dependent variable. There was a lower relapse risk for patients developing extensive chronic GVHD with a hazard of relapse of 0.40 (95% CI: 0.17 to 0.96, P= 0.039) in multivariate analysis.
Non-relapse mortality
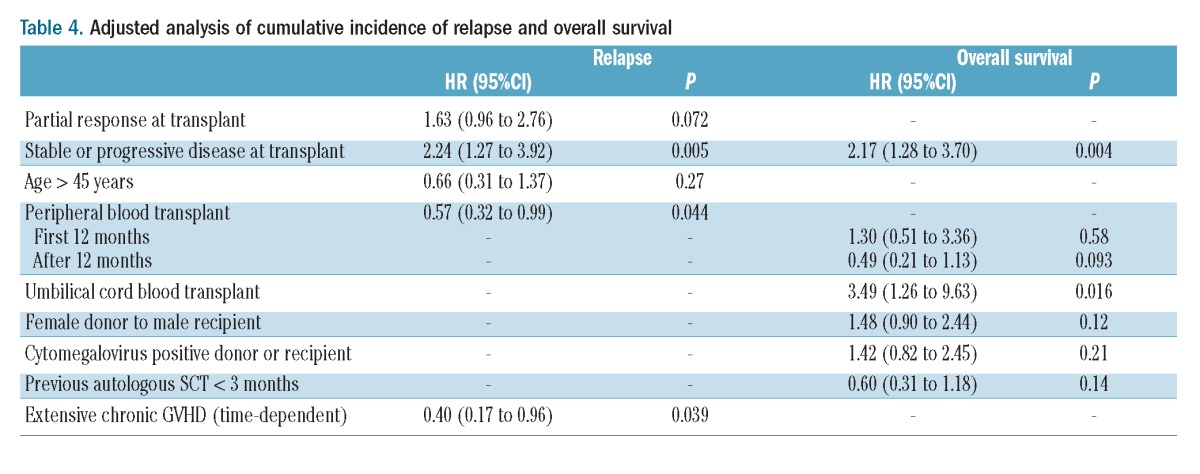
Thirty-two patients died free of relapse. The causes of death included infection (n=13), GVHD (n=6), pulmonary toxicity (n=2), multi-organ failure (n=2), cardiac toxicity (n=2), second neoplasia and miscellaneous other causes (n=7). The cumulative incidence of non-relapse mortality was 6% (95% CI: 3% to 10%) at 3 months and 16% (95% CI: 11% to 21%) at 3 years. In univariate analysis, two factors were associated with worse non-relapse mortality: chemoresistant disease (HR=2.71; 95% CI: 1.35 to 5.46; P=0.005) and presence of cytomegalovirus-positive serology [donor or recipient, HR=2.93 (1.13 to 7.62; P=0.027)] (Table 3). This translates into a 3-year non-relapse mortality rate of 26% (95% CI: 15% to 39%] for patients with chemoresistant disease compared to 12% (95% CI: 7% to 18%) for patients in complete response (Figure 1).
Table 3.
Association of covariates with hazards of events (univariate analysis).
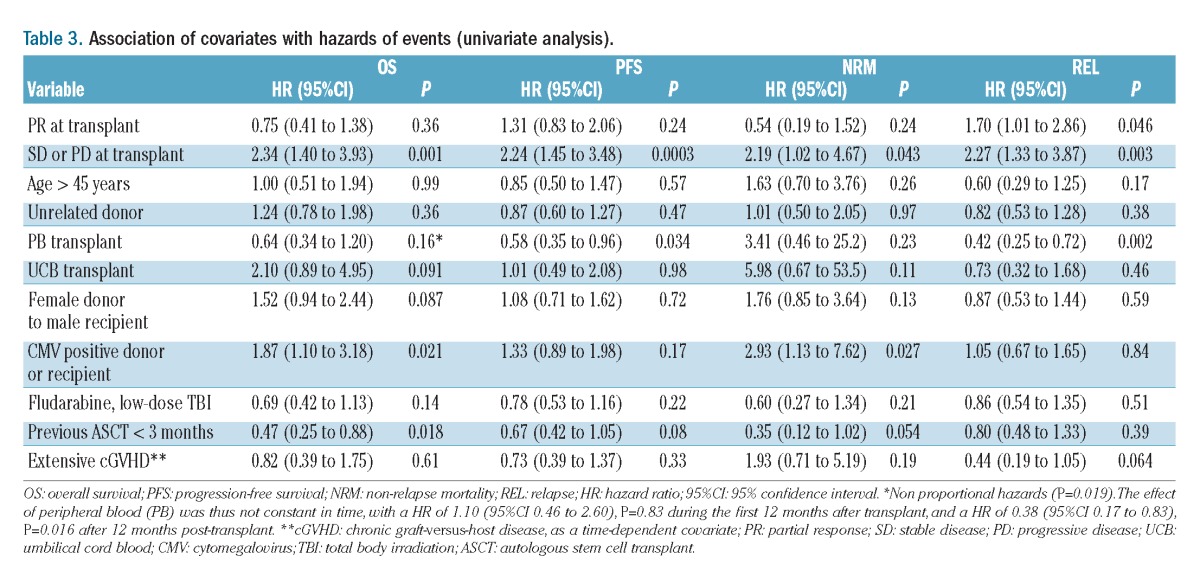
Figure 1.
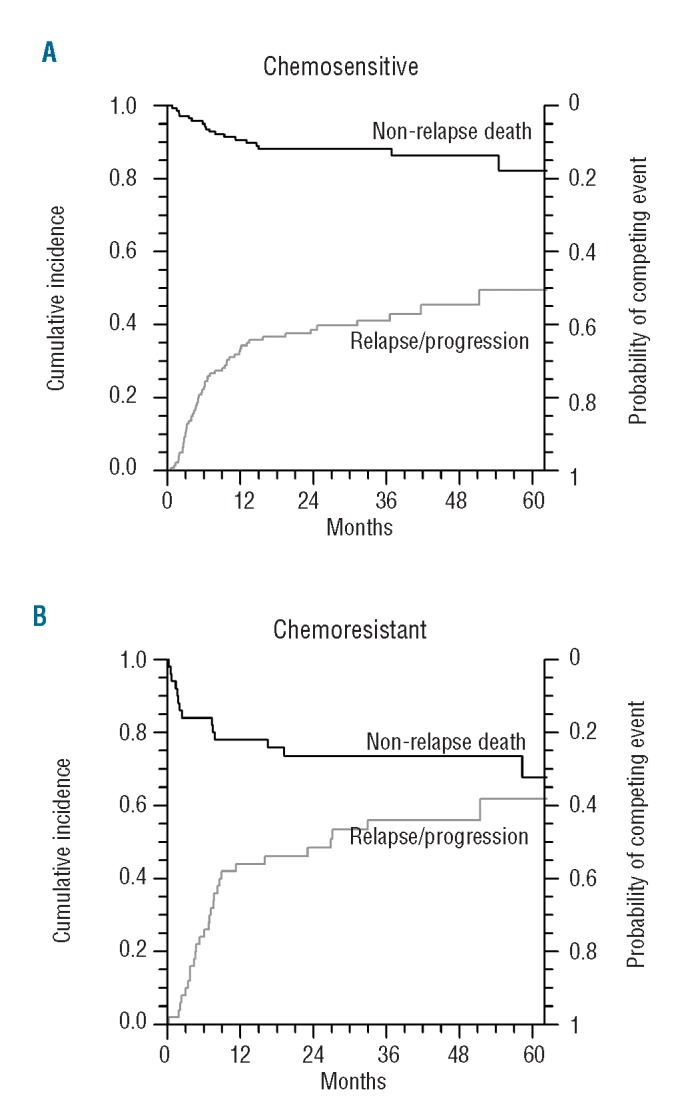
Non-relapse mortality and cumulative incidence of relapse according to disease status at transplant. (A) Chemosensitive disease. (B) Chemoresistant disease.
Relapse/progression
Eighty-five (45%) patients relapsed or progressed. The cumulative incidence of relapse/progression was 36% (95% CI: 29% to 43%) at 1 year and 46% (95% CI: 38% to 53%) at 3 years (Figure 1). In univariate analysis, two factors were associated with a higher cumulative incidence of relapse: chemoresistant disease (HR 2.27; 95% CI: 1.33 to 3.87; P=0.003) and partial response at the time of allogeneic SCT (HR 1.70; 95% CI: 1.01 to 2.86; P=0.046). In contrast, use of peripheral blood as the source of stem cells was associated with a lower relapse rate (HR=0.42; 95% CI: 0.25 to 0.72; P=0.002) (Table 3). In multivariate analysis, chemoresistant disease was associated with a higher cumulative incidence of relapse (HR=2.24; 95% CI: 1.27 to 3.92; P=0.005). Conversely, two factors were associated with a lower higher cumulative incidence of relapse: use of peripheral blood as the stem cell source (HR=0.57; 95% CI: 0.32 to 0.99; P=0.044) and extensive chronic GVHD (studied as a time-dependent factor, HR=0.40; 95% CI: 0.17 to 0.96; P=0.039) (Table 4).
Table 4.
Adjusted analysis of cumulative incidence of relapse and overall survival
Progression-free survival and overall survival
With a median follow up of 36 months, 116 patients remained alive. Seventy-five patients died, including 32 without relapse. The median overall survival was 55 months (lower 95% CI 44, upper 95% confidence limit unreached). The median progression-free survival was 13 months (95% CI: 9 to 27). At 36 months the overall survival rate was 63% (95% CI: 56 to 71) and the progression-free survival rate was 39% (95% CI: 32 to 47) (Figure 2).
Figure 2.
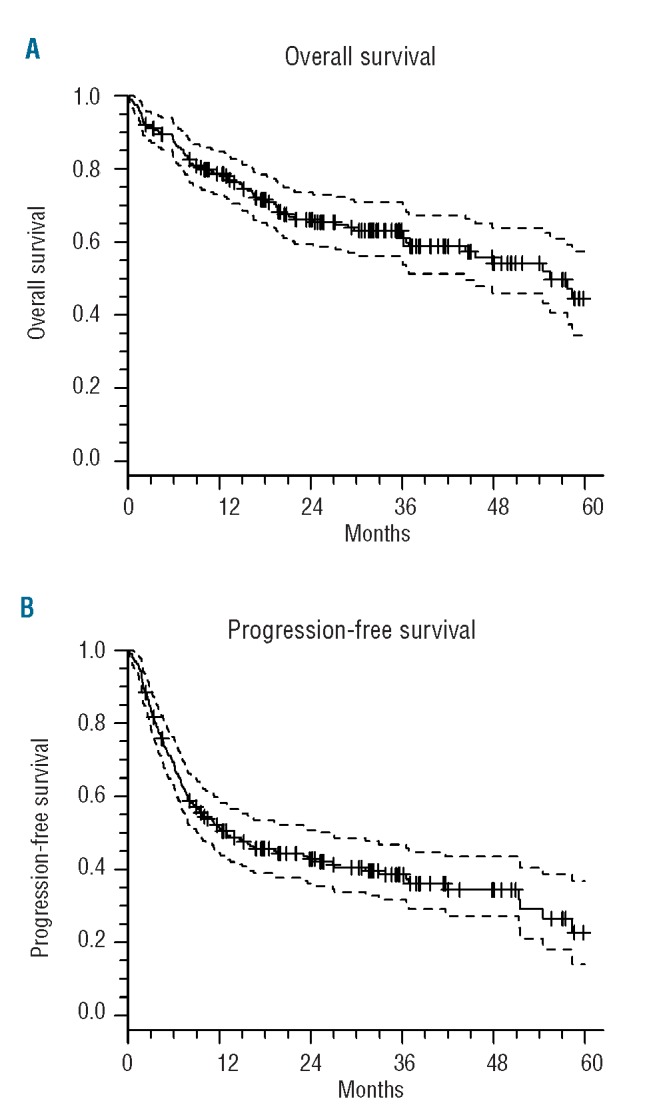
(A) Overall survival and (B) progression-free survival of the entire cohort.
In univariate analysis, the prognostic factors for progression-free survival were chemoresistant disease, which had an adverse impact (HR=2.24; 95% CI: 1.45 to 3.48; P=0.003) and the use of peripheral blood as the source of stem cells, which had a favorable impact (HR=0.58; 95% CI 0.35 to 0.96; P=0.034) (Table 3). Regarding overall survival, two factors were associated with a worse overall survival: chemoresistant disease (HR=2.34; 95% CI: 1.40 to 3.93; P=0.001) and the presence of positive cytomegalovirus serology (donor or recipient, HR=0.47; 95% CI: 0.25 to 0.88; P=0.08) (Table 3).
In summary, patients in complete or partial response had comparable progression-free survival [51% (95% CI: 40 to 64) and 42% (95% CI: 31 to 57) at 3 years; P=ns] and overall survival [70% (95% CI: 60 to 81) and 74% (95% CI: 63 to 87) at 3 years; P=ns). Patients with chemoresistant disease had lower progression-free survival and overall survival rates than patients in complete response [18% at 3 years (95% CI: 9% to 33%), P=0.0003 and 39% at 3 years (95% CI: 27% to 57%); P=0.001] (Figure 3).
Figure 3.
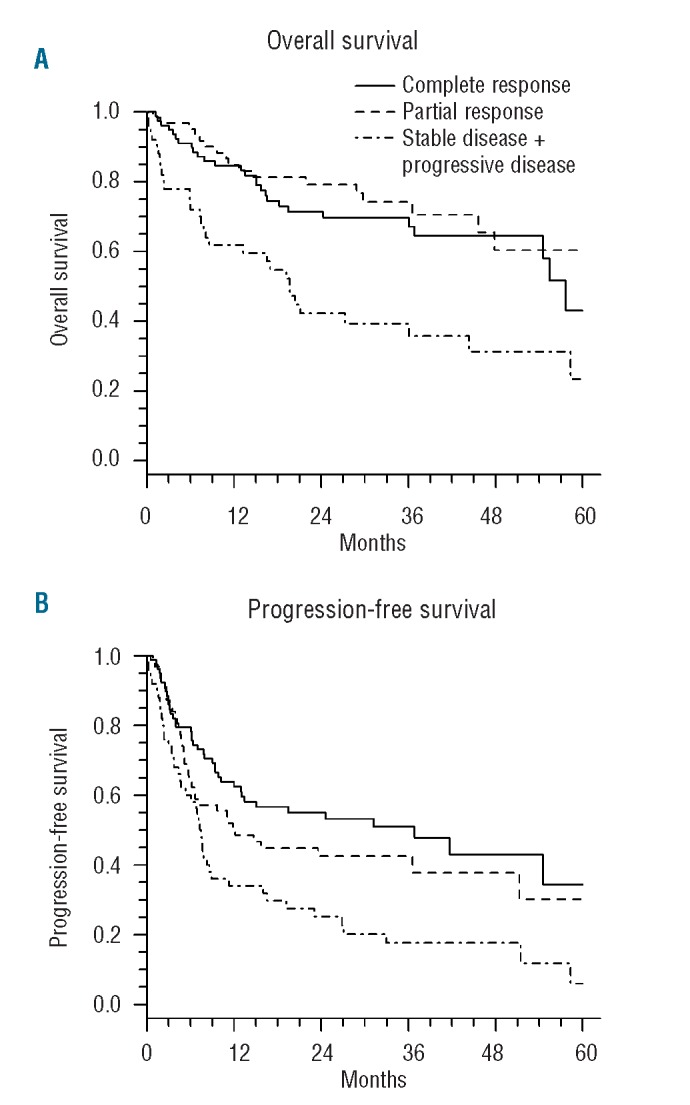
(A) Overall survival and (B) progression-free survival according to disease status at transplant.
In addition, the use of peripheral blood as the stem cell source appears to have a positive impact on overall survival in patients surviving beyond 1 year after SCT, with a lower hazard of death. Indeed, the effect of peripheral blood was not constant in time, with a HR of 1.10 (95% CI: 0.46 to 2.60; P=0.83) during the first 12 months after transplantation, and a HR of 0.38 (95% CI: 0.17 to 0.83; P=0.016) more than 12 months after transplantation (Table 3, Figure 4).
Figure 4.
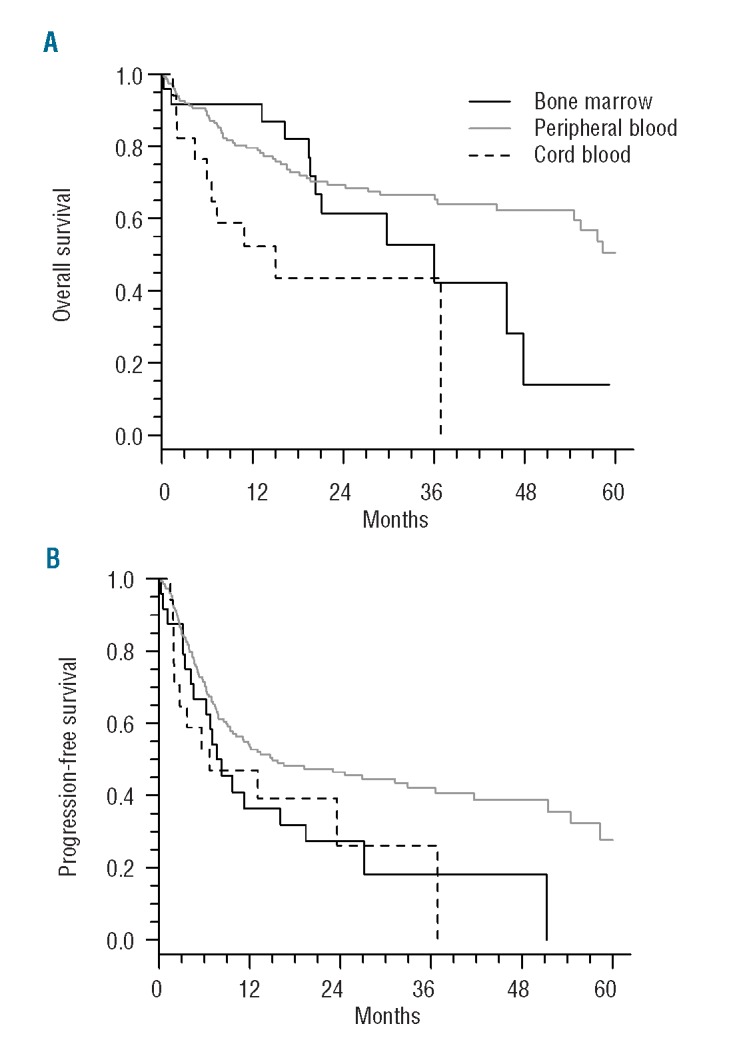
(A) Overall survival and (B) progression-free survival according to stem cell source.
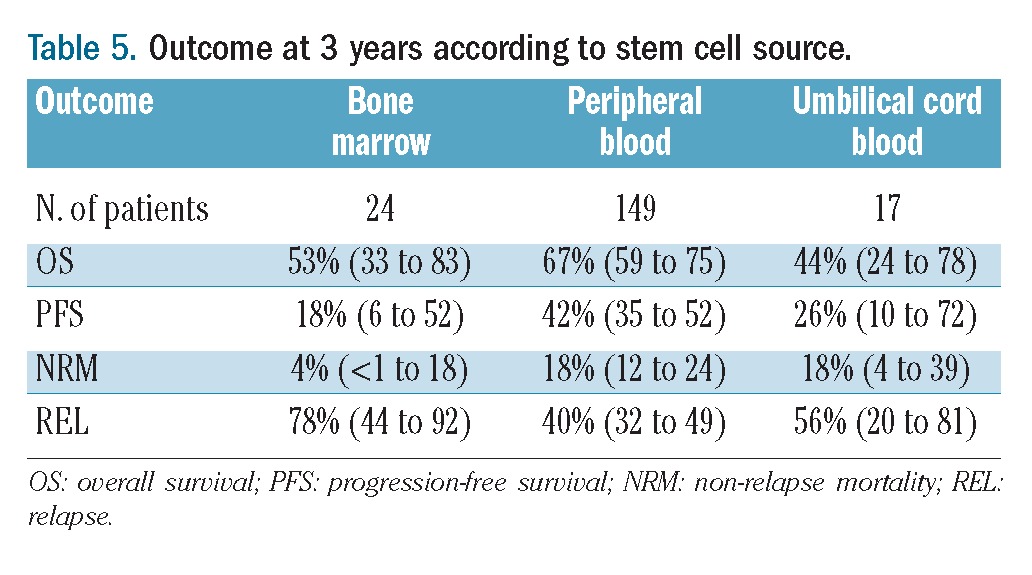
In multivariate analysis, two factors were associated with worse overall survival: chemoresistant disease at time of transplantation (HR= 2.17; 95% CI: 1.28 to 3.70; P=0.004) and the use of umbilical cord blood as the stem cell source (HR=3.49; 95% CI: 1.26 to 9.63; P=0.016) (Table 4). Of note, overall survival was similar between patients whose graft was from a matched unrelated donor or an HLA-identical sibling donor (Table 3). Results of outcome according to the stem cell source are listed in Table 5.
Table 5.
Outcome at 3 years according to stem cell source.
Donor lymphocyte infusions
Donor lymphocyte infusions were given to 47 patients at a median of 6 months post-transplant (range, 1–12 months), in 40 cases for persistent or progressive disease, and in seven cases for either mixed chimerism or as part of a pre-emptive strategy to prevent relapse. Thirty-nine patients received donor lymphocyte infusions alone, while eight received the donor lymphocyte infusions combined with chemotherapy. The median number of donor lymphocyte infusions per patient was two (range 1–4). The median dose of CD3+ cells infused per donor lymphocyte infusion was 1×107 (range, 1×104–1×108). Overall 29% of patients developed GVHD following the infusions. Information on the response to donor lymphocyte infusions was available for 30 patients: the response rate was 27% (3 complete responses and 5 partial responses). Of the 22 patients receiving donor lymphocyte infusion alone for whom response information was available, three achieved a complete response and two showed a partial response. At last follow-up, of the eight patients who responded to donor lymphocyte infusions, four had died of relapse or progression.
Discussion
This study reports the outcome of 191 patients with relapsed or refractory Hodgkin’s lymphoma who received an allogeneic SCT following reduced intensity conditioning as part of a salvage regimen with a median follow-up of 36 months after allogeneic SCT. The transplants were performed in 28 transplant centers in France between 1998 and 2008. Eighty percent of the patients were transplanted after 2002, which is mainly due to the development of reduced intensity conditioning during this period. As expected, this cohort of patients was a young and heavily pre-treated group. The median age was 31 years. Ninety-two percent (n=171) of the patients had undergone previous autologous SCT and 29% had chemoresistant disease at the time of the allogeneic SCT.
Despite this risk profile, non-relapse mortality was acceptable with a cumulative incidence of 16% at 3 years. This was especially true for patients with chemosensitive disease who had a non-relapse mortality at 3 years of 9%. This result is in keeping with the last EBMT study reporting on 285 patients allografted for Hodgkin’s lymphoma with reduced intensity conditioning. In this study, the non-relapse mortality rate was 21% at 3 years for the entire cohort and 12% for patients with a good performance status and chemosensitive disease.15
Disease progression was the main cause of treatment failure following allogeneic SCT with 85 (45%) patients relapsing or progressing during the follow-up. At 3 years, the cumulative incidence of relapse/progression was 46% which is comparable to that of other studies reporting relapse rates ranging between 43–55%.9,12,15 As expected, disease status at the time of allogeneic SCT was the major prognosis factor for the risk of relapse.
As a consequence of the high relapse rate; the progression-free survival rate remains low, as in other studies. Despite this low progression-free survival, overall survival was prolonged with a median of 55 months. Most of the patients who relapsed received salvage therapy including chemotherapy and/or donor lymphocyte infusions suggesting that salvage treatment after allogeneic SCT can prolong overall survival. The disease status at the time of allogeneic SCT was the major prognostic factor for outcome. Of note, the progression-free survival and overall survival were similar for patients in partial response and complete response. This was not the case for patients with chemoresistant disease, who have a worse outcome. Our results suggest than even for patients in partial response, allogeneic SCT should be proposed. Conversely, it does not seem reasonable for us to propose allogeneic SCT for patients with chemoresistant disease due to the dismal results in this setting.
The use of umbilical cord blood as the source of stem cells appears to be associated with a worse overall survival, which contrasts with the finding of another recent study,23 in which a small series of nine patients who underwent allogeneic SCT with umbilical cord blood were compared with 12 patients who received allogeneic SCT from an HLA-matched sibling donor. Progression-free and overall survival rates at 2 years were similar for both groups: 25% and 51% for the umbilical cord blood cohort and 20% and 48% for the HLA-matched sibling donor cohort, respectively. The 3-month transplant-related mortality rate was 11% for the entire cohort. The same institution has reported a larger series of 23 patients who underwent allogeneic SCT using umbilical cord blood as the stem cell source without any control group. With a median follow up of 23 months, overall survival, progression-free survival, incidence of relapse and transplant-related mortality rates were 43%, 34%, 43% and 13%, respectively.24
In our study, we report on 17 patients who were allografted with umbilical cord blood with a longer follow up. At 3 years, overall survival, progression-free survival, cumulative incidence of relapse and non-relapse mortality rates were 44%, 26%, 56% and 18%, respectively. These results are comparable in terms of overall survival but seem to be worse with regards to progression-free survival, mainly due to a higher relapse rate. However, when compared to other patients in our study, the results are more disappointing. Indeed the use of umbilical cord blood was associated with an increased risk of death (HR= 2.78; 95% CI: 1.05 to 7.36; P=0.040) in multivariate analysis. Even though the hazard ratio does not reach statistical significance, this excess of death seems to be due to an increased risk of both non-relapse mortality and relapse. Despite controlled disease at the time of allogeneic SCT with 15 patients of 17 having chemosensitive disease (7 in complete response and 8 in partial response), the outcome was worse in these patients than in those transplanted with stem cells from others sources. This led us to recommend that umbilical cord blood should not be used for this indication when possible.
The use of peripheral blood was associated with a better progression-free survival due to a lower risk of relapse. This result, due to a non-constant effect of peripheral blood, translated into better overall survival only for patients who were alive beyond 12 months, with the positive impact on overall survival demonstrated by a HR of 0.38 (95% CI: 0.17 to 0.83; P=0.016). The use of peripheral blood rather than bone marrow is associated with a lower relapse rate. However, the benefit of peripheral blood is counterbalanced by an excess rate of non-relapse mortality mainly due to more acute and chronic GVHD.25 Here, we observed that the use of peripheral blood was associated with a lower risk of relapse with no excess of non-relapse mortality and in consequence a better progression-free survival and overall survival for patients who survived beyond 12 months after transplantation. Of note, there was no excess of non-relapse mortality for the peripheral blood group during the first year after allogeneic SCT.
It has already been described that the use of an HLA-matched unrelated donor does not influence the outcome,12,15 while the use of low-dose total body irradiation has been reported to be associated with a worse outcome. Here, we were not able to show an adverse effect of total body irradiation and our study did not allow us to define the best conditioning regimen.11 Tandem autologous-allogeneic SCT has been reported to be an option for relapsed/refractory Hodgkin’s lymphoma.26 In our study, 47 patients received an allogeneic SCT within 3 months of autologous SCT (data not shown) but without indication of whether this strategy was part of a tandem autologous-allogeneic SCT program. These 47 patients had a better overall survival mainly due to a lower rate of non-relapse mortality but progression-free survival was not greater. This strategy, however, remains a matter of debate and the small number of patients in our study does not provide definitive information in this respect.
The existence of a graft-versus-lymphoma effect is also still debated. Historically, earlier studies suggested that there was a graft-versus-Hodgkin’s lymphoma effect based on the fact that the relapse rate was lower when allografting was compared to autologous SCT. More recently, two large EBMT studies showed that chronic GVHD was associated with a lower incidence of relapse but these results did not translate in better progression-free survival or overall survival mainly because of an increased non-relapse mortality.11,15 In these studies, the effect of GVHD on outcome was assessed by a landmark analysis approach and not as a time-dependent variable. Using the latter statistical approach, patients with extensive chronic GVHD had a lower risk of relapse with a HR of 0.40 (95% CI: 0.17 to 0.96; P= 0.039). Responses to donor lymphocyte infusion are often viewed as evidence of a graft-versus-lymphoma effect.9 Two studies have demonstrated this effect.27,28 In our study, among the 30 patients for whom response information was available, the response rate was 27%.
In conclusion, with all the limitations inherent to a retrospective registry analysis we confirm that reduced intensity conditioning allogeneic SCT is a valid therapeutic option for advanced Hodgkin’s lymphoma mainly for patients with disease that remains sensitive to salvage therapy. The results are dismal for those with chemoresistant disease and thus do not allow us to recommend reduced intensity conditioning allogeneic SCT for such patients. We show that the source of stem cell is of importance and that in this respect the use of peripheral blood stem cells should be recommended. Conversely, we show that using umbilical cord blood as the source of stem cells carries a high risk of failure and should be avoided outside prospective trials. The non-relapse mortality rate for patients with chemosensitive disease at the time of transplantation was less than 10% at 1 year. Having consistently reached these results with reduced intensity conditioning allogeneic SCT should ease prospective randomized trials aimed at comparing this type of treatment with other approaches in patients at high risk of poor response to standard salvage treatments. For example, it could be interesting to perform a prospective study comparing a double autologous SCT strategy versus a tandem autologous-allogeneic SCT strategy for patients with a high risk of relapse according to defined criteria.2 These include primary refractory status or more than two risk factors at first relapse among the following: time to relapse or progression less than 12 months after the end of first-line treatment, stage III or IV at relapse, and relapse in a previously irradiated site (<30 Gy) after combined-modality therapy.
Acknowledgments
The authors would like to thank the Société Française de Greffe de Moelle et de Thérapie cellulaire (SFGM-TC) for sharing their database and the data manager Nicole Raus. We also thank the Agence de la Biomedecine, especially Dr. Irina Ionescu and Dr. Federico Garnier, for kindly sharing their database for cord blood transplantation. We would like to acknowledge the physicians who took care of patients. We also thank Dr. Felipe Suarez and Pr. Olivier Hermine for their helpful discussions and editing the manuscript.
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359(9323):2065–71 [DOI] [PubMed] [Google Scholar]
- 2.Morschhauser F, Brice P, Fermé C, Diviné M, Salles G, Bouabdallah R, et al. Risk-adapted salvage treatment with single or tandem autologous stem-cell transplantation for first relapse/refractory Hodgkin’s lymphoma: results of the prospective multicenter H96 trial by the GELA/SFGM study group. J Clin Oncol. 2008;26(36): 5980–7 [DOI] [PubMed] [Google Scholar]
- 3.Moskowitz AJ, Perales M-A, Kewalramani T, Yahalom J, Castro-Malaspina H, Zhang Z, et al. Outcomes for patients who fail high dose chemoradiotherapy and autologous stem cell rescue for relapsed and primary refractory Hodgkin lymphoma. Br J Haematol. 2009;146(2):158–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson JE, Litzow MR, Appelbaum FR, Schoch G, Fisher LD, Buckner CD, et al. Allogeneic, syngeneic, and autologous marrow transplantation for Hodgkin’s disease: the 21-year Seattle experience. J Clin Oncol. 1993;11(12):2342–50 [DOI] [PubMed] [Google Scholar]
- 5.Gajewski JL, Phillips GL, Sobocinski KA, Armitage JO, Gale RP, Champlin RE, et al. Bone marrow transplants from HLA-identical siblings in advanced Hodgkin’s disease. J Clin Oncol. 1996;14(2):572–8 [DOI] [PubMed] [Google Scholar]
- 6.Milpied N, Fielding AK, Pearce RM, Ernst P, Goldstone AH. Allogeneic bone marrow transplant is not better than autologous transplant for patients with relapsed Hodgkin’s disease. European Group for Blood and Bone Marrow Transplantation J Clin Oncol. 1996;14(4):1291–6 [DOI] [PubMed] [Google Scholar]
- 7.Akpek G, Ambinder RF, Piantadosi S, Abrams RA, Brodsky RA, Vogelsang GB, et al. Long-term results of blood and marrow transplantation for Hodgkin’s lymphoma. J Clin Oncol. 2001;19(23):4314–21 [DOI] [PubMed] [Google Scholar]
- 8.Peniket AJ, Ruiz de Elvira MC, Taghipour G, Cordonnier C, Gluckman E, De Witte T, et al. An EBMT registry matched study of allogeneic stem cell transplants for lymphoma: allogeneic transplantation is associated with a lower relapse rate but a higher procedure-related mortality rate than autologous transplantation. Bone Marrow Transplant. 2003;31(8):667–78 [DOI] [PubMed] [Google Scholar]
- 9.Peggs KS, Hunter A, Chopra R, Parker A, Mahendra P, Milligan D, et al. Clinical evidence of a graft-versus-Hodgkin’s-lymphoma effect after reduced-intensity allogeneic transplantation. Lancet. 2005;365 (9475):1934–41 [DOI] [PubMed] [Google Scholar]
- 10.Corradini P, Dodero A, Farina L, Fanin R, Patriarca F, Miceli R, et al. Allogeneic stem cell transplantation following reduced-intensity conditioning can induce durable clinical and molecular remissions in relapsed lymphomas: pre-transplant disease status and histotype heavily influence outcome. Leukemia. 2007;21(11):2316–23 [DOI] [PubMed] [Google Scholar]
- 11.Sureda A, Robinson S, Canals C, Carella AM, Boogaerts MA, Caballero D, et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin’s lymphoma: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26(3): 455–62 [DOI] [PubMed] [Google Scholar]
- 12.Anderlini P, Saliba R, Acholonu S, Giralt SA, Andersson B, Ueno NT, et al. Fludarabine-melphalan as a preparative regimen for reduced-intensity conditioning allogeneic stem cell transplantation in relapsed and refractory Hodgkin’s lymphoma: the updated M.D. Anderson Cancer Center experience. Haematologica. 2008;93(2):257–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armand P, Kim HT, Ho VT, Cutler CS, Koreth J, Antin JH, et al. Allogeneic transplantation with reduced-intensity conditioning for Hodgkin and non-Hodgkin lymphoma: importance of histology for outcome. Biol Blood Marrow Transplant. 2008;14(4):418–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burroughs LM, O’Donnell PV, Sandmaier BM, Storer BE, Luznik L, Symons HJ, et al. Comparison of outcomes of HLA-matched related, unrelated, or HLA-haploidentical related hematopoietic cell transplantation following nonmyeloablative conditioning for relapsed or refractory Hodgkin lymphoma. Biol Blood Marrow Transplant. 2008;14(11):1279–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson SP, Sureda A, Canals C, Russell N, Caballero D, Bacigalupo A, et al. Reduced intensity conditioning allogeneic stem cell transplantation for Hodgkin’s lymphoma: identification of prognostic factors predicting outcome. Haematologica. 2009;94(2): 230–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarina B, Castagna L, Farina L, Patriarca F, Benedetti F, Carella AM, et al. Allogeneic transplantation improves the overall and progression-free survival of Hodgkin lymphoma patients relapsing after autologous transplantation: a retrospective study based on the time of HLA typing and donor availability. Blood. 2010;115(18):3671–7 [DOI] [PubMed] [Google Scholar]
- 17.Chen R, Palmer JM, Popplewell L, Shen J, Smith E, Delioukina M, et al. Reduced intensity allogeneic hematopoietic cell transplantation can induce durable remission in heavily pretreated relapsed Hodgkin lymphoma. Ann Hematol. 2011; 90(7):803–8 [DOI] [PubMed] [Google Scholar]
- 18.Sureda A, Canals C, Arranz R, Caballero D, Ribera JM, Brune M, et al. Allogeneic stem cell transplantation after reduced intensity conditioning in patients with relapsed or refractory Hodgkin’s lymphoma. Results of the HDR-ALLO study - a prospective clinical trial by the Grupo Espanol de Linfomas/Trasplante de Medula Osea (GEL/TAMO) and the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2011;97(2):310–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–9 [DOI] [PubMed] [Google Scholar]
- 20.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. J. Wiley; 2002 [Google Scholar]
- 21.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34(4): 541–54 [PubMed] [Google Scholar]
- 22.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3): 515–26 [Google Scholar]
- 23.Majhail NS, Weisdorf DJ, Wagner JE, Defor TE, Brunstein CG, Burns LJ. Comparable results of umbilical cord blood and HLA-matched sibling donor hematopoietic stem cell transplantation after reduced-intensity preparative regimen for advanced Hodgkin lymphoma. Blood. 2006;107(9):3804–7 [DOI] [PubMed] [Google Scholar]
- 24.Brunstein CG, Cantero S, Cao Q, Majhail N, McClune B, Burns LJ, et al. Promising progression-free survival for patients low and intermediate grade lymphoid malignancies after nonmyeloablative umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2009;15(2):214–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cutler C, Giri S, Jeyapalan S, Paniagua D, Viswanathan A, Antin JH. Acute and chronic graft-versus-host disease after allogeneic peripheral-blood stem-cell and bone marrow transplantation: a meta-analysis. J Clin Oncol. 2001;19(16):3685–91 [DOI] [PubMed] [Google Scholar]
- 26.Carella AM, Cavaliere M, Lerma E, Ferrara R, Tedeschi L, Romanelli A, et al. Autografting followed by nonmyeloablative immunosuppressive chemotherapy and allogeneic peripheral-blood hematopoietic stem-cell transplantation as treatment of resistant Hodgkin’s disease and non-Hodgkin’s lymphoma. J Clin Oncol. 2000;18(23):3918–24 [DOI] [PubMed] [Google Scholar]
- 27.Peggs KS, Sureda A, Qian W, Caballero D, Hunter A, Urbano-Ispizua A, et al. Reduced-intensity conditioning for allogeneic haematopoietic stem cell transplantation in relapsed and refractory Hodgkin lymphoma: impact of alemtuzumab and donor lymphocyte infusions on long-term outcomes. Br J Haematol. 2007;139(1):70–80 [DOI] [PubMed] [Google Scholar]
- 28.Peggs KS, Kayani I, Edwards N, Kottaridis P, Goldstone AH, Linch DC, et al. Donor lymphocyte infusions modulate relapse risk in mixed chimeras and induce durable salvage in relapsed patients after T-cell-depleted allogeneic transplantation for Hodgkin’s lymphoma. J Clin Oncol. 2011; 29(8):971–8 [DOI] [PubMed] [Google Scholar]


