Abstract
Elevated D-dimer levels are reportedly associated with higher risk of total mortality in patients with different diseases. We investigated whether a similar association could be found in a large, apparently healthy population. A large sample of individuals (N=17,359, 47% men, age ≥35 years) free of clinically recognized cardiovascular and cancer disease, for whom baseline D-dimer level was available, were studied within the MOLI-SANI cohort, randomly recruited from the general adult population of Southern Italy. The cohort was followed for a median of 4.2 years (73,807 person-years). D-dimer was measured in fresh citrated plasma by an automated latex-enhanced immunoassay. Hazard ratios were calculated using three Cox-proportional hazard models. Two hundred and eighty deaths were recorded. When modeled as a continuous variable, D-dimer level at baseline showed a non-linear association with mortality, whose incidence increased only in the upper quartile of the distribution (D-dimer ≥221 ng/mL). Thus, the group of individuals with D-dimer <221 ng/mL (75% of the population) acted as the reference group, while the remaining individuals were subdivided in tertiles and compared with the former group. Multivariable hazard ratios for mortality were 1.06, 1.45 and 1.97, respectively (P for trend <0.0001) across the three categories of increasing D-dimer concentration. The association was slightly attenuated, but still highly significant (P for trend 0.0002), after further adjustment for white blood cell count and C-reactive protein. In conclusion, Elevated D-dimer levels were independently associated with increased risk of death from any cause in an apparently healthy adult population.
Introduction
D-dimer, a high molecular weight fibrinogen derivative derived from the cleavage of cross-linked fibrin, reflects both thrombin production and activation of fibrinolysis.1–3 Among healthy individuals, there is significant between-person variability of D-dimer concentration within the normal range.4 Elevated D-dimer levels occur in various disorders in which the coagulation system is activated, such as acute venous thromboembolism, ischemic cardiovascular disease and cancer.5–17 A meta-analysis has suggested an independent 1.7-fold increased risk of coronary heart disease for people with the highest versus the lowest third of D-dimer levels.18 In this context, D-dimer may represent the summation of pro-coagulant balance or genetic factors, the extent of subclinical atherosclerosis, or the presence of underlying coagulation disorders that predispose to coronary thrombosis.10 Moderately elevated D-dimer levels reflect minor increases in blood coagulation, thrombin formation, and turnover of cross-linked intravascular fibrin and these increases may be relevant to coronary heart disease.10
Besides the cardiovascular setting, elevated D-dimer levels have been associated with higher risk of total mortality in apparently healthy subjects19 and in patients with different diseases.20,21 Systemic activation of hemostasis is frequently observed in cancer patients, even in the absence of thrombosis.22,23 Moreover, this activation has been implicated in cancer progression, angiogenesis and metastatic spread.22 Increased levels of D-dimer indicate a global activation of hemostasis and fibrinolysis, and have been associated with poor overall survival and increased mortality risk in cancer patients.17 These observations give plausibility to the hypothesis of a link between elevation of D-dimer and total mortality. However, the specificity of the relation of D-dimer with total mortality has been questioned, as the degree of correlation with cardiovascular risk factors and markers of inflammation is moderate.3,19
In order to better understand the relation of D-dimer with all-cause mortality, we analyzed the cohort of participants in the MOLI-SANI study.
Methods
Study participants
We included all individuals free of clinically recognized cardiovascular and cancer disease, recruited in the MOLI-SANI study.24–30 Briefly, the MOLI-SANI project is a cohort study that was started in March 2005. Participants, men and women aged ≥35, living in Molise, a region of Southern Italy, were randomly recruited from city-hall registries. Up to April 2010 24,325 subjects had been recruited. For the purposes of this study, we excluded participants with a previous personal history of cardiovascular disease (6%) or cancer (3%), those lacking information on D-dimer levels or other relevant variables (14%) and those lost to follow-up (5%). The final study sample consisted of 17,639 individuals. The cohort was followed-up for death from any cause for a median of 4.2 years (maximum 6.5 years). Follow-up based on the regional death register was from the baseline examination until death or 31 December 2011 for those patients who remained alive.
The ascertainment of vital status was carried out through linkage with demographic rosters, to identify the date of death. For deceased subjects, families were asked to confirm the death and its cause. Moreover, death certificates were retrieved; these reported the initial and underlying causes of death and were coded according to the International Classification of Diseases, ninth revision (ICD-9). In-hospital deaths were also checked through regional hospital discharge databases by record linkage to the MOLI-SANI database.
On 31/12/2011, a total of 596 deaths had occurred among the whole MOLI-SANI cohort (n=24,325 subjects). The number of deaths in the sample (n=17,639) included in the present study was 280.
The MOLI-SANI study complies with the Declaration of Helsinki and was approved by the Catholic University ethical committee. All participants enrolled provided written informed consent.
Risk factor ascertainment
D-dimer levels were measured on fresh citrated plasma by an automated latex-enhanced immunoassay (HemosIL-IL, Milan, Italy). Quality control was maintained using an internal laboratory standard in-house plasma pool. Inter and intra-day variability coefficients were 5.4% and 7.6%, respectively.
Statistical methods
Hazard ratios for death according to D-dimer level, modeled as quartiles or as a continuous variable, were calculated using the following Cox proportional hazard models: (i) crude (unadjusted), (ii) adjusted for baseline age and sex and (iii) additionally adjusted for other risk factors: baseline body mass index, hypertension, diabetic and dyslipidemic status, usual alcohol intake, smoking status, exercise, and social status score. The data were analyzed using SAS/STAT software (version 9.1.3 of the SAS System for Windows© 2009; SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics
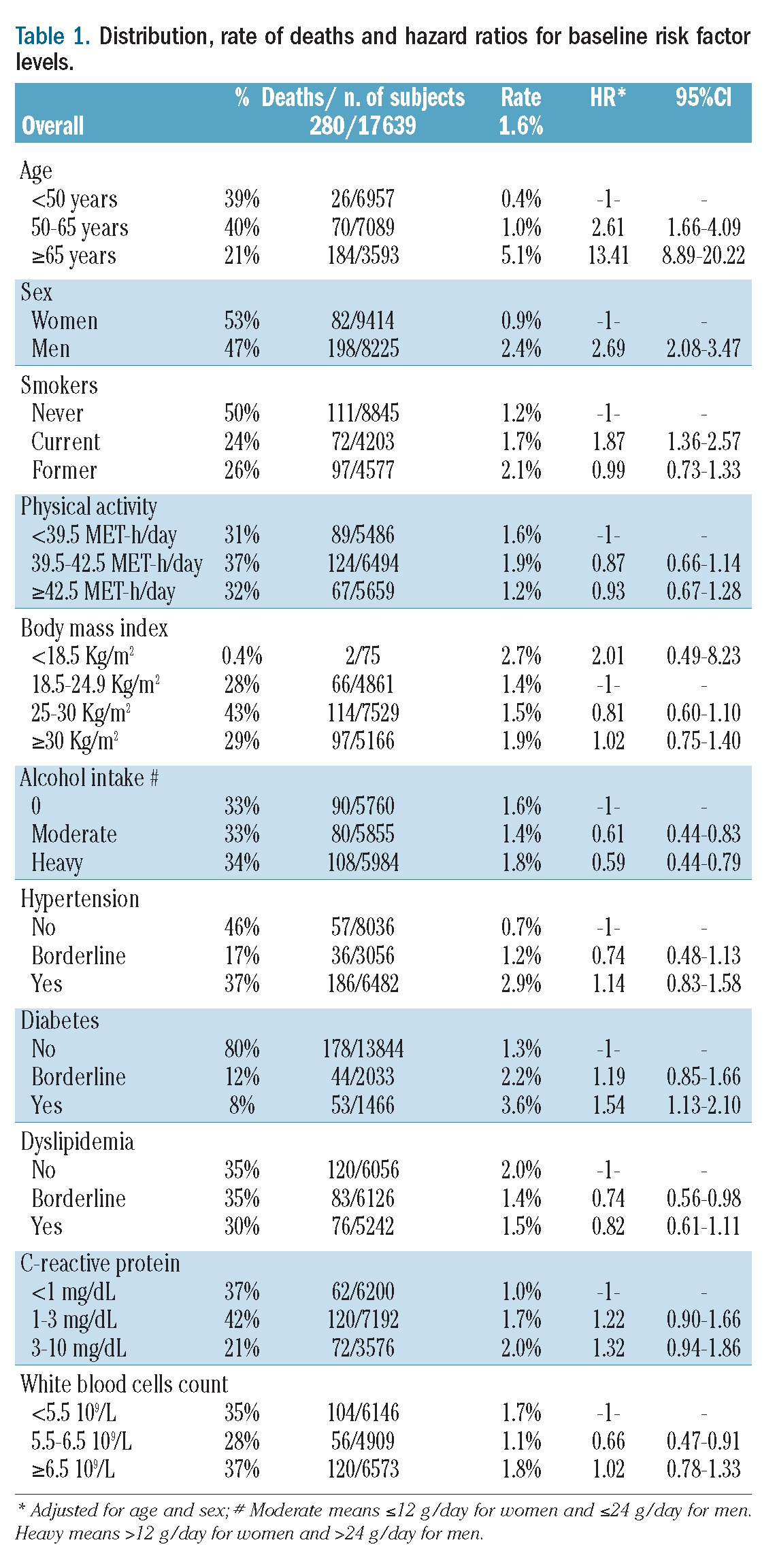
The baseline characteristics of the 17,639 subjects included in the analysis are reported, in relation to death, in Table 1. The incidence of death was lower in women and higher in older subjects; hazard ratios for death were higher for those with a higher prevalence of smoking, being underweight, having diabetes and abstaining from alcohol.
Table 1.
Distribution, rate of deaths and hazard ratios for baseline risk factor levels.
D-dimer and cardiovascular risk factors
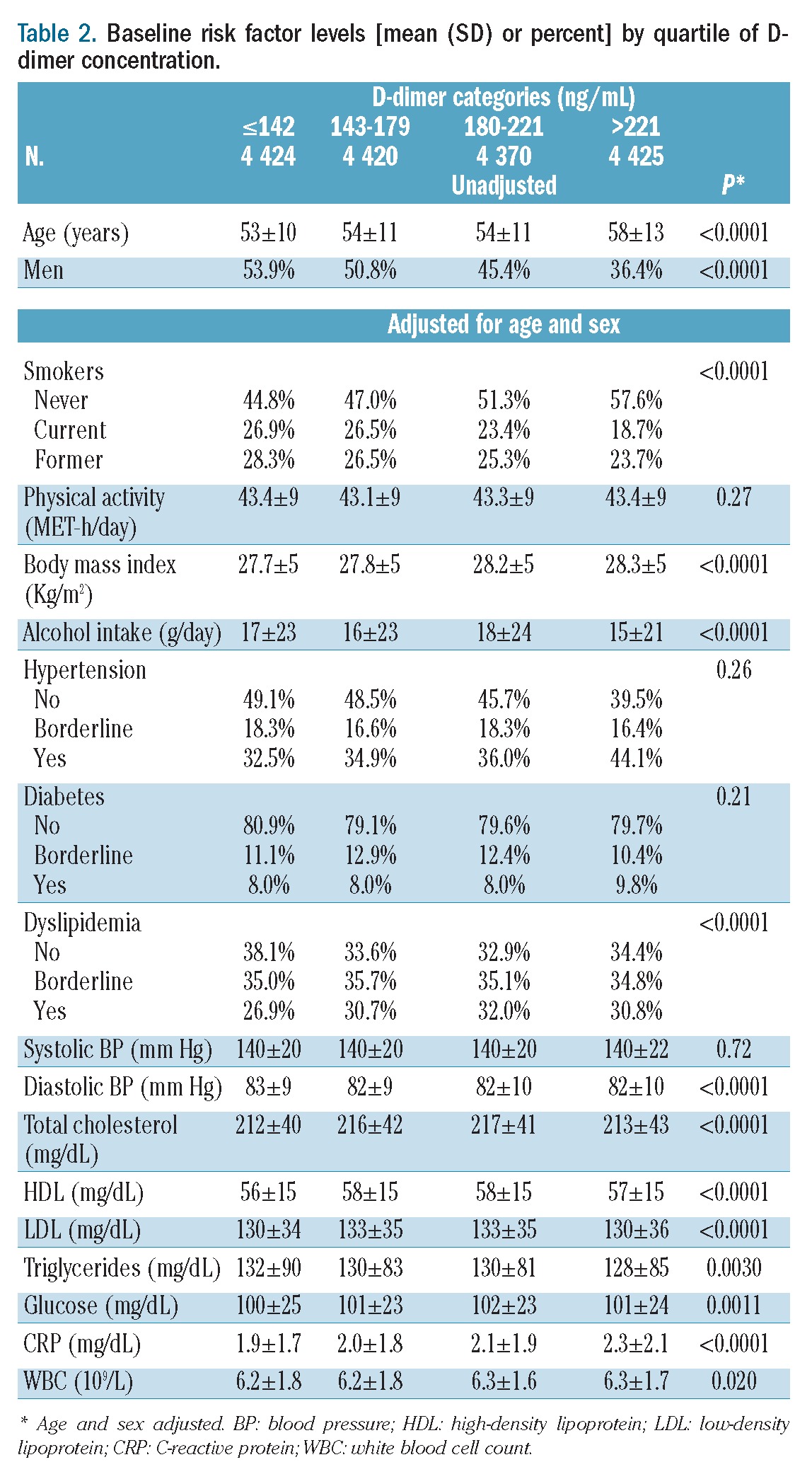
As shown in Table 2, D-dimer level was positively associated with age, was lower in men than in women, and varied with smoking, body mass index, alcohol intake and dyslipidemia. D-dimer levels were also modestly but significantly associated with diastolic blood pressure, lipids, glucose, C-reactive protein and white blood cell count.
Table 2.
Baseline risk factor levels [mean (SD) or percent] by quartile of D-dimer concentration.
D-dimer levels and deaths
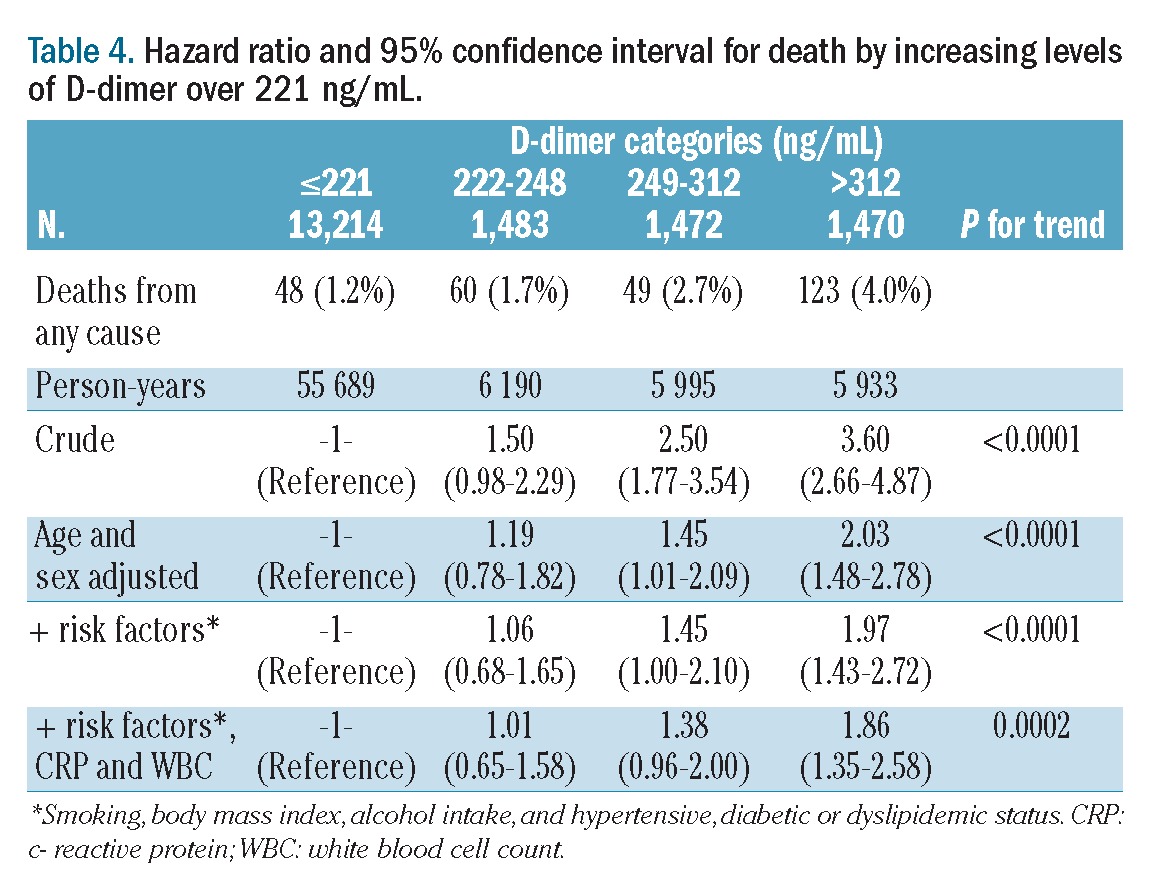
The incidence of death increased from 1.1% to 2.8% according to quartiles of D-dimer concentration (Table 3). In comparison with the death rate for subjects with values in the lowest quartile, the hazard ratio for death in those individuals who had D-dimer levels in the upper quartile was 2.86 (95% CI: 1.04 to 4.00) in univariable analysis, 1.54 (95% CI: 1.08 to 2.20) when adjusted for age and sex and 1.46 (95% CI: 1.02 to 2.10) in multivariable analysis adjusted for age, sex, smoking, body mass index, alcohol intake, hypertension, diabetes or dyslipidemic status. The increase in the risk of death was confined to the upper quartile in fact in none of the three models did the death rate in the second and the third quartiles differ from that in the first quartile. To better investigate the dose-response relationship between D-dimer level and incidence of death the population was divided into 12 categories according to increasing D-dimer levels and hazard ratios were calculated for each category with the lowest one acting as the reference. As illustrated in Figure 1, the relative risk of death was equal to one for all categories except the last three (when D-dimer was >221 ng/mL) in which it increased linearly. Following this observation, hazard ratios were re-calculated using the group of individuals with D-dimer <221 ng/mL acting as the reference group, while the remaining individuals were subdivided in tertiles (Table 4). Multivariable hazard ratios for mortality were 1.06, 1.45 and 1.97 across the categories of increasing D-dimer level (P for trend <0.0001). The association was slightly attenuated, but still statistically significant (P for trend 0.0002), after further adjustment for white blood cell count and C-reactive protein concentration (Table 4).
Table 3.
Hazard ratio and 95% confidence interval for death by quartile of D-dimer concentration.
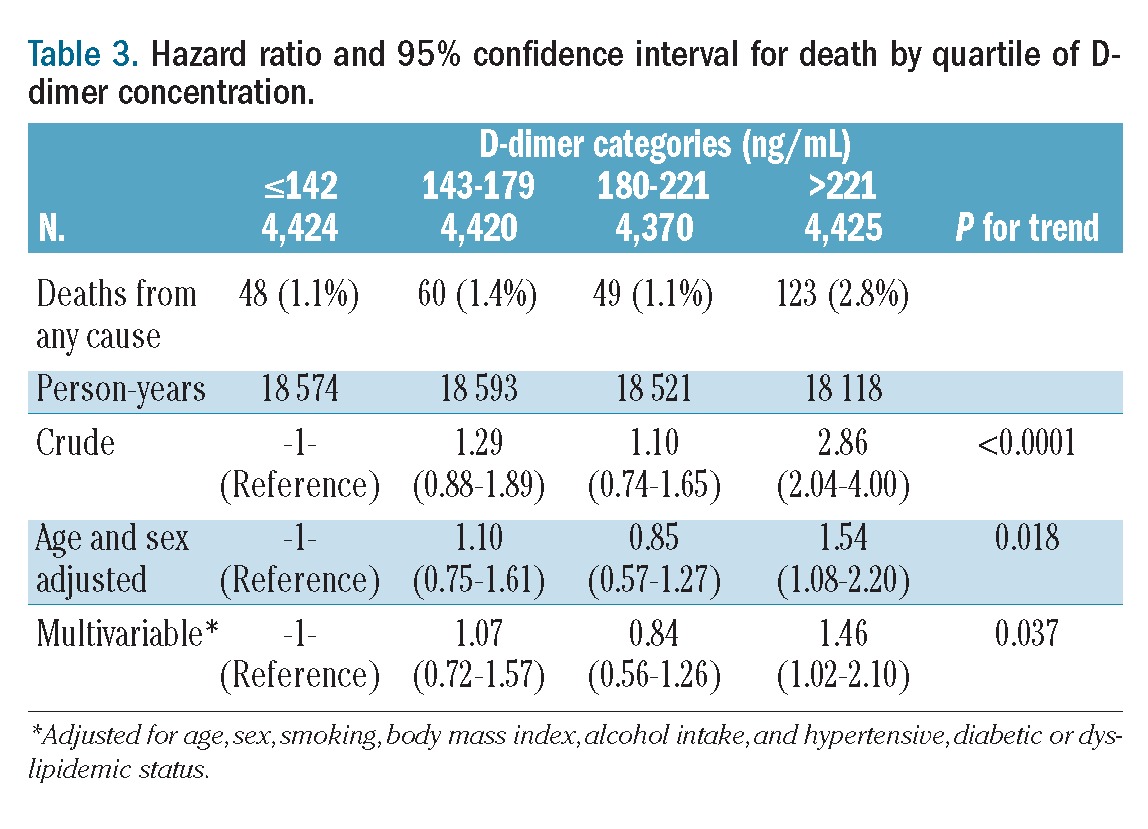
Figure 1.
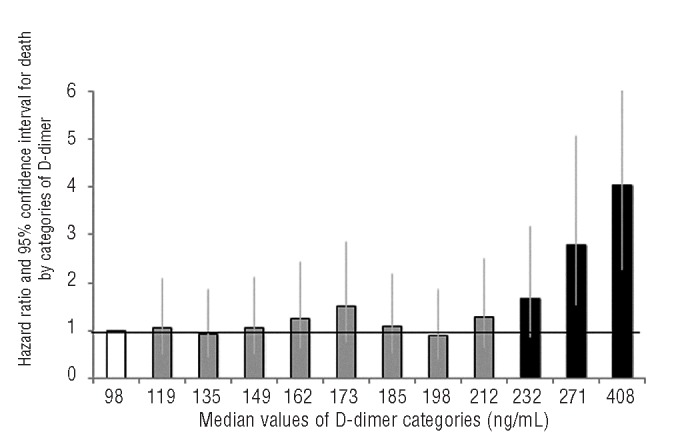
Hazard ratio and 95% confidence interval for death by categories of D-Dimer. Hazard ratios are adjusted for age, sex, smoking, body mass index, alcohol intake, and hypertensive, diabetic or dyslipidemic status. The reference group, with a median D-dimer con-centrarion of 98 ng/mL, is in white.
Table 4.
Hazard ratio and 95% confidence interval for death by increasing levels of D-dimer over 221 ng/mL.
We failed to observe any statistically significant interaction in regulating risk of death between elevated D-dimer level and any of the risk factors listed in Table 4 (data not shown). In particular, we tested for additive or multiplicative interactions of D-dimer with age or sex, and failed to observe statistically significant terms for interaction for any of the analyses performed. For example, in the model in Table 4 (adjusted for age, sex, smoking, body mass index, alcohol intake, hypertension, diabetes or dyslipidemic status), the terms of interaction had P=0.56 (sex) and P=0.83 (age).
Discussion
This study shows, in a large prospective cohort of apparently healthy adults, that a high D-dimer concentration at baseline was independently associated with incidence of mortality from any cause. The association was evident only for D-dimer values higher than 221 ng/mL (corresponding to the highest quartile of the distribution); starting from this level the relative risk of death increased linearly, doubling for values higher than 312 ng/mL. Previous smaller prospective studies also reported that higher levels of D-dimer are markers of increased total mortality.3,11,19–21,31 In particular our findings are in agreement with those of the MESA study performed in a multiethnic, general healthy population in the USA.19 In that cohort a statistically significant increase in the risk of total mortality was observed for D-dimer levels higher than 210 ng/mL. In the MESA study the value of 210 ng/mL corresponded to the median of the distribution, whereas in the MOLI-SANI study it approximately identified the highest quartile. This discrepancy may be attributed to the fact that subjects included in the USA study were older (mean age 62 years as compared to 55 years in MOLI-SANI), and D-dimer increases with age. Notably, in both studies the risk of death started to increase at values around 210–220 ng/mL, which are candidates to be the cut-off for defining a “high risk value” of D-dimer if D-dimer were to be considered in the future as a reliable biomarker of risk for total mortality.
In our population D-dimer levels showed a moderate correlation with several conventional risk factors and markers of inflammation. When adjusting for potential confounders (age, sex, smoking, baseline evidence of diabetes, hypertension or dyslipidemia, alcohol intake and inflammatory markers), the association was somewhat reduced but remained highly statistically significant (P for trend: 0.0002). The lack of a strong correlation between D-dimer levels and conventional risk factors for cardiovascular diseases and the fact that elevated D-dimer concentrations associate with total mortality independently of conventional risk factors suggest that D-dimer measurements should be considered as informative as other conventional risk factors, at least in a middle-aged Italian population.
The link between D-dimer and mortality from any cause in apparently healthy adults is not fully understood.2,10,17 It is reasonable to conceive that early elevation of D-dimer together with other inflammatory factors may contribute to the development or the severity of several chronic diseases, including cardiovascular disease and cancer, thus leading to augmented mortality. The impact of higher D-dimer levels on mortality from any cause leaves open the possibility that effects on cardiovascular and cancer deaths contribute to the observed result, as implied by the so-called “common soil hypothesis”, which recognizes a number of common mechanisms and risk factors for ischemic vascular disorders and hormone-dependent tumors.22,32,33 Indeed, cancer is frequently associated with activation of the hemostatic system and the extent of this activation has been reported to correlate with a more advanced tumor stage, with unfavorable outcomes and prognosis.32,33
Nonetheless we may also consider that D-dimer itself is not a causal factor but rather a marker for other factors related to the pathophysiology of thrombosis or cancer, and elevated D-dimer levels may reflect currently unknown hemostatic or cancer disorders. D-dimer has high heritability and this fact supports the notion that D-dimer levels reflect genetic factors.34 Nevertheless D-dimer might reflect other environmental risk factors for thrombosis, such as inflammatory markers.15 The possibility that D-dimer levels predict pro-thrombotic states is suggested by the rapid normalization of D-dimer values after car-dioversion35 or warfarin treatment36 in patients with atrial fibrillation.
Nonetheless, a reverse causality bias cannot be excluded, as tumors22,23,32 and other chronic conditions will increase intravascular coagulation or elevation of markers of hemostasis and inflammation.12
Lastly, residual confounding by factors not measured or adjusted for could explain the observed link between D-dimer levels and total mortality.
Limitations
Data from the MOLI-SANI study do not currently include information on specific cause of death or incidence of non-fatal events. These data need rigorous validation, which is ongoing. However, our findings concerning a clear association of high levels of D-dimer with total mortality, in agreement with a previous smaller study,19 deserve appropriate dissemination. Another limitation of our study is the relatively small number of events and the short follow-up period. Moreover, similarly to several previous epidemiological studies, we had only a single baseline measure of D-dimer; biological variability, related to imprecision of single measurements, usually tends to weaken measured associations.
Conclusions
In conclusion, in a apparently healthy population cohort of middle-aged men and women, elevated D-dimer levels at baseline were related to subsequent mortality from any cause, independently of conventional risk factors or markers of inflammation. Measurement of D-dimer may lead to a practical improvement in the current risk stratification criteria for mortality. Further studies are required to test this hypothesis.
Acknowledgments
The MOLI-SANI Project was partially supported by research grants from Pfizer Foundation (Rome, Italy), the Italian Ministry of University and Research (MIUR, Rome, Italy)–Programma Triennale di Ricerca, Decreto n.1588 and Instrumentation Laboratory, Milan, Italy and Project “Malattie cardiovascolari: ruoli di fattori genetici, acquisiti, nuovi approcci terapeutici e condizioni organizzative ottimali per la produzione delle conoscenze”, (D. MIUR n. 328 of 01/07/2010). Associazione Cuore-Sano Onlus contributed to the publication cost.
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Lorenzet R, Donati MB. Blood clotting activation, angiogenesis and tumor metastasis: any role for TFPI? Thromb Haemost. 2002;87(6):928–9 [PubMed] [Google Scholar]
- 2.Donati MB. Assays for fibrinogen-fibrin degradation products in biological fluids: some methodological aspects. Thromb Diath Haemorrh. 1975;34(3):652–60 [PubMed] [Google Scholar]
- 3.Meade TW, Mellows S, Brozovic M, Miller GJ, Chakrabarti RR, North WR, et al. Haemostatic function and ischaemic heart disease: principal results of the Northwick Park Heart Study. Lancet. 1986;2(8506):533–7 [DOI] [PubMed] [Google Scholar]
- 4.Gorog DA. Prognostic value of plasma fibrinolysis activation markers in cardiovascular disease. J Am Coll Cardiol. 2010;55(24): 2701–9 [DOI] [PubMed] [Google Scholar]
- 5.Fowkes FG, Lowe GD, Housley E, Rattray A, Rumley A, Elton RA, et al. Cross-linked fibrin degradation products, risk of coronary heart disease, and progression of peripheral arterial disease. Lancet. 1993;342(8863):84–6 [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Hennekens CH, Cerskus A, Stampfer MH. Plasma concentration of cross-linked fibrin degradation products (D-dimer) and the risk of future myocardial infarction among apparently healthy men. Circulation. 1994;90(5):2236–40 [DOI] [PubMed] [Google Scholar]
- 7.Smith FB, Lee AJ, Fowkes FGR, Rumley A, Lowe GDO. Hemostatic factors as predictors of ischemic heart disease and stroke in the Edinburgh Artery Study. Arterioscler Thromb Vasc Biol. 1997;17(11):3321–5 [DOI] [PubMed] [Google Scholar]
- 8.Lowe GD, Yarnell JW, Sweetnam PM, Rumley A, Thomas HF, Elwood PC. Fibrin D-dimer, tissue plasminogen activator, tissue plasminogen activator inhibitor, and the risk of major ischaemic heart disease in the Caerphilly Study. Thromb Haemost. 1998; 79(1):129–33 [PubMed] [Google Scholar]
- 9.Smith FB, Rumley A, Lee AJ, Leng GL, Fowkes FG, Lowe GD. Haemostatic factors and prediction of ischaemic heart disease and stroke in claudicants. Br J Haematol. 1998;100(4):758–63 [DOI] [PubMed] [Google Scholar]
- 10.Lowe GD, Rumley A. Use of fibrinogen and fibrin D-dimer in prediction of arterial thrombotic events. Thromb Haemost. 1999;82(2):667–72 [PubMed] [Google Scholar]
- 11.Moss AJ, Goldstein RE, Marder VJ, Sparks CE, Oakes D, Greenberg H, et al. Thrombogenic factors and recurrent coronary events. Circulation. 1999;99(19):2517–22 [DOI] [PubMed] [Google Scholar]
- 12.Cortellaro M, Cofrancesco E, Boschetti C, Mussoni L, Donati MB, Cardillo M, et al. Increased fibrin turnover and high PAI-1 activity as predictors of ischemic events in atherosclerotic patients. A case-control study. The PLAT Group Arterioscler Thromb. 1993;13(10):1412–7 [DOI] [PubMed] [Google Scholar]
- 13.De Lucia D, De Vita F, Orditura M, Renis V, Belli A, Conte M, et al. Hypercoagulable state in patients with advanced gastrointestinal cancer: evidence for an acquired resistance to activated protein C. Tumori. 1997;83(6):948–52 [DOI] [PubMed] [Google Scholar]
- 14.Cushman M, Lemaitre RN, Kuller LH, Psaty BM, Macy EM, et al. Fibrinolytic activation markers predict myocardial infarction in the elderly: the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1999;19(3): 493–8 [DOI] [PubMed] [Google Scholar]
- 15.Lowe GD, Yarnell JW, Rumley A, Bainton D, Sweetnam PM. C-reactive protein, fibrin D-dimer, and incident ischemic heart disease in the Speedwell study: are inflammation and fibrin turnover linked in pathogenesis? Arterioscler Thromb Vasc Biol. 2001;21 (4):603–10 [DOI] [PubMed] [Google Scholar]
- 16.Cushman M, Folsom AR, Wang L, Aleksic N, Rosamond WD, Tracy RP, et al. Fibrin fragment D-dimer and the risk of future venous thrombosis. Blood. 2003;101(4): 1243–8 [DOI] [PubMed] [Google Scholar]
- 17.Ay C, Dunkler D, Pirker R, Thaler J, Quehenberger P, Wagner O, et al. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica. 2012;97(8):1158–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, et al. Fibrin D-dimer and coronary heart disease: prospective study and meta-analysis. Circulation. 2001;103(19):2323–7 [DOI] [PubMed] [Google Scholar]
- 19.Folsom AR, Delaney JA, Lutsey PL, Zakai NA, Jenny NS, Polak JF, et al. Multiethnic Study of Atherosclerosis Investigators Associations of factor VIIIc, D-dimer, and plasmin-antiplasmin with incident cardiovascular disease and all-cause mortality. Am J Hematol. 2009;84(6):349–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcucci R, Gori AM, Giannotti F, Baldi M, Verdiani V, Del Pace S, et al. Markers of hypercoagulability and inflammation predict mortality in patients with heart failure. J Thromb Haemost. 2006;4(5):1017–22 [DOI] [PubMed] [Google Scholar]
- 21.Morange PE, Bickel C, Nicaud V, Schnabel R, Rupprecht HJ, Peetz D, et al. AtheroGene Investigators Haemostatic factors and the risk of cardiovascular death in patients with coronary artery disease: the AtheroGene study. Arterioscler Thromb Vasc Biol. 2006;26(12):2793–9 [DOI] [PubMed] [Google Scholar]
- 22.Donati MB. Thrombosis and cancer: a personal view. Thromb Haemost. 2007;98(1): 126–8 [PubMed] [Google Scholar]
- 23.Falanga A, Panova-Noeva M, Russo L. Procoagulant mechanisms in tumour cells. Best Pract Res Clin Haematol. 2009;22(1): 49–60 [DOI] [PubMed] [Google Scholar]
- 24.Iacoviello L, Bonanni A, Costanzo S, De Curtis A, Di Castelnuovo A, Olivieri M, et al. On behalf of the Moli-sani Project Investigators The Moli-Sani Project, a randomized, prospective cohort study in the Molise region in Italy; design, rationale and objectives. Italian J Pub Health. 2007;4:110–8 [Google Scholar]
- 25.Centritto F, Iacoviello L, di Giuseppe R, De Curtis A, Costanzo S, Zito F, et al. On behalf of Moli-sani Investigators Dietary patterns, cardiovascular risk factors and C-reactive protein in a healthy Italian population. Nutr Metab Cardiovasc Dis. 2009;19(10):697–706 [DOI] [PubMed] [Google Scholar]
- 26.di Giuseppe R, Di Castelnuovo A, Melegari C, De Lucia F, Santimone I, Sciarretta A, et al. On behalf of the Moli-sani Project Investigators Typical breakfast food consumption and risk factors for cardiovascular disease in a large sample of Italian adults. Nutr Metab Cardiovasc Dis. 2012;22(4):347–54 [DOI] [PubMed] [Google Scholar]
- 27.Di Castelnuovo A, Costanzo S, Persichillo M, Olivieri M, de Curtis A, Zito F, et al. Distribution of short and lifetime risks for cardiovascular disease in Italians Eur J Cardiovasc Prev Rehabil. 2012;19(4):723–30 [DOI] [PubMed] [Google Scholar]
- 28.Santimone I, Di Castelnuovo A, de Curtis A, Spinelli M, Cugino D, Gianfagna F, et al. White blood cells count, sex and age are major determinants of platelet indices heterogeneity in an adult general population: results from the MOLI-SANI project. Haematologica. 2011;96(8):1180–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonaccio M, Di Castelnuovo A, Costanzo S, De Lucia F, Olivieri M, Donati MB, et al. Mass media information and adherence to Mediterranean diet: results from the Moli-sani study. Int J Public Health. 2012;57(3): 589–97 [DOI] [PubMed] [Google Scholar]
- 30.Arcari A, Magnacca S, Bracone F, Costanzo S, Persichillo M, Di Castelnuovo A, et al. Relation between pulmonary function and 10-year risk for cardiovascular disease among healthy men and women in Italy: the Moli-sani Project. Eur J Prev Cardiol. 2012. May 18 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Folsom AR, Wu KK, Rosamond WD, Sharrett AR, Chambless LE. Prospective study of hemostatic factors and incidence of coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 1997;96(4):1102–8 [DOI] [PubMed] [Google Scholar]
- 32.Rickles FR, Falanga A. Molecular basis for the relationship between thrombosis and cancer. Thromb Res. 2001;102:V215–V224 [DOI] [PubMed] [Google Scholar]
- 33.Donati MB. The “common soil hypothesis”: evidence from population studies? Thromb Res. 2010;125 (Suppl 2):S92–5 [DOI] [PubMed] [Google Scholar]
- 34.Ariëns RA, de Lange M, Snieder H, Boothby M, Spector TD, Grant PJ. Activation markers of coagulation and fibrinolysis in twins: heritability of the prethrombotic state. Lancet. 2002;359(9307):667–71 [DOI] [PubMed] [Google Scholar]
- 35.Lip GY, Rumley A, Dunn FG, Lowe GD. Plasma fibrinogen and fibrin D-dimer in patients with atrial fibrillation. Int J Cardiol. 1995;51(3):245–51 [DOI] [PubMed] [Google Scholar]
- 36.Lip GY, Lip PL, Zarifis J, Watson RD, Bareford D, Lowe GD, et al. Fibrin D-dimer and beta-thromboglobulin as markers of thrombogenesis and platelet activation in atrial fibrillation: effects of introducing ultra-low-dose warfarin and aspirin. Circulation. 1996;94(3):425–31 [DOI] [PubMed] [Google Scholar]


