Abstract
Incomplete Sendai virus particles (I particles) interfered with the replication of several strains of infectious Sendai virions (standard virus) but not with the replication of Newcastle disease virus, mumps virus, or Sindbis virus. I particles did not induce interferon, and ultraviolet irradiation of I particles abolished their ability to interfere. Protein synthesis was not necessary to establish interference. The degree of interference depended on the interval between exposure of cells to the I particles and challenge by standard virus, and this was reflected in the degree of inhibition of virus-specific ribonucleic acid (RNA) synthesis in infected cells. The most dramatic change was decreased accumulation of 50S virus-specific RNA in infected cells. RNA species sedimenting slower than 50S were not as markedly reduced in total amount, but hybridization experiments showed that a substantial portion of these slowly sedimenting RNA species were plus strands, presumably representing replicas of the RNA species in I particles. When I particles in insufficient numbers to interfere were added to cells as late as 8 hr after standard virus, there were no obvious changes in virus-specific RNA species in the cells; however, significant amounts of 19 and 25S RNA species, representing progeny of the I particles, appeared in the culture medium. It was concluded that interference was an intracellular event affecting an early step in virus replication. Competition by I particles for cell sites or substrates needed by standard virus seemed a less likely mechanism of interference than competition for enzymes specified by standard virus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALUDA M. A. Loss of viral receptors in homologous interference by ultraviolet-irradiated Newcastle disease virus. Virology. 1959 Mar;7(3):315–327. doi: 10.1016/0042-6822(59)90201-6. [DOI] [PubMed] [Google Scholar]
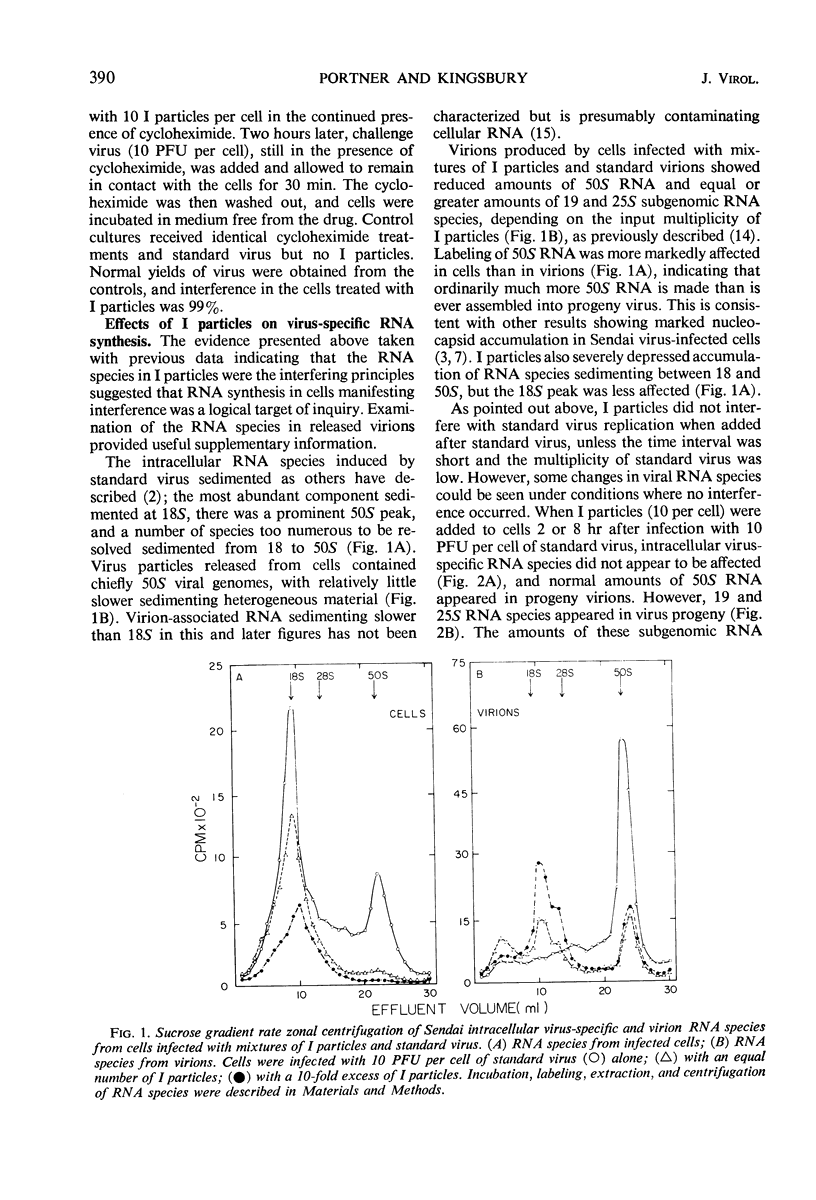
- Blair C. D., Robinson W. S. Replication of Sendai virus. I. Comparison of the viral RNA and virus-specific RNA synthesis with Newcastle disease virus. Virology. 1968 Aug;35(4):537–549. doi: 10.1016/0042-6822(68)90284-5. [DOI] [PubMed] [Google Scholar]
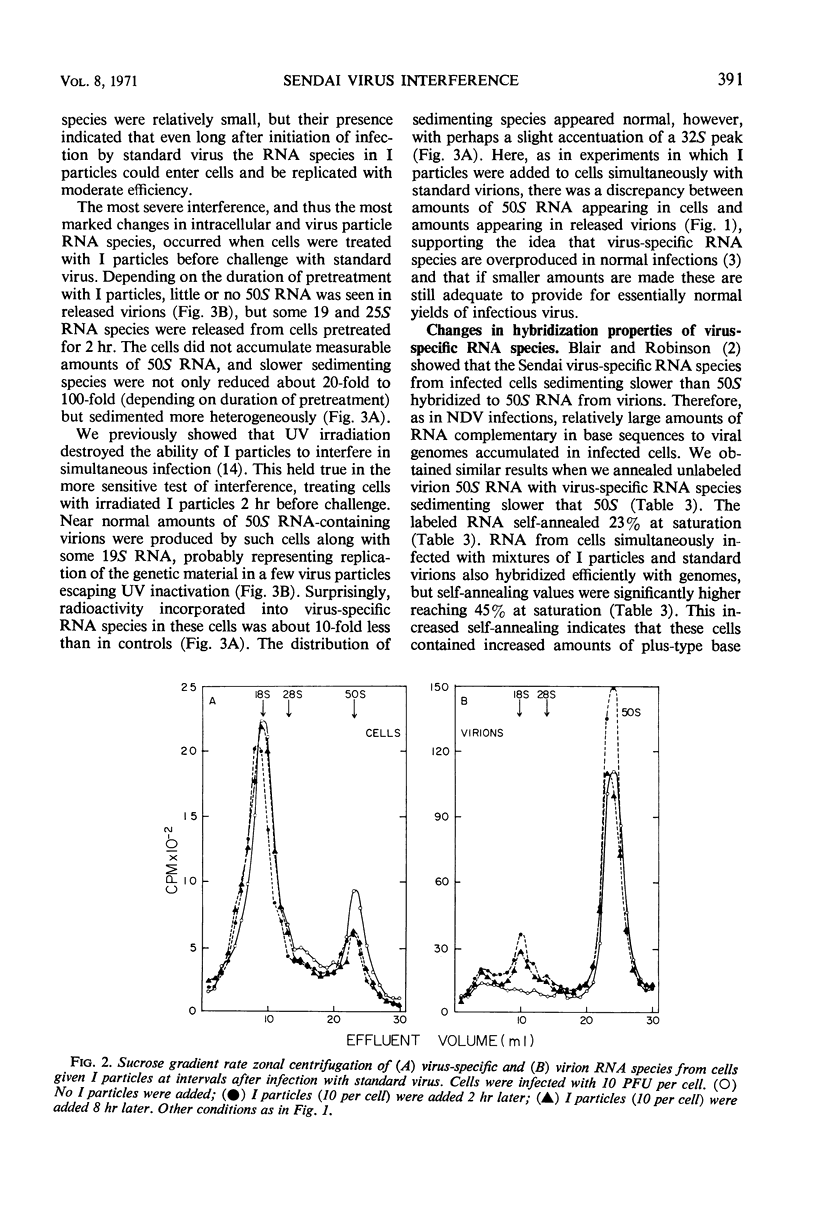
- Blair C. D., Robinson W. S. Replication of Sendai virus. II. Steps in virus assembly. J Virol. 1970 May;5(5):639–650. doi: 10.1128/jvi.5.5.639-650.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
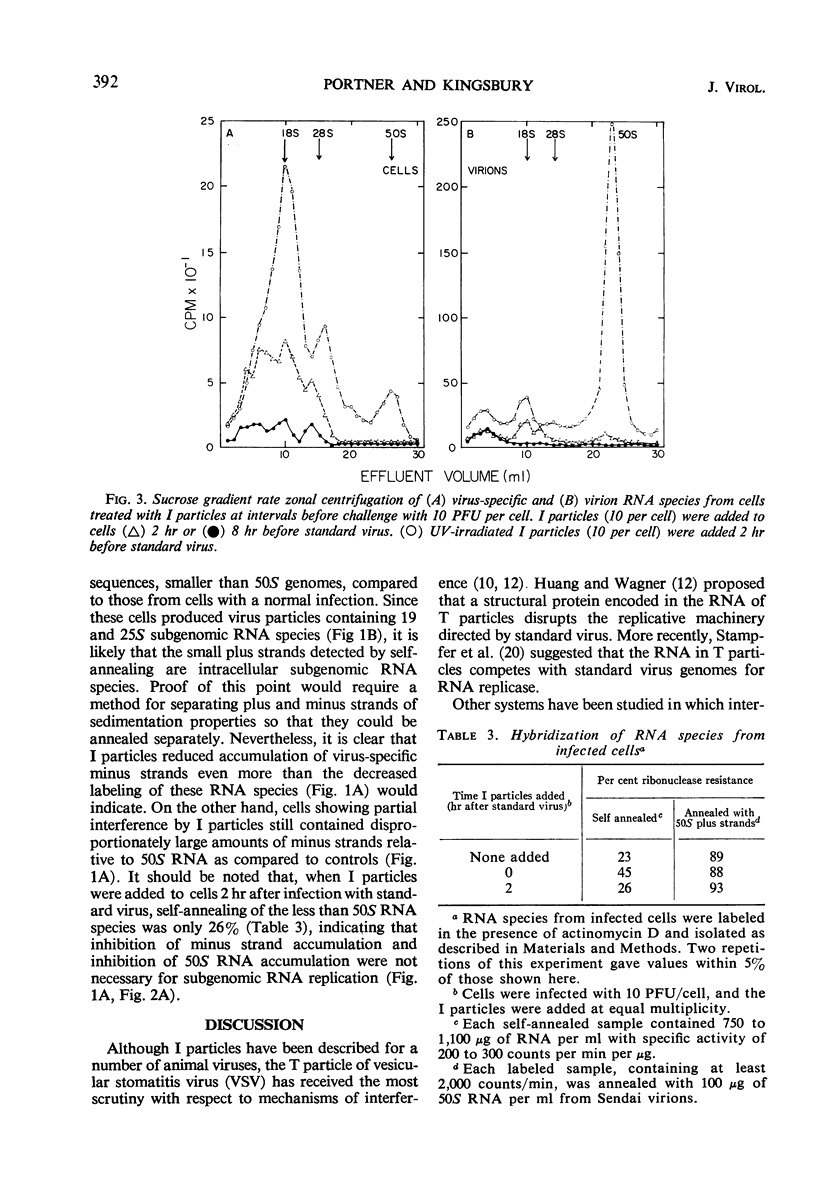
- CORDS C. E., HOLLAND J. J. INTERFERENCE BETWEEN ENTEROVIRUSES AND CONDITIONS EFFECTING ITS REVERSAL. Virology. 1964 Feb;22:226–234. doi: 10.1016/0042-6822(64)90007-8. [DOI] [PubMed] [Google Scholar]
- Crowell R. L. Specific cell-surface alteration by enteroviruses as reflected by viral-attachment interference. J Bacteriol. 1966 Jan;91(1):198–204. doi: 10.1128/jb.91.1.198-204.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington R. W., Portner A., Kingsbury D. W. Sendai virus replication: an ultrastructural comparison of productive and abortive infections in avian cells. J Gen Virol. 1970 Dec;9(3):169–177. doi: 10.1099/0022-1317-9-3-169. [DOI] [PubMed] [Google Scholar]
- East J. L., Kingsbury D. W. Mumps virus replication in chick embryo lung cells: properties of ribonucleic acids in virions and infected cells. J Virol. 1971 Aug;8(2):161–173. doi: 10.1128/jvi.8.2.161-173.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FROTHINGHAM T. E., GRANOFF A. Plaque formation with mumps virus. Virology. 1961 Oct;15:213–214. doi: 10.1016/0042-6822(61)90242-2. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D., Bratt M. A. Ribonucleic acid polymerase in virions of Newcastle disease virus: comparison with the vesicular stomatitis virus polymerase. J Virol. 1971 Mar;7(3):389–394. doi: 10.1128/jvi.7.3.389-394.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Defective T particles of vesicular stomatitis virus. II. Biologic role in homologous interference. Virology. 1966 Oct;30(2):173–181. doi: 10.1016/0042-6822(66)90093-6. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W. Newcastle disease virus RNA. II. Preferential synthesis of RNA complementary to parental viral RNA by chick embryo cells. J Mol Biol. 1966 Jun;18(1):204–214. doi: 10.1016/s0022-2836(66)80086-4. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W., Portner A., Darlington R. W. Properties of incomplete Sendai virions and subgenomic viral RNAs. Virology. 1970 Dec;42(4):857–871. doi: 10.1016/0042-6822(70)90335-1. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. W., Portner A. On the genesis of incomplete Sendai virions. Virology. 1970 Dec;42(4):872–879. doi: 10.1016/0042-6822(70)90336-3. [DOI] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., HUNTER H. S. PURIFICATION AND PARTIAL CHEMICAL ANALYSIS OF SINDBIS VIRUS. Virology. 1963 Jul;20:433–445. doi: 10.1016/0042-6822(63)90092-8. [DOI] [PubMed] [Google Scholar]
- POHJANPELTO P., COOPER P. D. INTERFERENCE BETWEEN POLIOVIRUSES INDUCED BY STRAINS THAT CANNOT MULTIPLY. Virology. 1965 Mar;25:350–357. doi: 10.1016/0042-6822(65)90054-1. [DOI] [PubMed] [Google Scholar]
- Roizman B. Abortive infection of canine cells by herpes simplex virus. 3. The interference of conditional lethal virus with an extended host range mutant. Virology. 1965 Sep;27(1):113–117. doi: 10.1016/0042-6822(65)90148-0. [DOI] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D., Huang A. S. Ribonucleic acid synthesis of vesicular stomatitis virus. I. Species of ribonucleic acid found in Chinese hamster ovary cells infected with plaque-forming and defective particles. J Virol. 1969 Aug;4(2):154–161. doi: 10.1128/jvi.4.2.154-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck F. T., Rubin H. The mechanism of interference between an avian leukosis virus and Rous sarcoma virus. II. Early steps of infection by RSV of cells under conditions of interference. Virology. 1966 Aug;29(4):642–653. doi: 10.1016/0042-6822(66)90288-1. [DOI] [PubMed] [Google Scholar]


