Abstract
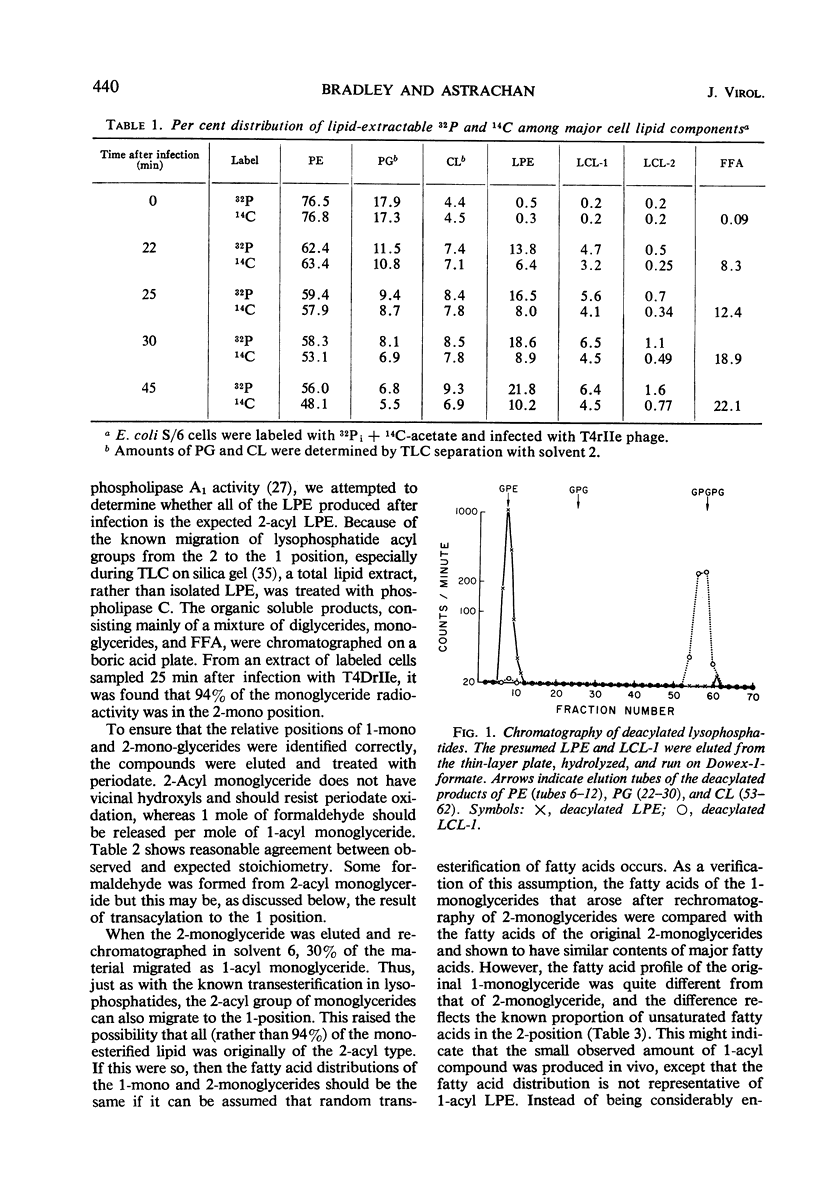
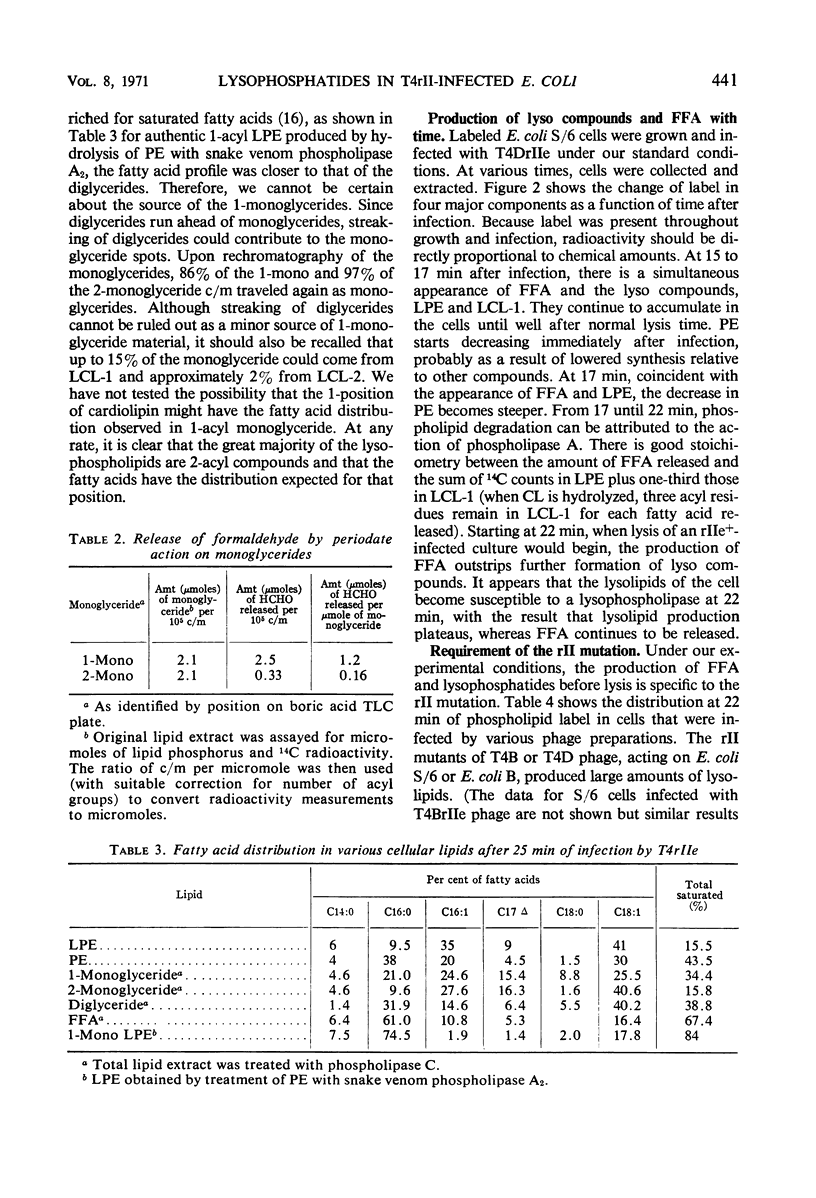
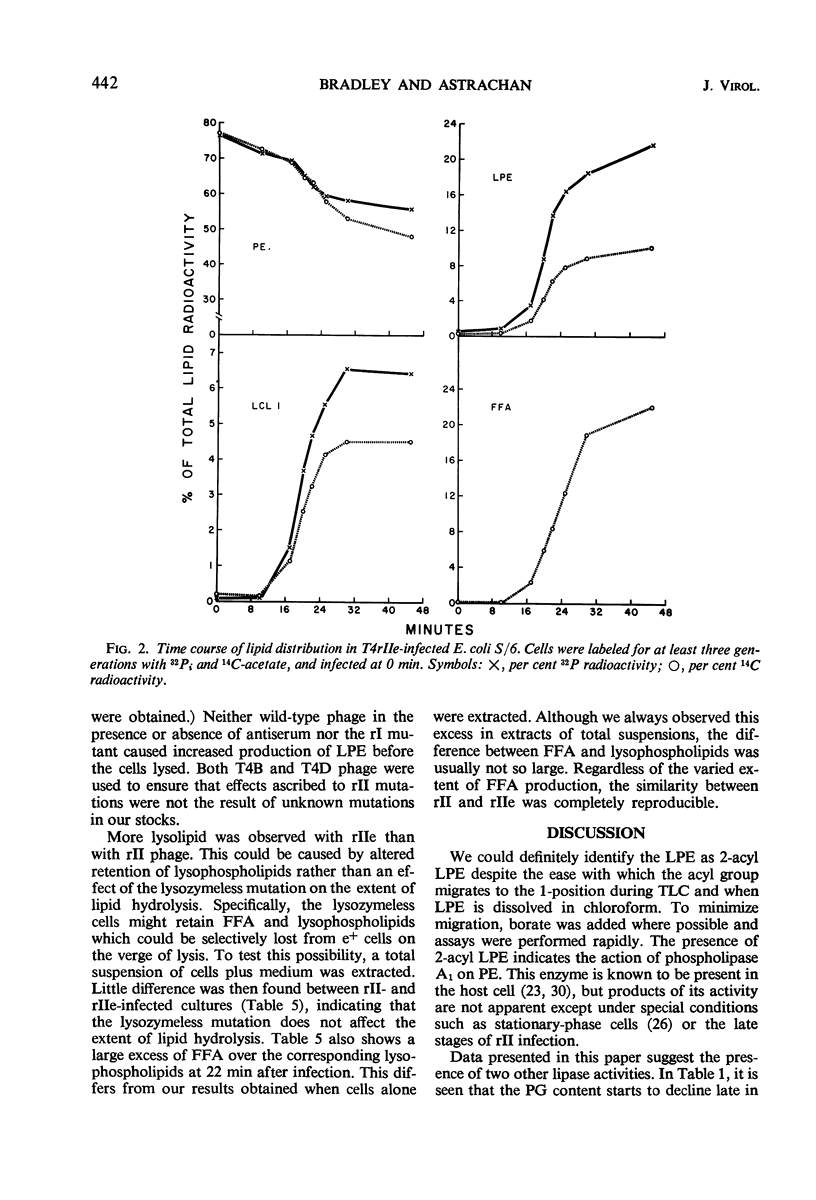
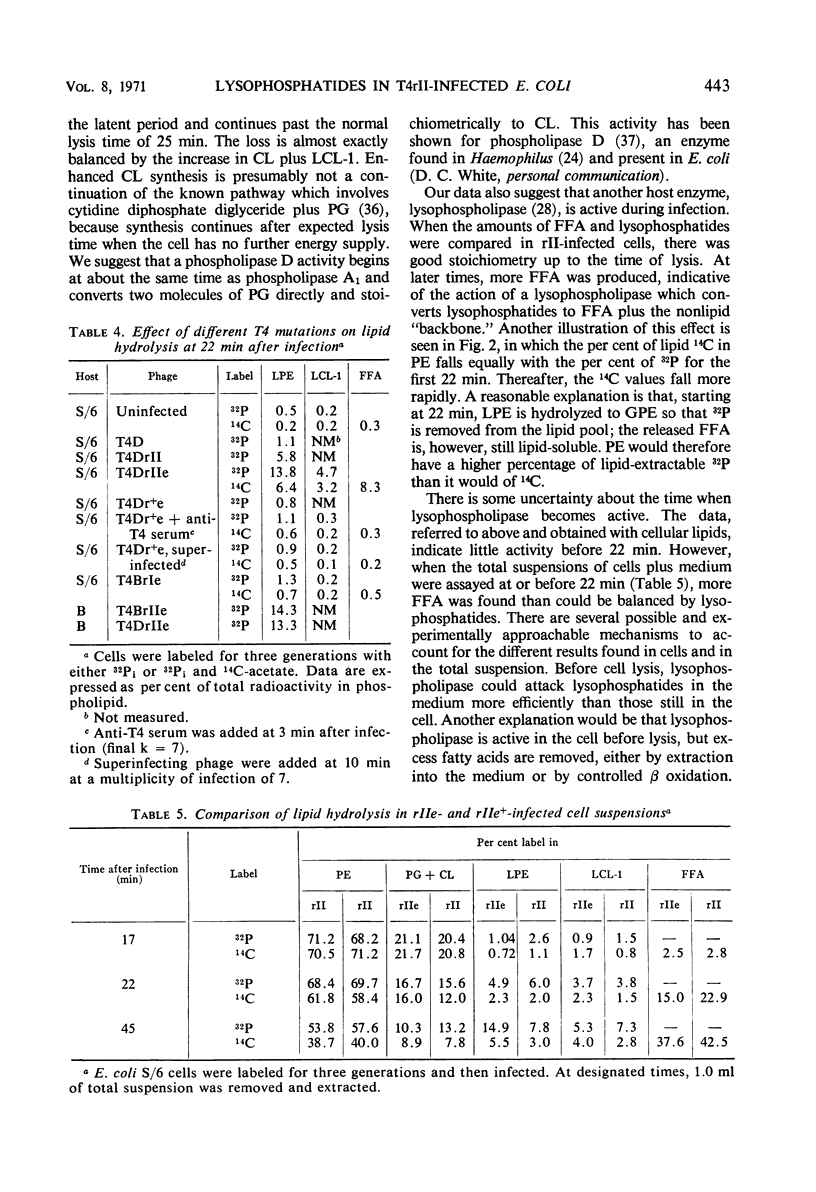
Hydrolysis of phospholipids was observed to start about 15 min after Escherichia coli S/6 cells were infected with T4rII bacteriophage mutants. Hydrolysis continued through the latent period and well past the time when cell lysis occurs. The hydrolytic products that accumulated were free fatty acids, 2-acyl lysophosphatidylethanolamine, and various lysocardiolipins. These products indicated the action of phospholipase A1. From 15 to 22 min after infection, there were equivalent amounts of fatty acids and lysophosphatides in extracts of cellular lipids. Thereafter, free fatty acids were produced in excess. This suggests that lysophospholipase was active at the later time. We also observed a stoichiometric relation between loss of phosphatidylglycerol and increase of cardiolipin plus lysocardiolipins. This continued well past the normal lysis time (25 min). The appearance of lipase activities during the latent period seems to be specific to infection with rII mutants. Neither the wild-type bacteriophage nor rI mutants produced similar activities by 22 min after infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bennett J., Glavinovich J., Liskay R., Wulff D. L., Cronan J. E., Jr Phospholipid hydrolysis in Escherichia coli infected with rapid lysis mutants of Phage T4. Virology. 1971 Feb;43(2):516–518. doi: 10.1016/0042-6822(71)90326-6. [DOI] [PubMed] [Google Scholar]
- Berger H., Kozinski A. W. Suppression of T4D ligase mutations by rIIa and rIIb mutations. Proc Natl Acad Sci U S A. 1969 Nov;64(3):897–904. doi: 10.1073/pnas.64.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller C. S., Astrachan L. Replication of T4rII bacteriophage in Escherichia coli K-12 (lambda). J Virol. 1968 Apr;2(4):298–307. doi: 10.1128/jvi.2.4.298-307.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan V. L., Shugar S., Ebisuzaki K. Intergenic suppression of amber polynucleotide ligase mutation in bacteriophage T4. Virology. 1970 Feb;40(2):403–406. doi: 10.1016/0042-6822(70)90418-6. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Wulff D. L. A role for phospholipid hydrolysis in the lysis of Escherichia coli infected with bacteriophage T4. Virology. 1969 Jun;38(2):241–246. doi: 10.1016/0042-6822(69)90365-1. [DOI] [PubMed] [Google Scholar]
- Emrich J. Lysis of T4-infected bacteria in the absence of lysozyme. Virology. 1968 May;35(1):158–165. doi: 10.1016/0042-6822(68)90315-2. [DOI] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- Furrow M. H., Pizer L. I. Phospholipid synthesis in Escherichia coli infected with T4 bacteriophages. J Virol. 1968 Jun;2(6):594–605. doi: 10.1128/jvi.2.6.594-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A. Physiological effects of rII mutations in bacteriophage T4. Virology. 1961 Jun;14:151–163. doi: 10.1016/0042-6822(61)90190-8. [DOI] [PubMed] [Google Scholar]
- Gellert M., Bullock M. L. DNA ligase mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1580–1587. doi: 10.1073/pnas.67.3.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERRIOTT R. M., BARLOW J. L. Preparation, purification, and properties of E. coli virus T2. J Gen Physiol. 1952 May;36(1):17–28. doi: 10.1085/jgp.36.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILDEBRAND J. G., LAW J. H. FATTY ACID DISTRIBUTION IN BACTERIAL PHOSPHOLIPIDS. THE SPECIFICITY OF THE CYCLOPROPANE SYNTHETASE REACTION. Biochemistry. 1964 Sep;3:1304–1308. doi: 10.1021/bi00897a020. [DOI] [PubMed] [Google Scholar]
- Josslin R. Physiological studies on the t gene defect in T4-infected Escherichia coli. Virology. 1971 Apr;44(1):101–107. doi: 10.1016/0042-6822(71)90157-7. [DOI] [PubMed] [Google Scholar]
- Josslin R. The effect of phage T4 infection on phospholipid hydrolysis in Escherichia coli. Virology. 1971 Apr;44(1):94–100. doi: 10.1016/0042-6822(71)90156-5. [DOI] [PubMed] [Google Scholar]
- Josslin R. The lysis mechanism of phage T4: mutants affecting lysis. Virology. 1970 Mar;40(3):719–726. doi: 10.1016/0042-6822(70)90216-3. [DOI] [PubMed] [Google Scholar]
- Karam J. D. DNA replication of phage T4 rII mutants without polynucleotide ligase (gene 30). Biochem Biophys Res Commun. 1969 Oct 22;37(3):416–422. doi: 10.1016/0006-291x(69)90931-0. [DOI] [PubMed] [Google Scholar]
- Kleiman J. H., Lands W. E. Purification of a phospholipase C from Bacillus cereus. Biochim Biophys Acta. 1969 Dec 17;187(4):477–485. doi: 10.1016/0005-2760(69)90044-7. [DOI] [PubMed] [Google Scholar]
- Krylov V. N. Possible function of rII genes of bacteriophage T4. Virology. 1970 Aug;41(4):711–717. doi: 10.1016/0042-6822(70)90435-6. [DOI] [PubMed] [Google Scholar]
- Okuyama H., Nojima S. The presence of phospholipase A in Escherichia coli. Biochim Biophys Acta. 1969 Jan 21;176(1):120–124. [PubMed] [Google Scholar]
- Ono Y., White D. C. Cardiolipin-specific phospholipase D activity in Haemophilus parainfluenzae. J Bacteriol. 1970 Jul;103(1):111–115. doi: 10.1128/jb.103.1.111-115.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. H., Buller C. S. Phospholipid metabolism in T4 bacteriophage infected Escherichia coli K-12 (lambda). J Virol. 1969 May;3(5):463–468. doi: 10.1128/jvi.3.5.463-468.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peypoux F., Michel G. Etude des phospholipides d'une souche sauvage et d'une souche mutante thermosensible d'Escherichia coli. Biochim Biophys Acta. 1970 Dec 15;218(3):453–462. [PubMed] [Google Scholar]
- Proulx P. R., Van Deenen L. L. Phospholipase activities of Escherichia coli. Biochim Biophys Acta. 1967 Aug 8;144(1):171–174. doi: 10.1016/0005-2760(67)90092-6. [DOI] [PubMed] [Google Scholar]
- Proulx P., Fung C. K. Metabolism of phosphoglycerides in E. coli. IV. The positional specificity and properties of phospholipase A. Can J Biochem. 1969 Dec;47(12):1125–1128. doi: 10.1139/o69-181. [DOI] [PubMed] [Google Scholar]
- Rampini C. Variations des phospholipides d'E. coli K 12 après infection soit par le phage T 2 soit par le phage T 6. C R Acad Sci Hebd Seances Acad Sci D. 1969 Nov 17;269(20):2040–2043. [PubMed] [Google Scholar]
- STEINBERG C. M., EDGAR R. S. A critical test of a current theory of genetic recombination in bacteriophage. Genetics. 1962 Feb;47:187–208. doi: 10.1093/genetics/47.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi M. Studies on the physiological defect in rII mutants of bacteriophage T4. J Mol Biol. 1966 Apr;16(2):503–522. doi: 10.1016/s0022-2836(66)80188-2. [DOI] [PubMed] [Google Scholar]
- Stanacev N. Z., Chang Y. Y., Kennedy E. P. Biosynthesis of cardiolipin in Escherichia coli. J Biol Chem. 1967 Jun 25;242(12):3018–3019. [PubMed] [Google Scholar]
- Stanacev N. Z., Stuhne-Sekalec L. On the mechanism of enzymatic phosphatidylation. Biosynthesis of cardiolipin catalyzed by phospholipase D. Biochim Biophys Acta. 1970 Jul 14;210(2):350–352. doi: 10.1016/0005-2760(70)90183-9. [DOI] [PubMed] [Google Scholar]
- VAN DEENENL, DE HAAS G. H. THE SUBSTRATE SPECIFICITY OF PHOSPHOLIPASE A. Biochim Biophys Acta. 1963 Oct 22;70:538–553. doi: 10.1016/0006-3002(63)90792-3. [DOI] [PubMed] [Google Scholar]
- Wells M. A., Dittmer J. C. A microanalytical technique for the quantitative determination of twenty-four classes of brain lipids. Biochemistry. 1966 Nov;5(11):3405–3418. doi: 10.1021/bi00875a004. [DOI] [PubMed] [Google Scholar]
- White D. C., Cox R. H. Indentification and localization of the fatty acids in Haemophilus parainfluenzae. J Bacteriol. 1967 Mar;93(3):1079–1088. doi: 10.1128/jb.93.3.1079-1088.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


