Abstract
A 79-year-old woman, a smoker, presented to the emergency department with a 2-week history of progressive dyspnoea and peripheral oedema, preceded by flu-like symptoms that had failed to improve despite antibiotics. Examination identified severe hypertension and signs consistent with severe fluid overload. Baseline chest X ray showed a right-sided abnormality. In the context of an extensive smoking history CT thorax was indicated which demonstrated a right-sided hilar mass and lymphadenopathy. Blood tests showed an unexplained metabolic alkalosis with partial respiratory compensation and hypokalaemia prompting endocrinological tests investigating Cushing's syndrome. Urinary cortisol was measured at 3174 nmol/L/24 h (normal<560), serum adrenocorticotropic hormone (ACTH) of 215 ng/L (normal<46). Pleural fluid cytology confirmed a diagnosis of small-cell lung cancer (SCLC). The patient's condition deteriorated despite intravenous diuretics and nitrates, metyrapone and non-invasive ventilation. Treatment was withdrawn 1 week after admission. The clinical course in the presence of biochemical derangement and SCLC is highly suggestive of paraneoplastic ectopic ACTH secretion.
Background
Recent evidence suggests that nearly one in four cancer diagnoses are made as a result of emergency admission to hospital, rising to 31% in the elderly population.1 It is a challenge to identify atypical cancer presentations in patients with multiple contributory comorbidities. This report describes Cushing's syndrome associated with metastatic malignancy manifesting with cardio-respiratory failure.
Case presentation
A 79-year-old woman presented acutely to the emergency department with a 2-week history of progressive dyspnoea, leg swelling and decreased exercise tolerance. She described 6 weeks of lethargy and flu-like symptoms. Her medical history included chronic obstructive pulmonary disease, hypertension and cerebrovascular disease. She was a current smoker with a 35 pack-year history. Her medications included bisoprolol, ramipril, bendroflumethiazide, aspirin, simvastatin and tiotropium inhaler.
On clinical assessment, the patient had a blood pressure (BP) of 220/100 mm Hg, respiratory rate of 25/min and oxygen saturations of 98% on 1 L inhaled oxygen. She was afebrile with a venous glucose of 9 mmol/L. Systemic examination was remarkable for gross peripheral oedema, an elevated jugular venous pressure, systolic murmur and dullness to percussion at the right lung base, with left basal crackles.
Investigations
On admission laboratory investigations demonstrated neutrophilia with normal inflammatory markers, marked hypokalaemia and profound metabolic alkalosis with partial respiratory compensation (table 1).
Table 1.
Blood results: admission investigations
| ABG (1 L oxygen) | Admission bloods |
|
|---|---|---|
| pH 7.54 | PT 12.6 | Na 141 |
| pCO2 7.19 | APTT 24.8 | K 2.0 |
| pO2 10.9 | Hb 15.2 | Creat 88 |
| BE 21.3 | MCV 92.0 | Bili 11 |
| HCO3 44.1 | WCC 14.6 | ALT 131 |
| Lactate 2.7 | Neut 12.8 | ALP 123 |
| Plts 147 | Alb 37 | |
| TSH 0.5 | CRP <0.5 | |
ABG, arterial blood gas; ALP, alanine phosphatase; ALT, alanine transaminase; APTT, activated partial thromboplastin time; BE, base excess; CRP, C-reactive protein; MCV, mean corpuscular volume; PT, prothrombin time; TSH, thyroid-stimulating hormone; WCC, white cell count; Plts, platelets.
Posterioanterior chest X ray (figure 1) showed a bulky right hilum, partial right middle and lower lobe collapse, and a right pleural effusion. Initial transthoracic echocardiogram showed normal left ventricular function. Despite this result the clinical examination revealed clear signs of fluid overload.
Figure 1.
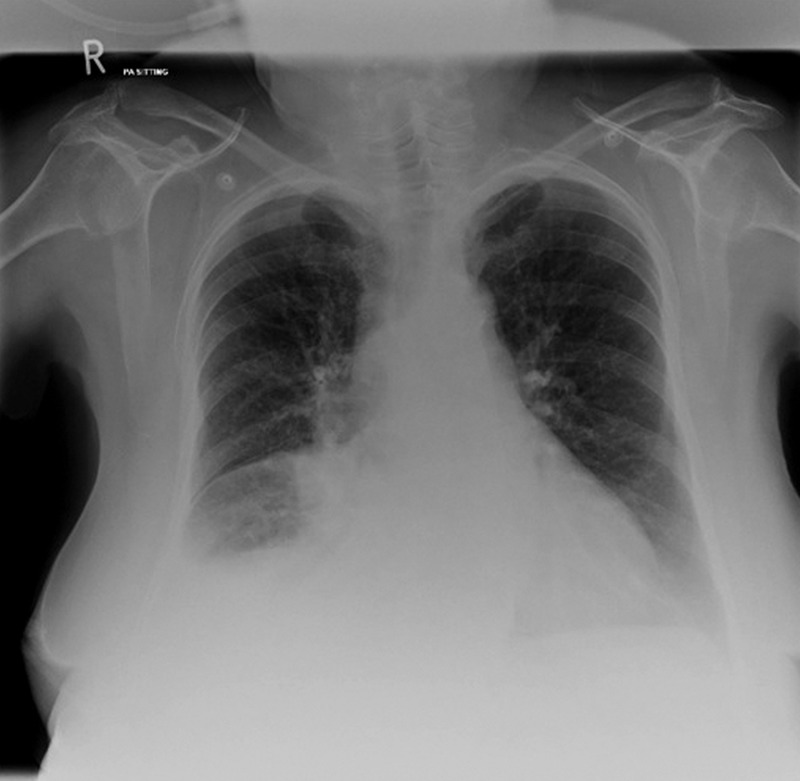
Chest X ray showing (1) bulky right hilum, (2) partial right middle lobe collapse, (3) right lower lobe consolidation and (4) right pleural effusion.
The abnormal chest X-ray raised the suspicion of underlying malignancy, and a subsequent respiratory consult prompted an urgent CT thorax (figure 2), demonstrating a right hilar mass with mediastinal lymphadenopathy.
Figure 2.
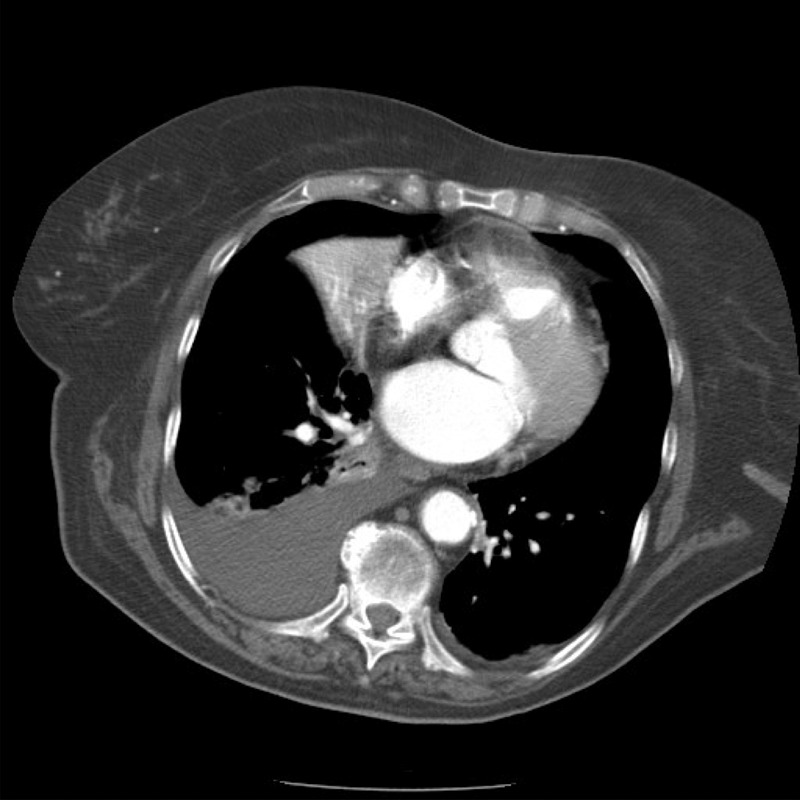
CT thorax showing right hilar mass and mediastinal lymphadenopathy.
Biochemical derangement prompted testing random cortisol, adrenocorticotropic hormone (ACTH) and 24-h urine collection (table 2).
Table 2.
Blood results: endocrine investigations
| Endocrine tests | |
|---|---|
| Random serum cortisol | 4432 nmol/L |
| 24-h urine cortisol | >3174 nmol/L (range: 0–560) |
| Serum ACTH | 215 ng/L (range: 0–46) |
ACTH, adrenocorticotropic hormone.
Differential diagnosis
Differential diagnosis included one or more of the following:
Acute hypertensive heart failure and pulmonary oedema
Lower respiratory tract infection
Conn's syndrome
Cushing's syndrome with or without underlying malignancy
Treatment
The patient was started on intravenous co-amoxiclav for pneumonia, intravenous furosemide and glyceryl trinitrate infusion for heart failure and hypertension. It was noted that the patient's BP was poorly controlled without nitrate support.
Oral metyrapone was started on advice of the endocrine team once biochemical investigations confirmed a diagnosis of Cushing's syndrome.
Following worsening pulmonary oedema and increasing dyspnoea, non-invasive ventilation was deemed the next appropriate step of management.
Outcome and follow-up
After 3 days of supportive care, the patient developed lateral ischaemia on ECG with a raised troponin of 8.5 μg/L. Worsening respiratory failure despite escalating medical care prompted discussion with the patient and her family about the likely diagnosis and prognosis. With their agreement active treatment was withdrawn 1-week postadmission.
Pleural fluid cytology subsequently confirmed a diagnosis of small-cell lung cancer (SCLC).
Discussion
Cushing's syndrome is most frequently caused by iatrogenic glucocorticoid administration. Other aetiologies can include glucocorticoid hypersectretion secondary to excess ACTH release or ACTH-independent secretion of cortisol. The majority of ACTH-dependent Cushing's syndrome are due to pituitary basophilic adenomas (approx. 80%) (Cushing disease, described by Harvey Cushing in 1932) and approximately 20% secondary to ectopic secretion of ACTH (ectopic Cushing's syndrome; ECS).2 3
ECS most commonly occurs in patients with lung and pancreatic tumours: 20% of cases are associated with bronchial carcinoid and 20% with SCLC.3–6 SCLC is characterised by its aggressive onset and most patients have metastatic disease at diagnosis. Presenting features are commonly due to intrapulmonary tumour bulk with compression and invasion of local structures (bronchi, oesophagus, superior vena cava and recurrent laryngeal nerves), in addition to symptoms of paraneoplastic phenomena.7
ECS is the second most common paraneoplastic phenomena associated with SCLC (2–5% of cases). Classical features of Cushing's syndrome include moon-facies, striae and fat redistribution. In contrast, patients with ECS associated with underlying malignancy can present with cachexia, pigmentation and peripheral oedema in addition to hypertension, hyperglycaemia and hypokalaemia.
Hypertension and hypokalaemic alkalosis associated with Cushing's syndrome is proportional to the degree of hypercortisolism.8 The pathophysiology is multifactorial. Cortisol binds to both mineralocorticoid and glucocorticoid receptors. At mineralocorticoid receptor rich sites, such as the renal cortex, 11b-hydroxysteroid dehydrogenase (11b-HSD) acts to inactivate cortisol and prevent its mineralocorticoid effects. In hypercortisolism, 11b-HSD activity is overwhelmed resulting in apparent mineralocorticoid excess.9 In addition, cortisol directly activates the renin–angiotensin system increasing vascular tone, promotes insulin resistance and mediates catecholamine sensitivity, collectively increasing BP.10 Hypersecretion of ACTH, in conditions such as ECS, leads to increased production of upstream products such as the mineralocorticoid hormone desoxycorticosterone as well as cortisol.11
This presentation and its associated mortality reflect the aggressive nature of the underlying pathology preventing classical features of Cushing's syndrome from developing. It is suspected that its atypical presentation contributes to the under-reporting of ECS and the delay in appropriate management.6
Other paraneoplastic syndromes diagnosed in SCLC include the syndrome of inappropriate antidiuretic hormone (SIADH; 10% cases)12 and Lambert Eaton syndrome (3%). Both SIADH and ECS infer a poorer prognosis in SCLC. Patients with SCLC and uncontrolled hypercortisolaemia have a significantly shorter survival than patients with a comparable stage of disease not complicated by paraneoplastic syndromes. The increased mortality in this group of patients is attributable to two factors: increased susceptibility to severe infections, both bacterial and opportunistic, and the greater incidence of thromboembolic phenomena.13–15 Median survival is 3–6 months.
Drugs inhibiting mineralocorticoid activity (metyrapone) and suppressors of adrenal steroidogenesis (ketoconazole) have been used with varying success in the acute management of hypercortisolaemia. Pharmacological adrenal blockade is advocated as first-line management in patients with metastatic disease and ECS.16 The literature advocates achieving biochemical homoeostasis before definitive management is attempted; this may improve mortality and morbidity, including susceptibility to infection.6
In this case of extensive-stage SCLC, the clinical course was suggestive of ECS. Therapies aimed at reducing hypercortisolaemia were not successful. Overwhelming failure of the cardiovascular and respiratory system resulted in rapid deterioration before chemotherapy could be initiated.
Learning points.
A common acute presentation such as cardio-respiratory failure can have an unexpected underlying cause, including malignancy.
Refractory hypertension with metabolic alkalosis and hypokalaemia should alert the clinician to the possibility of Cushing's syndrome.
Small-cell lung cancer is frequently complicated by paraneoplastic phenomena such as Cushing's syndrome with associated worsening of prognosis.
The classical signs and symptoms of Cushing's syndrome are often absent in presentations associated with malignancy and ectopic adrenocorticotropic hormone production.
Footnotes
Contributors: All authors were involved in the patient’s care. CBvS and CP drafted the article. All authors were involved in reviewing, revising and approving the final submitted version.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ellis-Brookes L, McPhail S, Ives A, et al. Routes to diagnosis for cancer—determining the patient journey using multiple routine data sets. Br J Cancer 2012;2013:1220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cushing H. The basophilic adenomas of the pituitary body and their clinical manifestations (pituitary basophilism). Bull Johns Hopkins Hosp 1932;2013:138–9521 [Google Scholar]
- 3.Boscaro M, Arnaldi G. Approach to the patient with possible Cushing's syndrome. JCEM 2009;2013:3121–31 [DOI] [PubMed] [Google Scholar]
- 4.Wajchenberg BL, Mendonça B, Liberman B, et al. Ectopic ACTH syndrome. J Steroid Biochem Mol Biol 1995;2013:139–51 [DOI] [PubMed] [Google Scholar]
- 5.Ilias I, Torpy DJ, Pacak K, et al. Cushing's syndrome due to ectopic corticotropin secretion. J Clin Endocrinol Metab 2005;2013:4955–62 [DOI] [PubMed] [Google Scholar]
- 6.Ejaz S, Vassilopoulou-Sellin R, Busaidy NL, et al. Cushing syndrome secondary to ectopic adrenocorticotropic hormone secretion. Cancer 2011;2013:4381–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Meerbeeck J, Fennell D, De Ruysscher D. Review: small-cell lung cancer. Lancet 2011;2013:1741–55 [DOI] [PubMed] [Google Scholar]
- 8.Christy NP, Laragh JH. Pathogenesis of hypokalemic alkalosis in Cushing's syndrome. New Engl J Med 1961;2013:1083. [DOI] [PubMed] [Google Scholar]
- 9.Ulick S, Wang JZ, Blumenfeld JD, et al. Cortisol inactivation overload: a mechanism of mineralocorticoid hypertension in ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab 1992;2013:963–7 [DOI] [PubMed] [Google Scholar]
- 10.Magiakou MA, Smyrnaki P, Chrousos GP. Hypertension in Cushing's syndrome. Best Pract Res Clin Endocrinol Metab 2006;2013:467–82 [DOI] [PubMed] [Google Scholar]
- 11.Schambelan M, Slaton PE, Jr, Biglieri EG. Mineralocorticoid production in hyperadrenocorticism. Role in pathogenesis of hypokalemic alkalosis. Am J Med 1971;2013:299–303 [DOI] [PubMed] [Google Scholar]
- 12.Krug L, Pietanza M, Kris M, et al. Small cell and neuroendocrine tumors of the lung. In: DeVitas VT, Lawrence TS, Rosenberg SA, eds. Cancer principle and practice of oncology. 9th edn. Philadelphia: Lippincott Williams & Wilkins, 2011:848–70. [Google Scholar]
- 13.Sarlis N, Chanock S, Nieman L. Cortisolemic indices predict severe infections in Cushing syndrome due to ectopic production of adrenocorticotropin. J Clin Endocr Soc 2000;2013:42–7 [DOI] [PubMed] [Google Scholar]
- 14.Kastelan D, Dusek T, Kraljevic I, et al. Hypercoagulability in Cushing's syndrome: the role of specific haemostatic and fibrinolytic markers. Endocrine 2009;2013:70–4 [DOI] [PubMed] [Google Scholar]
- 15.Dimopoulos M, Fernandez J, Samaan N. Paraneoplastic Cushing's syndrome as an adverse prognostic factor in patients who die early with small cell lung cancer. Cancer 1992;2013:66–71 [DOI] [PubMed] [Google Scholar]
- 16.Biller BM, Grossman AB, Stewart PM, et al. Treatment of adrenocorticotropin-dependent Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab 2008;2013:2454–62 [DOI] [PMC free article] [PubMed] [Google Scholar]


