Abstract
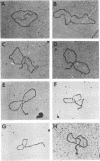
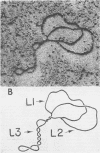
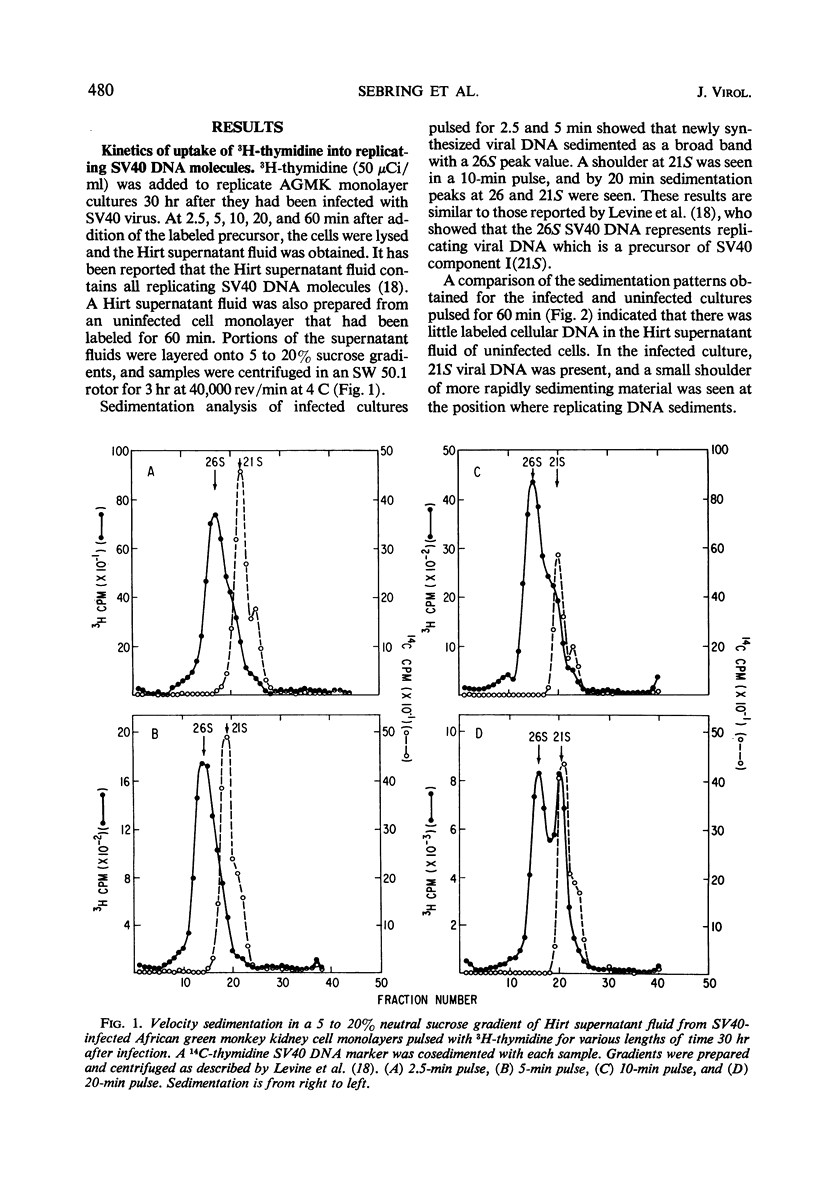
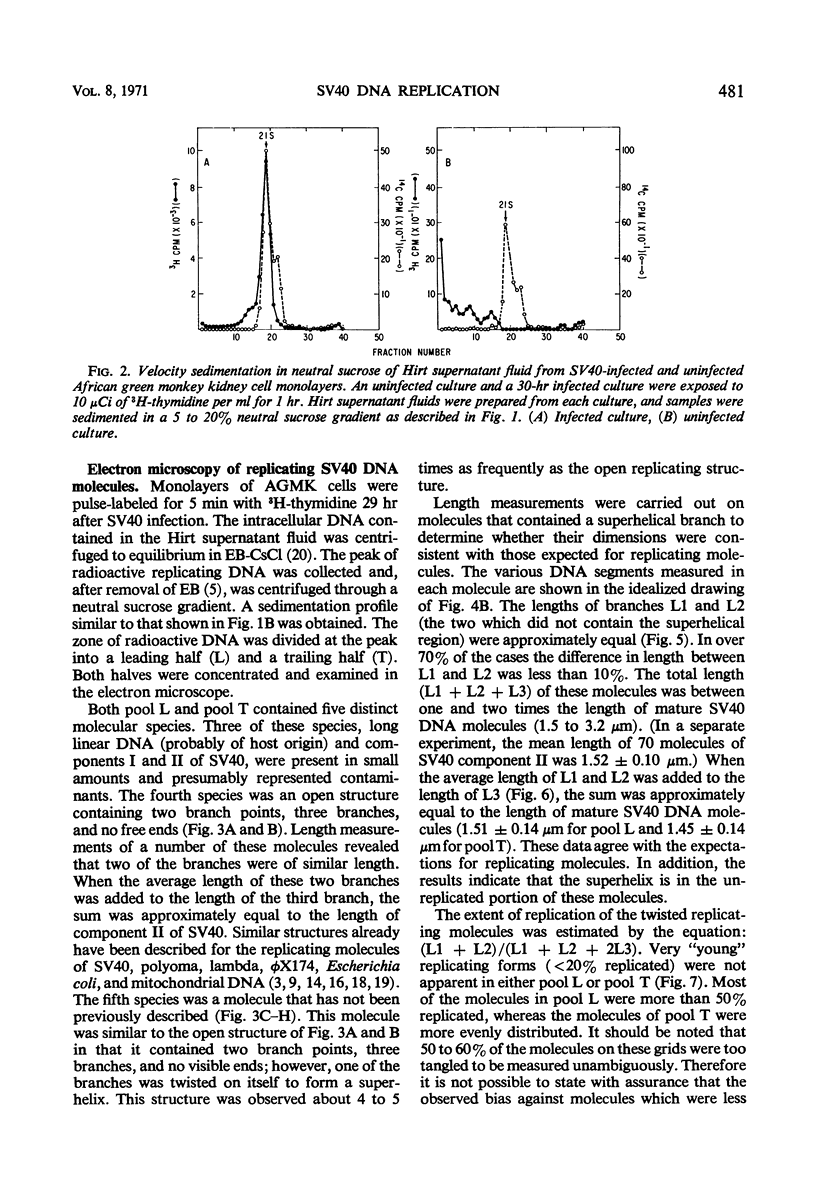
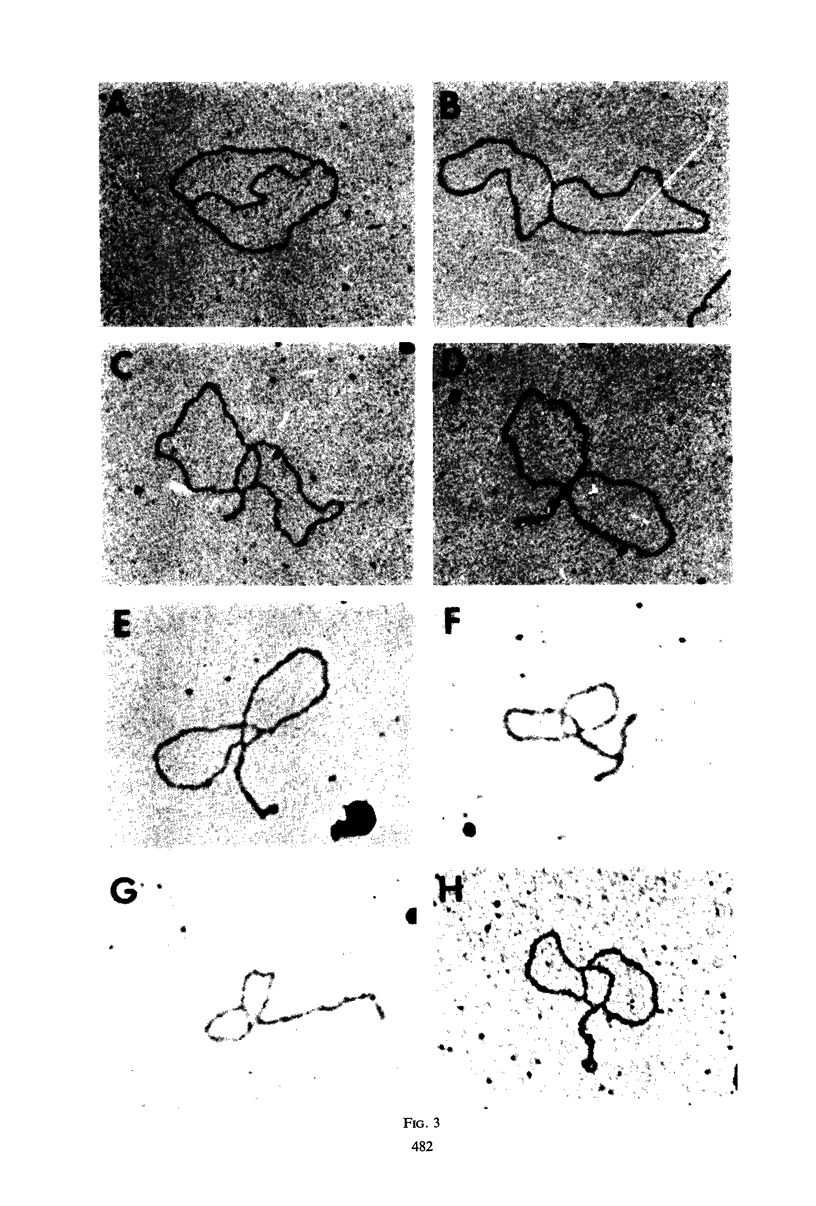
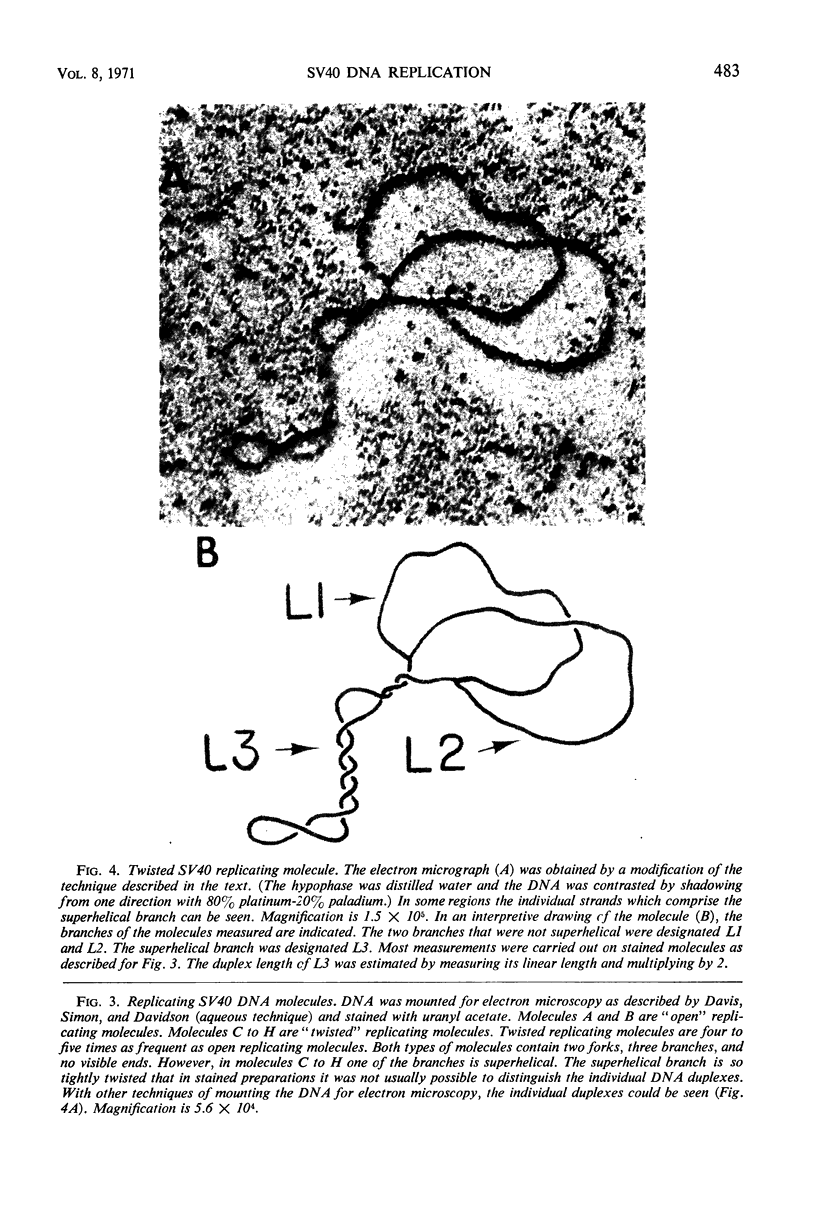
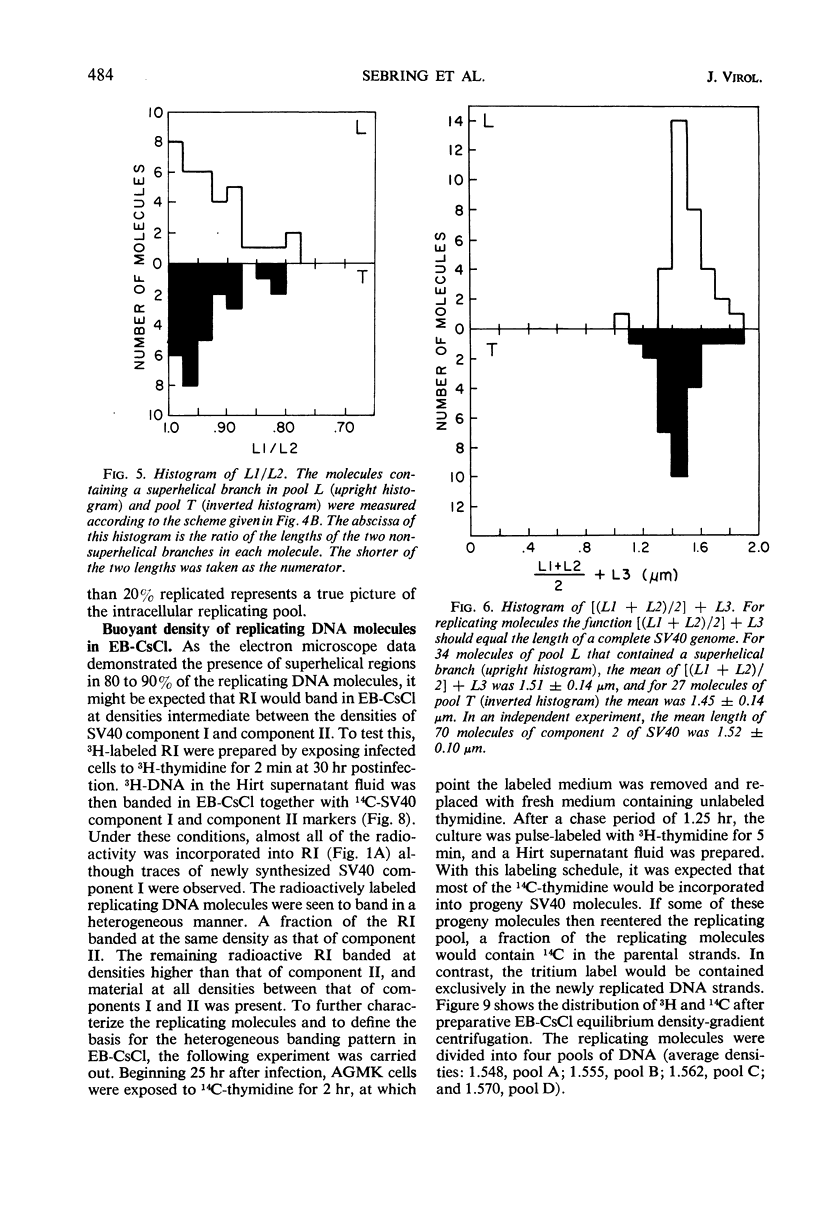
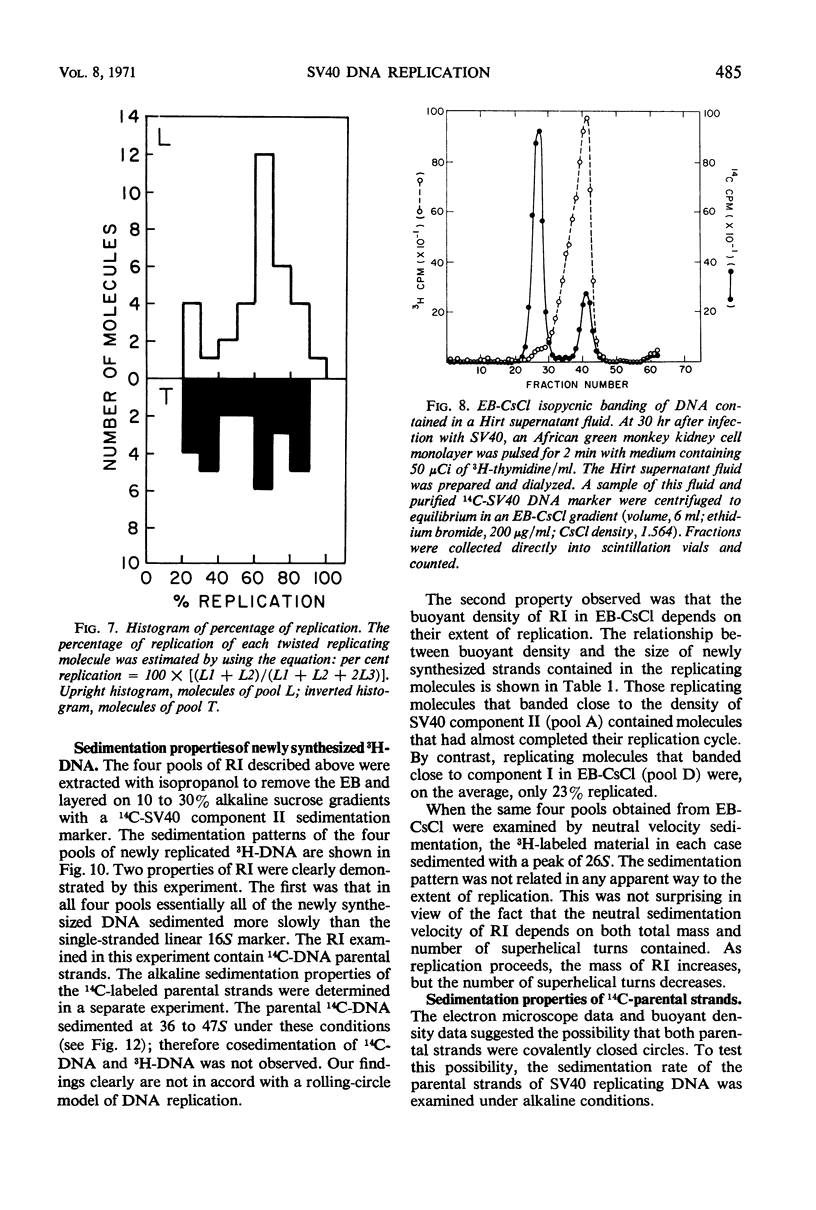
Properties of replicating simian virus 40 (SV40) deoxyribonucleic acid (DNA) have been examined by sedimentation analysis and by direct observation during a lytic cycle of infection of African green monkey kidney cells. Two types of replicating DNA molecules were observed in the electron microscope. One was an open structure containing two branch points, three branches, and no free ends whose length measurements were consistent with those expected for replicating SV40 DNA molecules. A second species had the same features as the open structure, but in addition it contained a superhelix in the unreplicated portion of the molecule. Eighty to ninety per cent of the replicative intermediates (RI) were in this latter configuration, and length measurements of these molecules also were consistent with replicating SV40 DNA. Replicating DNA molecules with this configuration have not been described previously. RI, when examined in ethidium bromide-cesium chloride (EB-CsCl) isopycnic gradients, banded in a heterogeneous manner. A fraction of the RI banded at the same density as circular SV40 DNA containing one or more single-strand nicks (component II). The remaining radioactive RI banded at densities higher than that of component II, and material was present at all densities between that of supercoiled double-stranded DNA (component I) and component II. When RI that banded at different densities in EB-CsCl were examined in alkaline gradients, cosedimentation of parental DNA and newly replicated DNA did not occur. All newly replicated DNA sedimented more slowly than did intact single-stranded SV40 DNA, a finding that is inconsistent with the rolling circle model of DNA replication. An inverse correlation exists between the extent of replication of the SV40 DNA and the banding density in EB-CsCl. Under alkaline conditions, the parental DNA strands that were contained in the RI sedimented as covalently closed structures. The sedimentation rates in alkali of the covalently closed parental DNA decreased as replication progressed. Based on these observations, some possible models for replication of SV40 DNA are proposed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgaux P., Bourgaux-Ramoisy D., Dulbecco R. The replication of the ring-shaped DNA of polyoma virus. I. Identification of the replicative intermediate. Proc Natl Acad Sci U S A. 1969 Oct;64(2):701–708. doi: 10.1073/pnas.64.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAIRNS J. The bacterial chromosome and its manner of replication as seen by autoradiography. J Mol Biol. 1963 Mar;6:208–213. doi: 10.1016/s0022-2836(63)80070-4. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V., BLACK P. H. THE NUCLEIC ACID OF SIMIAN VIRUS 40. Virology. 1964 Nov;24:388–392. doi: 10.1016/0042-6822(64)90176-x. [DOI] [PubMed] [Google Scholar]
- Cuzin F., Vogt M., Dieckmann M., Berg P. Induction of virus multiplication in 3T3 cells transformed by a thermosensitive mutant of polyoma virus. II. Formation of oligometric polyoma DNA molecules. J Mol Biol. 1970 Feb 14;47(3):317–333. doi: 10.1016/0022-2836(70)90305-0. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. EVIDENCE FOR A RING STRUCTURE OF POLYOMA VIRUS DNA. Proc Natl Acad Sci U S A. 1963 Aug;50:236–243. doi: 10.1073/pnas.50.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Hirt B. Replicating molecules of polyoma virus DNA. J Mol Biol. 1969 Feb 28;40(1):141–144. doi: 10.1016/0022-2836(69)90302-7. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Riggs A. D. On the mechanism of DNA replication in mammalian chromosomes. J Mol Biol. 1968 Mar 14;32(2):327–341. doi: 10.1016/0022-2836(68)90013-2. [DOI] [PubMed] [Google Scholar]
- KLEINSCHMIDT A. K., BURTON A., SINSHEIMER R. L. ELECTRON MICROSCOPY OF THE REPLICATIVE FORM OF THE DNA OF THE BACTERIOPHAGE PHI-X174. Science. 1963 Nov 15;142(3594):961–961. doi: 10.1126/science.142.3594.961. [DOI] [PubMed] [Google Scholar]
- Kiger J. A., Jr, Sinsheimer R. L. Vegetative lambda DNA. IV. Fractionation of replicating lambda DNA on benzoylated-naphthoylated DEAE cellulose. J Mol Biol. 1969 Mar 28;40(3):467–490. doi: 10.1016/0022-2836(69)90166-1. [DOI] [PubMed] [Google Scholar]
- Kiger J. A., Jr, Sinsheimer R. L. Vegetative lambda DNA. V. Evidence concerning single-strand elongation. J Mol Biol. 1969 Aug 14;43(3):567–579. doi: 10.1016/0022-2836(69)90359-3. [DOI] [PubMed] [Google Scholar]
- Kirschner R. H., Wolstenholme D. R., Gross N. J. Replicating molecules of circular mitochondrial DNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1466–1472. doi: 10.1073/pnas.60.4.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knippers R., Whalley J. M., Sinsheimer R. L. The process of infection with bacteriophage phiX174. XXX. Replication of double-stranded phiX DNA. Proc Natl Acad Sci U S A. 1969 Sep;64(1):275–282. doi: 10.1073/pnas.64.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark K. G. Initiation and control of DNA synthesis. Annu Rev Biochem. 1969;38:569–604. doi: 10.1146/annurev.bi.38.070169.003033. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Kang H. S., Billheimer F. E. DNA replication in SV40 infected cells. I. Analysis of replicating SV40 DNA. J Mol Biol. 1970 Jun 14;50(2):549–568. doi: 10.1016/0022-2836(70)90211-1. [DOI] [PubMed] [Google Scholar]
- Ogawa T., Tomizawa J. I., Fuke M. Replication of bacteriophage DNA, II. Structure of replicating DNA of phage lambda. Proc Natl Acad Sci U S A. 1968 Jul;60(3):861–865. doi: 10.1073/pnas.60.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Shatkin A. J. Polynucleotide ligase activity in cells infected with simian virus 40, polyoma virus, or vaccinia virus. J Virol. 1969 Nov;4(5):719–726. doi: 10.1128/jvi.4.5.719-726.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Position of branch points in replicating lambda DNA. J Mol Biol. 1970 Jul 14;51(1):61–73. doi: 10.1016/0022-2836(70)90270-6. [DOI] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Starting point and direction of replication in P2 DNA. J Mol Biol. 1971 Jan 14;55(1):31–38. doi: 10.1016/0022-2836(71)90278-6. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Ogawa T. Replication of phage lambda DNA. Cold Spring Harb Symp Quant Biol. 1968;33:533–551. doi: 10.1101/sqb.1968.033.01.061. [DOI] [PubMed] [Google Scholar]
- WEIL R., VINOGRAD J. THE CYCLIC HELIX AND CYCLIC COIL FORMS OF POLYOMA VIRAL DNA. Proc Natl Acad Sci U S A. 1963 Oct;50:730–738. doi: 10.1073/pnas.50.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG E. T., 2nd, SINSHEIMER R. L. NOVEL INTRA-CELLULAR FORMS OF LAMBDA DNA. J Mol Biol. 1964 Dec;10:562–564. doi: 10.1016/s0022-2836(64)80080-2. [DOI] [PubMed] [Google Scholar]