Abstract
Purpose
In chicks, ocular growth inhibition is associated with choroidal thickening and growth stimulation with choroidal thinning, suggesting a mechanistic link between the two responses. Because muscarinic antagonists inhibit the development of myopia in animal models by a non-accommodative mechanism, we tested the hypothesis that agonists would stimulate eye growth and thin the choroid. We also hypothesized that the effective growth-inhibiting antagonists would thicken the choroid.
Methods
Chicks, age 12–16 days, were used. In vivo: Agonists: Single intravitreal injections (20 µL) of oxotremorine (oxo), pilocarpine (pilo), carbachol (carb), or arecaidine (arec) were given to otherwise untreated eyes. A-scan ultrasonography was done prior to injections, and at 3, 24, 48 and 72 h. Antagonists: — 10D lenses were worn on one eye for 4 days. Atropine (atro), pirenzepine (pirz), oxyphenonium (oxy) or dicyclomine (dicy) were injected (20 µL) daily into lens-wearing eyes; saline injections were done as controls. Ultrasonography was done on d1 and on d4; on d4 measurements were done before and 3 h after injections.
In vitro
Paired eyecups of retinal pigment epithelium (RPE), choroid and sclera were made from 1-week old chicks. All drugs except atropine were tested on one eyecup, its pair in plain medium. Choroidal thickness was measured at various times over 48 h.
Results
Agonists: In vivo, oxotremorine caused an increase in the rate of axial elongation (drug vs saline: 24–72 h: 338 µm vs 250 µrn; p < 0.001). All except pilocarpine caused choroidal thinning by 24 h (oxo, carb and arec vs saline: −25, −35 and −46 µm vs 3 µm). In vitro, all agonists thinned choroids by 24 h (oxo: −6 vs 111 µm; pilo: 45 vs 212 µm; carb: −58 vs 65 µm; arec: 47 vs 139 µm; p < 0.05). Antagonists: Atropine, pirenzepine and oxyphenonium inhibited the development of myopia in negative lens-wearing eyes, and also caused choroidal thickening (drug vs saline: 42, 80, 88 vs 10 µm per 3 h). In vitro, pirenzepine thickened choroids by 3 h (77 vs 2 µm, p < 0.01).
Conclusions
Muscarinic agonists caused choroidal thinning in intact eyes and eyecups, supporting a role for acetylcholine in the choroidal response to hyperopic defocus or form deprivation. Only oxotremorine stimulated eye growth, which is inconsistent with a muscarinic receptor mechanism for antagonist-induced eye growth inhibition. The dissociation between choroidal thinning and ocular growth stimulation for the other agonists in vivo suggest separate pathways for the two.
Keywords: choroid, muscarinic, emmetropisation, eye growth
Introduction
Animal models have definitively shown that the eye uses visual feedback to guide its growth to achieve emmetropia, in which the length of the eye becomes matched to the front optics so that the image is focused on the retina (review: ref. 1). It is presumed that a signal cascade originating at the retina and involving the RPE and choroid results in changes in scleral extracellular matrix biosynthesis that determines the size of the eye.2–4 In chicks, the choroid plays an active role in emmetropisation by changing its thickness in response to retinal defocus, thickening to move the retina toward the image plane in the case of myopic defocus (image in front of the retina), and thinning to move it back in the case of hyperopic defocus (image behind the retina), thereby performing a temporary focusing mechanism that acts prior to the changes in scleral growth rates that alter the length of the eye.5,6 It has been hypothesized that the thickness of the choroid per se may influence scleral growth, either via a thickness-dependent secretion of growth factors, or by providing a mechanical barrier to the effects of growth factors from the retina or RPE, the efficacy of which may be thickness-dependent.7 If this is true, then determining the cellular and molecular mechanisms that mediate these changes in choroidal thickness would be crucial to elucidating this middle part of the signal cascade from retina to sclera.
The non-selective muscarinic antagonist atropine has been used clinically in parts of Asia since the 1970s to slow the progression of myopia in children.8–13 Its anti-myopiagenie effects were initially thought to be via its cycloplegic action, in keeping with the belief that excessive accommodation was the main stimulus driving the development of myopia, but this premise has been disproven by animal studies showing that atropine was effective in preventing form-deprivation myopia in chicks, whose ciliary muscle receptors are nicotinic,14–16 and in a non-accommodating mammal.17 Since then, the site of action of muscarinic receptor antagonists has been an issue of active debate, with about equal lines of evidence in support of a retinal site 18 vs non-retinal one.19–21 Another potential effector tissue is the choroid, the thickness of which is influenced by retinal defocus, as discussed above, and by drugs that alter ocular growth, such as dopamine agonists,22 and nitric oxide synthase inhibitors.23 To date, the effects of muscarinic antagonists on the choroid have not been tested.
The purpose of this study was two-fold. First, we tested the hypothesis that the visually-induced choroidal thinning in response to negative lens-wear or form deprivation may be mediated by a muscarinic cholinergic mechanism. Chick choroids contain both vascular and non-vascular smooth muscle 24–26 and the muscarinic receptor subtypes cm2, cm3 and cm4 27 have been reported throughout the tissue, although the staining was too diffuse to allow localisation to specific cell types. To address the first question, we examined the effects of four relatively non-selective muscarinic agonists on choroidal thickness in intact, non-device-wearing chick eyes and in eyecups of RPE, choroid and sclera. We also measured ocular growth rates in the intact eyes to ascertain whether any (or all) of the agonists stimulated eye growth, which would be expected if the growth-inhibiting effects of atropine and pirenzepine are indeed mediated via a muscarinic receptor mechanism.18 Second, we tested the effects of three muscarinic antagonists known to inhibit ocular growth in form deprived eyes,19 on chicks wearing negative lenses, to determine if the effects were similar in both paradigms, and to determine if the growth inhibitors caused choroidal thickening, which would be true if choroidal thickening was part of the signal cascade mediating ocular growth inhibition. We also tested dicyclomine, which was ineffective at growth inhibition in form-deprived eyes. Parts of this manuscript have been presented in Abstract form.28–31
Methods
Subjects
Subjects were White Leghorn chickens (Gallus gallus domesticus; Cornell University K-strain), hatched in an incubator and raised in temperature-controlled brooders. The light cycle was 12L/12D (in vivo experiments at the New England College of Optometry) or 14L/10D (in vitro experiments at The City College of CUNY). Food and water were supplied ad libitum. In all in vivo experiments, the right eye was treated and the left eye served as the untreated control. The concentrations of the drugs and the relative selectivities are shown in Table 1. Care and use of the animals conformed to the ARVO Resolution for the Care and Use of Animals in Research.
Table 1.
Receptor selectivity, doses, and concentrations of muscarinic agents in vivo and in vitro
|
In vivo |
In vitro |
|||||
|---|---|---|---|---|---|---|
| Category | Drugs | Affinities | Total dose in 20 µL | Concentration in syringe (mM) |
Concent, in vit chamber (mM)a |
Concent, in medium (mM)b |
| Muscarinic Agonists | Oxotremorine | Non-selective | 20 nmol | 1 | 0.07 | 0.6 |
| 200 nmol | 10 | 0.71 | ||||
| Pilocarpine | M3c35 | 200 nmol | 10 | 0.71 | 1.2 | |
| Carbachol | Non-selective | 20 nmol | 1 | 0.07 | 5.5 | |
| 200 nmol | 10 | 0.71 | ||||
| 2 µmol | 100 | 7.14 | ||||
| Arecaidine | M2c37 | 220 nmol | 11 | 0.78 | 2.3 | |
| Muscarinic Antagonists | Atropine | Non-selective | 18 nmol | 1 | 0.07 | – |
| Pirenzepine | M4> M2, M318 | 2 µmol | 100 | 7.14 | 5 | |
| Oxyphenonium | Non-selective | 0.2 µmol | 10 | 0.71 | 1 | |
| Dicyclomine | Non-selective | 0.2 µmol | 10 | 0.71 | 0.6 | |
The concentration in the vitreous chamber was calculated assuming the volume of the vitreous chamber to be about 260 µL
These concentrations were chosen to approximate the concentrations used in in vivo experiments.
Indicates that there is some evidence for relative selectivity in some systems.
In vivo experiments
Agonists in ‘untreated’ eyes
Chicks, aged 12–16 days, had a single 20 µL intravitreal injection into the right eye, around mid-morning, under isoflurane inhalation anaesthesia (1.0% in oxygen). Using a Hamilton syringe with a 30G needle, injections went through the skin of the lids over the superior temporal sclera, after removing the feathers and cleaning the skin with alcohol. The needle remained in place for 30 s before being slowly withdrawn while the skin around the site was held tightly together using a small forceps to minimise leakage.
All four agonists used are relatively non-selective, and the pharmacology has not been characterised in the eyes of chicks. However, there is evidence for action on smooth muscle for all of them. (1) Oxotremorine: (20 nmol, n = 11; 200 nmol, n = 10; data combined; Tocris). Doses were based on Matsumoto et al.32; our lowest dose is about 10-fold higher than the EC50. There is evidence for smooth muscle activation.33 (2) Pilocarpine: (200 nmol; n = 14; Sigma, www.sigmapharmceuticals.com). The dose used was approximated from Schwahn et al.34; our dose is about 10× higher than theirs. There may be some M3 selectivity,35 and there is evidence for smooth muscle activation.36 (3) Carbachol: (20 nmol, n = 6; 200 nmol, n = 21; 2 umol, n = 12; Sigma). Doses were approximated from Stone et al.14 and Lind et al.20 Our lowest dose is approximately the highest used by Stone et al., which in their hands did not affect ocular growth; our middle dose was the highest used by Lind et al.20, which had no effect on scleral glycosaminoglycan synthesis. The data shown in figures 2 and 3 are from the middle dose; the other doses were used to generate a dose-response curve. Carbachol is non-selective. (4) Arecaidine: (220 nmol; n = 10; Sigma). The dose chosen was that found to be effective for oxotremorine and carbachol. In a pilot study, 22 nmol was ineffective (data not shown). There may be some M2 selectivity, and there is evidence for smooth muscle activation.37 Saline injections (20 µL; n = 32) were done as controls in all experiments, and these data were combined. Axial dimensions were measured using high-frequency A-scan ultrasonography (details in 38) prior to the injections, and at 3, 24, 48 and 72 h later. For oxotremorine, a measurement was also done at 96 h.
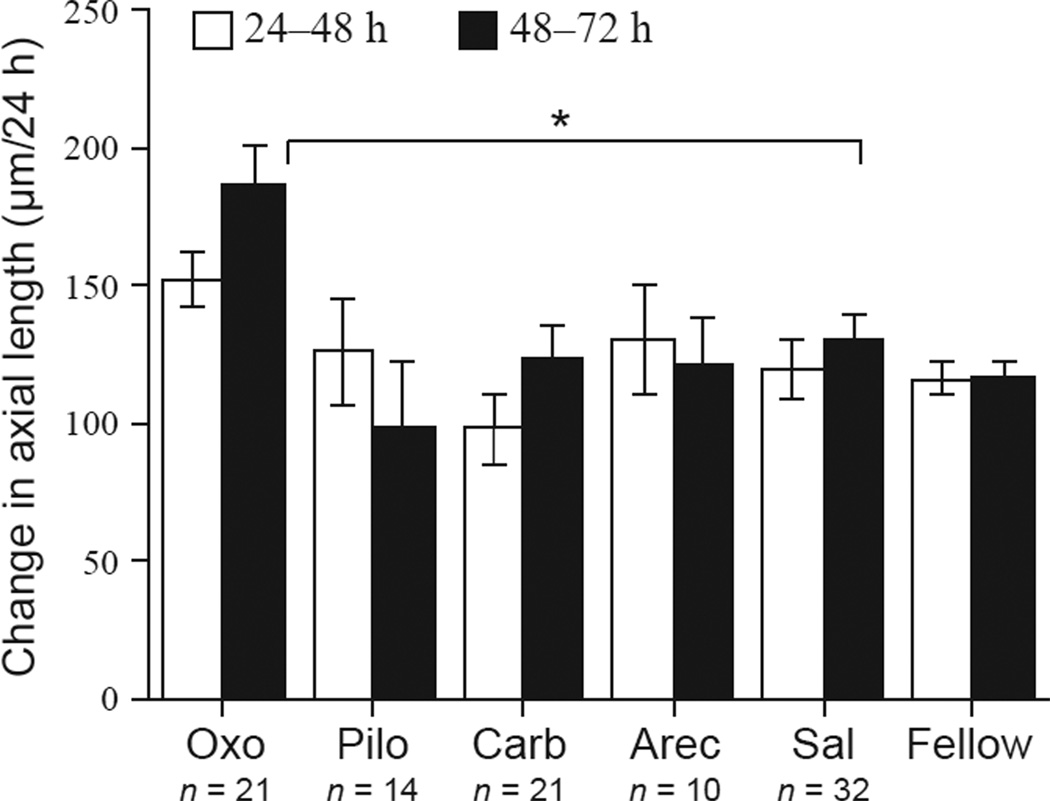
Figure 2.
Change in axial length in ′normal′ eyes injected with agonists, from 24–48 h (white bars) and 48–72 h (black bars). Numbers of animals are noted. Oxo: oxotremorine; Pilo: pilocarpine; Carb: carbachol; Arec: arecaidine; Sal: saline. ′Fellow′ denotes all untreated fellow eyes from all groups in all graphs. Error bars are standard errors of the mean in all graphs. *p < 0.0B.
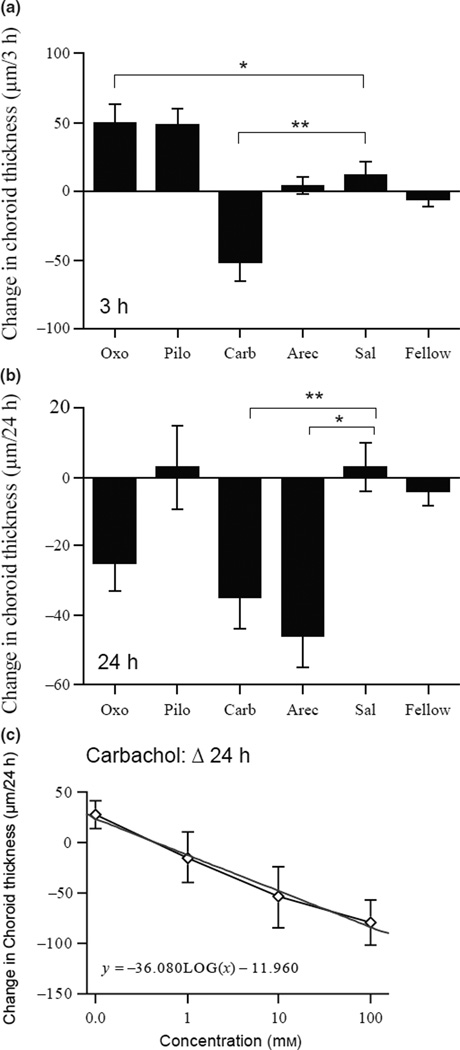
Figure 3.
Changes in choroidal thickness in eyes injected with agonists. (a) Changes in choroidal thickness 3 h after the injection. Note that oxotremorine and pilocarpine induced transient increases in choroidal thickness over this interval. (b) Changes in choroidal thickness 24 h after the injection. (c) Dose-response curve for carbachol at 24 h. Straight line is the linear fit to the data. *p < 0.05; **p < 0.005.
For all measurements, chicks were lightly anesthetised with isoflurane inhalation anaesthesia (1.0% in oxygen). Axial length is defined as the distance from the front of the cornea to the choroidal/scleral interface (front of sclera), and so reflect actual changes in eye length and not vitreous chamber depth. All data on ocular growth refer to the changes in axial length per unit of time, and so reflect growth rates of the eyes. All data on choroidal thickness refer to changes in thickness per unit time. Refractive errors were measured using a Hartinger’s refractometer (details in 39) at the end of the experiment.
Antagonists in eyes wearing negative lenses
At 12–16 days of age, −10 D lenses mounted on Velcro rings were attached to the matching ring that was glued to the feathers around one eye. At noon of each day for 4 days, intravitreal injections were given and the lenses were then replaced. We tried to use the same injection site for subsequent injections. The injections were 20 µL of the following drugs (dose): (1) Atropine (non-selective; 18 nmol, n = 10), (2) Pirenzepine (M4 > M2, M318; 2 µmol, n = 10), (3) Oxyphenonium (non-selective; 0.2 µmol, n = 13) and (4) Dicyclomine (non-selective; 0.2 µmol, n = 6). We chose three antagonists that were effective growth inhibitors, and dicyclomine, which was ineffective.19 For atropine, we used the sub-maximal dose reported by Schmid and Wildsoet to inhibit both lens-induced- and form-deprivation-induced myopia by approximately 50–60%.40 Doses chosen for the other three antagonists were the lower doses used by Luft et al.19 All antagonists were obtained from Sigma. Saline injections (n = 16) were done as controls. Axial dimensions were measured using A-scan ultrasonography at the start of lens wear, on day 4 immediately prior to the injections, and then again 3 h later, to assess any short term effects on the choroid. Refractive errors were measured at the end of the experiments using a Hartinger’s refractometer under isoflurane inhalation anaesthesia.
In vitro experiments
Dissections and measurements of choroidal thickness
The paired eyes of 1-week-old chicks were hemisected to make eyecups, and the retinas and vitreous removed. To retain the retinal pigment epithelium (RPE), we let 10 min elapse post-mortem before dissection. CO2-independent medium (Gibco) kept on ice, was used for the dissection, because it maintains a neutral pH in room air. Eyecups remained in medium for a maximum of 2 h during which we made the first measurements prior to drug exposure.
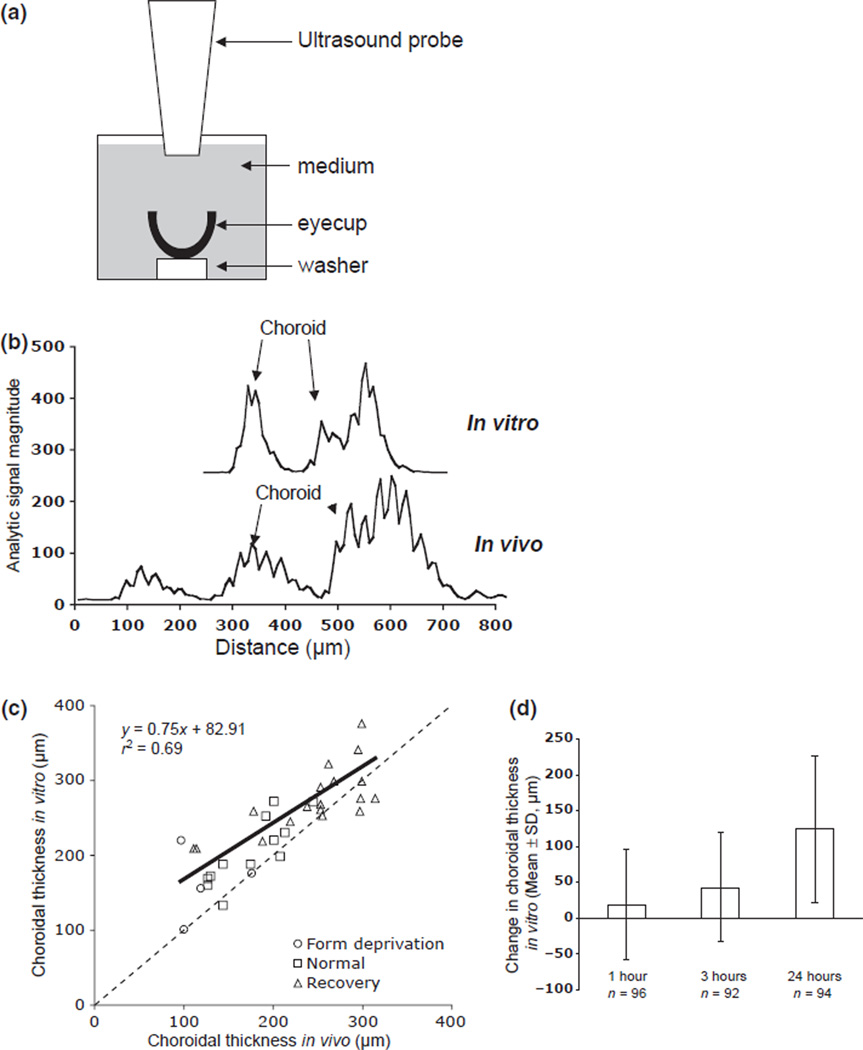
To measure choroidal thickness, eyecups were centred on plastic washers placed into wells of 12-well tissue culture plates; each well contained 5 mL medium. The ultrasound (same specifications as above) probe was positioned vertically, its tip was submerged in the medium and centred in the eyecup (Figure 1a). Choroidal thicknesses were measured at time 0, prior to drug exposure, and at 3 and 24 h. We had previously ascertained that choroidal thickness could be measured reliably in vitro by doing a repeated-measures study of 34 eyes, in which choroids were measured first in vivo and then in vitro (S.D. from repeated measurements = 9 µm; typical traces are shown in Figure 1b;31). To generate a range of thicknesses, 17 one-week-old chicks were either binocularly form-deprived (n = 2), previously form-deprived (n = 9), or untreated (n = 6), to provide thinner than normal, thicker than normal, and normal choroids, respectively. The eyes were measured before sacrifice and about 2 h after dissection. There was a significant positive correlation between the in vitro and in vivo measurements (Figure 1c: r2 = 0.69, p < 0.0001), despite a small amount of thickening that occurs in vitro over the 2 h (30.59 ± 37.69 µm, mean ± S.D.), probably due to the absence of intraocular pressure.
Figure 1.
Ultrasound measurements in vitro. (a) Schematic of the ultrasound set-up for the eyecup preparation in culture wells. (b) Sample ultrasound traces of an eyecup in vitro (top) and an intact eye in vivo (bottom), showing position of peaks used for choroidal thickness, and distance. Note that the 0 value on the x axis is an arbitrary starting point for distance; the front peaks in the intact eye traces have been removed. (c) Correlation between choroidal thickness measured in vivo and in vitro, 2 h post-mortem (r2 = 0.69, p < 0.0001). To generate a wide range of choroidal thicknesses, eyes were form-deprived (thin choroids), untreated (normal), or recovering from form-deprivation (thick choroids). Note that choroidal thickness in vitro is generally thicker than that measured in vivo, especially for thin choroids. (d) Change in choroidal thickness in eyecups cultured in plain medium over time.
In all experiments, one eyecup of each pair was cultured in medium (L-15) with the drug (treated eyecups), and the other in plain medium (untreated eyecups), at 37° in 5% CO2. Viability of the tissue was verified by the fact that glycosaminoglycan synthesis in the choroids after 24 h in culture was similar to that found in freshly harvested tissue (data not shown). Initial choroidal thicknesses were matched for each pair to control for possible differential effects as a function of ‘starting’ thickness. All drug effects were compared to their matched medium-only controls. There was some inter-experiment variability in the effect of culture conditions on control choroids, but in general, choroidal thickness increased with time. The mean changes were: 1 h = 19 µm (S.D. = 77 µm; n = 96), 3 h = 43 µm (S.D. = 76; n = 92), 24 h = 124 µm (S.D. = 103 µm; n = 94) (Figure 1d). The following drugs were tested (molar concentration in medium): Agonists: Oxotremorine (0.6 mM; n = 20), Pilocarpine (1.2 mM; n = 6), Carbachol (5.5 mM; n = 18) and Arecaidine (2.3 mM; n = 12). Antagonists: Pirenzepine (5 mM; n = 17), Oxyphenonium (1 mM; n = 13) and Dicyclomine (0.6 mM; n = 6). Atropine was not tested because we were interested in the relatively more selective drugs. Concentrations were chosen to approximate the vitreous concentrations in the in vivo experiments: The vitreous volume of one-week-old chicks was estimated to be 260 µL, therefore injecting 20 µL would cause an approximate 1:14 dilution.41
Statistics
For the in vivo data, the test for equal variances showed significance in one parameter, the anterior chamber depth for the antagonists (Table 2). We therefore used the more conservative non-parametric version of one-way anova (Kruskal–Wallis) with Bonferroni correction for post-hoc comparisons between any treatment groups and the saline group. For the other parameters, classical one-way anova was applied. The Dunnett’s corrections for comparisons between treatment groups and the saline group were used when the overall anova showed significant difference across groups. For the in vitro data, two-tailed Student’s t-tests are used to compare pairs of eyecups.
Table 2.
anova and Equal Variance p-values
| Group | Parameter |
|||
|---|---|---|---|---|
| Agonists | Axial 24–48 h |
Axial 48–72 h |
Choroid 3 h |
Choroid 24 h |
| anova | 0.0425 | 0.0028 | <0.001 | 0.0018 |
| Equal variance | 0.166 | 0.769 | 0.503 | 0.563 |
| Antagonists |
Axial 4 days |
Ant Cha 4 days |
Ref 4 days |
Choroid 3 h |
| anova | <0.001 | 0.0005 | <0.0001 | 0.0001 |
| Equal variance | 0.364 | 0.0099 | 0.619 | 0.099 |
Ant Cha, anterior chamber depth; Axial, axial length; Ref, refractive error.
Results
Agonists
In vivo
We tested four relatively non-selective muscarinic agonists for their effects on ocular growth and choroidal thickness in intact ‘normal’ eyes (Figure 2). All injected eyes, including saline controls, showed an ‘injection effect’ at 24 h, with eyes exhibiting lower-than normal growth compared to non-injected fellow eyes (data not shown). This effect was gone after the initial 24 h (compare ‘fellow’ to ‘saline’ for both intervals; 24–48 h, p = 0.76; 48–72 h, p = 0.26; two-sample Student’s t-test). Only oxtremorine had a significant effect on eye growth: on the third day (48–72 h) oxotremorine caused an increase in the rate of axial elongation compared to saline controls (Figure 2: anova p = 0.0028; 186 µm vs 130 µm; p = 0.012). Despite that the growth difference was not significant between 24–48 h (p = 0.26), the growth rate over the two-day period from 24–72 h was still significantly higher (338 µm vs 250 µm; p < 0.05; data not shown). There was no effect on the growth of the anterior chamber (drug vs saline at 48–72 h: 59 µm vs 45 µm; p = 0.55), therefore the change in axial elongation occurred in the posterior eye (lens to sclera). By 72–96 h, the growth rate in the oxotremorine eyes had returned to normal (168 µm vs 147 µm; p = 0.41). There was no effect on refractive error.
These agonists had varying effects on the choroids. Within 3 h of the injection, carbachol caused a significant thinning (Figure 3a: drug vs saline: −52 µm vs 11 µm per 3 h; p = 0.002; anova, p < 0.001). Interestingly, oxotremorine caused the opposite effect over this interval; choroids thickened instead of thinning (50 µm vs 11 µm per 3 h; p = 0.046). Pilocarpine also tended to cause thickening, but the difference was not statistically significant using the Dunnett’s test (48 µm vs 11 µm per 3 h; p = 0.23). By 24 h, choroids in both the carbachol- and arecaidine-injected eyes became significantly thinner than saline controls (Figure 3b: −35 and −46 µm vs 3 µm per 24 h; p = 0.004; p = 0.033 respectively; Dunnett’s correction); the initially thicker choroids in oxotremorine-injected eyes showed a similar trend (−25 µm vs 11 µm; p = 0.074; Dunnett’s correction; t-test 0; p = 0.005). There was no significant change in eyes injected with pilocarpine. The effect of carbachol on the choroids at 24 h was dose-dependent (Figure 3c). By 48 h after the injection, choroidal thickness had returned to baseline; there was no longer any difference between experimental and control groups (anova, p = 0.64).
In vitro
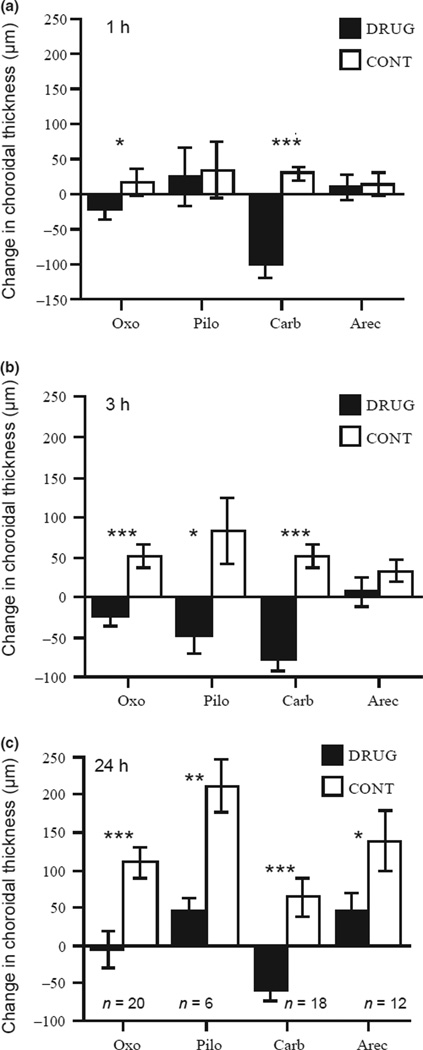
At 1 h of culture, both oxotremorine and carbachol resulted in significant choroidal thinning compared to those in medium controls (Figure 4a: Oxo: −23 µm vs 16 µm,p < 0.05; Carb: −100 µm vs 29 µm,p < 0.001). By 3 h, pilocarpine-treated choroids also became thinner than normal (Figure 4b: −48 µm vs 84 µm, p < 0.05) while choroids in oxotremorine and carbachol remained thin (−23 µm and −77 µm respectively; p < 0.001 for both comparisons with controls). At 24 h, all four agonists, including arecaidine, showed significant thinning (Figure 4c: Oxo: −6 µm vs 111 µm, p < 0.001; Pilo: 45 µm vs 212 µm, p < 0.01; Carb: −58 µm vs 65 µm, p < 0.001; Arec: 47 µm vs 139 µm, p = 0.05). The effects of both carbachol and oxtoremorine lasted for at least 48 h (Carb: −96 µm vs 62 µm, p < 0.01; Oxo: 8 µm vs 120 µm, p < 0.001; data not shown). To summarise, 3 of the 4 agonists, oxotremorine, carbachol and arecadinine, thinned choroids both in intact eyes and in eyecups; pilocarpine had no effect in intact eyes but thinned choroids in vitro.
Figure 4.
Changes in choroidal thickness in eyecups exposed to agonists (black bars) and paired medium-controls (white bars). (a) Changes in thickness after 1 h of culture. (b) Changes in thickness after 3 h of culture. (c) Changes in thickness after 24 h of culture, with numbers of eye-cups for all drugs. Note that choroidal thickness in controls increases over time in culture, probably because of the absence of intraocular pressure. Abbreviations same as in figure 3. *p < 0.05; **p < 0.01; ***p < 0.001.
Antagonists
In vivo
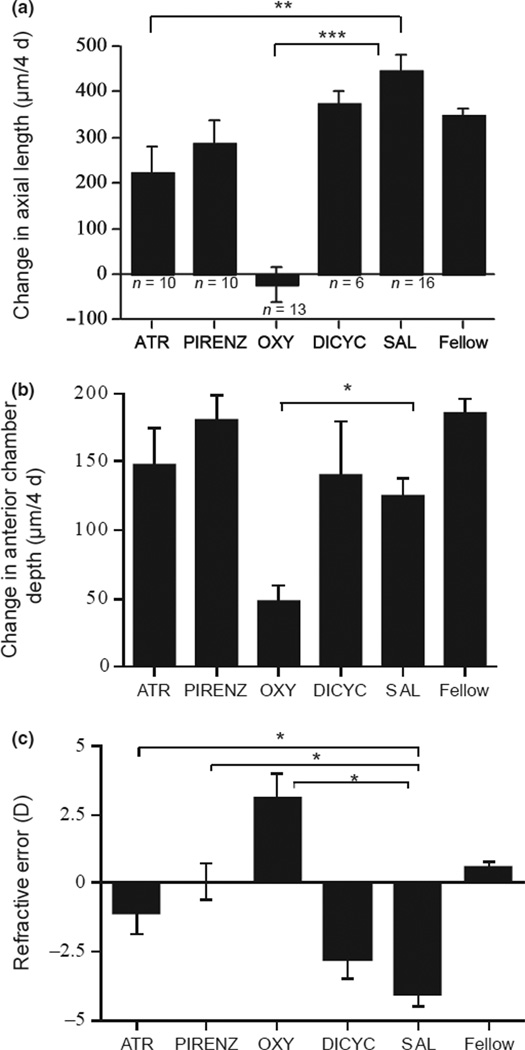
We tested four muscarinic antagonists for their effects on ocular growth rate and choroidal thickness in eyes wearing −10 D lenses. As expected from a study of these drugs on form-deprived eyes,19 atropine, pirenzepine and oxyphenonium inhibited ocular growth compared to saline controls (Figure 5a: 223, 290, and −23 µm vs 447 urn per 4 days; p = 0.005, p = 0.082, p < 0.0001, respectively; Dunnett’s correction). Dicyclomine was ineffective (drug vs saline: 396 µm vs 447 µm per 4 days). For atropine and pirenzepine, the effect was on the posterior eye (back of lens to front of sclera), because the growth of the anterior chamber was not affected (Figure 5b: 148 and 181 µm vs 125 µm per 4 days; p > 0.05). By contrast, oxyphenonium inhibited both anterior chamber growth (Figure 5b: 48 µm vs 125 µm per 4 days; p = 0.016) in addition to inhibition of posterior eye growth (lens to sclera: −87 µm vs 130 urn; p < 0.005; data not shown). This axial growth inhibition by atropine, pirenzepine and oxyphenonium reduced the development of refractive myopia (Figure 5c: anova, p < 0.001; respectively vs saline: −1.1, 0.1, 3.1 D vs −4.1 D; p < 0.05 for all). Dicyclomine had no effect on refractive error (−2.8 D vs −4.1 D).
Figure 5.
Changes in ocular dimensions and refractive errors in eyes wearing −10 D lenses and injected with antagonists for 4 days. (a) Changes in axial length, with numbers of animals. (b) Changes in anterior chamber depth. Note that oxyphenonium inhibits the growth of the anterior chamber. (c) End refractive errors. ATR: atropine; PIRENZ: pirenzepine; OXY: oxyphenonium; DICYC: dicyclomine; SAL: saline. *p < 0.05; **p < 0.005; ***p < 0.0005.
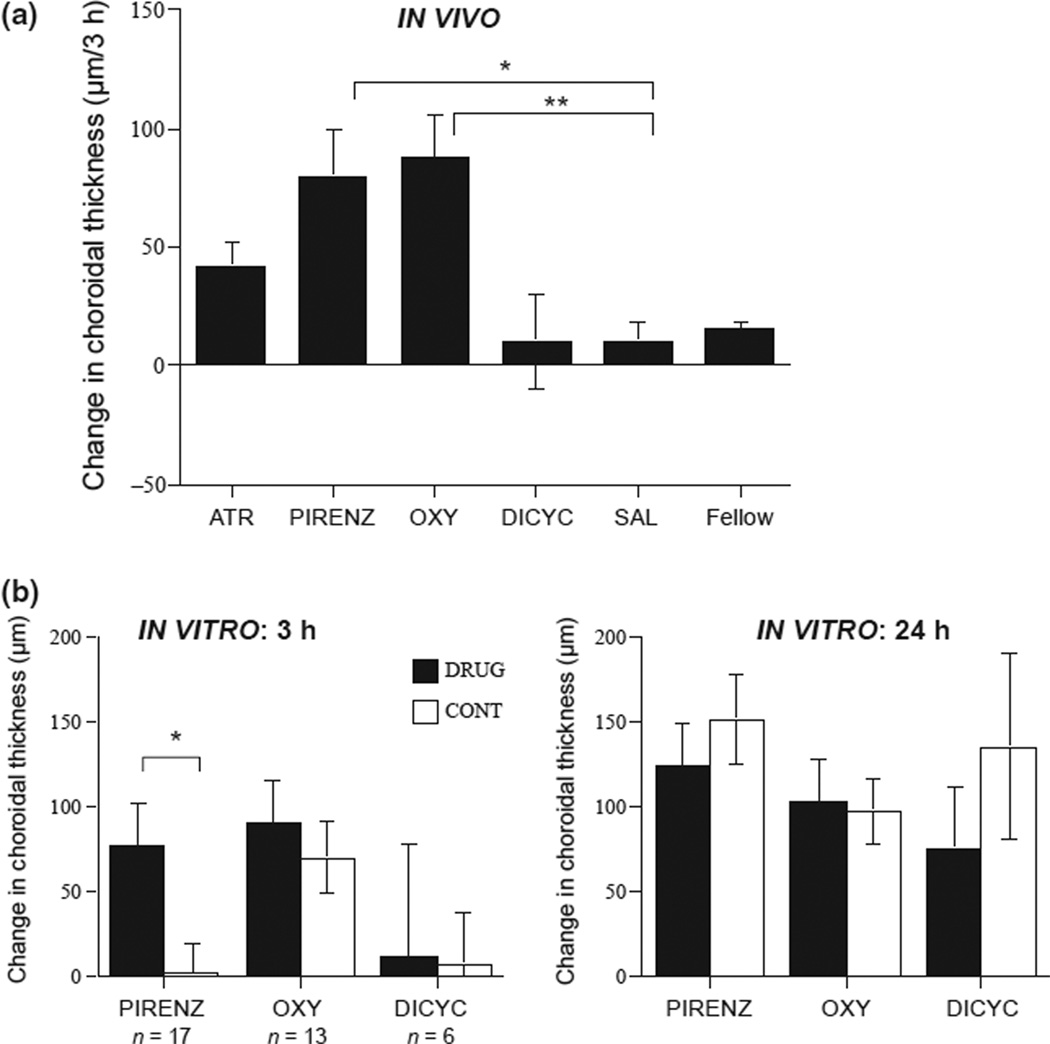
Our novel finding is that the three antagonists that effectively inhibited eye growth: atropine, pirenzepine and oxyphenonium, all caused increases in choroidal thickness by 3 h compared to saline controls (Figure 6a: 42 µm, 80 µm, 88 µm vs 10 µm per 3 h; p = 0.075, p = 0.0002, p < 0.0001 respectively; Dunnett’s correction), while the ineffective dicyclomine had no effect (10 µm vs 10 µm; p = 0.98).
Figure 6.
(a) Changes in choroidal thickness in intact eyes injected with antagonists for 4 days, and in eyecups cultured with antagonists. (a) Changes in choroidal thickness 3 h after the injection of antagonists on day 4, in eyes wearing negative lenses. (b) Changes in choroidal thickness in eyecups cultured with antagonists, for 3 h (left, black bars), and 24 h (right, black bars) compared to paired medium controls (white bars); ns are noted. Abbreviations are the same as in Figure 5. *p < 0.0005; **p < 0.0001.
In vitro
Similar to its effect in vivo, pirenzepine produced transient but robust choroidal thickening after 3 h of culture {Figure 6b, left: 77 urn vs 2 µm, p < 0.01) that was gone by 24 h (Figure 6b, right). Neither oxyphenonium (which was effective in vivo) nor dicyclomine (which was ineffective) had any effect in eyecups.
Discussion
We found that four muscarinic agonists, oxotremorine, pilocarpine, carbachol and arecaidine, produced choroidal thinning in eyecups without retinas; three of the four (not pilocarpine) caused thinning in intact eyes as well. Only oxotremorine stimulated eye growth. These results support a muscarinic mechanism in the visually-induced choroidal thinning that occurs in response to form deprivation and/or negative lenses, that does not require the retina. The lack of effectiveness of 3 non-selective agonists on eye growth is inconsistent with a muscarinic mechanism underlying the antagonist-induced ocular growth inhibition. Furthermore, three antagonists known to inhibit ocular growth in form deprived eyes 19 also inhibited growth in negative-lens-wearing eyes; they also caused a transient choroidal thickening, which is consistent with a link between the choroidal and scleral effects. Only pirenzepine thickened choroids in vitro. Taken together, our results suggest that (1) Choroidal thinning may be mediated by a muscarinic cholinergic mechanism that does not require the retina, and may or may not involve the RPE. (2) Choroidal thinning and ocular growth stimulation constitute separate responses to lens-induced hyperopic defocus and/or form deprivation. (3) Antagonist-induced choroidal thickening and ocular growth inhibition may be part of the same pathway.
Choroidal thinning may be mediated by a muscarinic mechanism
The choroid responds to hyperopic defocus and form deprivation by thinning; in the case of defocus it is a compensatory response, moving the retina toward the image plane. Our finding that four non-selective muscarinic agonists caused choroidal thinning in eyecups without retinas is consistent with a muscarinic mode of action on either the RPE or the choroid, both tissues of which reportedly express muscarinic cholinergic receptors.27 The rapidity of the response, with carbachol, oxotremorine and pilocarpine acting between 1–3 h, is consistent with a muscular mechanism,6,42 and is coherent with the time course (hours) for the thinning that occurs in response to both visual manipulations in vivo.43 The thinning effect in intact eyes is generally slower (except for carbachol), possibly due to the retina, or a more intact RPE, acting as a barrier through which the drugs must traverse to reach the target tissue.
We propose that these four cholinomimetics, all of which show evidence for action on smooth muscle, stimulate the non-vascular smooth muscle (NVSM) that spans the stroma of the choroid in birds, rabbits, and primates.24–26 In birds, NVSM are found in the suprachoroid and vascular layers, perpendicular to the surface, making them plausible effectors of the vision-induced changes in stromal thickness.6 Bird choroids contain cm2 and cm3 receptors,27 orthologues of M2 and M3, which predominate on mammalian smooth muscle,44–46 and cm3 receptors are found in bird ileal smooth muscle.47 While no data currently exist for the type of receptors expressed on choroidal NVSM, indirect evidence for cholinergic activation comes from a study showing that stimulation of explant ciliary postganglionics caused contraction of choroids that was blocked by atropine.24 Whether our effect is via a direct action on the choroid, or an indirect one via the RPE (which contains muscarinic receptors27) awaits experiments on eyecups without RPE.
Pilocarpine was the only drug that showed inconsistent effects between intact eyes and eyecups: In intact eyes, choroids initially tended to thicken before returning to baseline, while in eyecups they thinned within 3 h. Oxotremorine also caused an initial choroidal thickening in vivo, but, in contrast to the effect of pilocarpine, these choroids proceeded to become thinner than saline controls over the following 20 h. We speculate that the initial thickening induced by both drugs in intact eyes was mediated by an increase in aqueous outflow facility and concurrent drop in IOP, a clinically-used effect of pilocarpine.48,49 Alternatively, perhaps both drugs induced an increase in uveoscleral outflow, leading to an increase in choroidal thickness (although in monkeys, pilocarpine reduces uveoscleral outflow 50). That neither drug caused thickening in eyecups is consistent with either hypothesis. On the other hand, the pilocarpine-induced choroidal thinning in eyecups but not in intact eyes suggests either (1) the dose in vivo was too low to reach the choroid in effective amounts, or (2) its effect on the retina in intact eyes differed from that of the other drugs (this argument is only valid if the retina is part of the signal effecting thinning, a notion that the rest of the data contradicts). Specifically, this would imply that pilocarpine initiated a retina-mediated signal cascade that prevented choroidal thinning.
Ocular growth stimulation and choroidal thinning are likely via separate pathways
Form deprivation and hyperopic defocus both result in excessive ocular elongation and choroidal thinning, but it is uncertain whether the two effects are part of the same signal pathway, or whether they constitute separate responses to both stimuli. Our finding that of the four non-specific agonists, only oxotremorine increased ocular growth rate argues against a muscarinic mechanism mediating this growth enhancing effect, and by extension, is inconsistent with a muscarinic receptor mechanism being involved in the antagonist-induced growth inhibition. However, it is also possible that the ineffectiveness of the three agonists might be due to pharmacokinetic differences between them and oxotremorine, such as shorter half-lives, or to differences in their ability to diffuse from the vitreous to the site of action. While the mode of action of anti-muscarinics is currently under debate,21 several previous findings support a non-muscarinic role. First, higher doses of atropine and pirenzepine than would be indicative of a receptor-mediated mechanism are required to inhibit the development of myopia.19’51 Second, pirenzepine inhibits proteoglycan synthesis in isolated scleral chondrocytes in the absence of a cholinergic ligand,20 suggesting that perhaps it is working via another pathway altogether. Third, eliminating retinal cholinergic amacrine cells in chicks had no effect on the development of myopia, or on atropine’s anti-myopiagenic action.52 All these findings are supportive of a non-retinal site of action for these drugs as well.
Together these results suggest that ocular growth stimulation and choroidal thinning are two separate and distinct responses to form deprivation and/or negative lens wear, mediated by two separate mechanisms, the choroidal one muscarinic, and the one that influences eye growth non-muscarinic. Other studies consistent with separate mechanisms are the dissociation between the two responses that occurs when negative lenses are worn for very brief periods a few times per day in otherwise darkness: ocular growth rate does not increase, but the choroid still thins.53’54 Similarly, in eyes with double lesions of the parasympathetic pathways (ciliary ganglionectomy and N. VII to the pterygopalatine ganglia), form deprivation causes ocular growth inhibition rather than stimulation, but choroidal thinning remains unaffected.55
Ocular growth inhibition and choroidal thickening may be part of the same pathway
The growth inhibiting effects of the anti-muscarinics atropine and pirenzepine are well documented in animal models, but as already stated, the mode of action and effector tissue site(s) are as yet unknown. In our study in intact chicks, the three muscarinic antagonists that inhibited ocular elongation in response to form deprivation,19 atropine, pirenzepine and oxyphenonium, also did so in response to negative lenses, suggesting a similar mode of action for these drugs in the two paradigms, similar to that previously found for atropine.40,51 Furthermore, these antagonists also caused rapid, transient choroidal thickening, while the ineffective antagonist dicyclomine did not. This association between choroidal thickening and ocular growth inhibition is consistent with the hypothesis that the two might be mechanistically linked,7,56 despite the increasing evidence (above) that its converse, choroidal thinning and ocular growth stimulation, are not linked. A similar result is found with dopaminergics: the D2 receptor agonists that cause growth inhibition also cause transient choroidal thickening, while the Dl agonists, which do not affect eye growth, do not.22 Transient choroidal thickening is also found in response to various visual manipulations that retard eye growth, such as brief periods of vision or brief stroboscopic stimulation in form-deprived or negative lens-wearing eyes.57 We propose that choroidal thickening is part of the same pathway mediating growth inhibition, while by contrast, choroidal thinning is not part of the pathway mediating growth stimulation. Of course, these correlative findings are not definitive proof of either supposition.
In eyecups, only pirenzepine resulted in choroidal thickening, which was rapid and transient (within 3 h). If this effect is mediated by a muscarinic mechanism, the question of the ligand source is problematic. Neither RPE nor choroid is presumably cholinergic, so if pirenzepine is indeed acting as a muscarinic antagonist here, the source should be elsewhere: it is possible that axon terminals of parasympathetic origin remain in eyecups and ‘leaked’ the ligand that was antagonized by pirenzepine. This would be consistent with the transience of the effect. Alternatively, pirenzepine may be acting via a non-muscarinic mechanism, as has previously been suggested.19,20 We speculate that choroidal thickening and thinning are controlled by different mechanisms, with thinning via contraction of NVSM by acetylcholine, and thickening by another system, possibly dopaminergic21 or nitrergic56 (which is consistent with the possibility that pirenzepine acts via one of these non-muscarinic pathways). Further support for this notion is that lesions of the parasympathetic pathways inhibit the choroidal thickening in response to myopic defocus, but have no effect on choroidal thinning in response to hyperopic defocus or form deprivation.55
In conclusion, our results are consistent with a cholinergic muscarinic mechanism for the visually-induced choroidal thinning found in response to hyperopic defocus, and add to the evidence supporting a non-muscarinic mode of action for atropine and pirenzepine in ocular growth inhibition in animal models and perhaps in humans.
Acknowledgements
The authors acknowledge grant support: NIH-EY-013636 (DLN) and EY-02727 (XZ and JW). The authors thank Yekaterina Yusupova and Kristen Totonelly (NECO) for collecting some of the data on the agonists. We also thank Dr. Li Deng (NECO) for performing the statistical analyses required.
Footnotes
Disclosure
The authors report no conflicts of interest.
References
- 1.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Rada JA, McFarland AL, Cornuet PK, Hassell JR. Proteoglycan synthesis by scleral chondrocytes is modulated by a vision dependent mechanism. Curr Eye Res. 1992;11:767–782. doi: 10.3109/02713689209000750. [DOI] [PubMed] [Google Scholar]
- 3.Rada JA, Thoft RA, Hassell JR. Increased aggrecan (cartilage proteoglycan) production in the sclera of myopic chicks. Dev Biol. 1991;147:303–312. doi: 10.1016/0012-1606(91)90288-e. [DOI] [PubMed] [Google Scholar]
- 4.Nickla DL, Wildsoet C, Wallman J. Compensation for spectacle lenses involves changes in proteoglycan synthesis in both the sclera and choroid. Curr Eye Res. 1997;16:320–326. doi: 10.1076/ceyr.16.4.320.10697. [DOI] [PubMed] [Google Scholar]
- 5.Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995;35:1175–1194. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- 6.Wallman J, Wildsoet C, Xu A, et al. Moving the retina: choroidal modulation of refractive state. Vision Res. 1995;35:37–50. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- 7.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29:144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goss DA. Attempts to reduce the rate of increase of myopia in young people-a critical literature review. Am J Optom Physiol Opt. 1982;59:828–841. doi: 10.1097/00006324-198210000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Rubin ML, Milder B. Myopia–A treatable disease? Surv Ophthalmol. 1976;21:65–69. doi: 10.1016/0039-6257(76)90050-3. [DOI] [PubMed] [Google Scholar]
- 10.Bedrossian RH. The effect of atropine on myopia. Ann Ophthalmol. 1971;3:891–897. [PubMed] [Google Scholar]
- 11.Shih YF, Chen CH, Chou AC, Ho TC, Lin LL, Hung PT. Effects of different concentrations of atropine on controlling myopia in myopic children. J Ocul Pharmacol Ther. 1999;15:85–90. doi: 10.1089/jop.1999.15.85. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy RH, Dyer JA, Kennedy MA, et al. Reducing the progression of myopia with atropine: a long term cohort study of Olmsted County students. Binocul Vis Strabismus Q. 2000;15:281–304. [PubMed] [Google Scholar]
- 13.Kennedy RH. Progression of myopia. Trans Am Ophthalmol Soc. 1995;93:755–800. [PMC free article] [PubMed] [Google Scholar]
- 14.Stone RA, Lin T, Laties AM. Muscarinic antagonist effects on experimental chick myopia. Exp Eye Res. 1991;52:755–7588. doi: 10.1016/0014-4835(91)90027-c. [DOI] [PubMed] [Google Scholar]
- 15.McBrien NA, Moghaddam HO, Reeder AP. Atropine reduces experimental myopia and eye enlargement via a nonaccommodative mechanism. Invest Ophthalmol Vis Sci. 1993;34:205–215. [PubMed] [Google Scholar]
- 16.Cottriall CL, Truong HT, Gentle A, McBrien NA. Changes in scleral metabolism following in vivo application of pirenzepine to prevent axial myopia. Invest Ophthalmol Vis Sci (Suppl) 2000;41:S133. [Google Scholar]
- 17.McBrien NA, Moghaddam HO, New R, Williams LR. Experimental myopia in a diurnal mammal (Sciurus carolinensis) with no accommodative ability. J Physiol. 1993;469:427–441. doi: 10.1113/jphysiol.1993.sp019821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McBrien N, Arumugam B, Gentle A, Chow A, Sahebjada S. The M4 muscarinic antagonist MT-3 inhibits myopia in chick: evidence for site of action. Ophthalmic Physiol Opt. 2011;31:529–539. doi: 10.1111/j.1475-1313.2011.00841.x. [DOI] [PubMed] [Google Scholar]
- 19.Luft W, Ming Y, Stell W. Variable effects of previously untested muscarinic receptor antagonists on experimental myopia. Invest Ophthalmol Vis Sci. 2003;44:1330–1338. doi: 10.1167/iovs.02-0796. [DOI] [PubMed] [Google Scholar]
- 20.Lind GJ, Chew SJ, Marzani D, Wallman J. Muscarinic acetylcholine receptor antagonists inhibit chick scleral chondrocytes. Invest Ophthalmol Vis Sci. 1998;39:2217–2231. [PubMed] [Google Scholar]
- 21.McBrien NA, Stell WK, Carr B. Point-counterpoint. How does atropine exert its anti-myopia effects? Ophthalmic Physiol. 2013 Opt; doi: 10.1111/opo.12052. [DOI] [PubMed] [Google Scholar]
- 22.Nickla DL, Totonelly K, Dhillon B. Dopaminergic agonists that result in ocular growth inhibition also elicit transient increases in choroidal thickness in chicks. Exp Eye Res. 2010;91:715–720. doi: 10.1016/j.exer.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nickla D, Wildsoet C. The effect of the nonspecific nitric oxide synthase inhibitor NG-nitro-L-arginine methyl ester on the choroidal compensatory response to myopic defocus in chickens. Optom Vis Sci. 2004;81:111–118. doi: 10.1097/00006324-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Meriney S, Pilar G. Cholinergic innervation of the smooth muscle cells in the choroid coat of the chick eye and its development. J Neurosci. 1987;7:3827–3839. doi: 10.1523/JNEUROSCI.07-12-03827.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guglielmone R, Cantino D. Autonomic innervation of the ocular choroid membrane in the chicken. Cell Tissue Res. 1982;222:417–431. doi: 10.1007/BF00213222. [DOI] [PubMed] [Google Scholar]
- 26.Walls GL. Ed C l o Science. Bloomfield Hills, MI: Cranbrook Institute of Science; 1942. The Vertebrate Eye and its Adaptive Radiations. [Google Scholar]
- 27.Fischer A, McKinnon L, Nathanson N, Stell W. Identification and localization of muscarinic acetylcholine receptors in the ocular tissues of the chick. J Comp Neurol. 1998;392:273–284. [PubMed] [Google Scholar]
- 28.Nickla DL, Zhu X, Wallman J. A role for muscarinic agonists in the choroidal responses to defocus in chickens. 13th International Myopia Conference. 2010:33. [Google Scholar]
- 29.Nickla DL, Zhu X, Totonelly K, Bastian D, Wallman J. Muscarinic agonists can thin chick choroid and stimulate ocular elongation. 2010 ARVO E-Abstract #3676. [Google Scholar]
- 30.Nickla D. The middle of the signal cascade in emmetropization: The choroid and the effects of nitric oxide synthase (NOS) inhibitors, muscarinic antagonists and dopaminergic agonists. 12th International Myopia Conference. 2008:24. [Google Scholar]
- 31.Zhu X, Liu Y, Wallman J. Glucagon increases choroidal thickness of chick eyes by acting on the retinal pigment epithelium. 2005 ARVO E-Abstract#3338. [Google Scholar]
- 32.Matsumoto S, Yorio T, DeSantis L, Pang I. Muscarinic effects on cellular functions in cultured human ciliary muscle cells. Invest Ophthalmol Vis Sci. 1994;35:3732–3738. [PubMed] [Google Scholar]
- 33.Ishikawa H, DeSantis L, Patil P. Selectivity of muscarinic agonists including (+/−)-aceclidine and antimuscarinics on the human intraocular muscles. J Ocul Pharmacol Ther. 1998;14:363–373. doi: 10.1089/jop.1998.14.363. [DOI] [PubMed] [Google Scholar]
- 34.Schwahn HN, Kaymak H, Schaeffel F. Effects of atropine on refractive development, dopamine release, and slow retinal potentials in the chick. Vis Neurosci. 2000;17:165–176. doi: 10.1017/s0952523800171184. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Shi H, Lu Y, Yang B, Wang Z. Pilocarpine modulates the cellular electrical properties of mammalian hearts by activating a cardiac M3 receptor and a K+ current. Br J Pharmacol. 1999;126:1725–1734. doi: 10.1038/sj.bjp.0702486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wendt M, Glasser A. Topical and intravenous pilocarpine stimulated accommodation in anesthetized rhesus monkeys. Exp Eye Res. 2010;90:605–616. doi: 10.1016/j.exer.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barlow R, Weston-Smith P. The relative potencies of some agonists at M2 muscarinic receptors in guinea pig ilium, atria and bronchi. Br J Pharmacol. 1985;85:437–440. doi: 10.1111/j.1476-5381.1985.tb08879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nickla DL, Wildsoet C, Wallman J. Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Exp Eye Res. 1998;66:163–181. doi: 10.1006/exer.1997.0420. [DOI] [PubMed] [Google Scholar]
- 39.Wallman J, Adams II. Developmental aspects of experimental myopia in chicks: susceptibility, recovery and relation to emmetropization. Vision Res. 1987;27:1139–1163. doi: 10.1016/0042-6989(87)90027-7. [DOI] [PubMed] [Google Scholar]
- 40.Schmid KL, Wildsoet C. Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks. Optom Vis Sci. 2004;81:137–147. doi: 10.1097/00006324-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Zhu X, Wallman J. Opposite effects of glucagon and insulin on competition for spectacle lenses in chicks. Invest Ophthalmol Vis Sci. 2009;50:24–36. doi: 10.1167/iovs.08-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poukens V, Glasgow BJ, Demer JL. Nonvascular contractile cells in sclera and choroid of humans and monkeys. Invest Ophthalmol Vis Sci. 1998;39:1765–1774. [PubMed] [Google Scholar]
- 43.Kee C-s, Marzani D, Wallman J. Differences in time course and visual requirements of ocular responses to lenses and diffusers. Invest Ophthalmol Vis Sci. 2001;42:575–583. [PubMed] [Google Scholar]
- 44.Levey A. Immunological localization of ml-m5 muscarinic acetylcholine receptors in peripheral tissues and brain. Life Sci. 1993;52:441–448. doi: 10.1016/0024-3205(93)90300-r. [DOI] [PubMed] [Google Scholar]
- 45.Caufield M. Muscarinic receptors characterization, coupling, and function. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- 46.Matsui M, Motomura D, Karasawa H, et al. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci USA. 2000;97:9579–9584. doi: 10.1073/pnas.97.17.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Darroch S, Irving H, Mitchelson F. Characterization of muscarinic receptor subtypes in avian smooth muscle. Eur J Pharmacol. 2000;402:161–169. doi: 10.1016/s0014-2999(00)00489-1. [DOI] [PubMed] [Google Scholar]
- 48.Erickson K, Schroeder A. Direct effects of muscarinic agents on the outflow pathways in human eyes. Invest Ophthalmol Vis Sci. 2000;41:1743–1748. [PubMed] [Google Scholar]
- 49.Kaufman P, Barany E. Loss of acute pilocarpine effect on outflow facility following surgical disinsertion and retrodis-placement of the ciliary muscle from the scleral spur in the cynomolgus monkey. Invest Ophthalmol Vis Sci. 1976;15:793–807. [PubMed] [Google Scholar]
- 50.Bill A. Effects of atropine and pilocarpine on aqueous humour dynamics in cynomolgus monkeys (Macaca irus) Exp Eye Res. 1967;6:120–125. doi: 10.1016/s0014-4835(67)80062-9. [DOI] [PubMed] [Google Scholar]
- 51.Diether S, Schaeffel F, Lambrou GN, Fritsch C, Trendelenburg A. Effects of intravitreally and intraperitoneally injected atropine on two types of experimental myopia in chicken. Exp Eye Res. 2007;84:266–274. doi: 10.1016/j.exer.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 52.Fischer A, Miethke P, Morgan I, Stell W. Cholinergic amacrine cells are not required for the progression and atropine-mediated suppression of form deprivation myopia. Brain Res. 1998;794:48–60. doi: 10.1016/s0006-8993(98)00188-7. [DOI] [PubMed] [Google Scholar]
- 53.Winawer J, Wallman J. Temporal constraints on lens compensation in chicks. Vision Res. 2002;42:2651–2668. doi: 10.1016/s0042-6989(02)00300-0. [DOI] [PubMed] [Google Scholar]
- 54.Zhu X, Wallman J. Temporal properties of compensation for positive and negative spectacle lenses in chicks. Invest Ophthalmol Vis Sci. 2009;50:37–46. doi: 10.1167/iovs.08-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nickla DL, Schroedl F. Parasympathetic influences on emmetropization in chicks: evidence for different mechanisms in form deprivation vs negative lens-induced myopia. Exp Eye Res. 2012;102:93–103. doi: 10.1016/j.exer.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nickla D, Wilken E, Lytle G, Yom S, Mertz J. Inhibiting the transient choroidal thickening response using the nitric oxide synthase inhibitor L-NAME prevents the ameliorative effects of visual experience on ocular growth in two different visual paradigms. Exp Eye Res. 2006;83:456–464. doi: 10.1016/j.exer.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 57.Nickla D. Transient increases in choroidal thickness are consistently associated with brief daily visual stimuli that inhibit ocular growth in chicks. Exp Eye Res. 2007;84:951–959. doi: 10.1016/j.exer.2007.01.017. [DOI] [PubMed] [Google Scholar]