Abstract
Background: Crimean-congo hemorrhagic fever (CCHF) is an acute tick-borne viral, zoonotic disease with hemorrhagic manifestations and considerable mortality in humans. The purpose of this study was to introduce CCHF as a case report from Babol, north of Iran. It is known as an endemic pathogen in some regions of Iran.
Case presentation: We present a case of CCHF suffering from sudden onset of fever, headache, nausea, vomiting, melena and hemorrhagic manifestations like petechiae and epistaxis accompanied with evidence of ticks bite in a non-endemic area in the north of Iran. The laboratory dignosis was CCHF. He was treated with ribavirin and recovered completely.
Conclusion: CCHF may be seen in non- endemic regions and clinicians must be awarded about its diagnosis and treatment.
Key Words: Crimean-congo hemorrhagic fever, Ribavirin, Epidemiology, North of Iran
Crimean Congo hemorrhagic fever (CCHF) is a widely distributed lethal zoonotic disease. It is caused by an RNA virus in wild and domestic mammals, birds and ticks. This virus has been classified as a Nairovirus genus in the family of Bunyaviridae from Arbovirus group. Sheep, goats and cattle develop high titers of virus in blood, but tend not to fall ill. Human are usually infected with CCHF virus through a tick bite or close contact with viral contaminated tissues or with the blood of domestic animals or infected patients (1-3). In Iran, Hyalomma species probably play the main role in transmitting the infection from animals to humans (4). The incubation period of CCHF depends on the method of transmission. It can extend from 2 up to 7 days following a tick bite or 10 up to 14 days after blood transfusion. The onset of the illness is sudden, with fever, chills, severe muscle pains, headache, vomiting and pain in the epigastria and lumbar regions. A hemorrhagic state develops from the 3ed to 5th day and manifests with petechiae, purpura, epistaxis, hemoptysis, hematemesis, melena and hematuria (1-3). Clinical features usually include a rapid progression with 30% mortality rate and can develop up to 50% (1, 3, 5). In patients who recover, body temperature decreases within the 10th and 20th day and bleeding stops however, convalescence can last up to 4 weeks or longer. Usually, death occurs from massive hemorrhage and cardiac arrest, from the 7th to 9th day after the onset of the illness (3, 5, 6). In this paper, we present a case of CCHF in a hospital in Babol, North of Iran.
Case Presentation
A previously healthy, 34 year-old male was admitted to the Emergency department of a tertiary care center in Babol with sudden onset of fever, malaise, anorexia , myalgia, headache, skin eruptions with 4 day duration. He gave history of bleeding from mocusa (gingival bleeding) several times before admission and epistaxia occurred the time of admission without trauma.
The clinical features consist of severe nausea/vomiting and loose bloody defecation without abdominal pain. There was no corisa sign and the respiratory organs were seen intact without any sign and symptoms. He was a painter and lives in a rural area (a village near Babol city, North of Iran). There was not any history of working in the farm but he kept domestic animals (cows) near his house, in other words he was part time animal keeper.
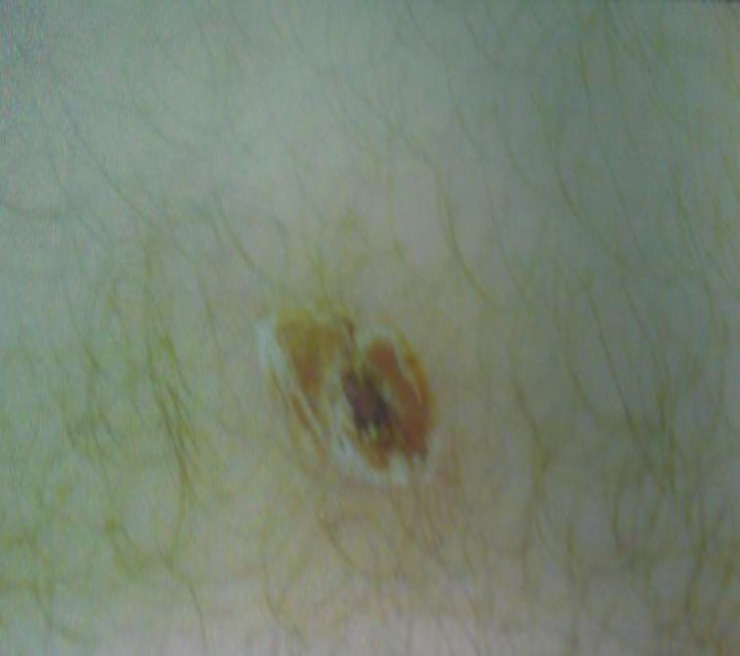
On initial examination, his vital signs included: blood pressure, 100/70 mmHg; pulse rate, 110 beats/min; respiratory rate, 22 cycle/min; body temperature, 38.7°c. The patient was oriented without neurological symptoms, but prostrated. In physical examination, head and neck were normal and sclera seen had normal color. Meningeal signs were slightly positive. The heart was in mild sinus tachyarrhythmia. Heart sound was normal accompanied with weak pulse tone. In the lungs, auscultation normal broncovesicular sound was heard. purpuric lesions on the trunk and upper limb was observed. A skin eruption (1×1 cm) with central ulcer was seen in the distal of left femur (figure 1). Broad-range antibiotic therapy was initiated and isolation in the ICU was recommended.
Figure 1.
skin eruption about 1*1 cm diameter on distal of our patient’s left femur area.
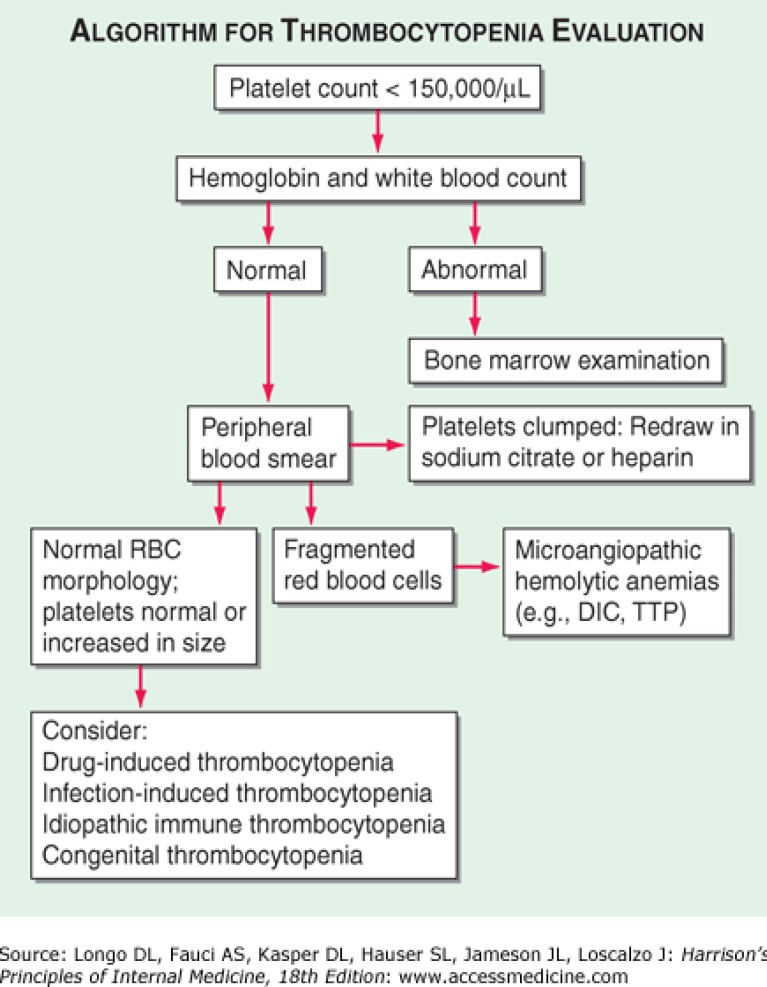
Paraclinical evaluation showed normal hemoglobin level (HB: 13mg/dL). WBC: 2900 (Poly 55%, Lymph 45%) and PLT: 31000 (Pro-thrombin time: 12 sec, Activated partial thromboplastin time: 47 sec, INR=1) were two prominent laboratory findings, so an approach to fever and thrombocytopenia was initiated (figure 2). A scanty rise in hepatic enzymes (alanine transaminase) and lactate dehydrogenase levels were reported.
Figure 2.
An Algorithmic approach to thrombocytopenia
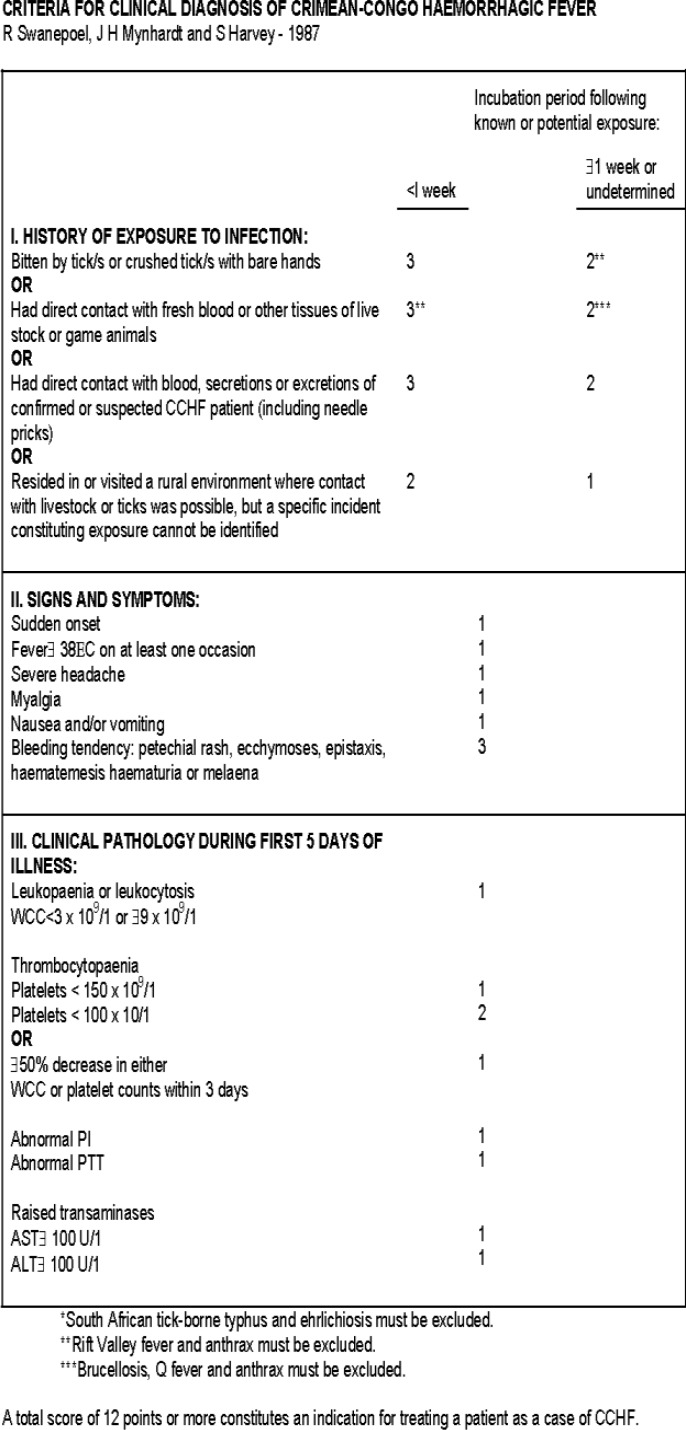
Intravenous antibiotics like Ceftriaxone supportive therapy consisted of hydration, blood product transfusion (5 unit platelets) and antipyretics were continued according to clinical suspicion to sepsis, meningitis since the patient came from an area in which leptospirosis was endemic. Although, CCHF had not been seen before in this province, ribavirin was prescribed based on criteria for clinical diagnosis of CCHF table created by Swanepoel, Mynhardt and Harvey-1987 (figure 3). Ribavirin 30 mg/kg as initials dose followed by 15 mg/kg every 6 hours for 4 days and 7.5 mg/kg every 8 hours for 6 days were administered to the patient. Complementary laboratory tests were requested and a serum sample for viral hemorrhagic fever was send.
Figure 3.
Criteria for clinical diagnosis of CCHF
Serological tests for hepatitis were negative whereas, serological (IgG and IgM using ELISA method) and virological assessments were positive for CCHF during the next 4 days and confirmed in Pasteur Institutes of Iran (PII). Forthy-eight hours from the start of treatment, the bleeding stopped, 48 hours later, the body temperature became normal and 96 hours later, he was transferred to the ward. He was treated and discharged from the hospital after a successful conservative therapy plus adequate dose of Ribavirin during the next 2 weeks. He was followed up every 2 weeks for about 2 months.
Discussion
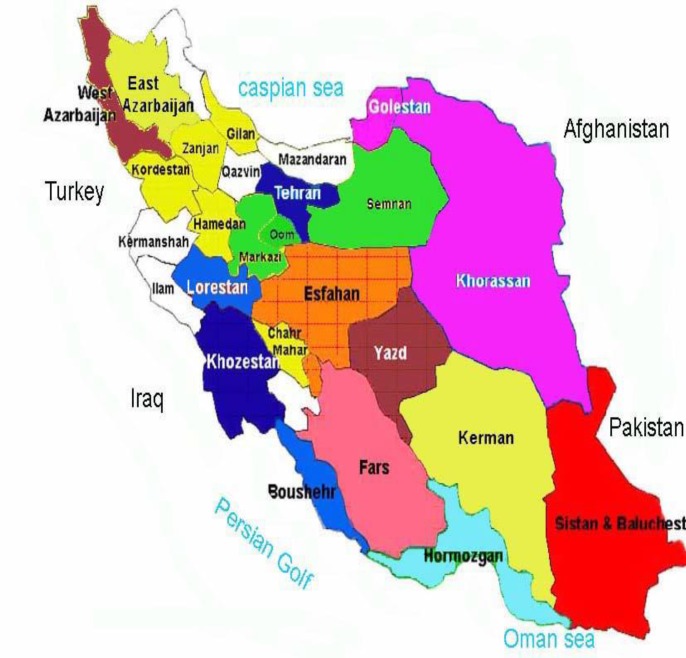
Infectious diseases still play a significant role in shaping the morbidity and mortality patterns in the developing countries. Physicians in these areas stand at crossroads because of the non-specific overlapping features of many infectious diseases (7). We described clinical features, laboratory findings, treatments and outcome for our patient who was diagnosed of Crimean-congo hemorrhagic fever in non-endemic region in the north of Iran. According to this confirmed CCHF appearance, the geographic distribution of CCHF in Iran can be revised because the last version of the complete distribution map of CCHF in Iran has been extensively identified by the laboratory of arboviruses and viral hemorrhagic fevers (Chinikar et al. Pasteur Institute of Iran- National Reference Laboratory and Center for Disease Control of Ministry of Health), Mazandran was considered a region without CCHF involvement (3, 4) (figure 4).
Figure 4.
Last version of geographic distribution of CCHF in Iran, based on the Center for Disease Control (CDC) at the Ministry of Health (MOH) and Pasteur Institute of Iran (PII) reports
CCHF outbreak as an expected event in the endemic areas should notify the clinicians in the neighborhood. They should be aware of the probability of the potential transmission of the virus via tick-infested and infected imported livestock. Febrile hemorrhagic syndromes encountered in non-endemic areas near endemic regions, such as other parts of Iran, should probably be considered to be viral hemorrhagic fever (8). Holding workshops and other training courses is appropriate to enhance the knowledge of medical staff because it significantly improves the outcome of CCHF. Ribavirin (a synthetic purine nucleoside analogue) has been shown to inhibit viral replication of the CCHF virus in vitro (9). The World Health Organization (WHO) currently recommends the administration of Ribavirin as a potential therapeutic drug for CCHF, but its efficacy in the treatment is controversial and some studies have shown that oral ribavirin treatment in CCHF patients do not affect on viral load or disease progression (1, 8, 10, 11).
Based on our clinical experience, we observed a real uncertainty exists over the benefit of Ribavirin prescription in our patient. This important fact that early use of Ribavirin in the beginning of illness has significant positive effect on survival which has also been emphasized in other reports (1, 2, 8, 10). Perhaps late diagnosis decreases the efficacy of treatment and aggravates the outcome of the disease. The only relevant outcome of CCHF is survival and there is not any long-term sequela or disability (8, 10, 11).
In summary, our case represented a complex clinical scenario whereby the patients showed the clinical features of the viral hemorrhagic fever, there was not a prior observation of CCHF in this area. Treatment and control strategy was established based on clinical observations and it made an appropriate outcome. As this case clearly demonstrated one of the biggest challenges we faced in the early recognition, control and treatment of our patient was our location in a non-endemic area.
Acknowledgments
The authors would like to thank their colleagues in the Infectious Ward of Ayatollah Rouhani Hospital of Babol for their cooperation, Dr. Jila Masrour Roudsari and Mrs. Soleimanian for their technical help, and Professor Mohammad Reza Hasanjani Roushan for his overall support.
Conflict of Interest:
The authors declare no competing interests
References
- 1.Jabbari A, Besharat S, Abbasi A, Moradi A, Kalavi KH. Crimean-congo hemorrhagic fever: case series from a medical center in Golestan province, northeast of Iran (2004) Ind J Med Sci. 2006;60:327–9. [PubMed] [Google Scholar]
- 2.Jabbari A, Besharat S, Abbasi A. Some evidences about Crimean-congo hemorrhagic fever. Pak J Med Sci. 2008;24:187–8. [Google Scholar]
- 3.Chinikar S, Ghiasi SM, Ghalyanchi- Langeroudi A, et al. An overview of Crimean Congo Hemorrhagic Fever in Iran. Iran J microbiol. 2009;1:7–12. [Google Scholar]
- 4.Chinikar S, Fayaz A, Mir Ahmadi R, et al. The specific serological investigation of suspected human and animals to have Crimean-Congo hemorrhagic fever in various parts of Iran using ELISA. Hakim. 2002;4:294–300. [Google Scholar]
- 5.Ergonul O. Crimean-Congo haemorrhagic fever. Lancet Infect Dis. 2006;6:203–14. doi: 10.1016/S1473-3099(06)70435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vorou R, Pierroutsakos IN, Maltezou HC. Crimean Congo hemorrhagic fever. Curr Opin Infect Dis. 2007;20:495–500. doi: 10.1097/QCO.0b013e3282a56a0a. [DOI] [PubMed] [Google Scholar]
- 7.Ali F, Salleem T, Khalid U, Mehmood SF, Jamil B. Crimean-Congo hemorrhagic fever in a dengue-endemic region: lessons for the future. J Infect Dev Ctries. 2010;4:459–63. doi: 10.3855/jidc.812. [DOI] [PubMed] [Google Scholar]
- 8.Jabbari A, Tabasi S, Abbasi A, Alijanpour E. Crimean-congo hemorrhagic fever: treatment and control strategy in admitted patients. Caspian J Intern Med. 2012;3:443–4. [PMC free article] [PubMed] [Google Scholar]
- 9.Bodur H, Erbay A, Akıncı E, et al. Effect of oral ribavirin treatment on the viral load and disease progression in Crimean-Congo hemorrhagic fever. Int J Infect Dis. 2011;15:e44–7. doi: 10.1016/j.ijid.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Ascioglu S, Leblebicioglu H, Vahaboglu H, Chan KA. Ribavirin for patients with Crimean–Congo haemorrhagic fever: a systematic review and metaanalysis. J Antimicrob Chemother. 2011;66:1215–22. doi: 10.1093/jac/dkr136. [DOI] [PubMed] [Google Scholar]
- 11.Ergonul O, Celikbas A, Dokuzoguz B, et al. The characteristics of Crimean Congo Hemorrhagic Fever in a recent outbreak in Turkey and the impact of oral ribavirin therapy. Clin Infect Dis . 2004;39:284–7. doi: 10.1086/422000. [DOI] [PubMed] [Google Scholar]