Abstract
Eight centers participated in Blood and Marrow Transplant Clinical Trials Network (BMT CTN) protocol 0303 to determine the effect of extensive T-cell depletion (TCD) on the outcome of HLA matched sibling donor transplant for acute myeloid leukemia. One goal of the study was to determine if TCD could be performed uniformly among study sites. TCD was achieved using the CliniMACS® CD34 Reagent System for CD34-enrichment. Processed grafts needed to contain ≥2.0 × 106 CD34+ cells/kg with a target of 5.0 × 106 CD34+ cells/kg and <105 CD3+ T cells/kg. Up to three collections were allowed to achieve the minimum CD34+ cell dose. In total 86 products were processed for 44 patients. Differences in the starting cell products between centers were seen in regards to total nucleated cells, CD34+ cells and CD3+ T cells which could in part be ascribed to a higher dose of G-CSF used for mobilization early in the trial. Differences between centers in processing outcomes were minimal and could be ascribed to starting cell parameters or to differences in graft analysis methods. Multivariate analysis showed that CD34+ cell recovery (66.1%±20.3%) was negatively associated with the starting number of CD34+ cells (p=0.02). Median purity of the CD34-enriched fraction was 96.7% (61.5 to 99.8%) with monocytes and B cells the most common impurity. All patients received the minimum CD34+ cell dose and 39 patients (89%) came within 10% or exceeded the target CD34+ cell dose without exceeding the maximum T cell dose. All patients proceeded to transplantation and all achieved initial engraftment. Products processed at multiple centers using the CliniMACS System for CD34-enrichment were comparably and uniformly highly enriched for CD34+ cells, with good CD34+ cell recovery and very low CD3+ T cell content.
INTRODUCTION
A number of single and multi-center trials have demonstrated favorable outcomes with the use of ex vivo T cell depletion (TCD) for acute myeloid leukemia (AML) patients in complete remission undergoing hematopoietic progenitor cell transplantation (HPCT) (1–6). These studies have shown a high rate of engraftment, minimal transplant-related complications, and, in contrast to recipients of T cell depleted grafts with chronic myelogenous leukemia, relapse rates that do not exceed that of recipients of non-TCD grafts. However, these studies have differed significantly both in the criteria for patient selection as well as the method and degree of TCD.
The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) undertook the BMT CTN 0303 trial, designed with defined patient selection criteria, common protocols for donor mobilization and a common preparative regimen (7). Further, the trial would use a single method of TCD utilizing the CliniMACS® CD34 Reagent System that would reduce T cells to the level that no post-HPCT pharmacological graft versus host disease (GVHD) prophylaxis was required. The CliniMACS System has been in clinical use in Europe since 1998 where it has consistently produced products that are highly depleted of CD3+ T cells with good recovery of CD34+ cells (8–10). However, prior studies were conducted at a limited number of specialized centers. Therefore, one of the objectives of BMT CTN 0303 was to evaluate the consistency of graft processing outcomes at multiple sites across the network, and to identify variables in trial design that might improve consistency for future studies.
Herein we report the characteristics of the products that were processed for patients enrolled on BMT CTN 0303, the composition of the grafts that were infused, and the consistency of processing among centers.
MATERIALS AND METHODS
Control of processing variables
The CliniMACS® CD34 Reagent System consisting of the CliniMACSplusInstrument, CliniMACS CD34+Reagent, CliniMACS PBS/EDTA buffer and CliniMACS Tubing Setswas used for CD34+ cell enrichment. All except one of the study site laboratories had previous training and experience using the CliniMACS system before the trial started. One center without previous experience underwent training by Miltenyi technical specialists prior to enrolling patients. Prior to initiation of the clinical trial, representatives from each of the processing laboratories identified critical steps in the processing procedure that should be harmonized to reduce variables that might affect processing outcome. It was agreed that procedures described in the CliniMACS Users’ Manual were to be strictly followed for the steps performed on the CliniMACS System. Hematopoietic progenitor cell apheresis products could be held overnight for processing provided that the cells were diluted to ≤2.0 × 108/mL using concurrent donor plasma or CliniMACS Buffer as the diluent and were stored in a controlled environment at 1–8°C. Products were to be allowed to warm to room temperature prior to processing. Platelet and antibody wash steps could be performed using a bag method as described in the CliniMACS Manual or by using a Cobe 2991 cell washer (CaridianBCT®, Lakewood, CO) as validated by the laboratories. Due to the limits recommended by the manufacturer, products that exceeded the limit of 1.2 × 1011 TNC of the CliniMACS Large Scale (LS) Tubing Set needed to be split and selected on two (or more) Tubing Sets. For products with fewer cells the regular scale set could be used with an upper limit of 6.0 × 1010 cells. Upper limits of 1.2 × 109 and 6.0 × 108 CD34+ cells were allowed for the LS Tubing and two bottles of CD34-Reagent, and regular Tubing Sets with one bottle of CD34-Reagent, respectively. All products were to be infused on the day they were processed after release test results were known. Products were to be assessed by flow cytometry for CD3+ and CD34+ cellular content prior to processing and for CD34+ cell content after antibody wash, prior to application to the tubing set. After CD34-enrichment, products were again assessed for cells expressing CD3 and CD34 in addition to CD56, CD19 or CD20 and CD14 to further characterize the infused product. All flow panels were to include the viability dye, 7-Amino-Actinomycin-D (7-AAD) to assess overall viability and to perform analysis only on viable cells. TNC together with flow analysis was to be used to determine absolute viable subset values (dual platform method) in the final CD34-enriched fraction.
The protocol allowed for split products to be pooled for final product analysis and release testing. Products that contained low cell numbers could be held overnight and pooled with the following days collection for processing. In such cases flow analysis was to be performed on the pooled product before and after processing.
Patients and Donors
A total of 47 patients were enrolled at eight centers between October 2005 and December 2008. Three patients did not proceed to transplant or product collection due to withdrawal of informed consent (N=2) or disease progression (N=1) and are not included in this manuscript. A full description of patient characteristics for this study, the cytoreduction regimen employed, and the protocol used to mobilize donor progenitor cells has been described (7). The goal was to infuse a target dose of 5.0 × 106 CD34+ cells/kg patient body weight containing not more than 1.0 × 105 CD3+ T cells/kg for each patient. Two apheresis products could be collected to achieve the target CD34+ cell dose, but up to three were allowed to reach the minimum dose of 2.0 × 106 CD34+ cells/kg without exceeding the CD3+ T cell dose. A total of 86 apheresis collections were obtained and processed for the 44 patients who underwent transplantation.
Statistical Analysis
Descriptive statistics (mean, standard deviation, median, minimum, maximum and number) for the parameters that were analyzed were performed on all products that were processed for CD34-enrichment. These included products obtained for 4 patients for whom three products were processed, 34 patients for whom two products were processed, and 6 patients for whom one product was processed. In two cases, two products were combined for processing on a single tubing set and these were counted as one procedure reducing the total number of processing procedures that were analyzed to 84. Processing outcome was assessed by evaluating the recovery of CD34+ cells from the starting product, the log reduction in CD3+ T cells, and the purity of the CD34-enriched fraction, in addition to the effect of the processing procedure on cell viability.
The evaluation of factors correlated with CD34+ cell recovery and log TCD considered the TNC processed for both outcomes, starting absolute CD34+ cells for CD34+ cell recovery, and starting absolute CD3+ T cells for log TCD. This analysis included the clinical center as a covariate. Analysis did not include 4 products that were split for selection since the CD34-enriched products were pooled prior to flow analysis and equal numbers of cells were not loaded onto the two tubing sets used for selection.
Data from 4 centers (centers 5, 6, 7 and 8) performing ≤4 procedures were pooled for comparison with centers performing > 4 procedures (range 9 to 34 procedures) when analyzing starting and ending product cell numbers and in comparing center outcomes for CD34+ cell recovery, CD34+ cell purity, log CD3+ T cell depletion, and ending product viability.
A linear mixed effect model was used to account for the repeated measures (≥2 procedures for most patients). An overall P-value of less than 0.05 indicated minimally one center differed from one other center in regards to the product characteristic tested. Pairwise comparison was then performed with Tukey-Kramer adjustment for multiple comparisons.
RESULTS
Starting Product Parameters
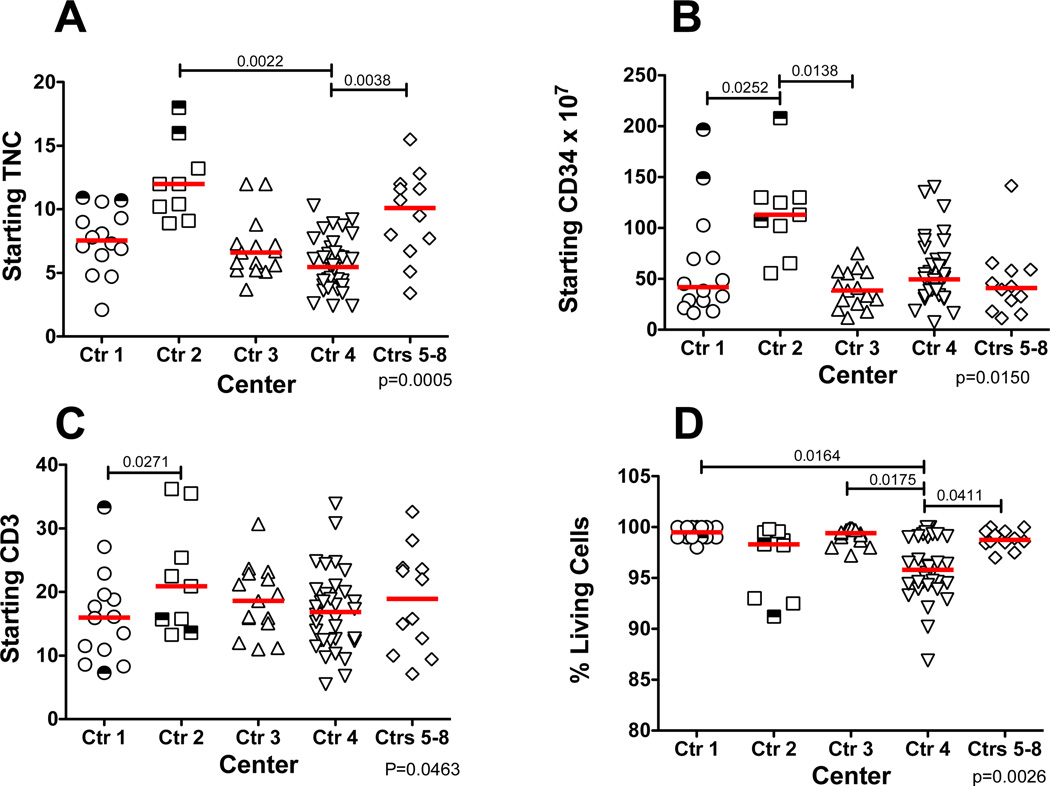
Individual centers were compared to determine if there were significant differences in the starting apheresis products that might have affected processing outcomes (Figure 1). Starting TNC was found to be significantly lower for center 4 as compared to center 2 and centers 5–8. (Figure 1A). Three of the 5 donors processed at center 2 underwent graft mobilization using 16 µg/kg/day of G-CSF rather than the 10 µg/kg/day that was later adopted. Along with the higher TNC for center 2, the products from this center also contained significantly more CD34+ cells than centers 1 or 3 and more CD3+ T cells than center 1 (Figure 1B and Figure 1C). The lower starting viabilities of the products from center 4 differed from all the other centers except center 2. Overall the starting products contained 0.84% ± 0.43% CD34+ cells and 27.2% ± 12.2% CD3+ cells.
Figure 1. Starting Product Characteristics by Center.
Cellular content of the individual apheresis products undergoing CD34-enrichment for each participating center. (A) Starting TNC. (B) Starting absolute CD34+ cells. (C) Starting absolute CD3+ T cells. (D) % viable cells as measured by 7-AAD. The bars represent the median for each center. The half open half closed symbols represent products that were subsequently split for CD34-enrichment. Only significant differences between centers are shown. The overall p value for the comparison is shown in the lower right of each panel.
Four products, two each from center 1 and center 2, were split for selection on two separate tubing sets and are indicated in Figure 1 by the half shaded symbols. In two cases, products were pooled for selection on a single tubing set, although in one of the two cases pooling resulted in more than the recommended number of cells being applied to the column within the tubing set.
Effect of Platelet and Antibody Wash and Wash Method on TNC and CD34+ Cell Content
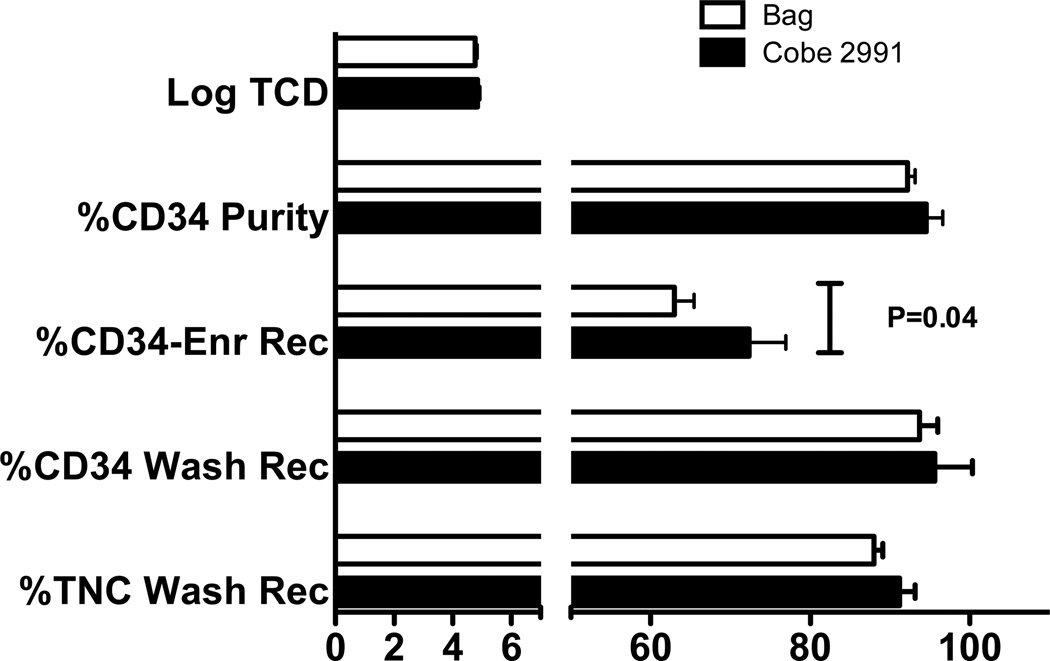
Samples were obtained from products just prior to loading the cells onto the tubing set to determine the effect of the wash steps required for platelet reduction and removal of unbound magnetic reagent on TNC and CD34+ cell content. Centers 1, 2 and 6 used the Cobe 2991 cell washer, while the other centers used blood transfer bags and a centrifuge (bag method) for the wash steps. Data from center 2 were available after the platelet wash, indicating that 65.4%±18.7% (N=10) of platelets are removed using the Cobe 2991 method. No significant difference was seen in post-wash TNC recovery between the Cobe and bag methods (91.2%±10.6% versus 88.0%±8.2%, respectively, p=0.09) or post wash CD34+ cell recovery (95.6%± 23.4% versus 93.8%±16.8%, respectively, p=0.43), (Figure 2, lower bars).
Figure 2. Effect of Wash Method Before and After CD34-Enrichment.
Data are from 27 products processed by 3 centers using a Cobe 2991 cell washer for the platelet and antibody wash steps and 57 products processed by 5 centers using a bag method. Results were compared for the effect of the wash method on TNC and CD34+ cell recovery prior to addition onto the tubing set, and for CD34+ cell recovery and purity post enrichment, as well as for Log TCD. The only significant difference based on wash method was a higher CD34+ cell recovery after CD34-enrichment. Data are the mean ± sem of the two groups with the Bag method represented by the white bars and the Cobe 2991 method by the black bars.
Outcome of Processing for CD34+ Cell-enrichment
CD34+ cell recovery post enrichment averaged 66.1±20.3%. The CD34-enriched fraction contained an average of 93.0% CD34+ cells and 0.23% CD3+ T cells, representing 4.8±0.6 log reduction of starting CD3+ T cells. Ending cell viability averaged 96.6% (Table 1).
Table 1.
Overall Outcomes of CD34-enrichment Procedures
| N=84 | %CD34 Recovery |
%CD34 Purity |
Log TCD | % Viability |
|---|---|---|---|---|
| Mean | 66.1 | 93.0 | 4.8 | 96.6 |
| SD | ±20.3 | ±8.3 | ±0.6 | ±3.8 |
| Median | 65.0 | 96.7 | 4.9 | 97.7 |
| Min | 29.9 | 61.5 | 3.2 | 74.0 |
| Max | 125.6 | 99.8 | 5.9 | 100.0 |
CD34+ cell recovery and Log TCDare calculated based on the starting apheresis product and the product as recovered from the tubing set. Viability was assessed by 7-AAD staining.
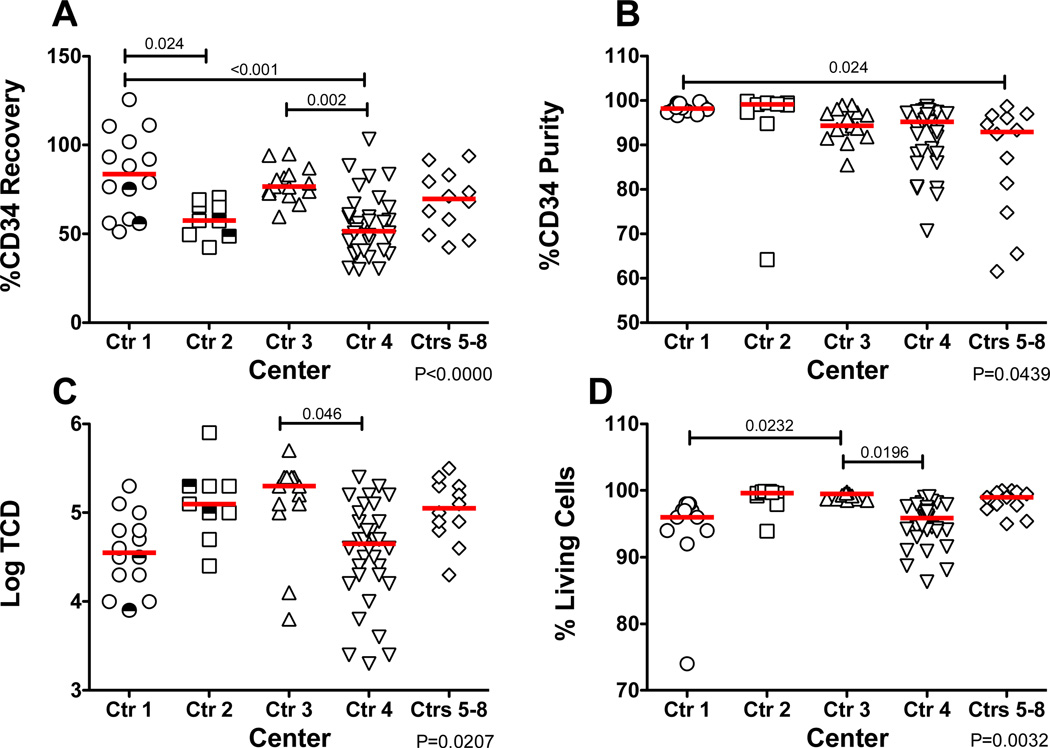
Of all of the outcomes, the most significant differences were seen for CD34+ cell recovery with the highest recoveries seen for centers 1 and 3 (Figure 3A). A significant difference in CD34+ cell purity was only seen between center 1, which had uniformly very high purities, and the combined centers 5–8 where there were more products with a lower purity (Figure 3B). Only centers 3 and 4 differed from each other in log TCD (Figure 3C). Center 3 reported the highest average final product viability that differed significantly from that reported by center 1 and center 4. All viabilities were well above the 70% limit for infusion ranging from 74% to 100% (Figure 3D).
Figure 3. Post Processing Outcomes by Center.
Outcome after CD34-enrichment. (A) Recovery of CD34+ cells from starting product. (B) % of the CD34-enriched fraction that are CD34+. (C) Log depletion from starting CD3+ T cells. (D) 7-AAD viability of the CD34-enriched fraction. The bars represent the median for each center. The half open half closed symbols represent products that were subsequently split for CD34-enrichment. Only significant differences between centers are shown. The overall p value for the comparison is shown in the lower right of each panel.
We additionally assessed if the wash method prior to CD34-enrichment affected final product CD34+ cell purity, CD34+ cell recovery, or Log TCD. The only significant difference between the two methods was for CD34+ cell yield which was higher for products washed using the Cobe 2991, 72.6±22.5% recovery using the Cobe 2991 compared to 63.0±18.5% recovery for bag-washed products (p=0.044). Figure 2 upper bars.
Evaluation of Starting Product Factors Correlating with CD34+ Cell Recovery and/or Log TCD
For the data analysis of the effect of starting cell numbers on final product outcome, we used the number of cells that started processing, rather than the number of cells from the pre-column assessments because the effect of cell numbers would include the labeling of the cells with the CD34-reagent. Four (5%) of products analyzed exceeded the recommended upper TNC limit for one or two CD34-Reagent vials. However only one of these 4 products was more than 10% over the limit.
Seven products (8.8%) (6 requiring two reagent vials) were over the specified upper limit for CD34+ cells with 3 of these >10% over the limit. Neither of the two patients for whom products were pooled exceeded the limit of CD34+ cells.
CD34+ cell recovery was significantly inversely correlated with starting numbers of CD34+ cells but not with starting TNC. In contrast log TCD was inversely correlated with starting TNC although the correlation was not significant (P=0.06) and Log TCD was higher with greater numbers of starting CD3+ T cells (P=0.05) (Table 2).
Table 2.
Predictors of CD34+ Cell Recovery and Log TCD
| Outcome | Predictor | Coefficient estimate of predictor |
P-value |
|---|---|---|---|
| CD34+cell recovery | Starting product TNC | 0.077 | 0.93 |
| CD34+ cell recovery | Starting product CD34+ cell count | −0.002 | 0.02 |
| Log10 T cell depletion | Starting product TNC | −0.047 | 0.06 |
| Log10 T cell depletion | Starting product CD3+ cell count | 0.016 | 0.05 |
Variables likely to affect the indicated outcome of products undergoing CD34-enrichment were assessed using a linear mixed effect model to adjust for repeated measurements. The clinical center was included in the model as a covariate.
CD34-enriched Product Purity
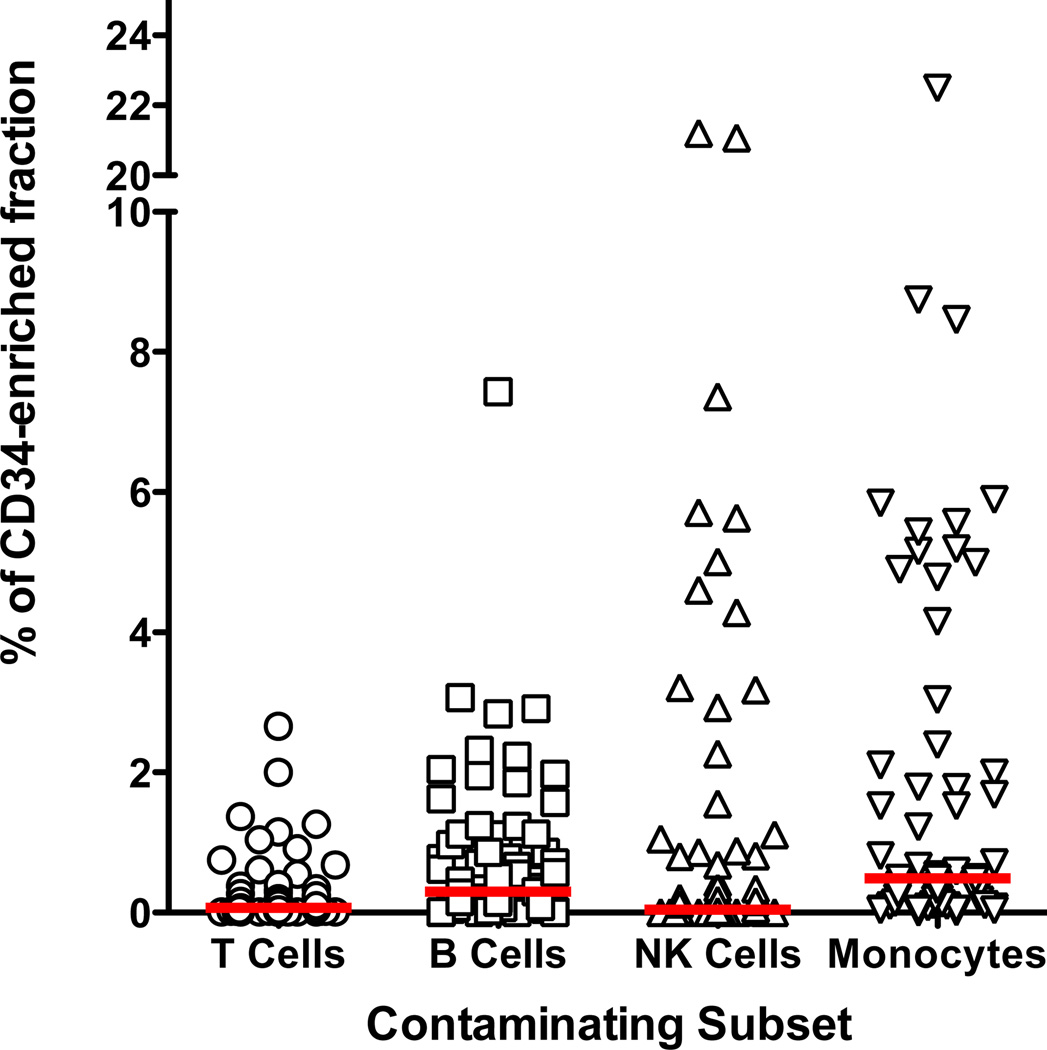
CD34-enriched products contained a median of 96.7% CD34+ cells (61.5 to 99.8%). Monocytes were the most common cellular subset other than CD34+ cells, but with a median content of only 0.5% (0 to 22.5%). B cells represented the second most common non-target cell type with a median of 0.30% (0.0 to 7.4%) of the final fraction. NK cells represented 0.05% (0 to 21.3%) of cells within the CD34-enriched fraction. The most infrequent cell subset was CD3+ T cells representing a median of 0.07% (0 to 2.6%) of the final fractions (Figure 4).
Figure 4. Other Cellular Subsets Found in the CD34-enriched products.
CD34-enriched fractions were assessed for other unwanted subsets including: CD3+ T cells (median 0.07%, range 0.0% to 2.6%, N=84), CD19 or CD20+ B Cells (median 0.28%, 0.0% to 7.4%, N=79), CD56+ NK cells (median 0.05%, range 0.0% to 21.3%, N=69), and CD14+ monocytes (median 0.5%, range 0.0% to 22.5%, N=65). The horizontal bar represents the median.
Cell Infusion Doses
For the study overall, 39 (88.6%) of patients received within 10% of the target CD34+ cell dose of 5.0 × 106 CD34+ cells/kg. Of these 19 (43.2%) required a single product, 18 (40.9%) required two products and 2 patients (4.5%) required 3 collections to reach this dose. Each of the 5 patients whose products did not contain minimally 90% of the of the target dose of CD34+ cells exceeded the minimum CD34+ cell dose of 2.0 ×106 CD34+ cells/kg specified by the protocol. Overall patients received a mean of 8.8 × 106 ± 5.2 × 106 CD34+ cells/kg, ranging from 2.4 to 30.3 × 106/kg. Given the high doses of CD34+ cells infused, no significant correlation could be seen between the dose of CD34+ cells infused and time to engraftment of neutrophils (data not shown). In no case did the CD3+ T cell dose exceed the upper limit of 105/kg. Patients received a mean of 1.5 × 104 ± 2.0 × 104 CD3+ cells/kg, ranging from 0.1 to 8.3 × 104. T cell dose did not correlate with the probability of developing acute GVHD (data not shown).
DISCUSSION
The BMT CTN trial BMT CTN0303 was designed to minimize inter-center variability of factors that might affect the outcome of recipients of T cell depleted HPC, apheresis grafts mobilized from HLA identical donors. Other goals were to test a TCD processing method sufficiently rigorous to eliminate the need for post-HPCT GVHD chemoprophylaxis to prevent clinically severe GVHD while allowing for the infusion of large numbers of CD34+ cells to promote rapid engraftment. These goals were achieved in that in the absence of post-transplant GVHD prophylaxis patients on BMT CTN0303 had a cumulative incidence of acute GVHD grades 2–4 of 22.7% with only 7.6% experiencing extensive chronic GVHD at two years and all patients engrafted neutrophils and platelets at a median of 12 days (range, 9–19 days), and 16 days (range, 13–159), respectively(7).
The CliniMACS CD34 Reagent System for CD34-enrichment has been shown to be highly effective in reducing T cell content such that GVHD incidence is extremely low even in the setting of HLA disparity(1, 2). The success of this device in producing graft products that have a very low T cell content and good recovery of CD34+ cells from the starting apheresis product has been demonstrated in multiple single center studies (11–13). However, to date there have been few published studies involving multiple institutions that demonstrate that this method for TCD is exportable and reproducible. We demonstrated that the results of 84 products processed in 8 different centers over the course of the BMT CTN 0303 clinical trial were consistent and that additional processing steps to further standardize the procedure were not necessary.
Differences in the starting products were seen for TNC, CD34+ cell content and viability. Significantly higher numbers of starting cells were mostly limited to a single center (center 2) where more of the products were collected early in the study from donors given G-CSF at 16 µg/kg/day as the mobilization stimulus. In part, due to the high cellular content of these first products, changes were made in the protocol to reduce the G-CSF dose to 10 µg/kg/day. The 7-AAD viability of the starting product was significantly lower for Center 4 compared to most of the other centers, most likely due to this center’s use of a flow cytometry staining method that does not utilize washes after antibody or lysing reagent additions as opposed to the other laboratories where wash steps that likely removed a portion of the dead cells were used.
The use of a cell washer method or a bag method to remove unwanted platelets and unbound magnetic bead reagent that might interfere with the selection process was an acceptable variation in the processing used by 3 of the 8 centers, We confirmed that there was no statistical difference in the recovery of TNC or CD34+ cells either immediately after the wash steps, nor were differences seen in log TCD or CD34+ cell purity post CD34-enrichment. However, there was improved recovery of CD34+ cells in centers using the cell washer. Previously published data using the CliniMACS CD34 Reagent System have shown a better recovery and purity of CD34-enriched products using a system that results in 95% to 99% platelets being removed compared to the 25% to 40% removal achieved using the bag method described in the CliniMACS Users’ Manual (13, 14). Platelets are thought to decrease CD34+ cell recovery by interfering with the binding of the immunomagnetic reagent to the CD34+ cells. Data from one of the three centers using the Cobe 2991 for the platelet wash step indicated that approximately 65% of the platelets are removed using this method, which may be sufficient for the improved CD34+ cell recovery that was observed.
The final CD34-enriched product is the best measure of processing consistency between centers. Significant differences between centers in CD34 recovery mostly involved center 1 where in 4 cases recovery exceeding 100% was reported. Careful review of the data did not reveal any errors in data recording or reporting to explain the results for those products. The comparability of the assessment of CD34+ cell content by flow in apheresis products between centers has been the subject of multiple studies most of which found significant differences between laboratories in how the flow testing and analysis was performed (15–17). The BMT CTN 0303 trial required that participating flow cytometry laboratories use a validated assay, be accredited by the College of American Pathologists, and participate in graded CD34 proficiency studies. However, a standard method for performing the flow assay was not mandated. Analysis of the final CD34-enriched product for CD34+ cell content does not suffer from the same technical difficulties associated with the starting product, namely issues of rare event analysis. Therefore it seems likely the post-processing assessment is the more accurate number and the starting CD34+ cell content may have been underestimated in cases where CD34+ cell recovery is greater than 100%. Variation in cell counting methods may have also contributed since a two-platform method for determining the absolute number of CD34+ cells was used by all centers for the final fraction, while the centers were divided in using a single versus a dual-platform method for the starting absolute CD34+ cell numbers.
CD34+ cell purity and log TCD were more consistent between the centers, though there was a higher purity of products produced by center 1 as compared to the pooled results from centers 5–8. After CD34+ cells monocytes were the highest frequency cell type, with one product containing 22% CD14+ monocytes and a second product with 8.7% monocytes. B cells were the second most frequent contaminant. It is likely that both monocytes and B cells may be nonspecifically captured during enrichment by binding of the antibody conjugated directly onto the magnetic beads through Fc receptors. Only center 3 and center 4 differed from one another in regards to log TCD for reasons that have not been identified. Center 3 reported final product viability that was very close to 100% for all products causing this center to differ significantly from center 1 and center 4 for this variable.
The association between poorer CD34+ cell recovery and the higher number of CD34+ cells loaded onto the column/tubing set revealed in the multivariate analysis was the major difference between centers that might be ascribed to the CliniMACS® CD34 Reagent System. The CliniMACS Users’ Manual recommends limiting the number of CD34+ cells that should be treated with the CliniMACS CD34 Reagent and the number that should be loaded onto a single Tubing Set. Seven of the products evaluated for factors affecting CD34+ cell recovery exceeded the recommended loading dose of CD34+ cells, three of which were more than 10% above this limit. Two of the three products that exceeded the loading limit by more than 10% had <50% recovery indicating that this upper limit for CD34+ cells should not be exceeded. Our observations are in agreement with a recently published analysis of a large number of products processed at multiple centers showing that TNC in excess of that recommended by the manufacturer does not affect device performance, whereas exceeding the recommended CD34+ cell limit reduces CD34+ cell recovery (10).
Even though differences among centers were seen in the statistical analysis, all centers were able to process final graft products that easily met the criteria of the study. The center differences in the processing outcomes mostly could be explained by differences in the flow cytometry methods that were used. Future trials may benefit from a higher degree of standardization of flow methods as well as requiring that the upper loading limit of CD34+ cells be strictly observed. We conclude that the use of the Miltenyi CliniMACS CD34 Reagent System for TCD in the setting of a multi-center trial results in products that are sufficiently uniform in regards to CD34+ cell content and contain very low numbers of CD3+ T cells.
ACKNOWLEDGMENTS
The Blood and Marrow Transplant Clinical Trials Network is supported in part by grant #U01HL069294 from the National Heart, Lung, and Blood Institute and the National Cancer Institute.
We additionally thank MiltenyiBiotec for support of this trial.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure Statement
S.M.D., S.C., R.J.S., and R.J.O. have served as advisors to MiltenyiBiotecIncorporated None of the other authors have a primary financial relationship with a company that has a direct financial interested in the subject matter or products discussed or with a company producing a competing product.
In addition to the first author, this study could not have been performed without the contributions of the other key laboratory personnel at the clinical sites including:
Grace Kao, MD- Dana Farber Cancer Institute
Lynn O’Donnell, PhD. –The Ohio State University
Nancy Collins, PhD-Memorial Sloan Kettering Cancer Center
Dave DiGiusto, PhD- City of Hope
Robert M. Fox, RN, ND- University Hospitals Case Medical Center
Joy Cruz, MT(ASCP)SBB- University of California-San Francisco
UnaO’Doherty, MD, PhD- Abramson Cancer Center of the University of Pennsylvania
REFERENCES
- 1.Aversa F, Reisner Y, Martelli MF. The haploidentical option for high-risk haematological malignancies. Blood Cells Mol Dis. 2008;40:8–12. doi: 10.1016/j.bcmd.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Handgretinger R, Klingebiel T, Lang P, et al. Megadose transplantation of purified peripheral blood CD34+progenitor cells from HLA-mismatched parental donors in children. Bone Marrow Transplant. 2001;27:777–783. doi: 10.1038/sj.bmt.1702996. [DOI] [PubMed] [Google Scholar]
- 3.Papadopoulos EB, Carabasi MH, Castro-Malaspina H, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood. 1998;91:1083–1090. [PubMed] [Google Scholar]
- 4.Soiffer RJ, Fairclough D, Robertson M, et al. CD6-depleted allogeneic bone marrow transplantation for acute leukemia in first complete remission. Blood. 1997;89:3039–3047. [PubMed] [Google Scholar]
- 5.Davies SM, Wang D, Wang T, et al. Recent decrease in acute graft-versus-host disease in children with leukemia receiving unrelated donor bone marrow transplants. Biol Blood Marrow Transplant. 2009;15:360–366. doi: 10.1016/j.bbmt.2008.12.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner JE, Thompson JS, Carter SL, Kernan NA. Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): a multi-centre, randomised phase II-III trial. Lancet. 2005;366:733–741. doi: 10.1016/S0140-6736(05)66996-6. [DOI] [PubMed] [Google Scholar]
- 7.Devine SM, Carter S, Soiffer RJ, et al. Low Risk of Chronic Graft Versus Host Disease and Relapse Associated with T-Cell Depleted Peripheral Blood Stem Cell Transplantation for Acute Myeloid Leukemia in First Remission: Results of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Protocol 0303. Biol Blood Marrow Transplant. 2011 doi: 10.1016/j.bbmt.2011.02.002. 10.1016/j.bbmt.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schumm M, Lang P, Taylor G, et al. Isolation of highly purified autologous and allogeneic peripheral CD34+ cells using the CliniMACS device. J Hematother. 1999;8:209–218. doi: 10.1089/106161299320488. [DOI] [PubMed] [Google Scholar]
- 9.Slaper-Cortenbach IC, Wijngaarden-du Bois MJ, de Vries-van Rossen A, et al. The depletion of T cells from haematopoietic stem cell transplants. Rheumatology (Oxford) 1999;38:751–754. doi: 10.1093/rheumatology/38.8.751. [DOI] [PubMed] [Google Scholar]
- 10.Braakman E, Schuurhuis GJ, Preijers FW, et al. Evaluation of 'out-of-specification' CliniMACS CD34-selection procedures of hematopoietic progenitor cell-apheresis products. Cytotherapy. 2008;10:83–89. doi: 10.1080/14653240701787650. [DOI] [PubMed] [Google Scholar]
- 11.Aversa F, Terenzi A, Tabilio A, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23:3447–3454. doi: 10.1200/JCO.2005.09.117. [DOI] [PubMed] [Google Scholar]
- 12.Ringhoffer M, Wiesneth M, Harsdorf S, et al. CD34 cell selection of peripheral blood progenitor cells using the CliniMACS device for allogeneic transplantation: clinical results in 102 patients. Br J Haematol. 2004;126:527–535. doi: 10.1111/j.1365-2141.2004.05062.x. [DOI] [PubMed] [Google Scholar]
- 13.Zinno F, Landi F, Aureli V, et al. Positive immunomagnetic CD34(+) cell selection in haplo-identical transplants in beta-thalassemia patients: removal of platelets using an automated system. Cytotherapy. 2010;12:60–66. doi: 10.3109/14653240903348301. [DOI] [PubMed] [Google Scholar]
- 14.Del Fante C, Perotti C, Viarengo G, et al. Immunomagnetic cell selection performed for HLA haploidentical transplants with the CliniMACS device: effect of additional platelet removal on CD34+ cell recovery. Stem Cells Dev. 2005;14:734–739. doi: 10.1089/scd.2005.14.734. [DOI] [PubMed] [Google Scholar]
- 15.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5:213–226. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 16.Keeney M, Chin-Yee I, Weir K, Popma J, Nayar R, Sutherland DR. Single platform flow cytometric absolute CD34+ cell counts based on the ISHAGE guidelines. International Society of Hematotherapy and Graft Engineering. Cytometry. 1998;34:61–70. [PubMed] [Google Scholar]
- 17.Baech J, Johnsen HE. Technical aspects and clinical impact of hematopoietic progenitor subset quantification. Stem Cells. 2000;18:76–86. doi: 10.1634/stemcells.18-2-76. [DOI] [PubMed] [Google Scholar]