Summary
The enzyme 5α-reductase (5αR) catalyzes the conversion of testosterone and other Δ4-3-ketosteroids into their 5α-reduced metabolites. Of the five members of the 5αR family the type 2 enzyme (5αR2) plays a key role in androgen metabolism, and is abundantly distributed in the urogenital system. Although 5αR2 has been reported to be highly expressed in the brain during early developmental stages, little is currently known on its anatomical and cellular distribution in the adult brain. Thus, the present study was designed to determine the detailed localization of 5αR2 in the adult rat brain, using a highly specific polyclonal antibody against this isoform. Parasagittal and coronal sections revealed 5αR2 immunoreactivity throughout most brain regions, with strong immunolabeling in the layers III and VI of the prefrontal and somatosensory cortex, olfactory bulb, thalamic nuclei, CA3 field of hippocampus, basolateral amygdala and Purkinje cell layer of cerebellum. Lower 5αR2 levels were detected in the hypothalamus and midbrain. Moreover, double labeling fluorescence with confocal laser scanning microscopy (CLSM) revealed that 5αR2 is localized in neurons, but not in glial cells. Specifically, the enzyme was documented in the pyramidal neurons of the cortex by CLSM analysis of simultaneous Golgi-Cox and immunofluorescent staining. Finally, low levels of 5αR2 expression were identified in GABAergic cells across the cortex, hippocampus and striatum. These findings show that, in the adult brain, 5αR2 is distributed in critical regions for behavioral regulation, suggesting that the functional role of this isoform is present throughout the entire lifespan of the individual.
Keywords: 5α-reductase, brain, immunohistochemistry, neurosteroids, androgens
1. Introduction
Steroid 5α-reductases (5αRs) are a family of enzymes catalyzing the saturation of the 4,5 double bond of the A ring of several Δ4-3-ketosteroid substrates, including progesterone, glucocorticoids, mineralocorticoids and androgens (see Russell and Wilson, 1994 and Paba et al, 2011). Of the five known 5αRs, only the types 1 (5αR1) and 2 (5αR2) are believed to be physiologically involved in steroidogenesis. Although these two isoenzymes share common genetic components (Langlois et al, 2010), similar size and catalytic activities (see Paba et al, 2011), their differences in substrate affinity and anatomical distribution suggest that they exert distinct physiological functions. In particular, 5αR2 is posited to convert testosterone into its metabolite 5α-androstan-17β-ol-3-one (dihydrotestosterone; DHT), the most potent androgen hormone, which stimulates the acquisition of the majority of secondary sexual traits in men (Breedlove, 1992).
In the central nervous system (CNS), 5αR catalyzes the main rate-limiting reaction for the synthesis of neurosteroids such as allopregnanolone (AP), a derivative of progesterone that regulates stress and anxiety responses by acting as a potent allosteric modulator of the γ-aminobutyric acid A (GABAA) receptor (Barbaccia et al, 2001; Girdler and Klatzkin, 2007). In addition to AP, other 5α-reduced neurosteroids have been associated with important functions in the brain; for example, DHT and its metabolite 5α-androstan-3α, 17β-diol (3α-diol), have been shown to play cardinal roles in the regulation of emotion and cognition, stimulation of myelination as well as development of sexually dimorphic areas in the central nervous system (Valencia et al, 1992; Goldstein and Sengelaub, 1994; Beyer and Hutchinson, 1997; Frye et al, 2001; Melcangi et al, 2003; Sato et al, 2004; Edinger and Frye, 2005).
Previous research has shown that numerous brain regions produce DHT from testosterone, suggesting the presence of 5αR2 in their neural tissues. Nevertheless, while several studies have shown that 5αR1 is abundantly expressed in the CNS throughout all developmental stages (Poletti et al, 1998), the brain distribution of 5αR2 was originally considered essentially limited to late fetal and early postnatal periods (Poletti et al, 1998). In contrast with this finding, subsequent studies have documented the presence of 5αR2 in brain regions of adult rodents and humans, albeit at lower levels than 5αR1 (Normington and Russell, 1992; Lephart 1993; Torres and Ortega, 2003, 2006; Kimoto et al, 2010; Bortolato et al, 2011). In humans, whereas 5αR1 immunoreactivity is present in both neurons and glia, 5αR2 distribution has been found only in pyramidal cells, but not in small neurons and glial cells, pointing to cell-specific patterns in the expression of this enzyme throughout the brain (Eicheler et al., 1994; Aumuller et al., 1996).
Recently, the whole localization of the 5αR2 transcript in the adult mouse brain was reported in the Allen Brain Atlas, showing that the molecule is indeed present in most brain regions, and particularly expressed in the olfactory lobe, neocortex, hippocampus and cerebellum (http://mouse.brain-map.org/gene/show/60858). In spite of these results, the complete anatomical and cellular distribution of 5αR2 protein in the brain remains elusive.
Here we report the detailed localization of 5αR2 in the brain of the adult rat, as detected by immunohistochemical analyses performed with a highly specific anti-5αR2 polyclonal antibody. In addition, the distribution of this enzyme in neurons, glia and GABAergic cells were carried by double-labeling immunostaining, analyzed by CLSM. Finally, we visualized the presence of 5αR2 in cortical pyramidal neurons by means of the simultaneous Golgi-Cox and immunofluorescence staining.
2. Methods
2.1. Animals
Male Sprague–Dawley rats (220-250 g; Charles River, Como, Italy) were used in all experiments. Animals were housed in groups of four at a temperature of 24 °C and with 60% humidity under a 12-h light/dark cycle (lights on from 0700 to 1900h). All experimental procedures were conducted between 0900h and 1300h, with methods aimed at minimizing environmental stress, in view of its impact on brain 5αR2 expression (Sanchez et al, 2009; Bortolato et al, 2011). Experiments were carried out in accordance with the guidelines of the European Communities Directive of 24 November 1986 (86/609/EEC) and the Italian Legislation (D.P.R. 116/92).
2.2. Brain tissue preparation
Rats were deeply anaesthetized with Equithesin (0.97 g pentobarbital, 2.1 g magnesium sulphate, 4.25 g chloral hydrate, 42.8 mL propylene glycol, 11.5 mL ethanol 90%, 5 mL·kg-1, intraperitoneal) and transcardially perfused with 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M phosphate-buffered saline (PBS, pH 7.4). Brains were rapidly removed and post-fixed in the same fixative for 6h. After repeated washing in 0.1 M PBS, brains were cryoprotected in 30% sucrose in PBS for 48h. Whole brains were cut with a cryostat either in coronal or parasagittal planes at levels containing the selected brain areas for subsequent free-floating immunostaining processing. Adjacent sections were collected and stained with Neutral Red to facilitate the identification of the selected brain areas.
2.3. 5αR2 immunofluorescent staining
Localization of 5αR2 in the rat brain was analyzed by single-labeling immunofluorescence. Pre-blocking of tissue sections was performed with 10% normal goat serum, 2% bovine serum albumin (BSA) and 0.3% Triton X-100 in PBS for 1h at room temperature. Sections were incubated for 48h at 4 °C with rabbit anti-5αR2 polyclonal antibody (1:1000) directed against the human carboxyterminal 25 amino acids of 5αR2 (amino acid residues 227-251) (Thigpen et al, 1993) and kindly supplied by Dr. Russell, Southwestern Medical Center Dallas, TX. The specificity and effectiveness of this polyclonal antibody, as well as its lack of cross-reactivity for 5αR1 in rats, have already been extensively validated by immunoblotting, Western blotting and immunohistochemical experiments (Thigpen et al., 1993; Silver et al., 1994; Levine et al., 1996; Patte-Mensah et al, 2004). After washing in PBS-0.3% Triton X-100, sections were incubated for 1h at room temperature with biotinylated goat anti-rabbit IgG (1:200, Vector Laboratories, Burlingame, CA, USA). Subsequently, sections were incubated with Streptavidin Alexa Fluor® 594 (1:1000) for 1h in the dark at room temperature.
2.4. Double immunofluorescence labeling
To characterize 5αR2-immunoreactive cells double-fluorescence labeling experiment was performed with Glial Fibrillary Acidic Protein (GFAP) as a glial marker, and Neuron-Specific Nuclear Protein (NeuN) as a neuronal marker. Glutamic acid decarboxylase-67 (GAD-67) was employed as a specific marker for GABAergic neurons and axons. To double-label 5αR2 immunoreactive cells, sections were incubated for 48h at 4 °C with a selected combination of primary antibodies including rabbit polyclonal antibody anti-5αR2 (1:1000) plus a mouse monoclonal antibody anti-GFAP (1:5000, Chemicon International, Temecula, CA, USA), or anti-NeuN (1:1000, Millipore, Chemicon, International, Temecula, CA, USA), or anti-GAD-67 (1:5000, Chemicon, International, Temecula, CA, USA). Sections were washed in PBS-0.3% Triton X-100 and then incubated for 1h at room temperature with biotinylated goat anti-rabbit IgG (1:200, Vector Laboratories, CA, USA), as second antibody to the 5αR2 antibody. Sections were subsequently incubated in a mixture containing Streptavidin Alexa Fluor® 594 (1:1000, Molecular Probes, Eugene, OR, USA) plus Alexa Fluor®/488 labeled goat anti-mouse (1:500, Molecular Probes, Eugene, Oregon, USA) for 2h in the dark at room temperature.
After incubations in secondary antisera, the tissue sections were rinsed and mounted with an antifading solution containing 200 mg/ml of 4′, 6-diamidino-2-phenylindole (DAPI) as a nuclei counterstain.
Standard control experiments were performed by omission of either the primary or secondary antibody, and yielded no cellular labeling.
2.5. Imaging
All observations were made using an Olympus IX 61 microscope equipped with 2.5, 4, 10, 20 and 60× planapochromatic oil immersion objectives. Images were taken with a 12-bit cooled F View II camera (Olympus, Hamburg, Germany). The digital resolution of images taken with the 60× objective was 0.1 μm/pixel. Excitation light was attenuated with a 6% transmittance neutral density filter. Color compositions were made using images of single antibodies as RGB channels. After being captured on the computer, images were analyzed using the Cell P AnalySIS® software module.
2.6. Golgi-Cox and immunofluorescence procedure
After perfusion, brains were carefully removed, postfixed in 4% paraformaldehyde (pH 7.4) overnight at 4 °C and processed as described in Spiga et al.(2011). At the end of the Golgi-Cox procedure, slices were collected in PBS for the following free-floating immunostaining.
Slices were rinsed in PBS (3 × 10 min). To prevent non-specific binding, slices were pre-incubated in 5% normal goat serum (NGS) containing 5% bovine serum albumin (BSA) and 0.5% Triton X-100 in PBS overnight at 4 °C. Slices were incubated for 48h at 4°C with rabbit polyclonal antibody anti-5αR2 (1:1000), then washed (3 × 10 min) in PBS and incubated with biotinylated anti-rabbit IgG antibody (1:200, Vector Laboratories, Burlingame, CA) in PBS for 4h at room temperature (RT). Following washing 3 × 10 min in PBS, slices were incubated in PBS for 4h at RT with Streptavidin Alexa Fluor® 594 (1:500, Molecular Probes, Eugene, Oregon, USA), washed 3 × 10 min in PBS and coverslipped with Vectashield (Vector Laboratories, Burlingame, CA).
2.7. Laser scanning confocal microscopy and image processing
Leica 4-D CLSM (Leica Microsystems, Heidelberg, Germany) with an Argon-Krypton laser was used to analyze the 5αR2-positive neurons and impregnated tissue. Co-localization analyses were performed between 5αR2, GFAP, NeuN and GAD67 (Bitplane Imaris 7.2). Confocal images were generated using PL Fluotar 10× (na. 0.3), 40 × oil (na. 1.00) and (e) 100 × oil (na. 1.3). Optical sections, usually at consecutive intervals of 0.5 μm in z-axis, were imaged through the depth of the labeled neurons and saved as image stacks as previously described (Spiga et al. 2005). Maximum intensity algorithm (ImageJ) was used for three-dimensional (3-D) reconstructions of 5αR2-, GAD67-, GFAP- and NeuN-immunolabelled cells, while extended focus algorithm was used for 3-D reconstructions of Golgi–Cox-stained neurons (Bitplane Imaris V7.2).
3. Results
3.1 Immunohistochemical and regional distribution of 5αR2
Parasagittal sections observed at low magnification and labeled for the 5αR2 antiserum revealed a widespread distribution of 5αR2 immunoreactivity throughout the rat brain (Fig. 1A). The immunolabeling completely disappeared in all brain areas after omission of the primary antibody (Fig. 1B). As shown both in parasagittal and coronal sections (Figs. 1, 2) the distribution of 5αR2 displayed marked heterogeneity among different brain areas. Strong 5αR2-immunolabeled cells were observed in all neocortical areas with the most intense labeling found in the frontal and somatosensory cortex (Figs. 1A, 2). Dense immunoreactivity was also present in the olfactory bulb, in the thalamic nuclei, in the basolateral amygdala and in the CA3 field of the hippocampus. Moderate to low 5αR2 levels were seen in the hypothalamus, in several midbrain structures, including the substantia nigra, as well as the pontine, medial and dorsal raphe nuclei. On the contrary, 5αR2 immunoreactivity was intense in the locus coeruleus and in the Purkinje cells of the cerebellum. A more detailed description of 5αR2 expression pattern is given in the following sections. The intensity of labeling of cell bodies throughout the CNS was scored as negative (-), low (+), moderate (++) and intense (+++) and summarized in Table 1 (see supplementary material).
Fig. 1. Photomicrographs showing 5αR2 immunoreactivity in parasagital section of the rat brain.
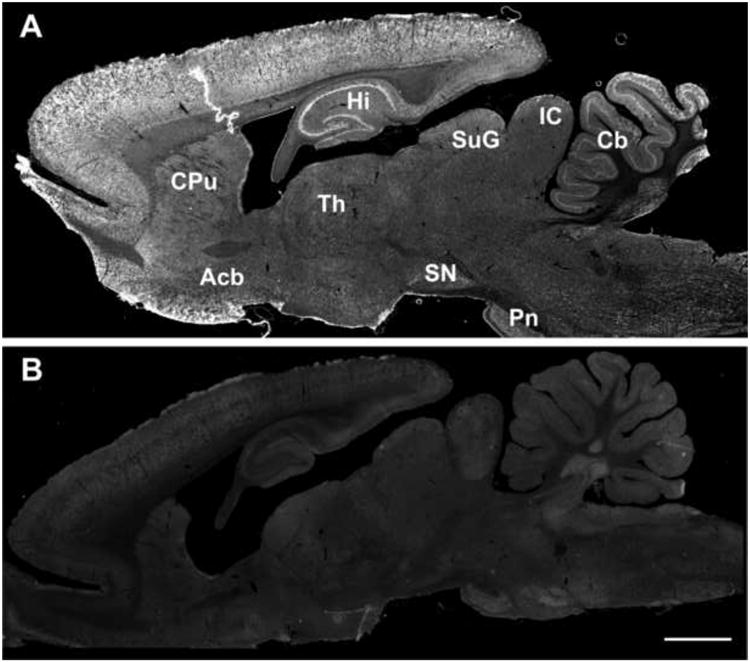
S5αR2 immunolabeling revealed a widespread distribution of 5αR2 through the brain (A); immunolabeling was completely abolished when the secondary antibody was omitted (B). Scale bars: 2000 μm.
Fig. 2. Comparison of 5αR2 immunoreactivity between different rat brain areas.
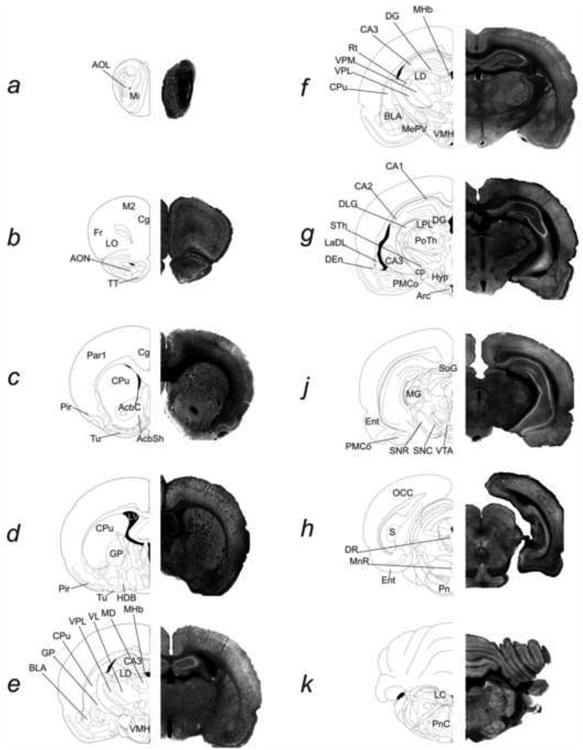
5αR2 immunolabelling showed intense immunoreactivity in the olfactory structures (a), in all cortical areas such as frontal, motor, cingulate (b), parietal, piriform (c,d) and entorhinal (j); intense 5αR2-IR is also observed in the CA3 field of the hippocampus (e,f), in the thalamic areas (e-j), in the locus coeruleus (k) and in the cerebellum (k). Moderate 5αR2 immunoreactivity is seen: in the basal ganglia (CPu, AcC andAcSh, GP) (c-e), the amygdaloid nuclei (e-j), the CA1-CA2 of fields of Ammon's horn (g, j), ventral tegmental area and the substantia nigra (J). As shown in panel j, 5αR2-IR is weak in the superior colliculus, SuG. For abbreviations, see list. (see supplementary material).
3.1.1 Cerebral cortex
5αR2 immunoreactivity was detected in neuronal somata throughout the neocortex and varied from moderate to strong intensity depending on the region analyzed, as well as on the particular layer (Fig. 2, panels b-j). A more intense cell-body 5αR2 immunoreactivity was expressed in orbital, frontal, cingulate area 1 and 3, parietal, piriform and enthorinal cortices, whereas motor and insular cortex showed a moderate 5αR2 staining. In layers II, III and V 5αR2 immunoreactivity was predominantly expressed on the cell bodies of larger pyramidal neurons (Fig. 3, panels A1, A2; Fig. 5, panels C3-C5), while few or no 5αR2-positive neurons were identified in layer I (Fig. 3, panels A1, A2).
Fig. 3. 5αR2 immunofluorescence in the rat prefrontal cortex (Cg1, Cg3), in the caudate-putamen and in the CA3 field of Ammon's horn.
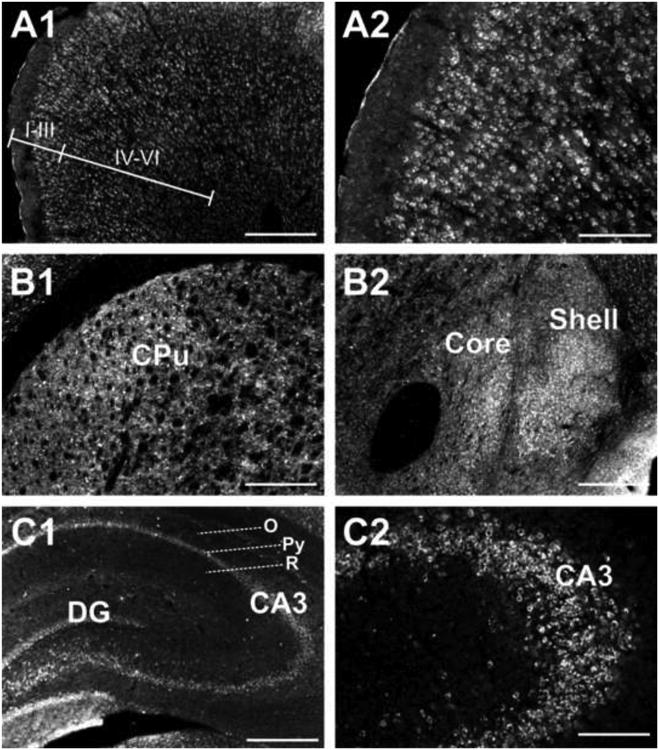
5αR2 immunoreactivity is detected in layers II-III and V pyramidal neurons, while little or no immunoreactivity is observed in layer I neurons. I to VI identify the cortical layers (A1); higher magnification of 5αR2 immunoreactivity in the Cg1 layer neurons (A2); photomicrographs at low magnification showing sparse 5αR2-immunolabeled neurons in the dorsal portion of CPu (B1) and in the core and shell of nucleus accumbens (B2); 5αR2 immunoreactivity is present throughout hippocampal regions (C1) with the CA3 showing the most intense staining in CA3 pyramidal neurons (C2). For abbreviations, see list. (see supplementary material). Scale bars: 500 μm in A1, B1, B2, C1; 200 μm in A2, C2.
Fig. 5. 5αR2 co-localizes in pyramidal neurons but not in glial cells of the rat prefrontal cortex.
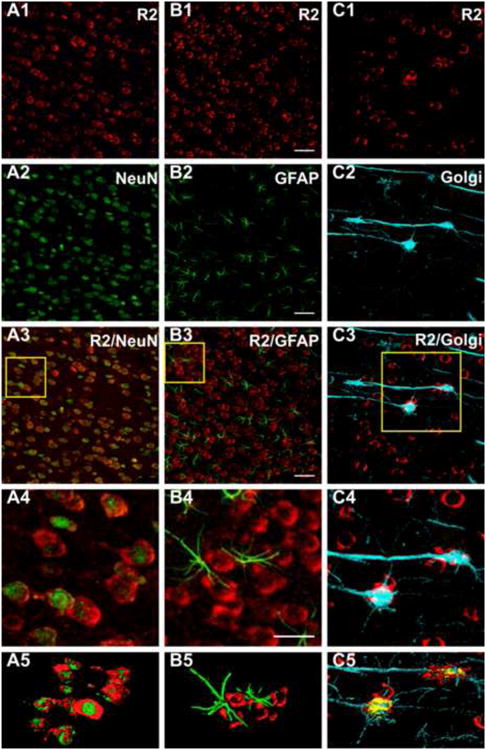
CLMS images showing strong 5αR2-immunolabeled neurons (R2) (red) (A1, B1, C1) and NeuN (A2-A5) and GFAP (B2-B5) (green) immunofluorescence in the prefrontal cortex (PFC). A3: merge of A1 and A2; B3: merge of B1 and B2; A4 and B4: higher power of the respective boxed area displaying the presence of 5αR2 immunoreactivity throughout the cytoplasm of neuronal cells (A4), but not in glial cells (B4). C2: Golgi-Cox impregnated pyramidal neurons. C3: merge of C1 and C2; C4: higher power of the boxed area displaying the co-localization of R2 with Golgi-Cox impregnated pyramidal neurons. A5, B5, C5 channels surface rendering for R2 and NeuN (A5), for R2 and GFAP (B5) and R2 immunofluorescence and Golgi-Cox (C5). Scale bars: 50 μm in A1-C3, 100 μm in A4, B4, C4. For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.
3.1.2 Basal ganglia
The basal ganglia exhibited low to moderate expression of 5αR2 cellular immunolabeling with the exception of the globus pallidus which displays strong 5αR2 immunoreactivity (Fig. 2, panels d-e). As shown in Figure 3 (panel B1), only few scattered positive 5αR2 cells were present in the dorsal portion of the caudate. In the nucleus accumbens, 5αR2 immunoreactivity was observed in the cell bodies with a more intense labeling in the shell rather than in the core (Fig. 3, panel B2).
3.1.3 Hippocampal formation
As shown in Figure 2 (panels e-j), the detection signal for 5αR2-immunolabeled cells was observed throughout the hippocampus. The most intense immunoreactive cells were in the pyramidal cell layer of the CA3 subfield of Ammon's horn, while only few positive cells were labeled in the strata oriens and radiatum (Fig. 3, panels C1, C2). The CA1-CA2 subfields showed moderate 5αR2 staining while in the dentate gyrus only faint immunostaining was seen.
3.1.4 Amygdala
The different nuclei of the amygdala expressed different intensities of 5αR2. The highest density was found in the basolateral anterior amygdaloid nuclei, the lowest in the lateral and cortical amygdaloid nuclei (Fig. 2, panels e-g; Fig. 4, panel A).
Fig. 4. 5αR2 immunofluorescence in selected brain regions.
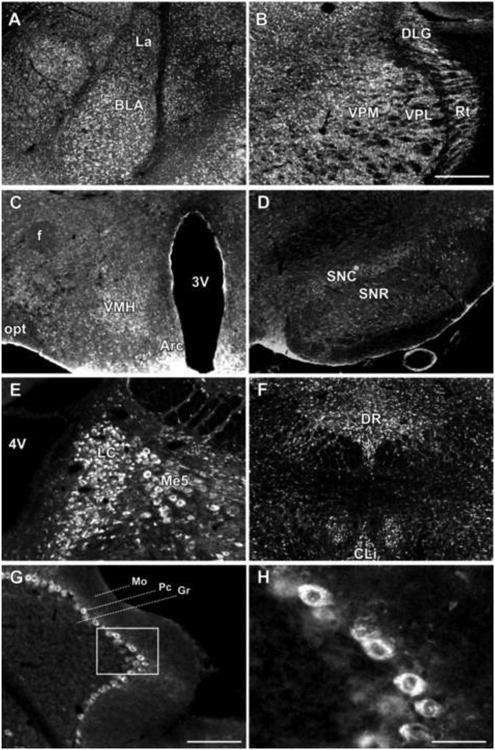
The lateral and basolateral amygdaloid nuclei are characterized by neurons displaying strong staining for 5αR2 (A); 5αR2-immunolabeled neurons are also expressed in the VPM, VPL, DLG and Rt thalamic nuclei (B), in VMH and Arc nuclei of hypothalamus (C), in SNC and SNR (D), in LC (E), in DR (F), and in the cerebellum (G). H, Higher power of the respective boxed area in G revealing 5αR2-immunolabeled Purkinje cells. For abbreviations, see list. (see supplementary material). Scale bars: 500 μm in A-F, 200 μm in G and 50 μm in H.
3.1.5 Thalamus
5αR2 immunoreactivity was particularly intense in the cell bodies throughout the thalamus (Fig. 2, panels e-g). Immunostaining was detected in the majority of nuclei, especially in the ventral postero-lateral and -medial nuclei, reticular nuclei and dorsal lateral geniculate nuclei (Fig 4, panel B). Somewhat less intense 5αR2 immunoreactivity was seen in the ventral, medial and dorsal nuclei.
3.1.6 Hypothalamus
Moderate 5αR2 immunoreactivity, consisting of cell body labeling, was observed in the paraventricular nucleus, ventromedial and arcuate nuclei (Fig. 4, panel C).
3.1.7 Midbrain
5αR2-staining was low to moderate in the superior and inferior colliculus as well as in the ventral tegmental area. In the substantia nigra, 5αR2 immunoreactivity was apparent in both the pars reticulata and pars compacta, where densely labeled cell bodies were observed (Fig. 4D).
3.1.8 Rhombencephalon and cerebellum
Intense 5αR2 labeling was seen in the cell somata of the locus coeruleus and, to a lesser extent, in the cell bodies of the mesencephalic trigeminal nucleus (Fig. 4, panel E). All vestibular nuclei, the olivary nuclei, and the medial and dorsal raphe nuclei exhibited moderate 5αR2-positive somatic immunostaining. Furthermore, labeling of 5αR2 revealed a clear pattern of staining throughout the cerebellar cortex. Strong staining was selectively localized in the somata of Purkinje neurons; in contrast, molecular and granule cell layers were mostly devoid of cell body labeling (Fig. 4, panels G, H,)
3.2 5αR2 cell type identification
To characterize the type of 5αR2-expressing cells, we combined double labeling experiments using specific markers for glial (anti-GFAP) and neuronal (anti-NeuN) cells, with CLSM analysis in the prefrontal cortex. Double immunofluorescence with anti-5αR2 and anti-NeuN antibodies demonstrated that 5αR2 was almost completely co-localized with NeuN (Fig. 5, panels A3, A4). Consistently, surface rendering analysis showed nuclear and cytoplasmatic immunofluorescence for NeuN and 5αR2, respectively. On the contrary, we failed to observe co-localization of 5αR2 with GFAP (Fig. 5, panels B3, B4), and surface rendering analysis did not reveal appreciable 5αR2 immunofluorescence in GFAP-positive cells.
Furthermore, to identify pyramidal neurons positive for 5αR2 we used an innovative procedure based on the application of Golgi-Cox impregnation and immunofluorescent staining on the same tissue sections. This approach allows the simultaneous visualization of neuronal structural details and the antigen's characterization (Spiga et al, 2011). As shown in Figure 5, both 5αR2-positive and Golgi-Cox-impregnated elements in the prefrontal cortex were simultaneously visualized by CLMS. Golgi-Cox impregnation offered a detailed representation of neuronal structures (Fig. 5, panel C2). As shown in panels C3-C5 of Figure 5, 5αR2 was found within the somata of pyramidal neurons.
Finally, double immunostaining with anti-5αR2 and anti-GAD67 showed the presence of 5αR2 in GABAergic neurons in the prefrontal cortex, caudate-putamen and stratum radiatum of the hippocampus. Contrary to the abundant presence of 5αR2 in pyramidal glutamatergic neurons, only few GABAergic neurons were positive for 5αR2 (Fig 6, panels A1-5, B1-5, C1-5)
Fig. 6. 5αR2 colocalizes in GABAergic neurons in rat prefrontal cortex, caudate-putamen and hippocampus.
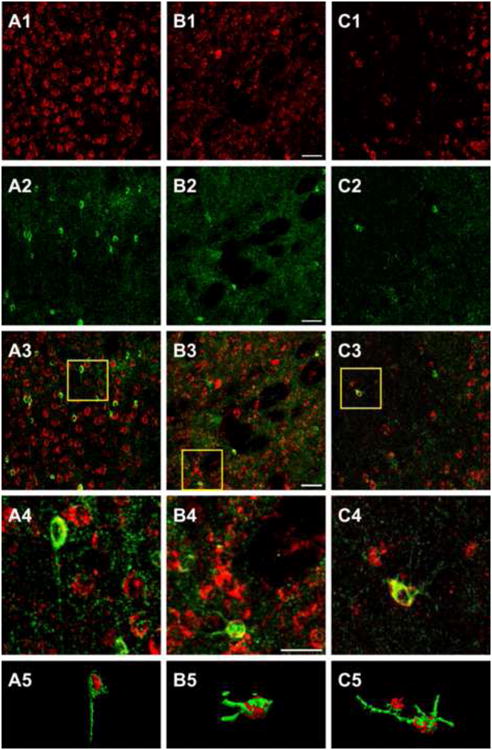
CLMS images of rat prefrontal cortex (A), caudate-putamen (B) and hippocampus, CA3 field of Ammon's horn (C), showing 5αR2-immunolabeled neurons (R2) (red) and GAD67 immunofluorescence (green). A3, B3 and C3: merge of A1-A2, B1-B2 and C1-C2, respectively. A4, B4 and C4 higher power of the respective boxed area displaying the co-localization of 5αR2-immunolabeled neurons with GAD67, a specific marker for GABAergic neurons. The R2-GAD67 positive neuron inside the box in C3 is localized in hippocampal stratum radiatum. A5, B5, C5 channels surface rendering for R2 and GAD67 immunofluorescence. Scale bars: 50 μm in A1-C3, 100 μm in A4, B4 and C4. For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.
4. Discussion
The findings of the present study indicate that the enzyme 5αR2 is widely distributed across most key regions of the adult rat brain, ranging from the forebrain to the brainstem and cerebellum. In particular, we found the highest 5αR2 immunoreactivity in the cortex, olfactory bulb, hippocampus and cerebellum. Our results substantially confirm the in situ hybridization data on 5αR2 distribution reported in the Allen Mouse Brain Atlas (http://mouse.brain-map.org/gene/show/60858). Additionally, these findings extend previous evidence documenting the expression of 5αR2 transcript or protein in specific brain regions of adult rats with a number of complementary methodological approaches, including Northern Blotting, RT-PCR, Western blotting and immunohistochemical techniques (Normington and Russell, 1992; Sanchez et al., 2008, 2009; Kimoto et al, 2010; Bortolato et al, 2011). Given that the content of 5αR2 is significantly lower than 5αR1 (Normington and Russell, 1992; Lephart, 1993), the detection of 5αR2 has been enabled by the employment of antisera with high specificity for this target, which had already been successfully used to localize it in the spinal cord and other steroidogenic tissues (Thigpen et al., 1993; Silver et al., 1994; Levine et al., 1996; Patte-Mensah et al, 2004).
Previous studies have shown that, although 5αR1 and 5αR2 are both able to catalyze the same reaction, the latter has a much higher affinity for androgens, and its physiological functions may specifically serve the conversion of androstenedione and testosterone to their 5α-reduced metabolites, 5α-androstanedione and DHT (Jin and Penning, 2001). The preference of 5αR2 for androgen metabolism is also indirectly suggested by converging lines of evidence, indicating that its transcription is facilitated by testosterone and DHT through activation of androgen receptors (Melcangi et al., 1998). Accordingly, the ontogenetic trajectory of brain 5αR2 expression has been shown to follow the secretory profile of testosterone and androgen receptors, with a peak in perinatal life followed by a time-dependent decline (Meaney et al, 1985; Poletti et al, 1998). Building on these premises, the expression of 5αR2 in multiple regions of the adult brain helps explain the occurrence of 5α-reduced androgens in cerebral tissues of vertebrates (Frye et al, 2001; Do Rego et al, 2009). Specifically, the localization of 5αR2 in the major output neurons of key corticolimbic structures, such as prefrontal cortex, amygdala, striatum and hippocampus, is in agreement with previous findings documenting the role of 5α-reduced androgens in the modulation of emotion, motivation and cognitive functions (Frye et al., 2002; Frye and Edinger, 2004; Edinger and Frye, 2005).
The localization of 5αR2 appears to largely overlap with those of other key enzymes for the synthesis and metabolism of androgens in the brain. Indeed, the enzyme cytochrome P450C17 (17α-hydroxylase/C17.20 lyase), which catalyzes the conversion of pregnenolone and progesterone into dehydroepiandrosterone (DHEA) and androstenedione, respectively, was documented in the pyramidal neurons in the CA1-CA3 hippocampal regions, granule cells of the dentate gyrus (Hojo et al, 2004; Kawato et al., 2002), as well as Purkinje cells of the cerebellar cortex (Zwain and Yen, 1999). Similarly, 3α-hydroxysteroid dehydrogenase (3α-HSD), the enzyme that converts DHT into 3α-diol, has been documented in the olfactory bulb, cortex, Purkinje cells of the cerebellum and hypothalamus (Compagnone and Mellon, 2000); furthermore, this enzyme has also been documented in the CA1-CA3 fields and granule cells of the dentate gyrus in the hippocampus in the adult mouse (Agis-Balboa et al, 2006).
The distribution of 5αR2 in the rat brain is also strikingly similar to that reported for aromatase, the enzyme that converts androstenedione and testosterone into estrone and estradiol, respectively. In rats, aromatase has been detected in the neocortex, amygdaloid structures, the CA1-CA3 region and dentate gyrus of the hippocampus and in the paraventricular and arcuate nuclei of the hypothalamus (Roselli et al, 1985; Sanghera et al, 1991; Jakab et al, 1993; Hojo et al, 2004). These findings suggest that aromatase and 5αR2 may be co-localized in the same region to finely regulate the metabolic pathways of androstenedione and testosterone towards the production of potent androgens or estrogens.
Conversely, the observed pattern of 5αR2 distribution in the rat brain lies in sharp contrast with that of 5αR1. While our results show that 5αR2 is consistently expressed in neurons and absent in glial cells, previous studies documented that, in rats, 5αR1 is typically localized in the cytosol of type I astrocytes and oligodendrocytes in the cerebral and cerebellar cortices, thalamus, hypothalamus and circumventricular organs (Pelletier et al. 1994; Tsuruo et al., 1996; Kiyokage et al., 2005). The stark anatomical dichotomy between glial 5αR1 and neuronal 5αR2 expressions may reflect the differential roles of these two isoenzymes in the modulation of brain functions. Furthermore, the sharp contrast between the distribution patterns of 5αR isoforms further shows the high specificity of the antibodies used in our study, in line with previous observations (Patte-Mensah et al, 2004).
Our CLSM analyses on double immunofluorescence and Golgi-Cox staining revealed that 5αR2 is localized mainly in cortical glutamatergic pyramidal neurons and few GABAergic neurons in the prefrontal cortex, in caudal portion of the caudate-putamen, and in the stratum radiatum of the hippocampus.
In addition to its role on androgen metabolism, 5αR2 may participate in the synthesis and metabolism of AP and other neurosteroids. These mediators are implicated in a wide array of functions, encompassing the regulation of survival and differentiation of neuronal and glial cells, modulation of neurotransmission and orchestration of behavioral responses (see Reddy, 2010). Our results highlight the possibility that the presence of 5αR2 across most brain regions may also reflect the contribution of this enzyme in the regulation of multiple brain and behavioral processes.
In particular, the localization of 5αR2 in the prefrontal cortex, basal ganglia, basolateral amygdala and hippocampus is consistent with a role of this enzyme in stress modulation (Girdler and Klatzkin, 2007). Accordingly, short-term stressors have been shown to enhance the synthesis of 5αR, as well as AP and other neurosteroids (Purdy et al., 1991; Barbaccia et al, 1996, 2001; Sanchez et al., 2008, 2009), while multiple chronic stress regimens yield opposite effects in corticolimbic regions (Dong et al., 2001; Agis-Balboa et al, 2007; Bortolato et al, 2011).
One of the most intriguing aspects of the present characterization is the potential mapping of the sites of action of finasteride, the prototypical 5αR2 inhibitor, in the brain. Recent human studies have shown that this drug has psychotropic effects, which may be harnessed in the therapy of several neuropsychiatric disorders, including Tourette syndrome (Bortolato et al, 2007) schizophrenia (Koethe et al, 2008) and pathological gambling in Parkinson's disease patients (Bortolato et al, 2012). The aforementioned clinical data parallel preclinical evidence in rodent models, indicating that the antipsychotic-like properties of this drug in rat models, which may be mediated by the attenuation of signaling cascades of postsynaptic dopaminergic receptors in the nucleus accumbens (Devoto et al, 2011 in press).
One of the major limitations of our study stems from our lack of data on the expression of 5αR2 in females as well as in other developmental stages. Indeed, we cannot exclude that 5αR2 patterns of distribution may exhibit marked gender and age differences; this possibility is supported by previous findings, showing 5αR2 up-regulation in response to activation of androgen receptors by testosterone and DHT. Although the analysis of 5αR2 distribution in females and early developmental stages remains outside the scope of the present study, future studies will be needed to address these critical issues. Furthermore, caution should be advocated in extending the present results to other species. For example, human 5αR1immunoreactivity has been documented not only in glia, but also in cortical pyramidal and granular neurons (Aumuller et al, 1996); conversely 5αR2 has been identified in pyramidal cells, but not in small neurons and glia (Eicheler et al, 1994; Aumuller et al, 1996). In mice, 5αR1 was only observed in neurons, but not glial cells, across the neocortex, hippocampus, striatum, thalamus and cerebellum (Agis-Balboa et al, 2006). Further studies on the specific functional roles of 5αRs in different mammalian species are warranted to further characterize the reciprocal roles and interactions of these isoenzymes in the regulation of steroid homeostasis.
In summary, the present findings highlight that the patterns of anatomical localization of 5αR2 in the adult brain are distinct from those of 5αR1, further supporting a possibly functional dichotomy between these two isoenzymes. Furthermore, the observed distribution pattern of 5αR2 appears to closely overlap with those of other key enzymes related to the synthesis and metabolism of androgens in the brain, providing further insight on the role of this molecule in the regulation of these neurosteroids throughout the entire lifespan of the individual. Future studies are warranted to explore the physiological role of 5αR2 in the modulation of brain functions, as well as its potential as a therapeutic target for neuropsychiatric disorders.
Supplementary Material
References
- Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci USA. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agís-Balboa RC, Pinna G, Pibiri F, Kadriu B, Costa E, Guidotti A. Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proc Natl Acad Sci USA. 2007;104:18736–18741. doi: 10.1073/pnas.0709419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumüller G, Eicheler W, Renneberg H, Adermann K, Vilja P, Forssmann WG. Immunocytochemical evidence for differential subcellular localization of 5 alpha-reductase isoenzymes in human tissues. Acta Anat. 1996;156:241–252. doi: 10.1159/000147852. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Bolacchi F, Concas A, Mostallino MC, Purdy RH, Biggio G. Stress-induced increase in brain neuroactive steroids: antagonism by abecarnil. Pharmacol Biochem Behav. 1996;54:205–210. doi: 10.1016/0091-3057(95)02133-7. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Serra M, Purdy RH, Biggio G. Stress and neuroactive steroids. Int Rev Neurobiol. 2001;46:243–272. doi: 10.1016/s0074-7742(01)46065-x. [DOI] [PubMed] [Google Scholar]
- Beyer C, Hutchinson JB. Androgens stimulate the morphological maturation of embryonic hypothalamic aromatase-immunoreactive neurons in the mouse. Dev Brain Res. 1997;98:74–81. doi: 10.1016/s0165-3806(96)00170-8. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Cannas A, Solla P, Bini V, Puligheddu M, Marrosu F. Finasteride Attenuates Pathological Gambling in Patients With Parkinson Disease. J Clin Psychopharmacol. 2012;32:424–425. doi: 10.1097/JCP.0b013e3182549c2a. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Devoto P, Roncada P, Frau R, Flore G, Saba P, Pistritto G, Soggiu A, Pisanu S, Zappala A, Ristaldi MS, Tattoli M, Cuomo V, Marrosu F, Barbaccia ML. Isolation rearing-induced reduction of brain 5α-reductase expression: relevance to dopaminergic impairments. Neuropharmacology. 2011;60:1301–1308. doi: 10.1016/j.neuropharm.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Muroni A, Marrosu F. Treatment of Tourette's syndrome with finasteride. Am J Psychiatry. 2007;164:1914–1915. doi: 10.1176/appi.ajp.2007.07060978. [DOI] [PubMed] [Google Scholar]
- Breedlove SM. Sexual dimorphism in the vertebrate nervous system. J Neurosci. 1992;12:4133–4142. doi: 10.1523/JNEUROSCI.12-11-04133.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- Devoto P, Frau R, Bini V, Pillolla G, Saba P, Flore G, Corona M, Marrosu F, Bortolato M. Inhibition of 5α-reductase in the nucleus accumbens counterssensorimotor gating deficits induced by dopaminergic activation. Psychoneuroendocrinology. 2011 doi: 10.1016/j.psyneuen.2011.09.018. http://dx.doi.org/10.1016/j.psyneuen.2011.09.018. [DOI] [PMC free article] [PubMed]
- Do Rego JL, Seong JY, Burel D, Luu-The V, Larhammar D, Tsutsui K, Pelletier G, Tonon MC, Vaudry H. Steroid biosynthesis within the frog brain: a model of neuroendocrine regulation. Ann N Y Acad Sci. 2009;1163:83–92. doi: 10.1111/j.1749-6632.2008.03664.x. [DOI] [PubMed] [Google Scholar]
- Dong E, Matsumoto K, Uzunova V, Sugaya I, Takahata H, Nomura H, Watanabe H, Costa E, Guidotti A. Brain 5alpha-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc Natl Acad Sci USA. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Testosterone's anti-anxiety and analgesic effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Psychoneuroendocrinology. 2005;30:418–430. doi: 10.1016/j.psyneuen.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Eicheler W, Tuohimaa P, Vilja P, Adermann K, Forssmann WG, Aumüller G. Immunocytochemical localization of human 5 alpha-reductase 2 with polyclonal antibodies in androgen target and non-target human tissues. J Histochem Cytochem. 1994;42:667–675. doi: 10.1177/42.5.8157936. [DOI] [PubMed] [Google Scholar]
- Frye CA, Edinger KL. Testosterone's metabolism in the hippocampus may mediate its anti-anxiety effects in male rats. Pharmacol Biochem Behav. 2004;78:473–481. doi: 10.1016/j.pbb.2004.04.019. [DOI] [PubMed] [Google Scholar]
- Frye CA, Park D, Tanaka M, Rosellini R, Svare B. The testosterone metabolite and neurosteroid 3alpha-androstanediol may mediate the effects of testosterone on conditioned place preference. Psychoneuroendocrinology. 2001;26:731–750. doi: 10.1016/s0306-4530(01)00027-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Rosellini R, Svare B. The nucleus accumbens as a site of action for rewarding properties of testosterone and its 5alpha-reduced metabolites. Pharmacol Biochem Behav. 2002;74:119–127. doi: 10.1016/s0091-3057(02)00968-1. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Klatzkin R. Neurosteroids in the context of stress: implications for depressive disorders. Pharmacol Ther. 2007;116:125–139. doi: 10.1016/j.pharmthera.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LA, Sengelaub DR. Differential effects of dihydrotestosterone and estrogens on the development of motoneuron morphology in a sexually dimorphic rat spinal cord. J Neurobiol. 1994;25:878–892. doi: 10.1002/neu.480250711. [DOI] [PubMed] [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab RL, Horvath TL, Leranth C, Harada N, Naftolin F. Aromatase immunoreactivity in the rat brain: gonadectomy-sensitive hypothalamic neurons and an unresponsive “limbic ring” of the lateral septum-bed nucleus-amygdala complex. J Steroid Biochem Mol Biol. 1993;44:481–498. doi: 10.1016/0960-0760(93)90253-s. [DOI] [PubMed] [Google Scholar]
- Jin Y, Penning TM. Steroid 5alpha-reductases and 3alpha-hydroxysteroid dehydrogenases: key enzymes in androgen metabolism. Best Pract Res Clin Endocrinol Metab. 2001;15:79–94. doi: 10.1053/beem.2001.0120. [DOI] [PubMed] [Google Scholar]
- Kawato S, Hojo Y, Kimoto T. Histological and metabolism analysis of P450 expression in the brain. Methods Enzymol. 2002;357:241–249. doi: 10.1016/s0076-6879(02)57682-5. [DOI] [PubMed] [Google Scholar]
- Kimoto T, Ishii H, Higo S, Hojo Y, Kawato S. Semicomprehensive analysis of the postnatal age-related changes in the mRNA expression of sex steroidogenic enzymes and sex steroid receptors in the male rat hippocampus. Endocrinology. 2010;151:5795–5806. doi: 10.1210/en.2010-0581. [DOI] [PubMed] [Google Scholar]
- Kiyokage E, Toida K, Suzuki-Yamamoto T, Ishimura K. Localization of 5alpha-reductase in the rat main olfactory bulb. J Comp Neurol. 2005;493:381–395. doi: 10.1002/cne.20760. [DOI] [PubMed] [Google Scholar]
- Koethe D, Bortolato M, Pomelli D, Leweke FM. Improvement of general symptoms in a chronic psychotic patient treated with finasteride: case report. Pharmacopsychiatry. 2008;41:115–116. doi: 10.1055/s-2008-1058110. [DOI] [PubMed] [Google Scholar]
- Langlois VS, Zhang D, Cooke GM, Trudeau VL. Evolution of steroid-5alpha reductases and comparison of their function with 5beta-reductase. Gen Comp Endocrinol. 2010;166:489–497. doi: 10.1016/j.ygcen.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Lephart ED. Brain 5alpha-reductase: cellular, enzymatic, and molecular perspectives and implications for biological function. Mol Cell Neurosci. 1993;4:473–484. doi: 10.1006/mcne.1993.1059. [DOI] [PubMed] [Google Scholar]
- Levine AC, Wang JP, Ren M, Eliashvili E, Russell DW, Kirschenbaum A. Immunohistochemical localization of steroid 5 alpha-reductase 2 in the human male fetal reproductive tract and adult prostate. J Clin Endocrinol Metab. 1996;81:384–389. doi: 10.1210/jcem.81.1.8550782. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Jensen LK, McGinnis MY, McEwen BS. Nuclear and cytosolic androgen receptor levels in the limbic brain of neonatal male and female rats. Brain Res. 1985;355:179–185. doi: 10.1016/0165-3806(85)90039-2. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Ballabio M, Cavarretta I, Gonzalez LC, Leonelli E, Veiga S, Martini L, Magnaghi V. Effects of neuroactive steroids on myelin of peripheral nervous system. J Steroid Biochem Mol Biol. 2003;85:323–327. doi: 10.1016/s0960-0760(03)00228-0. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Poletti A, Cavarretta I, Celotti F, Colciago A, Magnaghi V, Motta M, Negri-Cesi P, Martini L. The 5alpha-reductase in the central nervous system: expression and modes of control. J Steroid Biochem Mol Biol. 1998;65:295–299. doi: 10.1016/s0960-0760(98)00030-2. [DOI] [PubMed] [Google Scholar]
- Normington K, Russell DW. Tissue distribution and kinetic characteristics of rat steroid 5 alpha-reductase isozymes. Evidence for distinct physiological functions. J Biol Chem. 1992;267:19548–19554. [PubMed] [Google Scholar]
- Paba S, Frau R, Godar SC, Devoto P, Marrosu F, Bortolato M. Steroid 5α-reductaseas a novel therapeutic target for schizophrenia and other neuropsychiatric disorders. Curr Pharm Des. 2011;17:151–167. doi: 10.2174/138161211795049589. [DOI] [PubMed] [Google Scholar]
- Patte-Mensah C, Penning TM, Mensah-Nyagan AG. Anatomical and cellular localization of neuroactive 5 alpha/3 alpha-reduced steroid-synthesizing enzymes in the spinal cord. J Comp Neurol. 2004;477:286–299. doi: 10.1002/cne.20251. [DOI] [PubMed] [Google Scholar]
- Pelletier G, Luu-The V, Labrie F. Immunocytochemical localization of 5 alpha-reductase in rat brain. Mol Cell Neurosci. 1994;5:394–399. doi: 10.1006/mcne.1994.1049. [DOI] [PubMed] [Google Scholar]
- Poletti A, Coscarella A, Negri-Cesi P, Colciago A, Celotti F, Martini L. 5 alpha-reductase isozymes in the central nervous system. Steroids. 1998;63:246–251. doi: 10.1016/s0039-128x(98)00018-x. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 1991;15:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog Brain Res. 2010;186:113–137. doi: 10.1016/B978-0-444-53630-3.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Horton LE, Resko JA. Distribution and regulation of aromatase activity in the rat hypothalamus and limbic system. Endocrinology. 1985;117:2471–7. doi: 10.1210/endo-117-6-2471. [DOI] [PubMed] [Google Scholar]
- Russell DW, Wilson JD. Steroid 5α-reductase: two genes/two enzymes. Ann Rev Biochem. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- Sánchez P, Torres JM, Gavete P, Ortega E. Effects of swim stress on mRNA and protein levels of steroid 5alpha-reductase isozymes in prefrontal cortex of adult male rat. Neurochem Int. 2008;52:426–431. doi: 10.1016/j.neuint.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Sánchez P, Torres JM, Olmo A, O'Valle F, Ortega E. Effects of environmental stress on mRNA and protein expression levels of steroid 5alpha-Reductase isozymes in adult rat brain. Horm Behav. 2009;56:348–353. doi: 10.1016/j.yhbeh.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Sánchez P, Torres JM, Vílchez P, Del Moral RG, Ortega E. Effects of sulpiride on prolactin and mRNA levels of steroid 5alpha-reductase isozymes in adult rat brain. Neurochem Res. 2008;33:820–825. doi: 10.1007/s11064-007-9512-9. [DOI] [PubMed] [Google Scholar]
- Sanghera MK, Simpson ER, McPhaul MJ, Kozlowski G, Conley AJ, Lephart ED. Immunocytochemical distribution of aromatase cytochrome P450 in the rat brain using peptide-generated polyclonal antibodies. Endocrinology. 1991;129:2834–44. doi: 10.1210/endo-129-6-2834. [DOI] [PubMed] [Google Scholar]
- Sato T, Matsumoto T, Kawano H, Watanabe T, Uematsu Y, Sekine K, Fukuda T, Aihara K, Krust A, Yamada T, Nakamichi Y, Yamamoto Y, Nakamura T, Yoshimura K, Yoshizawa T, Metzger D, Chambon P, Kato S. Brain masculinization requires androgen receptor function. Proc Natl Acad Sci. 2004;101:1673–1678. doi: 10.1073/pnas.0305303101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver RI, Wiley EL, Thigpen AE, Guileyardo JM, McConnell JD, Russell DW. Cell type specific expression of steroid 5 alpha-reductase 2. J Urol. 1994;152:438–442. doi: 10.1016/s0022-5347(17)32758-1. [DOI] [PubMed] [Google Scholar]
- Spiga S, Acquas E, Puddu MC, Mulas G, Lintas A, Diana M. Simultaneous Golgi-Cox and immunofluorescence using confocal microscopy. Brain Struct Funct. 2011;216:171–182. doi: 10.1007/s00429-011-0312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga S, Puddu MC, Pisano M, Diana M. Morphine withdrawal-induced morphological changes in the nucleus accumbens. Eur J Neurosci. 2005;22:2332–2340. doi: 10.1111/j.1460-9568.2005.04416.x. [DOI] [PubMed] [Google Scholar]
- Thigpen AE, Cala KM, Russell DW. Characterization of Chinese hamster ovary cell lines expressing human steroid 5 alpha-reductase isozymes. J Biol Chem. 1993;268:17404–17412. [PubMed] [Google Scholar]
- Thigpen AE, Silver RI, Guileyardo JM, Casey ML, McConnell JD, Russell DW. Tissue distribution and ontogeny of steroid 5 alpha-reductase isozyme expression. J Clin Invest. 1993;92:903–910. doi: 10.1172/JCI116665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Differential regulation of steroid 5alpha-reductase isozymes expression by androgens in the adult rat brain. FASEB J. 2003;17:1428–1433. doi: 10.1096/fj.02-1119com. [DOI] [PubMed] [Google Scholar]
- Torres JM, Ortega E. Steroid 5alpha-reductase isozymes in the adult female rat brain: central role of dihydrotestosterone. J Mol Endocrinol. 2006;36:239–245. doi: 10.1677/jme.1.01907. [DOI] [PubMed] [Google Scholar]
- Tsuruo Y, Miyamoto T, Yokoi H, Kitagawa K, Futaki S, Ishimura K. Immunohistochemical presence of 5 alpha-reductase rat type 1-containing cells in the rat brain. Brain Res. 1996;722:207–211. doi: 10.1016/0006-8993(96)00188-6. [DOI] [PubMed] [Google Scholar]
- Valencia A, Collado P, Cales JM, Segovia S, Perez Laso C, Rodriguez Zafra M, Guillamon A. Postnatal administration of dihydrotestosterone to the male rat abolishes sexual dimorphism in the accessory olfactory bulb: a volumetric study. Dev Brain Res. 1992;8:132–135. doi: 10.1016/0165-3806(92)90255-u. [DOI] [PubMed] [Google Scholar]
- Zwain IH, Yen SS. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology. 1999;140:3843–3852. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


