Summary
Background
Chemotaxis, the ability to direct movements according to chemical cues in the environment, is important for the survival of most organisms. The vinegar fly, Drosophila melanogaster, displays robust olfactory aversion and attraction, but how these behaviors are executed via changes in locomotion remains poorly understood. In particular, it is not clear whether aversion and attraction bi-directionally modulate a shared circuit or recruit distinct circuits for execution.
Results
Using a quantitative behavioral assay, we determined that both aversive and attractive odorants modulate the initiation and direction of turns, but display distinct kinematics. Using genetic tools to perturb these behaviors, we identified specific populations of neurons required for aversion but not attraction. Inactivation of these populations of cells affected the completion of aversive turns but not their initiation. Optogenetic activation of the same populations of cells triggered a locomotion pattern resembling aversive turns. Perturbations in both the ellipsoid body and the ventral nerve cord, two regions involved in motor control, resulted in defects in aversion.
Conclusions
Aversive chemotaxis in vinegar flies triggers ethologically appropriate kinematics distinct from those of attractive chemotaxis, and requires specific motor-related neurons.
INTRODUCTION
All motile organisms are guided by environmental chemical cues. Escherichia coli aggregates at an attractant source by coupling the frequency of random changes in orientation to changes in attractant concentration over time [1]. Caenorhabditis elegans uses an analogous mechanism but biases the reorientation events according to the direction of the gradient [2]. Still more sophisticated mechanisms involving active sampling, specific orienting movements, and decision making underlie chemotactic strategies in Drosophila and mammals [3–6]. These strategies rely on complex neural processing, but the circuits for their execution remain unknown.
Adult Drosophila represent an outstanding model for investigating the neural control of chemotaxis. Flies are similar to vertebrates in the organization of their olfactory circuits [7], their bipartite central nervous systems comprising a brain and a ventral nerve cord (VNC)/spinal cord, and their complex repertoire of movements, made possible by articulated appendages. As a result, olfactory-motor transformations in flies are likely distinct from those in simpler organisms. Flies seek odorants indicative of food [8–11], and avoid many volatile chemicals especially at high concentrations [10–13]. Their olfactory behavior has been used extensively to study olfactory coding [3, 8–10] and neural plasticity [14]. However, beyond the circuits for sensing and processing olfactory information, the neurons that execute these behaviors are largely unknown. The genetic tools in Drosophila enable access to specific neural components [15]. Furthermore, recent advances in tracking of freely walking flies make it possible to screen for behavioral defects, and allow dissection of the temporal details of chemotactic behavior [16, 17].
Chemotactic behaviors can be elicited by dedicated olfactory receptor neurons (ORNs) [12, 13]. Moreover, distinct input channels were reported to mediate aversion and attraction to vinegar at different concentrations [8]. To what extent is this sensory separation preserved as information flows through the nervous system? One possibility is that attraction and aversion converge onto one circuit that is bi-directionally regulated; alternatively, these opposing tasks may each recruit distinct circuits for execution. Here we combine quantitative analysis of behavior with genetic perturbations to compare aversive and attractive chemotaxes. Our results demonstrate that these tasks are characterized by distinct kinematics, and that aversion engages specific neuronal populations for execution.
RESULTS
Aversion and attraction both enrich turns, but the kinematics differ
To observe adult fly chemotaxis with high temporal resolution and throughput, we simultaneously tested 15–20 flies in a four-quadrant arena [8], recording the trajectories of each individual [16]. A typical experiment began with 2.5 minutes of air delivery to all four quadrants (“control period”), and continued for 5 more minutes after an odorant entered one quadrant (“odorant period”). As previously reported, control flies were robustly repelled by 10% acetic acid (Figure 1A) as well as a number of other compounds at high concentration (Figure S1 and data not shown), and were attracted to apple cider vinegar (Figure 1B).
Figure 1.
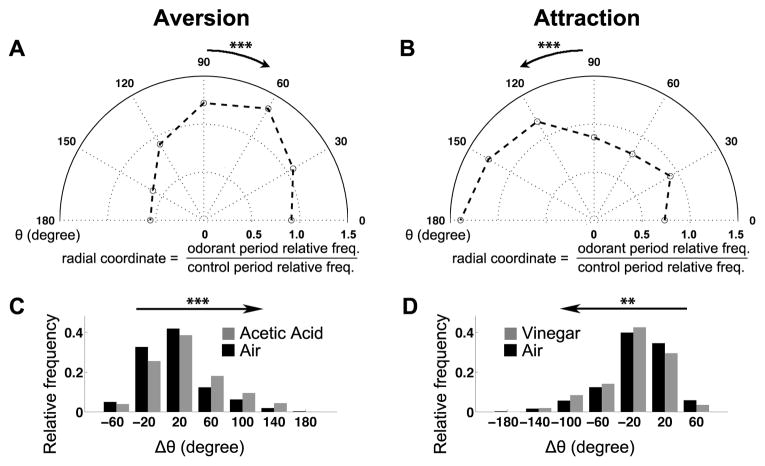
Aversive and attractive chemotaxes enrich turns, but differ in their kinematics. (A, B) Trajectories of UAS-shits1/+ flies with 10% acetic acid (A) or 2% vinegar (B) in the bottom right quadrant. Each black dot represents the appearance of one fly in one frame, and data were pooled over all periods as specified in Figure S3. (C) Definition of the orientation angle θ. Red dot: odorant source. Dashed arrow: approximated direction of the odorant gradient. Solid arrow: direction of the velocity. (D, E) Turn segments (red) flanking the aversive turning points in response to 10% acetic acid (D) or attractive turning points in response to 2% vinegar (E). Arrowhead: an exemplary turning point. Grey dots are the same as black dots in Figures 1A and 1B. The dashed lines specify the borders (2.5–7.5 cm in D and 5.5–9.5 cm in E) of areas for data collection in Figures 1H–1K. (F, G, F′, G′) The relation between the distance from the odorant source and the frequency of aversive (F, G′) or attractive (F′, G) turns in chemotactic aversion (F, F′) and attraction (G, G′). Compared to the air control, the aversive odorant (acetic acid) condition enriches aversive turning points (F), whereas the attractive odorant (vinegar) condition enriches attractive turning points (G). For each bin, the number of aversive turns against the total number of tracked fly position between the control and the odorant periods were compared with chi-square test, and only significantly different bins were indicated. (H–K) The temporal profiles of speed (H, J) and angular speed (I, K) around the aversive (H, I) or attractive (J, K) turning points. Solid traces represent mean of all individual data points over time; dashed traces represent s.e.m. The comparisons were made between mean speed or angular speed before turning (− 0.4 s to −0.2 s), and every time point after turning (0.17 s to 0.4 s), with the turning point as Time 0. Only significantly different time points were indicated. (Wilcoxon test, n = 387 for aversion, and n = 203 for attraction). Throughout the paper: * P < 0.05, ** P < 0.01, *** P < 0.001.
A variety of changes in the movements of individual flies might account for the changes in their spatial distribution during chemotaxis. Visual inspection suggested that flies approaching the aversive quadrant from an air quadrant turned back near the quadrant boundary, a behavior that could directly contribute to aversion (Figure 1D; Figure S1; Movie S1). Conversely, turns towards an attractive odorant source were also observed, although at a lower frequency (Figure 1E; Movie S2). To describe turns under both aversive and attractive conditions, we approximated the contour lines of odorant concentrations by concentric circles centered on the bottom right corner of the arena (“odorant source”, red dot in Figure 1C), based on direct visualization of airflow in the arena (Movie S3). By definition, the odorant gradient at any position points from that position to the “odorant source” (Figure 1C). We define the “aversive turning point” of a trajectory as the point closest to the odorant source (arrowhead in Figure 1D denotes one example), and the short segment of trajectory flanking the turning point was defined as an “aversive turn” (red segments in Figure 1D). Similarly, the “attractive turning point” of a trajectory was defined as the point farthest away from the odorant source, marking the corresponding trajectory segment as an “attractive turn” (red segments in Figure 1E). We examined how often such turns occurred at different distances from the odorant source, normalized by the total number of data points at the same distance. For both aversion and attraction, the corresponding turns were enriched within certain ranges (Figures 1F and 1G; Figure S1). As expected, attractive turns were not enriched in aversion (Figure 1F′; Figure S1), and aversive turns were not enriched in attraction (Figure 1G′).
To determine whether these turns represented quantitatively similar changes in behavior, we examined the fine structure of turning movements, based on the region where turns were enriched (between the dashed curves in Figures 1D, 1E, and Figure S1). We calculated the average walking speed and angular speed of aversive and attractive turns aligned by the “turning points” (as defined above). As expected, turns were associated with a reduction in speed (Figures 1H and 1J; Figure S1) and a peak in angular speed (Figures 1I and 1K; Figure S1), the magnitudes and durations of which were almost identical between aversion and attraction. However, after aversive turns, speed increased significantly (Figure 1H; Figure S1), and angular speed decreased significantly (Figure 1I; Figure S1), compared to the baseline values before turns. Thus, after aversive turns, flies moved more quickly and followed straighter paths. Neither of these modulations was observed in attractive turns, and the speed after a turn even decreased (Figures 1J and 1K). The difference between aversive and attractive turning kinematics was not simply due to the asymmetric geometry in our definition of turning points (see Methods), because “aversive turns” during the control period lacked the speed increase and angular speed decrease observed in aversive turns during the odorant period (Figure S1, compared to Figures 1H and 1I). Intriguingly, these odorant-free turns were qualitatively similar to attractive turns, implying that attraction, unlike aversion, might not induce specific types of turns. Finally, we note that despite our selection of a region of interest, none of these conclusions were sensitive to the precise borders chosen, because those chosen included 45% of aversive turns (red segments in Figure 1D) and 64% of attractive turns (red segments in Figure 1E). As a result, adding or removing a few turn segments by shifting the borders did not significantly alter the ensemble statistics. Thus, both aversion and attraction increase the frequency of turning events as flies transition into or out of the odorant quadrant, respectively, but these turns are quantitatively different, suggesting different underlying executive programs.
Odorants modulate turn initiation and direction
We envisioned two mechanisms that could contribute to the enrichment of aversive turns in aversion (or attractive turns in attraction). First, a fly could modulate the frequency of turn initiation depending on whether it is moving up or down an odorant gradient. Second, when a fly is turning, it could utilize the spatial odorant gradient to determine its turn direction. We tested whether either or both of these two mechanisms were used.
To test for modulation of the frequency of turn initiation, we identified all turns (see Methods), and classified them by the direction of fly movement prior to turn initiation. To determine the direction of movement, we calculated the angle θ between each fly’s velocity and the odorant gradient (Figure 1C, 0° ≤ θ ≤ 180°). When θ=0°, the fly was walking exactly towards the odorant source; at θ=180°, the fly was moving exactly in the opposite direction. We found that in the aversion assay, flies were more likely to be moving towards the odorant source (up the gradient) right before turns began (θ <90°, Figure 2A; Figure S2), while in the attraction assay, flies were more likely to be moving away from the source (θ >90°, Figure 2B). Odorant concentration changes thus modulate the frequency of turn initiation in both attractive and aversive contexts.
Figure 2.
Odorant gradients modulate turn initiation and direction. (A, B) Distribution of velocity orientations in the border area (see Figures 1D and 1E) upon turn initiation in aversion (A, n = 2954 for the control period, and n = 2276 for the odorant period) or attraction (B, n = 4642 for the control period, and n = 4238 for the odorant period) in the polar coordinate system. Radial coordinate represents the ratio of the relative frequency during the odorant period and the relative frequency during the control period. Angular coordinate represents θ (defined in Figure 1C; in degree) upon turn initiation. The velocity orientation upon turn initiation is biased towards the odorant source in aversion (arrow pointing to the right in A) and away from the odorant source in aversion (arrow pointing to the left in B), compared to their control periods respectively. (C) Distribution of Δθ after turning, for events initiated when the flies were moving towards the “odorant source” (red dot in Figure 1C) during the control (n = 1368) and aversive odorant (n = 1322) period. The arrow pointing to the right indicates that the whole distribution during the aversive period is biased to align the flies against the odorant gradient (> 0°). (D) Distribution of Δθ after turning, for events initiated when the flies were moving away from “odorant source” (red dot in Figure 1C) during the control (n = 1887) and attractive odorant (n = 2081) period. The arrow pointing to the left indicates that the whole distribution during the attractive period is biased to align the flies with the odorant gradient (> 0°). All statistical significance in this figure was assessed by Wilcoxon test. The comparisons were between the entire distributions of θ in the control period and the odorant period in A, B, and the entire distribution of Δθ in the control period and the odorant period in C, D. The genotype is UAS-Shi/+, which also serves as a control for Figure 4.
We next tested whether the direction of induced turns were influenced by the direction of the odorant gradient. We generated the metric Δθ by subtracting the absolute value of θ at turn initiation from that at turn termination. A positive (or negative) Δθ means that the turn shifts the walking direction away from (or towards) the odorant source, respectively. For turns initiated when the flies were moving towards the aversive odorant source, we compared the Δθ distribution to that observed in the control period of the same experiment. Given that we selected the turns whose initial θ was smaller than 90°, the Δθ distribution was inherently biased to be above 0°. However, the frequency of positive Δθ was further increased under the aversive condition compared with controls, demonstrating that the local odorant gradient indeed modulated the flies’ turn direction (Figure 2C; Figure S2). An attractive odorant gradient modulated turn direction in a similar fashion, enriching negative Δθ in turns triggered while moving down the gradient (Figure 2D). Thus, odorant gradients modulate the direction of turning in both aversion and attraction.
In summary, turn evoked during chemotaxis involve both mechanisms proposed at the beginning of this section. When a fly walks towards an aversive odorant (or away from an attractive odorant), it is more likely to start turning, and the turn direction is biased away from the aversive odorant (or towards the attractive odorant).
A screen for neurons necessary for aversion
Aversion and attraction could reflect different dynamical states of the same motor control circuit, or could engage distinct circuits for execution. To distinguish between these possibilities, we manipulated the underlying circuit(s). Under our experimental conditions, aversion was robust while attraction was sensitive to genetic background. We therefore screened for neurons necessary for aversion to 10% acetic acid. We chose 103 GAL4 lines from an InSITE enhancer trap collection [18], each of which allows transgene expression in specific neuronal populations. These lines were selected for relatively sparse expression and against expression in olfactory receptor neurons or projection neurons. To impair synaptic output from targeted neurons, we expressed UAS-shibirets1 (UAS-shits1), which acutely abolishes synaptic transmission at the restrictive temperature used in our assays [19].
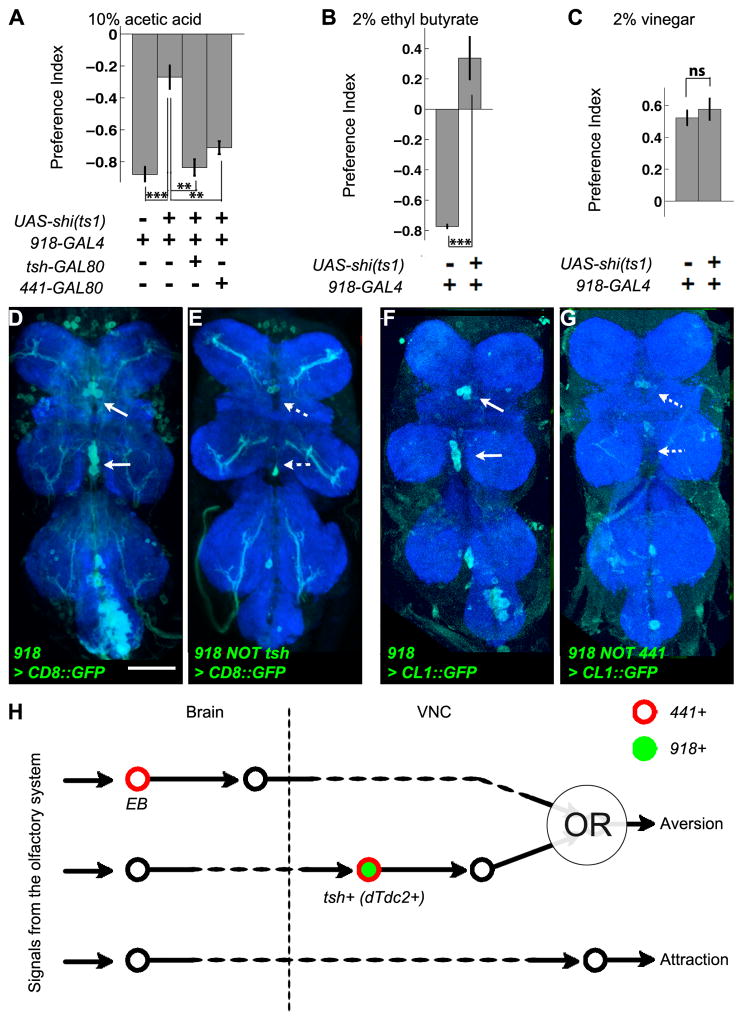
The metrics described above were data intensive and might not capture every aspect of chemotaxis, making them impractical for a screen. We therefore defined a Preference Index (PI) as a comprehensive chemotaxis indicator (Figure 3A). A PI of 1 means that the flies always remain within the odorant quadrant, a PI of 0 means that they spend as much time in the odorant quadrant as in the air quadrants, and a PI of –1 means that they never enter the odorant quadrant. We identified four lines that strongly impaired aversion (Figure S3), and characterized in detail two of these, 441-GAL4 and 918-GAL4.
Figure 3. 441 > shits1.
affects aversive but not attractive chemotaxis. (A) Definition of the Preference Index (PI). Each number represents all positions flies visit in a particular quadrant counted over a defined period of time. For aversion, the PI was calculated 2.5–5 minutes after the odorant onset, when the index reached a steady state (Figure S3). For attraction, the PI was calculated 1–3 minutes after the odorant onset to reduce the impact of habituation (Figure S3). (B) Strong aversion to 10% acetic acid in control animals is almost completely abolished in 441 > shits1 flies. (C) 441 > shits1 abolishes aversion to 2% ethyl butyrate. (D) 441 > shits1 abolishes learned aversion. To associate the naturally attractive/neutral odorant (0.1% ethyl butyrate) with a negative valence, it was repetitively delivered to the flies coupled with electric shocks right before testing in the arena. (E) 441 > shits1 does not affect the PI to 2% vinegar. Bars are for mean PIs of multiple independent runs; error bars represent s.e.m. (n ≥ 4). B, C, E: t-test with Holm-Bonferroni post hoc correction; D: two-way ANOVA testing the significance of the interaction between genotype and conditioning.
441-targeted silencing affects aversion but not attraction
Expression of UAS-shits1 under the control of 441-GAL4 (441 > shits1) almost completely abolished aversion to 10% acetic acid as measured with the PI (Figure 3B). This line therefore allowed us to address whether the circuit elements necessary for acetic acid aversion are shared by other chemotactic behaviors. We found that 441 > shits1 flies also abolished aversive responses to both 2% ethyl butyrate (Figure 3C) and 0.1% E2-hexenal (Figure S3). Flies can also be conditioned to avoid naturally attractive or neutral odorants through their induced association with electric shocks. Despite its distinct origin from innate aversion, learned aversion to 0.1% ethyl butyrate was also abolished by 441 > shits1 expression (Figure 3D). 441+ cells are thus broadly required for olfactory aversion. Interestingly, the attractive response to vinegar odor as measured by PI was completely unaffected (Figure 3E). These results implied a separation of neural circuits for olfactory aversion and attraction.
441-GAL4+ cells regulate the completion of aversive turning
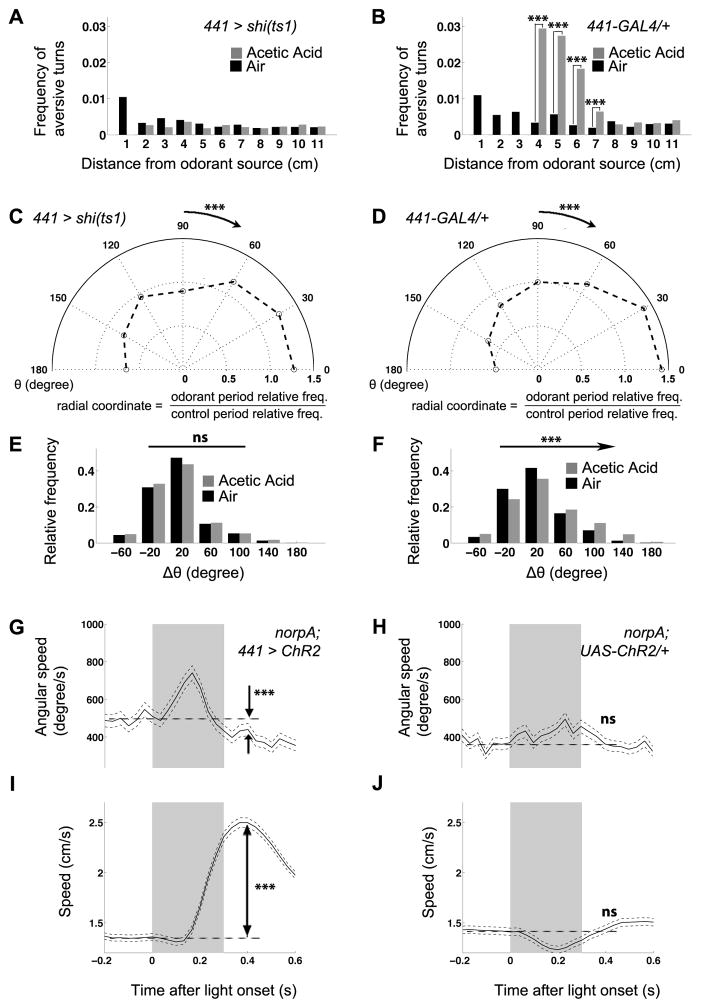
We next examined the possible behavioral mechanisms underlying the abolition of aversion in 441 > shits1 flies using our kinematic metrics. Unlike control flies, which increased aversive turns in response to 10% acetic acid (Figures 1F and 4B), 441 > shits1 flies did not (Figure 4A). To understand how 441 > shits1 disrupts aversive turns, we analyzed turn initiation and direction. Intriguingly, while 441 > shits1 flies were still biased to initiate turning when moving towards the aversive odorant source just as in controls (Figure 4C, compared with Figures 2A and 4D), they lost all directional modulation of Δθ (Figure 4E, compared to Figures 2C and 4F). 441 > shits1 flies thus still sensed the aversive odorant and initiated turns, but failed to complete the late stages of the kinematic pattern necessary to appropriately reorient the animal.
Figure 4.
441-GAL4 defines neurons for the completion of aversive turns. (A, B) The enrichment of aversive turns in response to 10% acetic acid is abolished in 441 > shits1 (A) compared to 441-GAL4 (B) and UAS-shits1/+ (Figure 1F) controls. (C, D) The modulation of turn initiation, as measured by the distribution of θ upon turn initiation in the border area, still persists in 441 > shits1 (C, n = 2765 for the control period, and n = 3341 for the odorant period) compared to 441-GAL4/+ (D, n = 2552 for the control period, and n = 1897 for the odorant period) and UAS-shits1/+ (Figure 2A) controls. (E) Distribution of Δθ after turning, for events initiated when 441 > shits1 flies were moving towards the “odorant source” (red dot in Figure 1C) during the control (n = 1142) and aversive odorant (n = 1631) period. The modulation of direction is lost compared to 441-GAL4/+ (F) and UAS-shits1/+ (Figure 2C) controls. (G, H) Angular speed peaks during illumination and then falls below baseline level in 441 > ChR2 flies (G, n = 1016 before illumination, and n = 989 after illumination; same for speed) but not in control flies (H, n = 890 before illumination, and n = 852 after illumination; same for speed). (I, J) Speed increases after illumination in 441 > ChR2 (I) but not control (J) flies. Grey bar represents the light on period. A–B: same comparison as in the corresponding panels in Figures 1F and 1G; C–F: same comparison as in the corresponding panels in Figures 2A–2D: Wilcoxon tests between the average angular speeds (G, H) or speeds (I, J) before (−0.2s to −0.1s) and after (0.4 s) illumination, with the light onset being Time 0.
If 441-GAL4 labeled circuit components necessary for the execution of an aversion-specific motor program, artificial activation of these cells might trigger elements of this program. To test this, we expressed Channelrhodopsin2 with 441-GAL4 (441 > ChR2), allowing 441+ neurons to be activated by light [20], while incorporating a norpA mutation to block phototransduction. In these blind flies, angular speed peaked during blue light illumination (mimicking turning), and then dropped below baseline levels (mimicking turn suppression, Figure 4G, compared to Figure 4H). Moreover, this turn suppression coincided with a speed increase (Figure 4I, compared to Figure 4J). These kinematic changes are strikingly similar to those accompanying natural aversive turns (Figures 1H and 1I). Both resting and walking 441 > ChR2 flies transitioned into fast walking after illumination (data not shown), indicating that the artificially induced locomotion overrode the flies’ endogenous locomotion status. We note, however, that the light-induced pattern of neuronal activity was unlikely to completely mimic the natural pattern due to its synchrony and uniformity, which might account for the lack of an initial speed decrease during illumination. Alternatively, since 441+ circuits are not necessary for turn initiation (Figures 4C and 4D), it is possible that the speed decrease at the beginning of turns is controlled by neurons upstream to those labeled by 441-GAL4. Thus, 441-GAL4 targets circuit components that are not only necessary for completion of aversive turns, but also sufficient to induce aspects of locomotion patterns characteristic of aversive turns.
The spatial distribution of 441 > shits1 flies in attraction appeared normal (Figure S4, also compared to Figure 1B), although the attractive turns were no longer enriched (Figure S4, also compared to Figure 1G) and the bias of turn initiation was changed (Figure S4, also compared to Figure 2B). Thus, aversion and attraction display both different turn kinematics, and distinct dependence on turning. We infer that odorants may generate attraction through another behavioral mechanism.
441-GAL4 targets motor-related neural structures
We sought to identify the 441+ cells responsible for aversion using intersectional strategies. 441-GAL4 labels glia and neurons in both the brain and the VNC (Figures 5A and 5A′). We first tested whether neuronal expression of shits1 was sufficient to cause the phenotype with a split GAL4 strategy [21]. We replaced the GAL4 in 441-GAL4 with the VP16 activation domain (AD), and introduced a second transgene expressing the GAL4 DNA-binding domain (DBD) with a pan-neuronal promoter elav. A functional GAL4 was therefore only reconstituted in 441+elav+ cells when we combined 441-VP16AD and elav-GAL4DBD to express shits1. This manipulation still abolished aversion (Figure S5), indicating that neuronal expression of shits1 in 441+ cells is responsible for the aversion deficit. To assess the contributions of 441-GAL4 expressions in the brain versus the VNC, we introduced a VNC-specific suppressor of GAL4, tsh-GAL80 [22]. tsh-GAL80 greatly reduced 441-GAL4 expressions in both neurons and glia in the VNC, but not in the brain (Figures 5B and 5B′). In behavioral experiments, adding tsh-GAL80 to 441 > shits1 flies restored the PI to about half of the PI observed in controls (Figure 5C), indicating that 441+tsh+ neurons were necessary for aversion. These data also suggest that either tsh-GAL80 was not strong enough to completely block 441-GAL4, or that additional 441+tsh- neurons contribute to aversion.
Figure 5.
441-GAL4 targets redundant neurons necessary for the execution of aversion. (A, A′) 441-GAL4 expression is visualized with a membrane-tagged GFP in the brain (A) and the VNC (A′). Arrowhead: the ellipsoid body. (B, B′) tsh-GAL80 suppresses VNC expression of 441-GAL4. (C) Aversion in 441 > shits1 flies is partially restored with tsh-GAL80 or EB-GAL80. The first bar represents the same data as the last bar in Figure 3B. Bars are mean PIs, and error bars represent s.e.m. (n ≥ 4); t-test with Holm-Bonferroni post hoc correction. (D, D′) R13C06-GAL80 suppresses EB expression of 441-GAL4, as indicated by the open arrowhead. (E–F′) Average 441-GAL4 expression without (E–E′) or with (F–F′) tsh-GAL80 is visualized with a dendritic marker in the ventral (E–F) and dorsal (E′-F′) halves of the VNC after image registration against a standard VNC, shown with neuropil counterstaining of VNC (E1-F1′) or without (E2-F2′). Dashed boxes: abdominal ganglion; arrows: ventromedial cell bodies (E, suppression indicated by dashed arrows in F) and the corresponding dorsal-bilateral projections (E′ suppression indicated by dashed arrows in F′); arrowhead: projection along the midline (E′, suppression indicated by open arrowhead in F′). Scale bars: 50 μm.
In the brain, 441-GAL4 has prominent expression in the ellipsoid body (EB, arrowhead in Figure 5A), which was reported to be necessary for olfactory aversion in a different assay [23]. To examine the role of these neurons, we generated two EB-GAL80s using enhancer fragments from a large collection of enhancer-GAL4 fusions [24, 25] (Figure S5 and data not shown). R13C06-GAL80 effectively blocked 441-GAL4 in the EB (Figure 5D, open arrowhead), and partially restored aversion in 441 > shits1 flies (Figure 5C). Similar results were obtained using an independent EB-GAL80 driven by a second enhancer R11F03 (Figure 5C). These data indicate that inactivation of EB neurons also contributed to loss of aversion when all 441+ neurons were inactivated. However, EB-GAL4s with the same two enhancers did not affect aversion when expressing shits1 (Figure S5), suggesting that 441+ EB neurons mediate aversive chemotaxis redundantly with other 441+ neurons. Finally, R13C06-GAL80 and tsh-GAL80 together did not restore the PI beyond the level of single GAL80 (Figure 5C). This could be due to incomplete suppression of 441-GAL4 in the EB or tsh+ neurons by GAL80; alternatively, 441 > shits1 may disrupt a third aversion-related circuit not accounted for by either GAL80 line.
Given the implied importance of 441+ VNC neurons, we directly tested their sufficiency to induce locomotion in decapitated 441 > ChR2 flies [26]. Headless 441 > ChR2 flies immediately started to swing their legs upon illumination (Figure S5; Movie S4), a behavioral change that was never observed in controls lacking 441-GAL4 (Figure S5; Movie S5) or with a GAL4 labeling many motor neurons (data not shown). Doubling the intensity of light stimulation caused nearly half of the headless flies to transition from leg swinging to non-directional displacements, jumps, or forward walking (Figure S5; Movie S6). Although these headless flies did not maintain steady walking, perhaps due to the absence of brain-mediated coordination, this result demonstrated that activation of 441+ VNC neurons generated new locomotor patterns, even in sluggish, headless flies, reminiscent of the transition from resting to fast walking in intact flies.
We also attempted to narrow down the most likely VNC neurons involved in aversion using morphological analysis. 441 > mCD8-GFP only weakly labeled neuronal processes, and detailed studies of these neurons were confounded by glial labeling. We therefore expressed a strong dendritic marker (DenMark) with 441-GAL4, and removed glia expression using repo-GAL80. To facilitate comparison between individual VNCs, we took multiple image stacks under the same conditions both with and without tsh-GAL80, registered them to a standard VNC [27], and averaged the expression intensity of DenMark within each genotype (Figures 5E–5F1′, and Figure S5). tsh-GAL80 suppressed 441 > DenMark expression in the abdominal ganglia (dashed boxes in Figures 5E––5E1′, compared to Figures 5F––5F1′), a region of the VNC that does not control locomotion. Outside the abdominal ganglia, 441 > DenMark expression was only suppressed by tsh-GAL80 in neurites extending from ventromedial cell bodies (arrows in Figures 5E, 5E1, and Figure S5, compared to the dashed arrows in Figures 5F and 5F1) to beneath the dorsal surface of the VNC (arrows in Figures 5E′ and 5E1′, compared to the dashed arrows in Figures 5F′ and 5F1′) and those along the midline within the VNC (arrowheads in Figures 5E′, 5E1′, and Figure S5, compared to the open arrowhead in Figures 5F′ and 5F1′).
In summary, we identified two circuit element candidates, the EB and small subsets of VNC neurons, which are causally linked to aversive chemotaxis, and are likely involved in motor control (see Discussion).
918-GAL4+ neurons are also required for aversion and overlap with 441+ VNC neurons
The second line identified in our genetic screen (Figure S3), 918-GAL4, strongly supported the importance of the VNC neural elements defined by 441-GAL4 for aversion. 918 > shits1, like 441 > shits1, attenuated aversion but not attraction (Figures 6A–6C), a deficit that was fully rescued by tsh-GAL80 (Figure 6A). 918-GAL4 appeared to label the 441+ ventromedial neurons as well, based on the stereotypic locations of their cell bodies (arrows in Figure 6D compared to those in Figure 5E). Labeling of these ventromedial neurons was also suppressed by tsh-GAL80 (Figure 6E, dashed arrows). In the brain, tsh-GAL80 only suppressed 918 expression in some local interneurons in the antennal lobes (Figure S6).
Figure 6.
Analyses of 918-GAL4 support the role of specific 441+tsh+ neurons in aversion. (A) 918 > shits1 attenuates aversion to 10% acetic acid, and this attenuation is suppressed by tsh-GAL80 or 441-GAL80. (B) 918 > shits1 abolishes aversion to 2% ethyl butyrate. (C) 918 > shits1 does not affect attraction to 2% vinegar. Bars in (A–C) are mean PIs of multiple independent runs, and error bars represent s.e.m. (n ≥ 3); t-test with Holm-Bonferroni post hoc correction. (D) 918-GAL4 expression in the VNC is visualized with a membrane-tagged GFP. Arrows point to the ventromedial cell bodies similar to those in Figure 5E. (E) tsh-GAL80 suppresses VNC expression of 918-GAL4, including the ventromedial class as indicated by the dashed arrows. (F) 918-GAL4 expression is visualized with a destabilized GFP. Arrows point to the ventromedial cell bodies. (G) 441-GAL80 suppressed the expression of 918-GAL4 in the ventromedial class, as indicated by the dashed arrows. Scale bars: 50 μm. (H) A schematic summary of the functionality of neuronal populations underlying aversive chemotaxis inferred from our genetic analysis. Each circle and the line attached to it represent a neuronal population and the direction of information flow; dashed lines represent potential intermediate layers of relay neurons. The OR logic gate near the end of the aversive circuit reflects the redundant roles of the pathways mediated by EB and dTdc2+ VNC neurons.
To test whether 918-GAL4 and 441-GAL4 label the same VNC neurons, and, if so, whether these neurons play essential roles in aversion, we replaced the GAL4 in 441-GAL4 with GAL80 at exactly the same genomic locus [18]. Indeed, 441-GAL80 rescued the aversion deficit in 918 > shits1 flies (Figure 6A). We used UAS-mCD8-GFP to visualize the pattern of 918-GAL4 after 441-GAL80 suppression, but did not find any apparent effect (data not shown). We reasoned that the intersectional effect might be sufficient to alter behavior, but insufficient to cause a change of expression visualized using a stable marker like mCD8-GFP. To sensitize our expression analysis, we utilized UAS-CL1-GFP, an unstable GFP with a degradation tag. The ventromedial cell bodies were labeled in 918 > CL1-GFP VNCs (Figure 6F, arrows), and the reporter expression was markedly reduced by 441-GAL80 (Figure 6G, dashed arrows; Figure S6). The intersection between 441 and 918 thus confirmed their overlapping expression patterns, and indicated that the neurons they label in common are necessary for aversion.
The ventromedial cell bodies and dorsal projection patterns are also characteristic of tyraminergic/octopaminergic neurons, which express tyrosine decarboxylase 2 (dTdc2) and can be targeted by dTdc2-GAL4 [28] (Figure S6). We therefore combined 441-GAL4 and dTdc2-GAL4 in the same fly, and found that two GAL4s in combination labeled as many neurons in the posterior cluster as dTdc2-GAL4 alone, and slightly more than 441-GAL4 alone, confirming that the 441+tsh+ ventromedial neurons are also dTdc2+ (Figure S6). dTdc2 > shits1 did not affect aversion (Figure S6), which could either be caused by redundancy similar to the case of EB > shits1, or imply the involvement of other 441+918+ VNC neurons in aversion.
DISCUSSION
To our knowledge, this is the first direct demonstration that odorants modulate turn initiation and direction in freely walking insects. Moreover, aversive and attractive turns involve distinct kinematics. Intuitively, our quantitative analyses reveal that flies speed up and follow straighter trajectories after turning away from a noxious smell, which should shorten their exposure to potential harm. Such a strategy is not employed for attraction. Chemotaxis has been studied in tethered adult flies, paradigms in which mimicking the olfactory inputs a freely moving fly would encounter proved challenging. For example, in a “fly-on-the-ball” paradigm, aversion was not triggered even using a strong repellent [4, 29]. In another study, flying flies responded symmetrically to aversive and attractive odorants [30]. Our more naturalistic approach provided new insights into the relationship between aversive and attractive chemotaxes.
In bacteria [1], aversion and attraction are achieved through bidirectional modulation of the same mechanism. Similarly, in C. elegans, aversion and attraction are thought to utilize a push-pull mechanism on one set of antagonizing command neurons [2]. Our genetic inactivation experiments suggest that, in flies, aversion is executed through specific neurons distinct from attraction. We identified two candidate circuit components, the EB and a subset of the VNC neurons, which appear redundantly necessary for aversive chemotaxis (Figure 6H). The EB is part of the central complex, defects in which are associated with uncoordinated walking [31]. In grasshoppers and cockroaches, activating central complex neurons induces specific kinematics [32, 33]. In the VNC, dTdc2+ neurons are prominent candidates for mediating aversion, although other 441+918+ neurons might be involved. These neurons are homologous to dorsal/ventral unpaired median neurons in other insects because they are octopaminergic and show similar projection patterns [34]. The activity in these neurons is correlated with specific aspects of locomotion in locusts, crickets, and moths [34]. Given that 441 > shits1 flies display defects in aversive turn completion but not initiation, we postulate that our genetic manipulation does not interfere with the perception, processing, decision-making, or even initiation steps of aversive chemotaxis, but rather the execution of motor programs specifically necessary for this behavior. Our discoveries bridge the extensive investigation of olfactory processing in insects such as honeybees and moths [35] with studies focused on motor control mechanisms in species such as cockroaches and stick insects [36].
It is intriguing that part of the aversion-specific circuit resides in the VNC, and can be artificially activated to generate a pattern similar to aversive turns. Although the larval VNC is sufficient for substrate exploration [37], VNC autonomy in motor pattern generation in adult flies has only been established for escape flight [26] and courtship song [22], both highly specialized for certain ethological functions. When a fly continuously explores the environment and updates its walking pattern, the division of labor between the brain and the VNC is less clear. Conceptually, one possibility is that circuit modules in the VNC only encode basic elements of locomotion. For example, right turns may always involve the same VNC circuit, and the only difference is their embedding within different sequences of actions based on the combination of descending signals from the brain. Alternatively, VNC circuit modules could be task-specific; once a descending signal specifies the task, the details of the motor output will unfold according to a pre-wired VNC circuit. Our findings support the latter possibility in the context of aversive chemotaxis. In both vertebrates and invertebrates, artificial activation of neurons in the spinal cord or the VNC generates specific motor outputs, but rarely have these neurons been demonstrated to be necessary for specific sensory-driven tasks [36]. It would be interesting to test the generality of having autonomous motor-related circuits specifically responsive to certain sensory triggers.
Experimental Procedures
Chemotaxis Paradigm
The four-quadrant behavioral arena was 16.5 cm by 16.5 cm, and 1 cm deep [8]. It was placed inside a 33 °C box in complete darkness. The airflow was filtered and saturated with water, entered each quadrant at a rate of 40 mL/min, and left through the central hole in the arena floor. One branch of airflow was controlled by solenoid valves through the LabView software (National Instruments), so that for each trial air passed directly into one quadrant for 2.5 min, and then was switched to bubble through 5 mL of water (for vinegar and acetic acid) or paraffin oil (for all the other odorants tested) containing an odorant with a specified concentration. The odorant source was replenished for each experiment.
Behavioral Data Analysis
All analyses were performed with Matlab (Mathworks). For aversion and attraction, all analyses were done with data from the time windows described in Figure S3. The only exception was learned aversion, where the first 2.5 min were analyzed to avoid memory extinction. The data points within 0.5 cm of the arena walls were excluded except for PI calculation to avoid the confounding effects of arena edges.
Due to the asymmetry of the odorant contours, which curve towards the odorant source, a direct comparison between the frequencies of aversive and attractive turns was confounded by the difference in control frequencies (note the y axis scales differ between Figures 1F and 1G′, and Figures 1F′ and 1G). That is because a turn towards the “odorant source” does not contribute an “attractive turn” unless it is more curved than the local odorant contour.
For the temporal profiles of turns, speeds and angular speeds (in the arena-based coordinate system) were collected from 24 flanking frames (0.8 s) in each trajectory, aligned by the turning points, and averaged. For the analysis of turn initiation and direction, we partitioned each trajectory into forward walking and turning segments. Any data point with an angular speed above 400 degrees/sec was annotated as turning, and the rest as forward walking. The threshold was set based on the basal angular speed in Figures 1I and 1K. The angular speed was the absolute value of the orientation difference between two adjacent frames divided by the frame duration (1/30 s). The distribution was skewed such that the mean angular speed was near 400 deg/s, but the median was only 130 deg/s. But even for 400 deg/s, the orientation difference between two adjacent frames was just 400/30 = 13.3 deg. Thus, the apparently high baseline angular speed reflects the fact that the flies never follow truly straight trajectories. The first frame of one continuous segment of turning points was defined as its initiation, and the last frame its termination.
Supplementary Material
Highlights.
Both olfactory aversion and attraction modulate turn initiation and direction
Aversive and attractive turns show distinct kinematics
Specific motor-related neurons are necessary for aversion but not attraction
Activation of these neurons triggers a locomotion pattern resembling aversive turns
Acknowledgments
We thank D. Luginbuhl and J. Brown for technical assistance; R.L. Davis, S.E.J. de Vries, G.S.X.E. Jefferis, M. Prakash, and T. Rohlfing for advice; B.D. Pfeiffer, A. Jenett, G.M. Rubin, and J. Simpson from the Janelia Farm for generously sharing unpublished reagents; Bloomington Stock Center for flies; Addgene for plasmids; C.J. Guenthner, W. Joo, T.J. Mosca, O. Riabinina, A. Ward, and B.C. Weissbourd for critiques. X.J.G. is supported by an Enlight Foundation Bio-X Interdisciplinary Fellowship. D.M.G was supported by a Ruth L. Kirschstein NRSA Postdoctoral Fellowship (F32EY020040) from the National Eye Institute. M.S. is supported by a postdoctoral fellowship from the Jane Coffin Childs Memorial Fund for Medical Research. C.J.P. was an Associate, and L.L. is an investigator, of the Howard Hughes Medical Institute. This study was also supported by NIH grants R01-DC005982 (L.L.), R01-EY022638 (T.R.C.), and a NIH Director’s Pioneer Award DP1 OD003530 (T.R.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berg HC. Chemotaxis in Bacteria. Annual Review of Biophysics and Bioengineering. 1975;4:119–136. doi: 10.1146/annurev.bb.04.060175.001003. [DOI] [PubMed] [Google Scholar]
- 2.Faumont S, Lindsay TH, Lockery SR. Neuronal microcircuits for decision making in C. elegans. Curr Opin Neurobiol. 2012;22:580–591. doi: 10.1016/j.conb.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis M, Huber T, Benton R, Sakmar TP, Vosshall LB. Bilateral olfactory sensory input enhances chemotaxis behavior. Nat Neurosci. 2008;11:187–199. doi: 10.1038/nn2031. [DOI] [PubMed] [Google Scholar]
- 4.Gaudry Q, Hong EJ, Kain J, de Bivort BL, Wilson RI. Asymmetric neurotransmitter release enables rapid odour lateralization in Drosophila. Nature. 2013;493:424–428. doi: 10.1038/nature11747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajan R, Clement JP, Bhalla US. Rats smell in stereo. Science. 2006;311:666–670. doi: 10.1126/science.1122096. [DOI] [PubMed] [Google Scholar]
- 6.Porter J, Craven B, Khan RM, Chang SJ, Kang I, Judkewitz B, Volpe J, Settles G, Sobel N. Mechanisms of scent-tracking in humans. Nat Neurosci. 2007;10:27–29. doi: 10.1038/nn1819. [DOI] [PubMed] [Google Scholar]
- 7.Bargmann CI. Comparative chemosensation from receptors to ecology. Nature. 2006;444:295–301. doi: 10.1038/nature05402. [DOI] [PubMed] [Google Scholar]
- 8.Semmelhack JL, Wang JW. Select Drosophila glomeruli mediate innate olfactory attraction and aversion. Nature. 2009;459:218–223. doi: 10.1038/nature07983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asahina K, Louis M, Piccinotti S, Vosshall LB. A circuit supporting concentration-invariant odor perception in Drosophila. J Biol. 2009;8:9. doi: 10.1186/jbiol108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreher SA, Mathew D, Kim J, Carlson JR. Translation of sensory input into behavioral output via an olfactory system. Neuron. 2008;59:110–124. doi: 10.1016/j.neuron.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Chiang AS, Xia S, Kitamoto T, Tully T, Zhong Y. Blockade of Neurotransmission in Drosophila Mushroom Bodies Impairs Odor Attraction, but Not Repulsion. Current Biology. 2003;13:1900–1904. doi: 10.1016/j.cub.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 13.Stensmyr Marcus C, Dweck Hany KM, Farhan A, Ibba I, Strutz A, Mukunda L, Linz J, Grabe V, Steck K, Lavista-Llanos S, et al. A Conserved Dedicated Olfactory Circuit for Detecting Harmful Microbes in Drosophila. Cell. 2012;151:1345–1357. doi: 10.1016/j.cell.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 14.Davis RL. Olfactory learning. Neuron. 2004;44:31–48. doi: 10.1016/j.neuron.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Venken KJ, Simpson JH, Bellen HJ. Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron. 2011;72:202–230. doi: 10.1016/j.neuron.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katsov AY, Clandinin TR. Motion processing streams in Drosophila are behaviorally specialized. Neuron. 2008;59:322–335. doi: 10.1016/j.neuron.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Branson K, Robie AA, Bender J, Perona P, Dickinson MH. High-throughput ethomics in large groups of Drosophila. Nat Methods. 2009;6:451–457. doi: 10.1038/nmeth.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gohl DM, Silies MA, Gao XJ, Bhalerao S, Luongo FJ, Lin CC, Potter CJ, Clandinin TR. A versatile in vivo system for directed dissection of gene expression patterns. Nature Methods. 2011;8:231–237. doi: 10.1038/nmeth.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 20.de Vries SE, Clandinin TR. Loom-sensitive neurons link computation to action in the Drosophila visual system. Curr Biol. 2012;22:353–362. doi: 10.1016/j.cub.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luan H, Peabody NC, Vinson CR, White BH. Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. Neuron. 2006;52:425–436. doi: 10.1016/j.neuron.2006.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clyne JD, Miesenbock G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell. 2008;133:354–363. doi: 10.1016/j.cell.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 23.Joseph RM, Devineni AV, King IF, Heberlein U. Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila. Proc Natl Acad Sci U S A. 2009;106:11352–11357. doi: 10.1073/pnas.0901419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfeiffer BD, Jenett A, Hammonds AS, Ngo TT, Misra S, Murphy C, Scully A, Carlson JW, Wan KH, Laverty TR, et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc Natl Acad Sci U S A. 2008;105:9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jenett A, Rubin GM, Ngo TT, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, et al. A GAL4-Driver Line Resource for Drosophila Neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lima SQ, Miesenbock G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Jefferis GS, Potter CJ, Chan AM, Marin EC, Rohlfing T, Maurer CR, Jr, Luo L. Comprehensive maps of Drosophila higher olfactory centers: spatially segregated fruit and pheromone representation. Cell. 2007;128:1187–1203. doi: 10.1016/j.cell.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, Hirsh J. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes. Journal of Biological Chemistry. 2005;280:14948–14955. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- 29.Borst A. Computation of Olfactory Signals in Drosophila-Melanogaster. Journal of Comparative Physiology. 1983;152:373–383. [Google Scholar]
- 30.Wasserman S, Lu P, Aptekar JW, Frye MA. Flies dynamically anti-track, rather than ballistically escape, aversive odor during flight. J Exp Biol. 2012;215:2833–2840. doi: 10.1242/jeb.072082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strauss R. The central complex and the genetic dissection of locomotor behaviour. Current Opinion in Neurobiology. 2002;12:633–638. doi: 10.1016/s0959-4388(02)00385-9. [DOI] [PubMed] [Google Scholar]
- 32.Heinrich R, Wenzel B, Elsner N. Pharmacological brain stimulation releases elaborate stridulatory behaviour in gomphocerine grasshoppers--conclusions for the organization of the central nervous control. J Comp Physiol A. 2001;187:155–169. doi: 10.1007/s003590100188. [DOI] [PubMed] [Google Scholar]
- 33.Bender JA, Pollack AJ, Ritzmann RE. Neural activity in the central complex of the insect brain is linked to locomotor changes. Curr Biol. 2010;20:921–926. doi: 10.1016/j.cub.2010.03.054. [DOI] [PubMed] [Google Scholar]
- 34.Braunig P, Pfluger HJ. The unpaired median neurons of insects. Advances in Insect Physiology. 2001;28:185–266. [Google Scholar]
- 35.Martin JP, Beyerlein A, Dacks AM, Reisenman CE, Riffell JA, Lei H, Hildebrand JG. The neurobiology of insect olfaction: sensory processing in a comparative context. Prog Neurobiol. 2011;95:427–447. doi: 10.1016/j.pneurobio.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Pearson KG. Common Principles of Motor Control in Vertebrates and Invertebrates. Annual Review of Neuroscience. 1993;16:265–297. doi: 10.1146/annurev.ne.16.030193.001405. [DOI] [PubMed] [Google Scholar]
- 37.Berni J, Pulver SR, Griffith LC, Bate M. Autonomous circuitry for substrate exploration in freely moving Drosophila larvae. Curr Biol. 2012;22:1861–1870. doi: 10.1016/j.cub.2012.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.