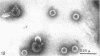
Abstract
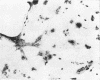
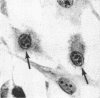
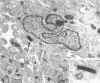
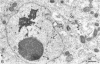
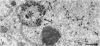
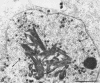
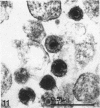
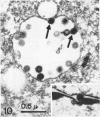
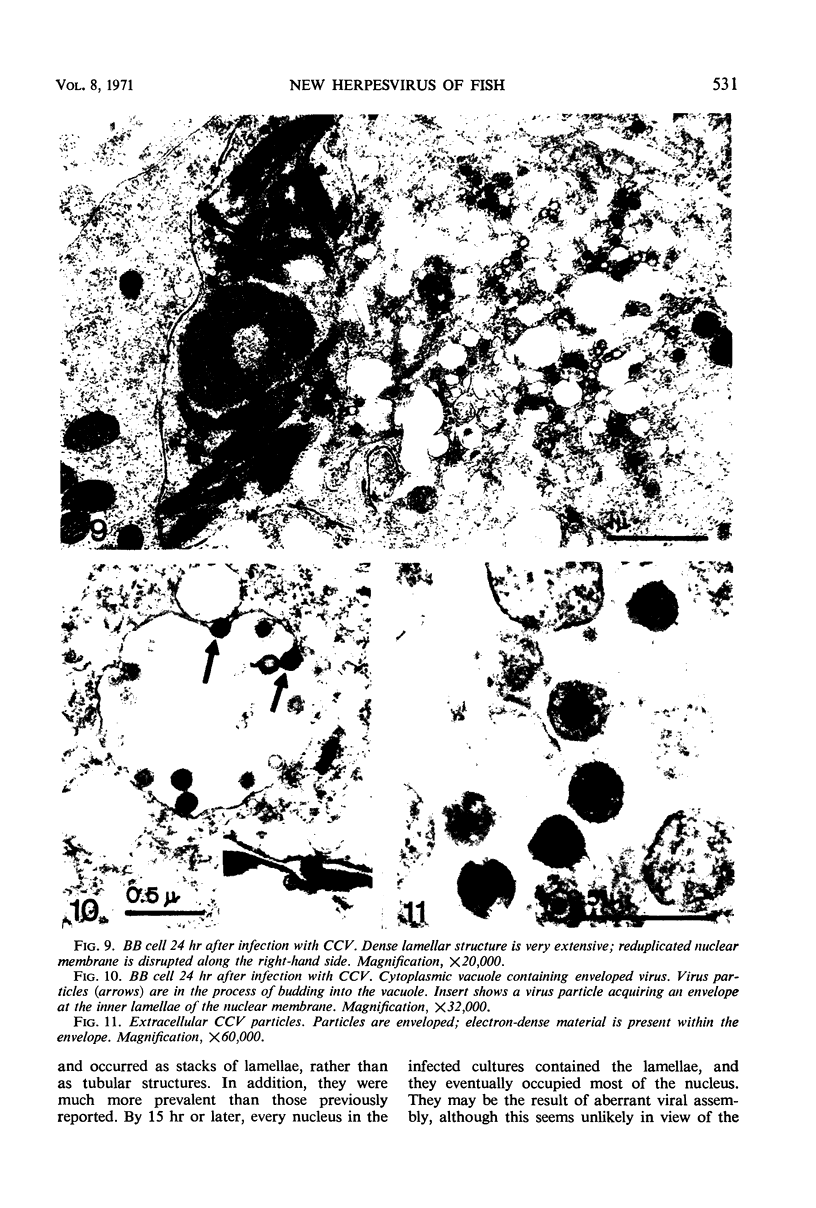
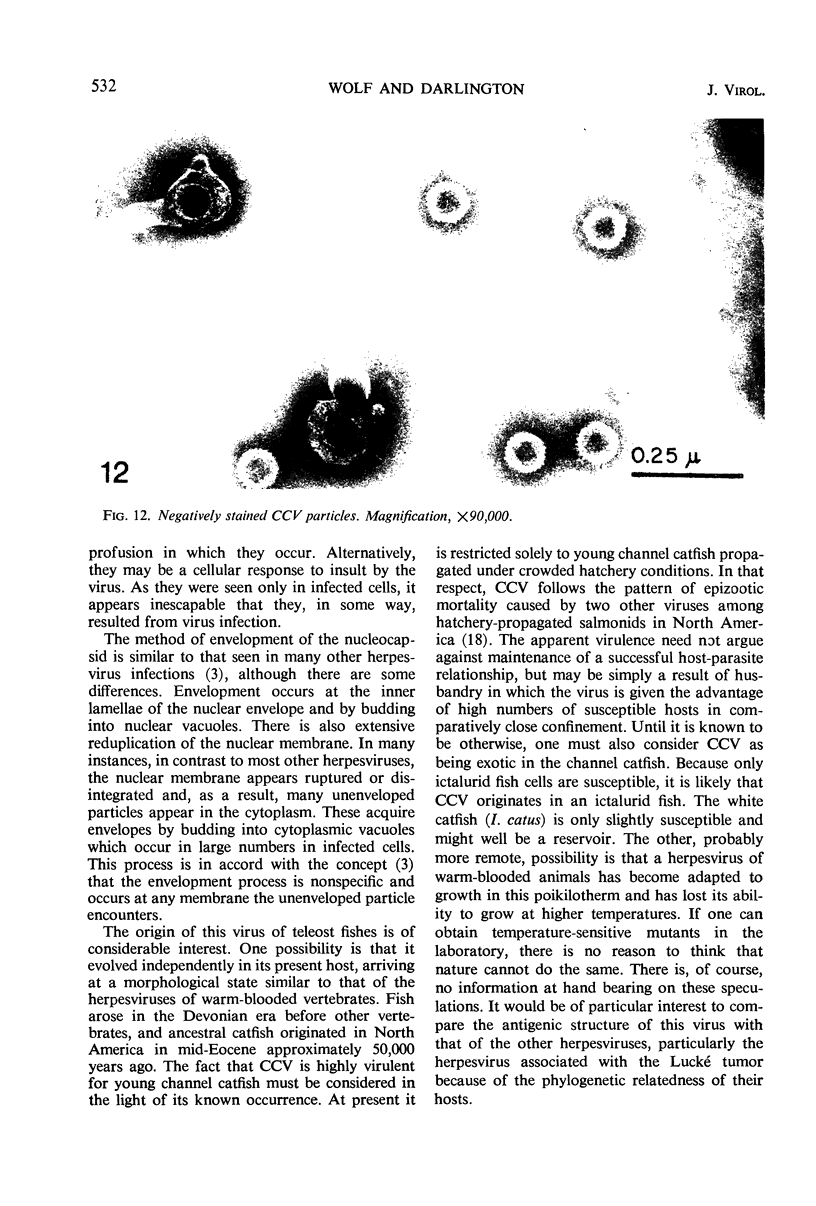
Channel catfish virus was studied in ictalurid fish cell culture, the only system of fish, amphibian, avian, and mammalian cells found to be susceptible. Channel catfish virus infection resulted in intranuclear inclusions and extensive syncytium formation. Replication occurred from 10 to 33 C, but not higher. Best growth was from 25 to 33 C, and the amount of virus released nearly equalled the amount which remained cell-associated. The virus was labile to lipid solvents, and indirect determinations with labeled precursors and a metabolic inhibitor showed evidence of deoxyribonucleic acid. Electron microscopy showed progeny virus, about 100 nm in diameter, in various stages of development in cell nuclei by 4 hr. Present also were nuclear masses of exceptionally electron-dense lamellar material, with a unit dimension of 10 to 15 nm. Virus was enveloped at the nuclear membrane and in cytoplasmic vacuoles, resulting in virions having a diameter of 175 to 200 nm. Negative staining demonstrated icosehedral symmetry and 162 capsomeres. Our data indicate that channel catfish virus is a herpesvirus.
Full text
PDF
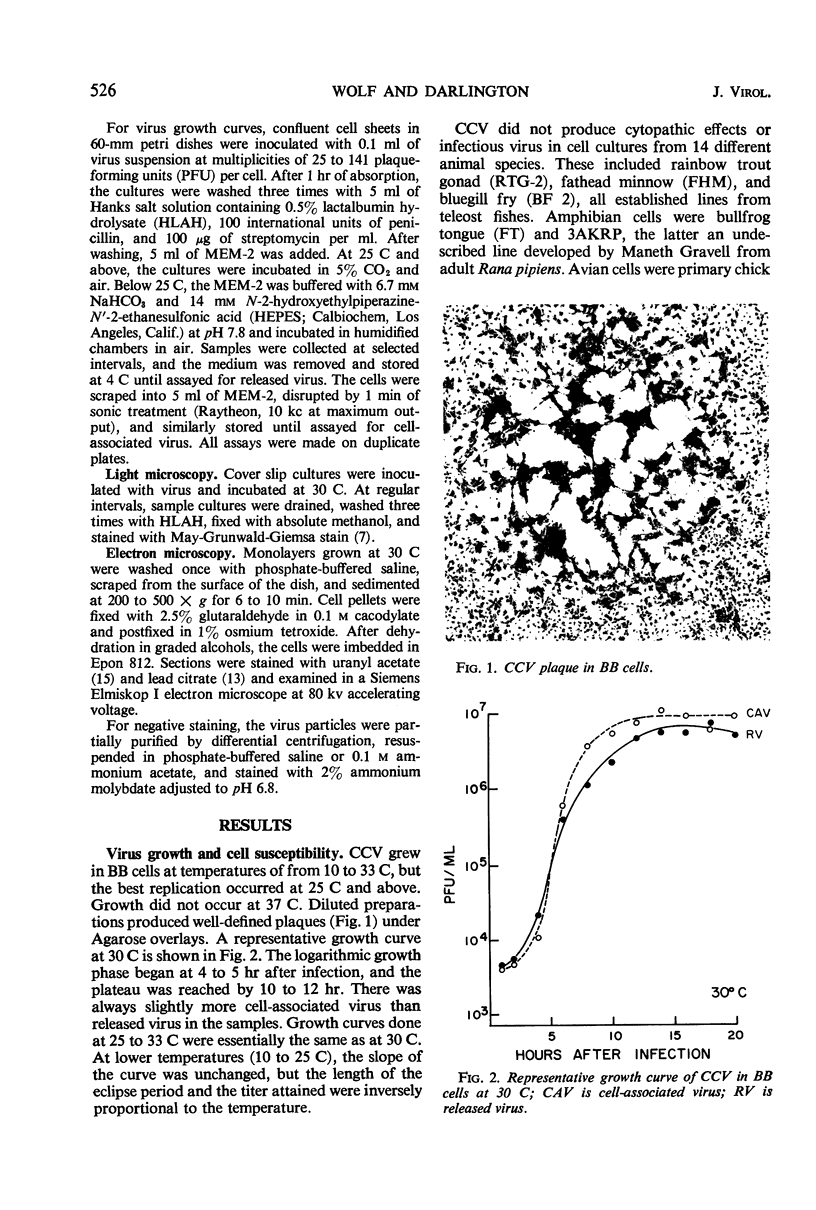

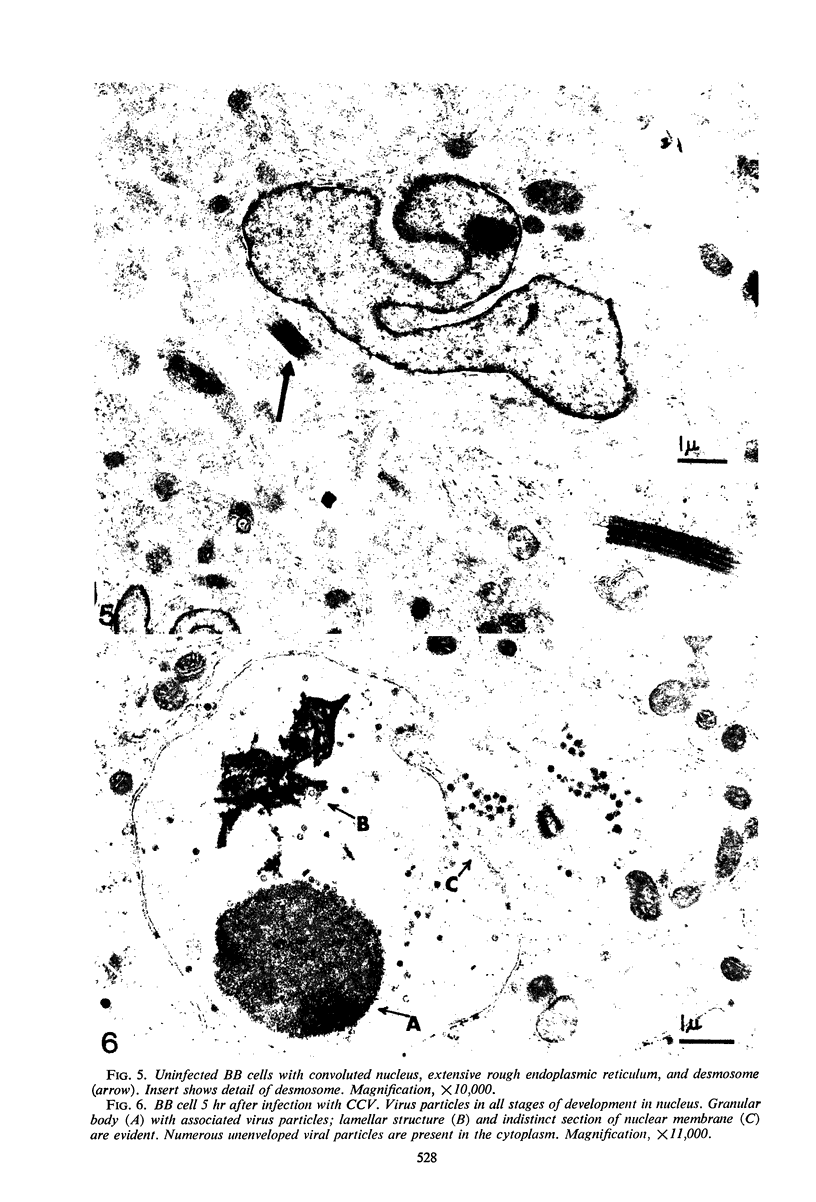
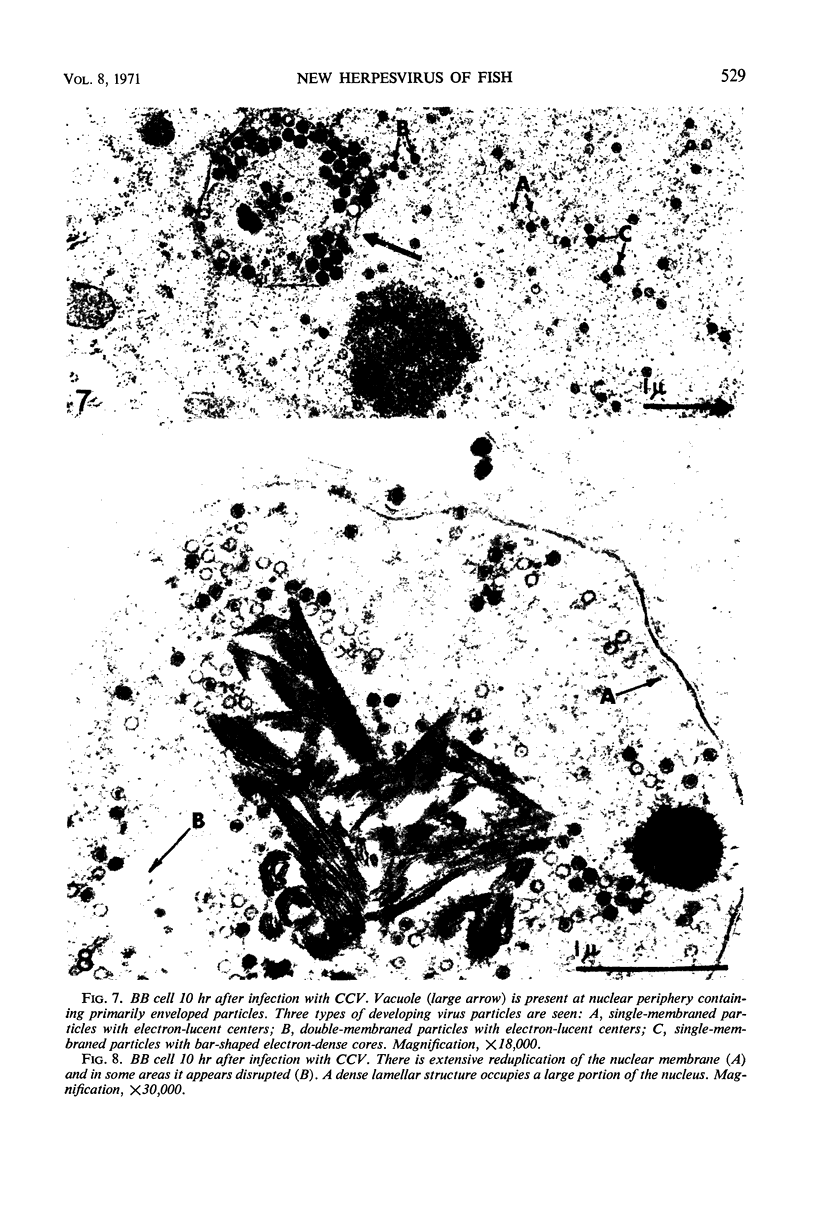


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Porat T., Coto C., Kaplan A. S. Unstable DNA synthesized by polyoma virus-infected cells. Virology. 1966 Sep;30(1):74–81. doi: 10.1016/s0042-6822(66)81011-5. [DOI] [PubMed] [Google Scholar]
- Couch E. F., Nahmias A. J. Filamentous structures of type 2 Herpesvirus hominis infection of the chorioallantoic membrane. J Virol. 1969 Feb;3(2):228–232. doi: 10.1128/jvi.3.2.228-232.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington R. W., Moss L. H., 3rd The envelope of Herpesvirus. Prog Med Virol. 1969;11:16–45. [PubMed] [Google Scholar]
- Lunger P. D. Cytoplasmic filaments and associated Lucké viruses in the frog renal adenocarcinoma. J Morphol. 1967 Sep;123(1):63–69. doi: 10.1002/jmor.1051230106. [DOI] [PubMed] [Google Scholar]
- MELNICK J. L., MIDULLA M., WIMBERLY I., BARRERA-ORO J. G., LEVY B. M. A NEW MEMBER OF THE HERPESVIRUS GROUP ISOLATED FROM SOUTH AMERICAN MARMOSETS. J Immunol. 1964 Apr;92:596–601. [PubMed] [Google Scholar]
- MORGAN C., ROSE H. M., HOLDEN M., JONES E. P. Electron microscopic observations on the development of herpes simplex virus. J Exp Med. 1959 Oct 1;110:643–656. doi: 10.1084/jem.110.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss L. H., 3rd, Gravell M. Ultrastructure and sequential development of infectious pancreatic necrosis virus. J Virol. 1969 Jan;3(1):52–58. doi: 10.1128/jvi.3.1.52-58.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F. A., Harrison A. K., Whitfield S. G. Intranuclear formation of filaments in herpesvirus hominis infection of mice. Arch Gesamte Virusforsch. 1967;21(3):463–468. doi: 10.1007/BF01241746. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reczko E., Böhm H. O., Straub O. C. Zur Feinstruktur des Rhinopneumonitisvirus der Pferde. Arch Gesamte Virusforsch. 1965;17(2):231–250. [PubMed] [Google Scholar]
- Ruebner B. H., Hirano T., Slusser R., Osborn J., Medearis D. N., Jr Cytomegalovirus infection. Viral ultrastructure with particular reference to the relationship of lysosomes to cytoplasmic inclusions. Am J Pathol. 1966 Jun;48(6):971–989. [PMC free article] [PubMed] [Google Scholar]
- STEMPAK J. G., WARD R. T. AN IMPROVED STAINING METHOD FOR ELECTRON MICROSCOPY. J Cell Biol. 1964 Sep;22:697–701. doi: 10.1083/jcb.22.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackpole C. W., Mizell M. Electron microscopic observations on herpes-type virus-related structures in the frog renal adenocarcinoma. Virology. 1968 Sep;36(1):63–72. doi: 10.1016/0042-6822(68)90117-7. [DOI] [PubMed] [Google Scholar]
- WATRACH A. M. Intranuclear filaments associated with infectious laryngotracheitis virus. Virology. 1962 Oct;18:324–327. doi: 10.1016/0042-6822(62)90020-x. [DOI] [PubMed] [Google Scholar]
- Weil R., Michel M. R., Ruschmann G. K. Induction of cellular DNA synthesis by polyoma virus. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1468–1475. doi: 10.1073/pnas.53.6.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K. The fish viruses. Adv Virus Res. 1966;12:35–101. doi: 10.1016/s0065-3527(08)60846-5. [DOI] [PubMed] [Google Scholar]