Abstract
Silver nanoparticles (AgNPs) are widely used nanoparticles and they are mainly used in antibacterial and personal care products. In this study, we evaluated the effect of AgNPs on cell death induction in the murine dendritic cell line DC2.4. DC2.4 cells exposed to AgNPs showed a marked decrease in cell viability and an induction of lactate dehydrogenase (LDH) leakage in a time- and dose-dependent manner. In addition, AgNPs promoted reactive oxygen species (ROS)-dependent apoptosis and AgNP-induced ROS triggered a decrease in mitochondrial membrane potential. The activation of the intracellular signal transduction pathway was also observed in cells cultured with AgNPs. Taken together, our data demonstrate that AgNPs are able to induce a cytotoxic effect in DCs through ROS generation. This study provides important information about the safety of AgNPs that may help in guiding the development of nanotechnology applications.
Keywords: Silver nanoparticle, Dendritic cells, Reactive oxygen species (ROS), Apoptosis, Intracellular signaling
INTRODUCTION
AgNPs are widely utilized nanoparticles, and they are mainly used in antibacterial and personal care products. Despite the rapidly increasing applications of AgNPs worldwide, their effects on humans and the environment are still under debate. It has been reported that AgNPs can induce toxic effects in various cells and animal models. AgNPs inhibited the activities of mitochondrial respiratory chain complexes in the brain, skeletal muscle, heart, and liver of rats (Costa et al., 2010). AgNPs had the most toxic effect in developing zebrafish embryos among silver, gold, and platinum nanoparticles (Asharani et al., 2011). AgNPs suppressed cell proliferation and promoted apoptosis in keratinocytes, liver cells, lung cells, macrophages, and Jurkat T cells (Yen et al., 2009; Eom and Choi, 2010; Lee et al., 2011; Piao et al., 2011; Zanette et al., 2011; Lim et al., 2012). Moreover, AgNPs induced DNA damage and increased the release of cytokines, including IL-6, IL- 8, and VEGF in human mesenchymal stem cells (Hackenberg et al., 2011). When mice were exposed to AgNPs by repeated oral administration, the level of various inflammatory cytokines and IgE in serum and the B cell proportion in whole blood were increased (Park et al., 2010).
However, AgNP application could suggest a new therapeutic possibility in several diseases. For example, AgNPs are able to promote neovascularization via the promotion of angiogenesis and VEGFR signaling (Kang et al., 2011). AgNPs have also been reported to trigger anti-tumor effects in Dalton’s lymphoma ascites tumor models, mouse fibroblast cells, and human hepatoma cells (Kim et al., 2009; Nallathamby and Xu, 2010; Sriram et al., 2010). Therefore, it is important to provide more extensive information regarding AgNP effects in living cells and organisms.
Dendritic cells (DCs) are the most potent antigen-presenting cells, and they reside in almost all tissues, including blood and lymphoid organs (Banchereau and Steinman, 1998; Banchereau et al., 2000; Blanco et al., 2008). DCs function as a sentinel of the immune system and an initiator of innate and adaptive immune responses. Mature DCs that have recognized antigen in peripheral tissue migrate to secondary lymphoid tissues and present the antigen to naïve T cells. Consequently, T cell responses are initiated (Heath et al., 2004). Activated dendritic cells are able to secret cytokines, such as IL-1, IL-12, TNF-α, and TGF-β. DCs also induce the activation of naïve T cells (Blanco et al., 2008). Given that AgNPs have been widely investigated in areas of drug delivery and targeting (Elechiguerra et al., 2005; Boucher et al., 2008), it is important to study the effect of AgNPs on dendritic cells which are able to orchestrate both innate and adaptive immune responses.
A number of reports have indicated that various nanoparticles applied to DCs play a significant role in the cytotoxicity, maturation, and function of DCs. Exposure to quantum dots (QD655-COOH) triggered the decreased expression of CD80/86 after LPS stimulation in DCs (Zhang et al., 2011). However, zinc oxide and carbon black nanoparticles increased the expression of CD80/86 and zinc oxide nanoparticles induced the production of infl ammatory cytokines in DCs (Koike et al., 2008; Heng et al., 2011). Silica nanoparticles and titanium dioxide nanoparticles induced an increase in reactive oxygen species (ROS) production in DCs (Winter et al., 2011). In this paper, for the first time, we evaluated the effect of Ag- NPs on DCs. DCs exposed to AgNPs (average size 2.3 nm) showed a decrease in cell viability and an induction of lactate dehydrogenase (LDH) leakage in a time- and dose-dependent manner. In addition, AgNPs promoted ROS-dependent apoptosis. AgNP-induced ROS triggered a decrease in mitochondrial membrane potential and an increase in the expression and activation of signaling proteins involved in intracellular signal transduction. In summary, our data demonstrate that AgNPs have a cytotoxic effect on DCs through ROS generation.
MATERIALS AND METHODS
Cell lines
The DC2.4 cell line, which was established as a murine dendritic cell line, was provided by Dr. K. Rock of Harvard Medical School (Shen et al., 1997). Cells were cultured in RPMI 1640 (Gibco/Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated FBS (Gibco/Invitrogen).
Preparation of AgNPs
PVP-capped AgNPs with an average size of 2.3 nm were synthesized in the laboratory of Prof. K. Lee (Department of Chemical Engineering, Yonsei University) by the polyol process, which was described in our previous report (Kang et al., 2011). To demonstrate DC uptake of AgNPs, DC2.4 cells were cultured with various concentrations of AgNPs for 12 h. Subsequenlty, the mean intensity of the side scatter channel (SSC) was determined using a FACSCanto™II flow cytometer (BD Biosciences, Bedford, MA).
Cell counting kit-8 (CCK-8) assay
A total of 1×104 DC2.4 cells were plated in 96-well plates and cultured in RPMI 1640 medium containing 10% FBS in the presence of various concentrations of AgNPs. After 24 h and 48 h of incubation, 10 μl of CCK-8 solution (Dojindo Laboratories, Kumamoto, Japan) was added to each well. After incubation for 3 h at 37℃, the absorbance was determined using a VICTOR3TM plate reader (PerkinElmer, Waltham, MA).
Trypan blue exclusion assay
A total of 1×105 DC2.4 cells were plated in RPMI 1640 medium containing 10% FBS in the presence of various concentrations of AgNPs. The cells were harvested after 24 h and 48 h, stained with 0.4% trypan blue and counted using a hematocytometer.
LDH leakage assay
DC2.4 cells were exposed to various concentrations of AgNPs for 48 h. LDH levels in cell culture supernatants were determined using a LDH cytotoxicity detection kit (Takara Bio Inc., Shiga, Japan). The high control was prepared by treatment with 1% Triton X-100. LDH leakage was obtained through the following equation: LDH leakage (%) = (exp. sample - untreated control/high control - untreated control) ×100.
Measurement of ROS levels
DC2.4 cells were pre-treated with 10 mM NAC (N-acetyl- L-cysteine, Sigma–Aldrich, St. Louis, MO) for 30 min. Cells were then exposed to AgNPs for 30 min. Next, 10 μM DCFDA (2′-7′-dichlorofluorescein diacetate, Sigma-Aldrich) was added to the cells for 15 min before analysis. The presence of the fluorescence product DCF, an indicator of ROS, was analyzed using a FACSCantoTMII flow cytometer.
Analysis of cell death by annexin V and PI staining
DC2.4 cells were pre-treated with 10 mM NAC for 30 min. Cells were then cultured with AgNPs for 1 h. Subsequently, the cells were stained with annexin V (BD Biosciences) and PI (BD Biosciences). Cell death was analyzed by flow cytometry using a FACSCantoTMII flow cytometer.
Mitochondrial membrane potential assessment
DC2.4 cells were pre-treated with 10 mM NAC for 30 min. The cells were exposed to AgNPs for 1 h. After the cells were washed, they were treated with the fluorescence probe 5,5’,6,6’-tetrachloro-1,1’,3,3’-tetraethylbenzimidazolcarbocyanine iodide (JC-1; Biotium, Inc., Hayward, CA) at 37℃ for 15 min. Subsequently, the fluorescence intensity was measured using a FACSCantoTMII flow cytometer, and the cells were imaged using an Olympus fluorescence microscope (Model IX71 with fluorescence system, Olympus Corp. Tokyo, Japan).
Western blot analysis
Cells were lysed in protein extraction solution (iNtRON Biotechnology). The proteins were separated on an SDS-polyacrylamide gel and blotted onto a PVDF membrane, which was then blocked by incubation with TBST (Tris-buffered saline and 0.05% Tween-20) containing 5% skim milk. Membranes were incubated with the appropriate antibodies. Antibodies against p44/42 MAPK, phospho-p44/42 MAPK, phospho-p38 MAPK, phospho-SAPK/JNK, SAPK/JNK, phospho-c-Jun, and IκBα were purchased from Cell Signaling Technology (Beverly, MA), and antibodies against p38 MAPK, p53, p21, MMP9 and α-actinin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Statistical analysis
The data represent the mean ± SD of more than two samples, and all of the experiments were performed more than three times. Statistical analysis was performed using Student's t-test (SigmaPlot Version 10, Systat Software Inc., San Jose, CA).
RESULTS
AgNPs uptake in DC2.4 cells
First, we evaluated whether AgNPs have a cytotoxic effect in murine dendritic cells. AgNPs coated with PVP to stabilize the NPs against aggregation have been previously described
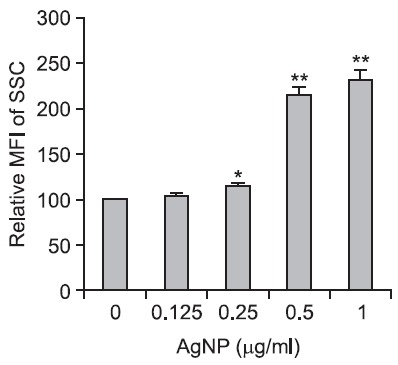
(Kang et al., 2011), and their average size was 2.3 nm. DC2.4 cells exposed to AgNPs showed a concentration-dependent increase in the mean intensity of the side scatter channel (SSC) in FACS analysis, suggesting the cellular uptake of nanoparticles (Fig. 1).
Fig. 1. AgNPs uptake in DC2.4 cells. DC2.4 cells were exposed to various concentrations of AgNPs for 12 h. Subsequently, the cellular uptake of AgNPs was detected using a FACSCantoTMII flow cytometer. The results represent the mean ± SD from triplicates. *p<0.05, **p<0.01.
AgNPs have cytotoxic activity in DC2.4 cells
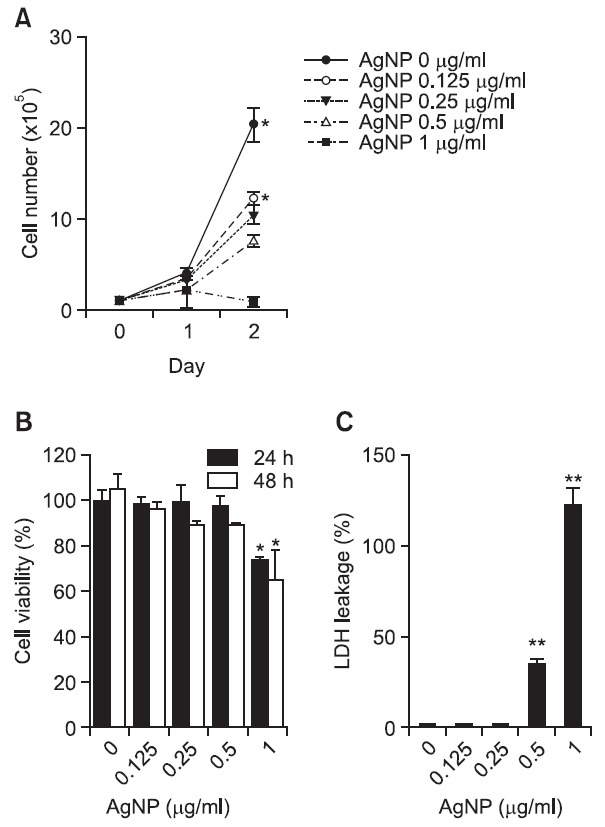
Next, we assessed the cytotoxic effect of AgNPs in DC2.4 cells. After cells were exposed to AgNPs for 24 h or 48 h, the number of cells was counted using a trypan blue exclusion assay. Cell proliferation was markedly decreased after exposure to AgNPs in a time- and dose-dependent manner (Fig. 2A). With a CCK-8 assay measuring water-soluble formazan dye produced by the dehydrogenase activity of live cells, we confirmed that cell viability was also suppressed after exposure to AgNPs in a time- and dose-dependent manner (Fig. 2B). In addition, culture supernatants from DC2.4 cells exposed to AgNPs for 48 h were evaluated for LDH leakage, because lactate dehydrogenase (LDH) is released after cell membrane damage. LDH leakage after exposure to AgNPs in DC2.4 cells was dramatically elevated at concentrations greater than 0.5 μg/ml (Fig. 2C). Thus, these results suggest that AgNPs can induce a cytotoxic effect in dendritic cells.
Fig. 2. AgNPs have cytotoxic effects on DC2.4 cells. (A) A total of 1×105 DC2.4 cells were cultured in the presence of various concentrations of AgNPs. After 24 h and 48 h, live cells were counted using a hematocytometer. (B) A total of 1×104 DC2.4 cells were cultured with various concentrations of AgNPs. After 24 h and 48 h incubation, 10 μl of CCK-8 solution was added to each well. After incubation for 3 h at 37℃, the absorbance was measured at 450 nm. (C) DC2.4 cells were exposed to various concentrations of AgNPs for 48 h. LDH in the cell culture supernatants was determined using an LDH cytotoxicity detection kit. The high control was prepared by treatment with 1% Triton X-100. LDH leakage was obtained through the following equation: LDH leakage (%) = (exp. sample - untreated control/high control - untreated control) X 100. The results represent the mean ± SD from triplicates. *p<0.05, **p<0.01.
AgNPs promote reactive oxygen species (ROS) production in DC2.4 cells
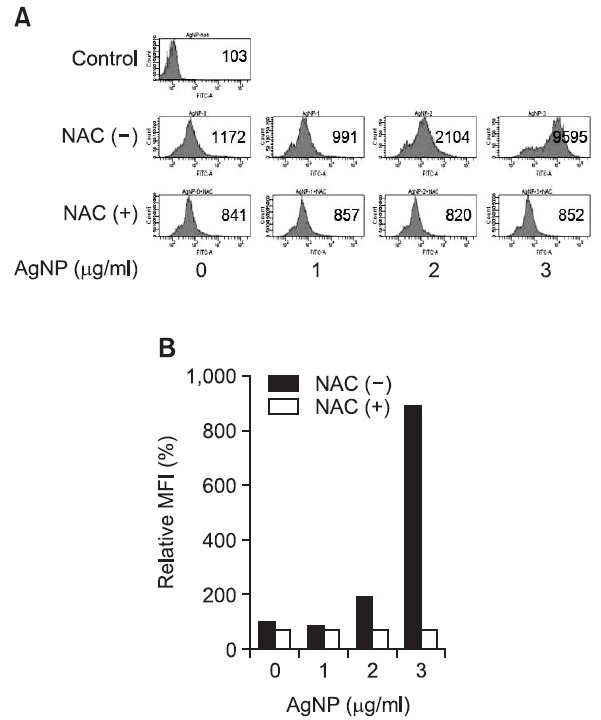
Because ROS are general mediators of nanoparticle-induced cytotoxicity, we evaluated whether AgNPs generated intracellular ROS in DC2.4 cells. As shown in Fig. 3, ROS levels were immediately increased in response to AgNPs, and AgNP-induced ROS were suppressed by pre-treatment with a synthetic antioxidant, N-acetyl-L-cysteine (NAC). Thus, these results indicate that AgNPs generate cellular oxidative stress, as revealed by elevated ROS production, in DC2.4 cells.
Fig. 3. AgNPs promote ROS production in DC2.4 cells. (A, B) DC2.4 cells were pre-treated with 10 mM NAC for 30 min. They were then exposed to AgNPs for 30 min. After 10 μM DCFDA was added to the cells for 15 min, the presence of the fluorescence product DCF was analyzed using a FACSCantoTMII flow cytometer. (A) The number indicates the mean fluorescence intensity (MFI). (B) The relative mean fluorescence intensity compared with the untreated control is plotted.
AgNPs promote ROS-induced apoptosis in DC2.4 cells
Next, we evaluated the effect of AgNPs on apoptosis in DC2.4 cells. After cells were exposed to various concentrations of AgNPs for 1 h, cell death was measured through double staining with propidium iodide (PI) and annexin V. DC2.4 cells showed an increase in cell death after being exposed to AgNPs in a concentration-dependent manner (Fig. 4). However, the increased cell death after exposure to AgNPs was
Fig. 4. AgNPs promote ROS-induced apoptosis. (A, B) DC2.4 cells were pre-treated with 10 mM NAC for 30 min. The cells were then cultured with AgNPs for 1 h. Subsequently, the cells were stained with annexin V (x-axis) and PI (y-axis). Cell death was analyzed by flow cytometry using a FACSCantoTMII, and the percentages of apoptotic cells are shown in each quadrant (A). (B) The total annexin V-positive cells were quantified.
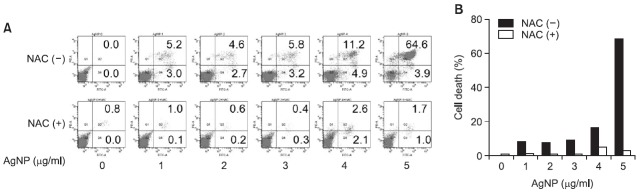
almost completely prevented by pre-treatment with NAC. Our data collectively show that AgNPs have a cytotoxic effect in DC2.4 cells and that this effect is triggered through ROS production.
Modulation of the mitochondrial membrane potential by AgNPs
Because mitochondrial permeability transition is an important aspect in the induction of apoptosis, the decrease in the mitochondrial membrane potential is a hallmark for apoptosis. Therefore, we investigated whether AgNPs affected the mitochondrial membrane potential in DCs. DC2.4 cells showed an increase in green fluorescence intensity after exposure to Ag- NPs in a concentration-dependent manner (Fig. 5A). Similarly, the percentage of depolarized cells was increased after exposure to AgNPs (Fig. 5B). When examined by fluorescence microscopy, the cells cultured without AgNPs showed intense red fluorescence and less green fluorescence. However, the cells treated with AgNPs showed intense red and green fluorescence, indicating depolarization of the mitochondrial fraction (Fig. 5C). Moreover, pre-treatment with NAC prevented the loss of mitochondrial membrane potential induced by Ag- NPs (Fig. 5). Thus, these results indicate that AgNPs induce the changes in mitochondrial membrane potential through ROS generation.
Fig. 5. AgNP treatment induces changes in mitochondrial membrane potential. DC2.4 cells were pre-treated with 10 mM NAC for 30 min. Subsequently, the cells were exposed to AgNPs for 1 h and treated with JC-1 for 15 min. (A) The green fluorescence was detected using a FACSCantoTMII. Filled black histogram, -NAC; tinted black histogram, +NAC. (B) The percentage of depolarized cells was determined by flow cytometry using a FACSCantoTMII. (C) Fluorescence images of AgNP-induced changes in mitochondrial membrane potential were obtained using fluorescence microscopy.

AgNPs activate ROS-mediated intracellular signals
ROS elicit intracellular signal transduction. To investigate whether AgNPs activated ROS-mediated intracellular signaling, western blot analysis for signaling mediators was performed in DC2.4 cells exposed to AgNPs. AgNPs led to the phosphorylation of ERK1/2 and SAPK/JNK and increased the expression of p53, p21, and MMP9 in DC2.4 cells in a concentration-dependent manner. In contrast, there was no significant change in the phosphorylation level of p38 or c-Jun, or the degradation of IκBα (Fig. 6A and B).
Fig. 6. AgNPs activate ROS-mediated intracellular signaling. (A, B) DC2.4 cells were exposed to various concentrations of AgNPs for 24 h. Western blot analysis was performed using specific antibodies in equal amounts of whole lysates. (C, D) DC2.4 cells were pretreated with 10 mM NAC for 30 min. After cells were exposed to 1 μg/ml AgNPs for 24 h, western blot analysis was performed.
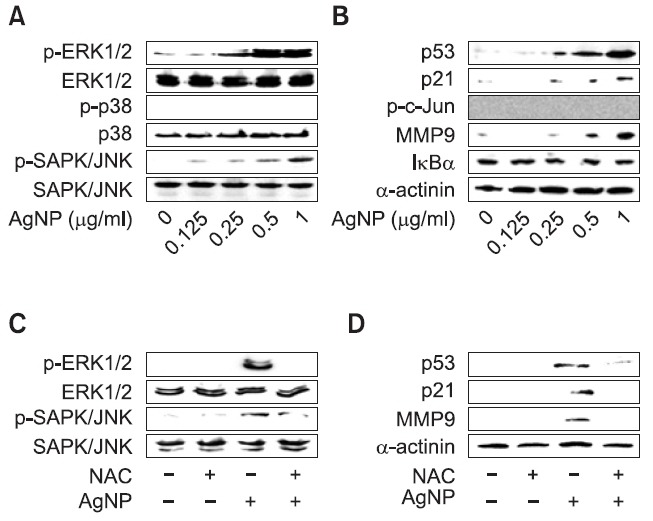
Next, we investigated whether the AgNP-induced activation of intracellular signaling in DC2.4 cells was promoted through the production of ROS. After the cells were pre-treated with NAC, they were exposed to AgNPs for 24 h. The activated levels of ERK1/2, SAPK/JNK, p53, p21, and MMP9 after exposure to AgNPs were inhibited by the pre-treatment with NAC, suggesting the involvement of ROS in intracellular signal transduction in DC2.4 cells (Fig. 6C and D). Thus, these data suggest that AgNP treatment results in the activation of ROS-mediated intracellular signaling pathways.
DISCUSSION
Although AgNPs are widely utilized nanoparticles in antibacterial and personal care products, their effects in living cells and organisms are poorly understood. According to previous publications, AgNPs suppress cell proliferation and induce apoptosis in various cells, including keratinocytes, liver cells, lung cells, macrophages, and Jurkat T cells (Yen et al., 2009; Eom and Choi, 2010; Lee et al., 2011; Piao et al., 2011; Zanette et al., 2011). Additionally, AgNPs have been widely investigated in areas of drug delivery and targeting (Elechiguerra et al., 2005; Boucher et al., 2008). Because of their potential application for drug delivery, it is also important to study the effect of AgNPs on immune cells.
In the present study, we first evaluated the effect of AgNPs on DCs which are the most potent antigen-presenting cells. Similar to our previous study (Kang et al., 2011), AgNPs (2.3 nm) were coated with PVP to prevent aggregation. AgNPs inhibited cell proliferation and viability and increased LDH leakage in a time- and concentration-dependent manner in DC2.4 cells (Fig. 2). Furthermore, we confirmed that AgNPs promot-
ed apoptosis in DC2.4 cells (Fig. 4). The main cause of AgNPinduced cell death was late apoptosis, and less than 5% of cell death induced by AgNPs was early apoptosis. There was no significant effect of AgNPs on early apoptosis induction, whereas late apoptotic or necrotic cell death was clearly induced by AgNPs. Because PVP alone did not result in any toxicity in DC2.4 cells (data not shown), it was evident that the observed toxicity was triggered by AgNP alone. Recently, data of Lim et al. demonstrated that 5 nm PVP-coated AgNPs induced destruction or swelling of mitochondria or nuclei and double-layered membrane structures in U937 macrophage cell line, meaning a possibility of necrotic changes by AgNPs (Lim et al., 2012). Therefore, although we showed changes in mitochondrial potential shortly after treatment with AgNPs, the possibility of AgNP affecting both apoptosis and necrosis should be considered.
ROS target mitochondria and cause apoptosis and DNA damage. Several nanoparticles have been reported as inducers of ROS in a variety of cells (AshaRani et al., 2009; Eom and Choi, 2009a; Eom and Choi, 2009b; Park and Park, 2009; Wang et al., 2009; Wu et al., 2010; Kang et al., 2011; Shukla et al., 2011).
However, the role of ROS generated by AgNPs was unclear in DCs. The exposure of DCs to AgNPs could result in the production of ROS, which could explain outcomes of metabolic and toxicological problems (Fig. 3). In fact, Ag- NPs elicited ROS-dependent apoptosis (Fig. 4) and the inhibition of mitochondrial function (Fig. 5). In the present study, the LDH leakage induced by AgNPs was examined when cells were treated with AgNPs for 48 h. ROS production and ROSinduced apoptosis were observed using cells treated with Ag- NPs for 30 min to 1 h. Although ROS was also produced from cells treated with AgNPs for 24 h and 48 h, we detected ROS level in early phase to determine ROS generation by AgNPs exactly. In addition, ROS-mediated intracellular signaling molecules, including ERK1/2, JNK, p53, p21, and MMP9, were activated in response to AgNPs, and their activation was inhibited by a pre-treatment with NAC (Fig. 6). In a previous paper, it was reported that PVP-coated AgNPs induced the DNA damage marker p53 in larval tissue of Drosophila melanogaster (Ahamed et al., 2010). AgNPs also activated JNK-dependent mechanisms involved in the mitochondrial pathway in NIH3T3 cells (Hsin et al., 2008). We also confi rmed that the activation of intracellular signaling in response to AgNPs was induced through the production of ROS, which was blocked by NAC (Fig. 6C and D). It is worth speculating that AgNPs might interact with some components that are able to generate ROS. Thus, further study on the mechanisms of AgNPs leading to the generation of ROS is required.
The toxic effect of NPs is triggered by inflammation resulting from oxidative stress (Carlson et al., 2008; Su et al., 2009). Alveolar macrophages have been shown to secrete TNF-α, MIP-2, and IL-1β after exposure to AgNPs (Carlson et al., 2008). In the mouse macrophage cell line RAW264.7, AgNPs also promoted the induction of COX-2, TNF-α, and IL-6 (Nishanth et al., 2011). Moreover, the level of IL-1, TNF-α, and IL-6 in serum was increased when mice were treated with Ag- NPs, and increased levels of Th2 cytokines were dominant compared with Th1 cytokines (Park et al., 2010). Results of Lim et al. showed that IL-8 production induced by AgNPs was inhibited in response to NAC in U937 cells (Lim et al., 2012). However, in our study using DC2.4 cells, AgNPs exhibited no significant effects on the expression and production of inflammatory cytokines such as TNF-α or IL-1β (data not shown).
In conclusion, our findings suggest that AgNPs have a cytotoxic effect through production of ROS in DCs. Moreover, this study provides important information about the safety of AgNPs that may help in guiding the development of nanotechnology applications.
Acknowledgments
This work was supported by the Nano R&D Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology of Korea (2010-0019159) and partly by the Sookmyung Women's University Research Grant 2010.
References
- 1.Ahamed M., Posgai R., Gorey T. J., Nielsen M., Hussain S. M., Rowe J. J. Silver nanoparticles induced heat shock protein 70, oxidative stress and apoptosis in Drosophila melanogaster. Toxicol. Appl. Pharmacol. (2010);242:263–269. doi: 10.1016/j.taap.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Asharani P. V., Lianwu Y., Gong Z., Valiyaveettil S. Comparison of the toxicity of silver, gold and platinum nanoparticles in developing zebrafish embryos. Nanotoxicology. (2011);5:43–54. doi: 10.3109/17435390.2010.489207. [DOI] [PubMed] [Google Scholar]
- 3.AshaRani P. V., Low Kah Mun G., Hande M. P., Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS. Nano. (2009);3:279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J., Steinman R. M. Dendritic cells and the control of immunity. Nature. (1998);392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau J., Briere F., Caux C., Davoust J., Lebecque S., Liu Y. J., Pulendran B., Palucka K. Immunobiology of dendritic cells. Annu. Rev. Immunol. (2000);18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 6.Blanco P., Palucka A. K., Pascual V., Banchereau J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. (2008);19:41–52. doi: 10.1016/j.cytogfr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boucher W., Stern J. M., Kotsinyan V., Kempuraj D., Papaliodis D., Cohen M. S., Theoharides T. C. Intravesical nanocrystalline silver decreases experimental bladder inflammation. J. Urol. (2008);179:1598–1602. doi: 10.1016/j.juro.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 8.Carlson C., Hussain S. M.,, Schrand A. M., Braydich-Stolle L. K., Hess K. L., Jones R. L., Schlager J. J. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J. Phys. Chem. B. (2008);112:13608–13619. doi: 10.1021/jp712087m. [DOI] [PubMed] [Google Scholar]
- 9.Costa C. S., Ronconi J. V., Daufenbach J. F., Goncalves C. L., Rezin G. T., Streck E. L., Paula M. M. In vitro effects of silver nanoparticles on the mitochondrial respiratory chain. Mol. Cell Biochem. (2010);342:51–56. doi: 10.1007/s11010-010-0467-9. [DOI] [PubMed] [Google Scholar]
- 10.Elechiguerra J. L., Burt J. L., Morones J. R., Camacho-Bragado A., Gao X., Lara H. H., Yacaman M. J. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnology. (2005);3:6. doi: 10.1186/1477-3155-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eom H. J., Choi J. Oxidative stress of CeO2 nanoparticles via p38-Nrf-2 signaling pathway in human bronchial epithelial cell, Beas-2B. Toxicol. Lett. (2009a);187:77–83. doi: 10.1016/j.toxlet.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 12.Eom H. J., Choi J. Oxidative stress of silica nanoparticles in human bronchial epithelial cell, Beas-2B. Toxicol. In Vitro. (2009b);23:1326–1332. doi: 10.1016/j.tiv.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Eom H. J., Choi J. p38 MAPK activation, DNA damage, cell cycle arrest and apoptosis as mechanisms of toxicity of silver nanoparticles in Jurkat T cells. Environ. Sci. Technol. (2010);44:8337–8842. doi: 10.1021/es1020668. [DOI] [PubMed] [Google Scholar]
- 14.Hackenberg S., Scherzed A., Kessler M., Hummel S., Technau A., Froelich K., Ginzkey C., Koehler C., Hagen R., Kleinsasser N. Silver nanoparticles: evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol. Lett. (2011);201:27–33. doi: 10.1016/j.toxlet.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Heath W. R., Belz G. T., Behrens G. M., Smith C. M., Forehan S. P., Parish I. A., Davey G. M, Wilson N. S., Carbone F. R., Villadangos J. A. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol. Rev. (2004);199:9–26. doi: 10.1111/j.0105-2896.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- 16.Heng B. C., Zhao X., Tan E. C., Khamis N., Assodani A., Xiong S., Ruedl C., Ng K. W., Loo J. S. Evaluation of the cytotoxic and infl ammatory potential of differentially shaped zinc oxide nanoparticles. Arch. Toxicol. (2011);85:1517–1528. doi: 10.1007/s00204-011-0722-1. [DOI] [PubMed] [Google Scholar]
- 17.Hsin Y. H., Chen C. F., Huang S., Shih T. S., Lai P. S., Chueh P. J. The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol. Lett. (2008);179:130–139. doi: 10.1016/j.toxlet.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Kang K., Lim D. H., Choi I. H., Kang T., Lee K., Moon E. Y., Yang Y., Lee M. S., Lim J. S. Vascular tube formation and angiogenesis induced by polyvinylpyrrolidone-coated silver nanoparticles. Toxicol. Lett. (2011);205:227–234. doi: 10.1016/j.toxlet.2011.05.1033. [DOI] [PubMed] [Google Scholar]
- 19.Kim S., Choi J. E., Choi J., Chung K. H., Park K., Yi J., Ryu D. Y. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol. In Vitro. (2009);23:1076–1084. doi: 10.1016/j.tiv.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Koike E., Takano H., Inoue K., Yanagisawa R., Kobayashi T. Carbon black nanoparticles promote the maturation and function of mouse bone marrow-derived dendritic cells. Chemosphere. (2008);73:371–376. doi: 10.1016/j.chemosphere.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 21.Lee Y. S., Kim D. W., Lee Y. H., Oh J. H., Yoon S., Choi M. S., Lee S. K., Kim J. W., Lee K., Song C. W. Silver nanoparticles induce apoptosis and G2/M arrest via PKCζ-dependent signaling in A549 lung cells. Arch. Toxicol. (2011);85:1529–1540. doi: 10.1007/s00204-011-0714-1. [DOI] [PubMed] [Google Scholar]
- 22.Lim D. H., Jang J., Kim S., Kang T., Lee K., Choi I. H. The effects of sub-lethal concentrations of silver nanoparticles on inflammatory and stress genes in human macrophages using cDNA microarray analysis. Biomaterials. (2012);33:4690–4699. doi: 10.1016/j.biomaterials.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Nallathamby P. D., Xu X. H. Study of cytotoxic and therapeutic effects of stable and purified silver nanoparticles on tumor cells. Nanoscale. (2010);2:942–952. doi: 10.1039/c0nr00080a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishanth R. P., Jyotsna R. G., Schlager J. J., Hussain S. M., Reddanna P. Inflammatory responses of RAW 264.7 macrophages upon exposure to nanoparticles: role of ROS-NFκB signaling pathway. Nanotoxicology. (2011);5:502–516. doi: 10.3109/17435390.2010.541604. [DOI] [PubMed] [Google Scholar]
- 25.Park E. J., Park K. Oxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitro. Toxicol. Lett. (2009);184:18–25. doi: 10.1016/j.toxlet.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Park E. J., Bae E., Yi J., Kim Y., Choi K., Lee S. H., Yoon J., Lee B. C., Park K. Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ. Toxicol. Pharmacol. (2010);30:162–168. doi: 10.1016/j.etap.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Piao M. J., Kang K. A., Lee I. K., Kim H. S., Kim S., Choi J. Y., Choi J., Hyun J. W. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol. Lett. (2011);201:92–100. doi: 10.1016/j.toxlet.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Shen Z., Reznikoff G., Dranoff G., Rock K. L. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J. Immunol. (1997);158:2723–2730. [PubMed] [Google Scholar]
- 29.Shukla R. K., Sharma V., Pandey A. K., Singh S., Sultana S., Dhawan A. ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol. In Vitro. (2011);25:231–241. doi: 10.1016/j.tiv.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Sriram M. I., Kanth S. B., Kalishwaralal K., Gurunathan S. Antitumor activity of silver nanoparticles in Dalton's lymphoma ascites tumor model. Int. J. Nanomedicine. (2010);5:753–762. doi: 10.2147/IJN.S11727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su H. L., Chou C. C., Hung D. J., Lin S. H., Pao I. C., Lin J. H., Huang F. L., Dong R. X., Lin J. J. The disruption of bacterial membrane integrity through ROS generation induced by nanohybrids of silver and clay. Biomaterials. (2009);30:5979–5987. doi: 10.1016/j.biomaterials.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 32.Wang F., Gao F., Lan M., Yuan H., Huang Y., Liu J. Oxidative stress contributes to silica nanoparticle-induced cytotoxicity in human embryonic kidney cells. Toxicol. In Vitro. (2009);23:808–815. doi: 10.1016/j.tiv.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Winter M., Beer H. D., Hornung V., Krämer U., Schins R. P., Förster I. Activation of the infl ammasome by amorphous silica and TiO2 nanoparticles in murine dendritic cells. Nanotoxicology. (2011);5:326–340. doi: 10.3109/17435390.2010.506957. [DOI] [PubMed] [Google Scholar]
- 34.Wu J., Sun J., Xue Y. Involvement of JNK and P53 activation in G2/M cell cycle arrest and apoptosis induced by titanium dioxide nanoparticles in neuron cells. Toxicol. Lett. (2010);199:269–276. doi: 10.1016/j.toxlet.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Yen H. J., Hsu S. H., Tsai C. L. Cytotoxicity and immunological response of gold and silver nanoparticles of different sizes. Small. (2009);5:1553–1561. doi: 10.1002/smll.200900126. [DOI] [PubMed] [Google Scholar]
- 36.Zanette C., Pelin M., Crosera M., Adami G., Bovenzi M., Larese F. F., Florio C. Silver nanoparticles exert a long-lasting antiproliferative effect on human keratinocyte HaCaT cell line. Toxicol. In Vitro. (2011);25:1053–1060. doi: 10.1016/j.tiv.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L. W., Bäumer W., Monteiro-Riviere N. A. Cellular uptake mechanisms and toxicity of quantum dots in dendritic cells. Nanomedicine (Lond) (2011);6:777–791. doi: 10.2217/nnm.11.73. [DOI] [PMC free article] [PubMed] [Google Scholar]


