Abstract
Repeated stress induces corticosterone release. However, it is not clear that stress results in further elevation of corticosterone levels, and the roles of released corticosterone to aggravate stress-related symptoms are also not clear. This study investigated whether neuronal modulation was induced in the amygdala after two kinds of stress, that is, such as electric shock and corticosterone injection. It was found that stress by electric shock decreased the expression of tyrosine hydoroxylase (TH) in the amygdala while the expression of pERK was increased. However, there is no difference in the expressions of TH and pERK in the frontal cortex compared with those of the control group. The level of corticosterone was significantly increased in the serum after stress. To determine the effect of corticosterone on the induction of anxiety and the expression of TH, the rats received corticosterone (20 mg or 40 mg/kg i.p.) for 1 day, 1 week, 2 weeks and 3 weeks, respectively. The spent time in open arms of the EPM (elevated plus maze) test was significantly decreased after 1 week, 2 weeks and 3 weeks. The time spent in open arms of the EPM test after repeated injections of corticosterone was significantly decreased in a dose-dependent manner. The expression of TH in the amygdala was reduced after following repeated corticosterone treatment for 2 weeks and 3 weeks. Collectively, this study suggests that corticosterone has a major role in the induction of anxiety and the modulation of TH expression, at least, in the amygdala.
Keywords: Amygdala, Stress, Anxiety, Corticosterone, TH
INTRODUCTION
Stress is defined as a state of threatened homeostasis that causes a variety of changes in the central nervous system, endocrine, immune systems and in peripheral tissue’s metabolism (Kopin, 1995). Stresses are strong physical pressures applied to an object. Many stressors exist such as immobilization (Nankova et al., 1996), electric shock (Chida et al., 2004), pain stress (Oztaş et al., 2004) and cold water stress (Feng et al., 2005). Among those stressors, immobilization and electric shock are commonly used and they are easy to implement. The data on electric shock stress is similar to the data on immobilization stress and both elevate the corticosterone levels (Van de Kar et al., 1991; Iwasaki-Sekino et al., 2009) and induce anxiety (Hogg, 1996). In addition, there is a stress model using corticosterone as an indirect stressor, and corticosterone is known to be as a hormone closely related to induced stress (Gregus et al., 2005). Zhao suggested that the repeated corticosterone injection paradigm provides a useful, reliable mouse model of stress (Zhao et al., 2008). It was shown that repeated corticosterone injection (20 mg/kg, 40 mg/kg) for up to 5 weeks increased immobility behavior on the forced swim test in a time-dependent manner (Gregus et al., 2005; Zhao et al., 2008). However, it is not clear whether prolonged repeated immobilization stress and foot shock stress results in further elevations in circulating glucocorticoid levels or if there is some kind of adaptation of corticosterone release due to chronic stress.
Lechin and collegues measured the levels of plasma neurotransmitters and hormone levels in order to investigate the role of stress on many types of diseases. Noradrenaline, adrenaline, dopamine, serotonin, growth hormone and cortisol, which are major stress hormones, were determined during both exacerbation and improvement periods (Lechin et al., 1994; Wong, 2006). The behavioral and physical responses to stress are especially initiated by activation of the hypothalamic-pituitary-adrenal (HPA) axis, and this results in the release of catecholamines and stress hormones such as corticosterone from the adrenal glands (McEwen, 2000). This commonly includes activation of the neuroendocrine cascade of events whereby ACTH is released into the general circulation to stimulate production and release of glucocorticoids from the adrenal cortex. Stress-related sensory information is conveyed to the corticotrophin-releasing hormone (CRH) secreting neurons in the brain. The CRH directly stimulates corticosterone secretion and this is a major product of the stress response (Andreis et al., 1991). The amygdala is known to play a central function in aversive learning, responses to stress, and modulation of memory by stressful experiences (Stutzmann and LeDoux, 1999). Restraint stress rapidly and selectively increases serotonin release in the central nucleus of the amygdala by the activation of CRH receptors (Mo et al., 2008).
Repeated immobilization stress induces release of corticosterone (Yoshihara and Yawaka, 2008). Accumulating evidence has suggested that repeated corticosterone injections to male rats produce changes in emotional behavior that correspond to symptoms of clinical depression (Gregus et al., 2005). In addition, corticosterone receptor is involved in the suppressive effects of high levels of corticosterone on the 5-HT1A receptor- dependent regulation of serotonin’s neuronal activity in the rat dorsal raphe nucleus.
One of the most consistent adaptations is an increase of tyrosine hydroxylase (TH) expression in the ventral tegmental area after exposure to stress. Tyrosine hydroxylase is considered to be the rate-limiting enzyme in the synthesis of catecholamine in both the central and peripheral nervous systems. Tyrosine hydroxylase activity in the adrenal gland has been shown to increase following a number of stresses, including immobilization (Kvetnansky et al., 1970) and electroconvulsive shock (Masserano et al., 1981). It was reported that immobilization induced a signifi cant reduction of TH activity in brains (Gilad and McCarty, 1981). In view of this relatively precise knowledge of the anatomy of the catecholamine pathways in the brain, many investigators have examined the regulation of TH activity within the specific catecholamine cell body regions of the brain and in the areas to which they project in an attempt to understand the function of the catecholamine systems in the CNS and their interactions with other putative neurotransmitter systems.
The mitogen-activated protein (MAP) kinase pathway is an intracellular signaling cascade that has been implicated in learning, memory, immune system and stress (Gourley et al., 2008; Wu et al., 2008). ERK1/2 has been documented in response to certain physiological stimuli, such as ischemia, visceral pain and electric shock stress. Shen demonstrated that restraint stress activates the ERK and MAPK pathways (Shen et al., 2004). ERK1/2 phosphorylation has been hypothesized to be an intracellular signaling mechanism for mediating antidepressant efficacy in models of depression, yet the supporting evidence is largely limited to studies in otherwise naïve rodents or in vitro models (Hisaoka et al., 2001; Tiraboschi et al., 2004; Fumagalli et al., 2005) despite the evidence from postmortem studies of depressed suicide subjects (Dwivedi et al., 2006). It is not known if ERK1/2 is involved in reversal of the depression-like phenotype by antidepressant administration and if this is so, whether phosphorylation of both kinase isoforms is uniformly regulated throughout the cortico-limbic circuits, which have been implicated in depression (Gourley et al., 2008). In this experiment, we determined the effect of repeated cortocosterone treatment on anxiety and the modulation of TH and pERK expressions in the amygdala.
MATERIALS AND METHODS
Animals and chemicals
Sprague-Dawley rats (male, 260-280 g) at 7 weeks of age were obtained from Daehan Biolink (Eumsung, Korea). All rodents were maintained on a 12 h light-dark cycle at a constant temperature of 24 ± 3℃ and they were given lab chow and water ad libitum. These rodents were housed in plastic cages and they were allowed to adapt to the new environment for 1 week before use in the experiments. All procedures involving rodents were performed according to the guidelines approved by the Animal Care and Committee of the School of Medicine, Ewha Womans University.
Corticosterone (Sigma-Aldrich, St. Louis, USA) was dissolved in dimethyl sulfoxide (DMSO) and then diluted in 0.9% saline to reach the appropriate concentration. The final concentration of DMSO was 0.1% in saline. These doses were selected on the basis of previous experiments (Gregus et al., 2005). We assessed the exploratory behavior and neurochemical changes in rats injected with saline or corticosterone (20 or 40 mg/kg/day, i.p.) which was administered daily at a volume of 1.0 ml/kg at 9:00 AM for 1 day, 1 week, 2 weeks and 3 weeks, respectively.
Electric shock stress
Rats received inescapable 5 mA electric shocks from a 300 volt shock source and the shocks were delivered to the grid floor by a constant current shocker that used a cycle timer (Jungdo BNP, Seoul, Korea). Each stimulation lasted 1s and, the shocks were delivered with an intershock interval of 19 s for a total of 5 min. The experiments were started at 1 h after administration of glucocorticoid. The control group was placed in their original cages in an undisturbed home environment, which was the same as the stress experimental room.
Anxiety measurement with EPM
The elevated plus maze (EPM) test is a suitable rodent model of anxiety and this model has been extensively used in the discovery of novel anxiolytic agents and for investigating the psychological and neurochemical basis of anxiety (Santarelli et al., 2003). The EPM was made of black plexiglass and was elevated to 50 cm from the floor. The apparatus consisted of four arms (50×10 each) positioned at right angles to one another. Two of the arms had 20 cm high walls (enclosed arms) and the other arms had no walls (open arms). The illumination at the center was adjusted to 50 lux. Each rat was initially placed on the central platform and allowed to explore the arms for 5 min. The time of entry and staying in the open arms was recorded. A home video system was used to track the rats and we measured the amount of time spent stationary. Animals were sacrificed 3 h after the last session of stress. After decapitation, the brain was removed and each tissue (frontal cortex and amygdala) was excised from the brain.
HPLC analysis of glucocorticoid
The concentrations of corticosterone in plasma were determined by a modified high performance liquid chromatography (HPLC) procedure. Trunk blood was obtained to determine the corticosterone plasma levels. Corticosterone was extracted from plasma with a modification of the method reported by Woodward and Emery (Yamano et al., 2004). Corticosterone was extracted into 5 ml of diethyl ether-dicloromethane (60:40
v/v) by vortex mixing and immediately centrifuging for 5 min. Supernatant was vortex mixed with 1 ml of HPLC-grade water. After centrifugation, supernatant (3 ml) was evaporated at room temperature under nitrogen. The residue was redisolved in 100 ml of methanol water (60:40 v/v). The guard column (Symmetry C18, particle size 3.5 μm, column size 2.1×10 mm; Waters Corp., Milford, MA, USA) was equilibrated using HPLC-grade acetonitrile-water (65:35 v/v) at a flow rate of 0.4 ml/min. Separations were done at a temperature of 40℃, in a Waters Symmetry C18 column (particle size: 5 μm, column size: 2.0×150 mm, Waters Corp., Milford, MA, USA). A Waters 600-MS system controller was used to flush the mobile phase and the steroids were assessed with a 486 Waters UV absorbance detector (fitted at 250 nm). The results were analyzed using the Maxima 820 Chromatography Workstation, obtained from Waters. A series of standards covering the range of 0-50 μg/dl were used in the daily work. The detection limit of the assay for corticosterone was 0.05 μg/dl with using 1 ml of sample and a signal to noise ratio of 2:1. This detection limit is lower than the levels of corticosterone in adrenalectomized rats (0.5 μg/dl).
Statistical analysis
All the values were expressed as means ± standard error (SE). The results were subjected to an analysis of the variance (ANOVA) using the Newman-Keuls multiple comparison test. Differences with *p<0.05 and **p<0.01 were considered as statistically significant to analyze the difference.
RESULTS
Electric shock induced anxiety-like behavior
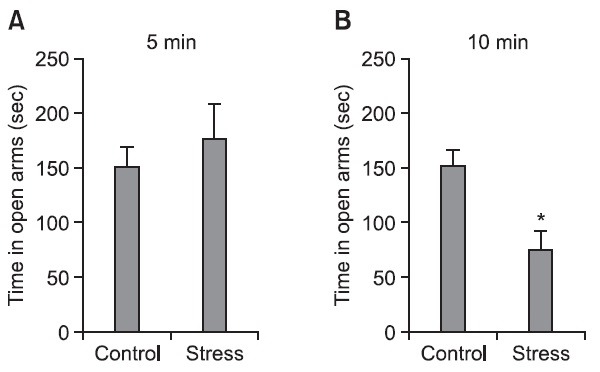
Rats were given stress with two different durations of electric shock, and induction of anxiety was determined by the elevated plus maze (EPM) at 2 hr after the final stress. The
stressed group with electric shock during 10 min for 7 days spent significantly less time (p<0.05) in the open arms compared with that of the control group (Fig. 1B), but the stressed group with electric shock for 5 min did not show difference of spending time in the open arms (Fig. 1A). Therefore, this study selected the electric shock period as 10 min to induce anxiety.
Fig. 1. The effect of electric shock on the anxiety of rat. Rats were individually tested in the elevated plus-maze (EPM) at 2 hr after the final stress was administered. Stress was delivered to the rats by daily electric shock for 7 days. The current of the shock was 5 mA, and the duration of shock was 1 s. Shocks were given according to an interval schedule, with an average of administering shock every 20 sec over a period of 5 min. Each rat was initially placed on the central platform and it was left to explore the arms for 5 min. The stress protocol consisted of (A) 5 min and (B) 10 min of footshock exposure. The staying time in the open arms was recorded. The time spent in the open arms was less for the stressed rats. *p<0.05 indicates a significant difference compared with the control group (n=7-8 rats/group).
Changes of the expression of TH and pERK in the amygdala and frontal cortex after stress
The effect of stress on the expressions of TH and pERK in the amygdala and frontal cortex was determined by immunoblot analysis at 2 hr after stress given. It was found that stress by electric shock for 10 min significantly decreased the expression of TH in the amygdala (p<0.01) while the expression of pERK was increased (p<0.01) (Fig. 2A, B). However, there is no difference in the expressions of TH and pERK in
Fig. 2. The effect of repeated electric shock on the expressions of TH and pERK in the amygdala (A, B) and frontal cortex (C, D). Representative immunoblots of tyrosine hydroxylase (TH) and the pERK levels in the rats. Each of the brain tissues of the amygdala or frontal cortex was collected at 2 hr after daily electric shock to the feet of the rats for 10 min for 7 days. The levels of the TH and pERK expressions were examined by Western immunoblot analysis. To verify the equality of loading, the immunoblots were reexamed with anti GAPDH antibody and ERK antibody. **p<0.01 indicates a significant difference compared with the control group (n=6 rats/group).
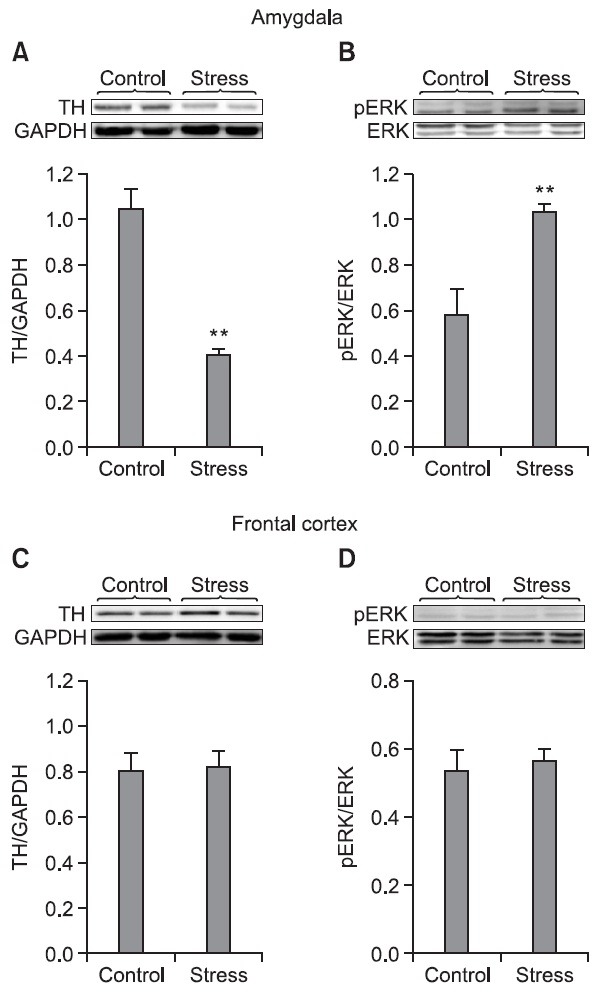
the frontal cortex as compared with those expressions in the control (non-stress) group (Fig. 2C, D).
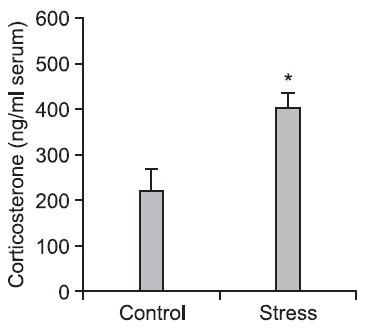
Elevation of corticosterone in the serum after electric shock stress
The serum was extracted from the blood which was collected from trunk of rats subjected to electric shock stress at 2 h after stress (10 min, electric shock) in order to analyze the corticosterone using HPLC. The level of corticosterone was significantly increased (p<0.05) in the serum of the stress group (Fig. 3).
Fig. 3. The level of corticosterone in the serum of the trunk of rats subjected to electric shock stress. The serum was separated from the blood that was collected 2 h after electric shock stress, and corticosterone was measured by using HPLC. *p<0.05 indicates a significant difference compared with the control (non-stress) group (n=7-8 rats/group).
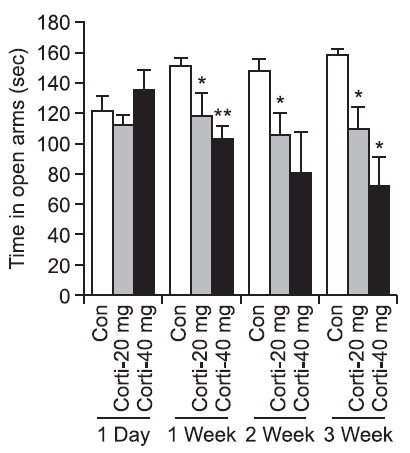
Measurement of anxiety on the EPM test after corticosterone injection
Rats were received corticosterone (20 mg or 40 mg/kg i.p.) daily for 1 day, 1 week, 2 weeks or 3 weeks, respectively. The time spent in the open arms during the EPM test was signifi-
cantly decreased after 1 week ((p<0.05, 20 mg/kg; (p<0.01, 40 mg/kg), 2 weeks (p<0.05, 20 mg/kg) and 3 weeks ((p<0.05, 20 mg/kg; (p<0.05, 40 mg/kg), while there was no significant difference between the 1-day corticosterone treatment group and the control group (Fig. 4). The time spent in open arms on the EPM test was 150 sec, 120 sec and 103 sec in the control group, the 20 mg/kg corticosterone group and the 40 mg/kg corticosterone group after 1 week treatment, respectively. Similarly, those were 148 sec, 108 sec and 81 sec after 2 weeks treatment, while those are 160 sec sec, 110 sec and 72 sec after 3 weeks treatment, respectively (Fig. 4). The time spent in open arms on the EPM test was significantly decreased in a dose-dependent manner after repeated injections of corticosterone.
Fig. 4. The effect of repeated injections of corticosterone on the induction of anxiety. Corticosterone (20 or 40 mg/kg/day) was administered (i.p.) daily at a volume of 1.0 ml/kg for 1 day, 1 week, 2 weeks and 3 weeks. The time spent in the open arms was measured for 5 min. The corticosterone group exhibited reduced exploration of the open arms. Each bar represents the mean ± SEM of 6 rats. P values for the group comparisons were obtained by one way ANOVA followed by the Student Newman Keuls test. *p<0.05, **p<0.01 indicates a significant difference compared with each control group (n=6 rats/group).
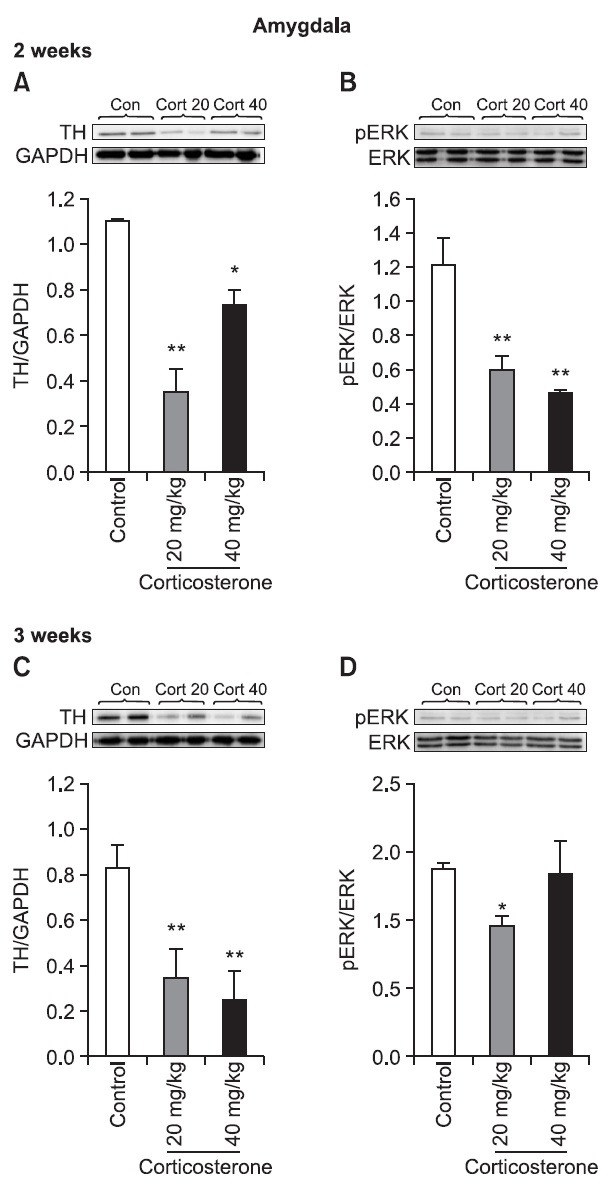
Changes of the expressions of TH and pERK in the amygdala after corticosterone treatment
The expression of TH in the amygdala was reduced following repeated corticosterone treatment for 2 weeks ((p<0.01, 20 mg/kg; (p<0.05, 40 mg/kg) and 3 weeks ((p<0.01, 20 mg/kg, 40 mg/kg) (Fig. 5). The expression of pERK in the amygdala was strongly decreased ((p<0.01) following repeated corticosterone treatment with 20 mg/kg or 40 mg/kg for 2 weeks, while it was recovered with a higher dose of corticosterone (40 mg/kg) in the 3-week treatment group.
Fig. 5. The effect of repeated corticosterone administration on the expressions of TH and pERK in the amygdala. Representative immunoblots show the effects of repeating corticosterone injection on the TH and pERK expression levels in the amygdala of rats. Corticosterone (20 or 40 mg/kg/day) was administered (i.p.) at a volume of 1.0 ml/kg for 2 weeks (A, B) or 3 weeks, respectively (C, D). The amygdala was collected 2 hr after the final corticosterone injection. *p<0.05, **p<0.01 indicate a significant difference compared with each control group (n=6 rats/group).
DISCUSSION
Stress is known to cause structural alterations in central nervous system and especially in the brain. The amygdala is known to play a central function in aversive learning, responses to stress, and modulation of memory due to stressful experiences (Mo et al., 2008). It is known that TH was expressed in the process of responding to stresses (Qian and Kim, 2007). It is also shown that the expression of TH in the amygdala of the stressed group was signifi cantly decreased while the expression of TH was not changed in the frontal cortex after applying immobilization and electric shock stress for 1 week in our previous experiment (Jang et al., 2011). Therefore, it could be understood that the decreased TH expression in the amygdala could be a significant marker of stress. Musacchio observed that the TH expressions in the frontal cortex were affected by electric shock stress (Musacchio et al., 1969), while there is no significant change in the TH and pERK expressions in the frontal cortex after exposing electric shock stress in our study. Musacchio observed rats after exposing to electroconvulsive shock twice daily for one week, while our study observed rats exposed to electroconvulsive shock once daily for one week. Electric shock is usually used as a stressor in animal models such as conditioned fear stress (Yamano et al., 2004). The 10-min electric shock stress reduced the time spent exploring the open arms as an indication of enhanced anxiety, while the 5-min shock group did not change the time spent in the open-arms compared to that of the control group in this experiment. These results suggest that the time duration of electric shock is an important factor to induce anxiety. In this present study, the effect of electric shock on the change of anxiety was observed by using the EPM, and the expressions of signal proteins in the amygdala were measured after exposing stress. The stress group spent less time in the open arms compared to that of the control group, and stress downregulated the level of the TH expression in the amygdala but not in the frontal cortex.
Stressor activates the hypothalamus–pituitary–adrenal (HPA) axis and this leads to the secretion of stress hormones including corticosterone. Corticosterone plays important roles in organizing the stress-related response by binding to corticosterone receptors in the peripheral tissue and brain (Mitra and Sapolsky, 2008). Several recent studies have indicated that repeated corticosterone treatment affected animal behavior during the forced-swim test. The repeated corticosterone injections increased immobility behavior (depression) during the forced-swim test (Zhao et al., 2008). We have verified that corticosterone was elevated in the serum after electric shock stress. The plasma corticosterone level was elevated for several hrs during restraint stress at days 1, 3, 7, 14 and 21 in rats (Galea et al., 1997). This result suggested that the acute and chronic stress caused the secretion of corticosterone into the circulating blood. There is an interesting report that daily injections of 40 mg/kg corticosterone in rats for 21 days led to increased immobility time on a forced-swim test. It was suggest that repeated corticosterone injections increase the depression-like behavior in a dose-dependent manner (Gregus et al., 2005). We also used corticosterone injection (20 mg/kg, 40 mg/kg) as a stressor and measured the spent time in the open-arms of the EPM and we found the induction of anxiety and changes of the expressions of stress-related signal proteins. A single day injection of corticosterone did not induce anxiety, yet the longer treatment of corticosterone, over 1 week, induced anxiety in the time-dependent manner. These results showed the possibility that corticosterone could be released during stress and the released corticosterone had a positive feedback role for the induction and enhancement of anxiety.
In this study, the effect of corticosterone on the expressions of TH and pERK in the amygdala was determined. TH is a rate limiting enzyme in the dopamine and noradrenergic systems (Glavin, 1985). In animal studies, the expression of TH was changed in different brain regions of rodents after repeated exposure to stress or corticosterone administration (Rastogi and Singhal, 1978). The expression of TH in the amygdala was decreased while the expression of TH was not changed in the frontal cortex after giving immobilization and electric shock stress for 7 days (Jang et al., 2011). The amygdala is composed of several nuclei and they perform different functions. The lateral and the basolateral nuclei of the amygdala funnel and integrate sensory input from the thalamus, and cognitive information from the frontal cortex and hippocampus (Van de Kar et al., 1991). The central amygdaloid nucleus is involved in behavioral, autonomic and endocrine responses. Activation of dopaminergic neurons in the amygdala activates the noradrenergic system in the locus coeruleus and in turn this activates sympathetic nerves (Carrasco and Van de Kar, 2003). Down-regulation of TH in the amygdala may have an inhibitory role on noradrenergic activation in the locus coeruleus, which results in decreasing the blood pressure and inhibiting metabolic responses during stress and after stress.
The main purpose of our experiment was to determine whether consecutive corticosterone injections have timerelated effects on the expression of TH. We found that the decreased level of the TH expression in the amygdala was maintained after corticosterone treatment for 2 weeks and 3 weeks. ERK1/2 phosphorylation is known as intracellular signaling that mediates the effi cacy of antidepressant in depressed humans and animal models. However, the supporting evidence is largely limited to rodents or in vitro models (Hisaoka et al., 2001), and some studies have showed that exposure to a stressor induced lower ERK phosphorylation in the amygdala (Wu et al., 2008). It has also been observed that corticosterone injection significantly decreased the pERK expression in the amygdala in our experiment. However, there is an inverse dose response relationship of the effect of corticosterone on the TH expression with 2-week treatment and the pERK/ERK expression with 3-week treatment. These results suggest that the signal system of TH and pERK/ERK is independently regulated by glucocorticoid. Since ERK1/2 is common effector of multiple signaling pathways, the phosphorylated state of ERK1/2 results from a balance of activating or inhibiting systems that operate simultaneously. Activation of ERK requires its phosphorylation by activated MEK, and dephosphorylation by a wide variety of protein phophatases is thought to play a crucial role in the control of ERK activity (Sweatt, 2001). It is also possible that short-term exposure to glucocorticoid activates phosphatases that can inhibit, at some point, the sequential MAPK kinase cascade. It has been reported that applying acute electroconvulsive seizures induces MAPK phosphatases in the limbic areas of rat (Kodama et al., 2005). The change of pERK expression is not always same direction followed by the stress. Experiments in rodents show that acute restraint stress increases pERK2 in hippocampus and frontal cortex (Meller et al., 2003), whereas chronic swim stress decreases pERK2 in these areas (Qi et al., 2006).
Interestingly, it was found that short-term and long-term corticosterone treatment induced different effects on anxiety behavior and the expressions of TH and pERK in the amygdala. It was found that corticosterone injections for 2 weeks and 3 weeks suppressed the expression of TH, while a single corticosterone injection did not change the TH expression (data not shown). These results suggested that the treatment period of corticosterone is one of important factor to affect the anxiety behavior and expression of TH (and pERK) in the amygdala. In the stress studies, the expressions of signal proteins were affected by the period of stress exposure. For example, the TH levels was increased after repeated stress exposure of corticosterone subcutaneous implantation for 16 days (Ortiz et al., 1995), while no change was noticed after implantation of corticosterone for 7 days (Makino et al., 2002). On the other hand, the expression of TH was decreased in the locus coeruleus with mild stress given for 3 weeks (Duncko et al., 2001; Gregus et al., 2005). Taken together, our results support that short-term corticosterone and long-term corticosterone treatment cause different alterations in anxiety behavior and the signal protein expressions such as TH and ERK.
We have used stress models such as electric shock or repeated corticosterone treatment. On the EPM test, the lesser spent time in the open arms in the stress group means the stressors induced anxiety. The expression of TH in the amygdala was strongly suppressed with repeated corticosterone administration. By administration of repeated injections of corticosterone, we showed almost identical results compared to that of chronic immobilization stress. This implies that corticosterone is released during stress and in turn the released corticosterone might have a positive feedback role for the induction of anxiety in rats that are under stress.
Acknowledgments
This work was supported by the Korea Research Foundation Grant (MRC, 2010-0029355) funded by the Korean Government (MEST).
References
- 1.Andreis P. G., Neri G., Nussdorfer G. G. Corticotropin-releasing hormone (CRH) directly stimulates corticosterone secretion by the rat adrenal gland. Endocrinology. (1991);128:1198–1200. doi: 10.1210/endo-128-2-1198. [DOI] [PubMed] [Google Scholar]
- 2.Carrasco G. A., Van de Kar L. D. Neuroendocrine pharmacology of stress. Eur. J. Pharmacol. (2003);463:235–272. doi: 10.1016/S0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- 3.Chida Y., Sudo N., Motomura Y., Kubo C. Electric footshock stress drives TNF-alpha production in the liver of IL-6-deficient mice. Neuroimmunomodulation. (2004);11:419–424. doi: 10.1159/000080153. [DOI] [PubMed] [Google Scholar]
- 4.Duncko R., Kiss A., Skultétyová I., Rusnák M., Jezová D. Corticotropin-releasing hormone mRNA levels in response to chronic mild stress rise in male but not in female rats while tyrosine hydroxylase mRNA levels decrease in both sexes. Psychoneuroendocrinology. (2001);26:77–89. doi: 10.1016/S0306-4530(00)00040-8. [DOI] [PubMed] [Google Scholar]
- 5.Dwivedi Y., Rizavi H. S., Conley R. R., Pandey G. N. ERK MAP kinase signaling in post-mortem brain of suicide subjects: differential regulation of upstream Raf kinases Raf-1 and B-Raf. Mol. Psychiatry. (2004);11:86–98. doi: 10.1038/sj.mp.4001744. [DOI] [PubMed] [Google Scholar]
- 6.Feng Q., Cheng B., Yang R., Sun F. Y., Zhu C. Q. Dynamic changes of phosphorylated tau in mouse hippocampus after cold water stress. Neurosci. Lett. (2005);388:13–16. doi: 10.1016/j.neulet.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 7.Fumagalli F., Molteni R., Calabrese F., Frasca A., Racagni G., Riva M. A. Chronic fluoxetine administration inhibits extracellular signal-regulated kinase 1/2 phosphorylation in rat brain. J. Neurochem. (2005);93:1551–1560. doi: 10.1111/j.1471-4159.2005.03149.x. [DOI] [PubMed] [Google Scholar]
- 8.Galea L. A., McEwen B. S., Tanapat P., Deak T., Spencer R. L., Dhabhar F. S. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. (1997);81:689–697. doi: 10.1016/S0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- 9.Gilad G. M, McCarty R. Difference in choline acetyltransferase but similarities in catecholamine biosynthetic enzymes in brains of two rat strains differing in their response to stress. Brain Res. (1981);206:239–243. doi: 10.1016/0006-8993(81)90124-4. [DOI] [PubMed] [Google Scholar]
- 10.Glavin G. B. Stress and brain noradrenaline: a review. Neurosci. Biobehav. Rev. (1985);9:233–243. doi: 10.1016/0149-7634(85)90048-X. [DOI] [PubMed] [Google Scholar]
- 11.Gourley S. L., Wu F. J., Kiraly D. D., Ploski J. E., Kedves A. T., Duman R. S., Taylor J. R. Regionally specifi c regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol. Psychiatry. (2008);63:353–359. doi: 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gregus A., Wintink A. J., Davis A. C., Kalynchuk L. E. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav. Brain Res. (2005);156:105–114. doi: 10.1016/j.bbr.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Hisaoka K., Nishida A., Koda T., Miyata M., Zensho H., Morinobu S., Ohta M., Yamawaki S. Antidepressant drug treatments induce glial cell line-derived neurotrophic factor (GDNF) synthesis and release in rat C6 glioblastoma cells. J. Neurochem. (2001);79:25–34. doi: 10.1046/j.1471-4159.2001.00531.x. [DOI] [PubMed] [Google Scholar]
- 14.Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol. Biochem. Behav. (1996);54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 15.Jang S., Kim D., Lee Y., Moon S., Oh S. Modulation of sphingosine 1-phosphate and tyrosine hydroxylase in the stressinduced anxiety. Neurochem. Res. (2011);36:258–267. doi: 10.1007/s11064-010-0313-1. [DOI] [PubMed] [Google Scholar]
- 16.Iwasaki-Sekino A., Mano-Otagiri A., Ohata H., Yamauchi N., Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. (2009);34:226–237. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Kodama M., Russell D. S, Duman R. S. Electroconvulsive seizures increase the expression of MAP kinase phosphatases in limbic regions of rat brain. Neuropsychopharmacology. (2005);30:360–371. doi: 10.1038/sj.npp.1300588. [DOI] [PubMed] [Google Scholar]
- 18.Kopin I. J. Definitions of stress and sympathetic neuronal responses. Ann. N. Y. Acad. Sci. (1995);771:19–30. doi: 10.1111/j.1749-6632.1995.tb44667.x. [DOI] [PubMed] [Google Scholar]
- 19.Kvetnansky R., Gewirtz G. P., Weise V. K., Kopin I. J. Effect of hypophysectomy on immobilization-induced elevation of tyrosine hydroxylase and phenylethanolamine-N-methyl transferase in the rat adrenal. Endocrinology. (1970);87:1323–1329. doi: 10.1210/endo-87-6-1323. [DOI] [PubMed] [Google Scholar]
- 20.Lechin F., van der Dijs B., Lechin A., Orozco B., Lechin M., Báez S., Rada I., León G., Acosta E. Plasma neurotransmitters and cortisol in chronic illness: role of stress. J. Med. (1994);25:181–192. [PubMed] [Google Scholar]
- 21.Makino S., Smith M. A., Gold P. W. Regulatory role of glucocorticoids and glucocorticoid receptor mRNA levels on tyrosine hydroxylase gene expression in the locus coeruleus during repeated immobilization stress. Brain Res. (2002);943:216–223. doi: 10.1016/S0006-8993(02)02647-1. [DOI] [PubMed] [Google Scholar]
- 22.Masserano J. M., Takimoto G. S., Weiner N. Electroconvulsive shock increases tyrosine hydroxylase activity in the brain and adrenal gland of the rat. Science. (1981);214:662–665. doi: 10.1126/science.6117127. [DOI] [PubMed] [Google Scholar]
- 23.McEwen B. S. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. (2000);886:172–189. doi: 10.1016/S0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 24.Meller E., Shen C., Nikolao T. A., Jensen C., Tsimberg Y., Chen J., Gruen R. J. Region-specifi c effects of acute and repeated restraint stress on the phosphorylation of mitogen-activated protein kinases. Brain Res. (2003);979:57–64. doi: 10.1016/S0006-8993(03)02866-X. [DOI] [PubMed] [Google Scholar]
- 25.Mitra R., Sapolsky R. M. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc. Natl. Acad. Sci. USA. (2008);105:5573–5578. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mo B., Feng N., Renner K., Forster G. Restraint stress increases serotonin release in the central nucleus of the amygdala via activation of corticotropin-releasing factor receptors. Brain Res. Bull. (2008);76:493–498. doi: 10.1016/j.brainresbull.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musacchio J. M., Julou L., Kety S. S., Glowinski J. Increase in rat brain tyrosine hydroxylase activity produced by electroconvulsive shock. Proc. Natl. Acad. Sci. USA. (1969);63:1117–1119. doi: 10.1073/pnas.63.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nankova B., Kvetnansky R., Hiremagalur B., Sabban B., Rusnak M., Sabban E. L. Immobilization stress elevates gene expression for catecholamine biosynthetic enzymes and some neuropeptides in rat sympathetic ganglia: effects of adrenocorticotropin and glucocorticoids. Endocrinology. (1996);137:5597–5604. doi: 10.1210/en.137.12.5597. [DOI] [PubMed] [Google Scholar]
- 29.Ortiz J., DeCaprio J. L., Kosten T. A., Nestler E. J. Strainselective effects of corticosterone on locomotor sensitization to cocaine and on levels of tyrosine hydroxylase and glucocorticoid receptor in the ventral tegmental area. Neuroscience. (1995);67:383–397. doi: 10.1016/0306-4522(95)00018-E. [DOI] [PubMed] [Google Scholar]
- 30.Oztaş B., Akgül S., Arslan F. B. Influence of surgical pain stress on the blood-brain barrier permeability in rats. Life Sci. (2004);74:1973–1979. doi: 10.1016/j.lfs.2003.07.054. [DOI] [PubMed] [Google Scholar]
- 31.Qi X., Lin W., Li J., Pan Y., Wang W. The depressive-like behaviors are correlated with decreased phosphorylation of mitogen- activated protein kinases in rat brain following chronic forced swim stress. Behav. Brain Res. (2006);175:233–240. doi: 10.1016/j.bbr.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 32.Qian Y. R., Kim Y. S. Effect of immobilization stress on the expression of TH, BDH and CRH gene in rat brain. J. Genet. Med. (2007);4:179–185. [Google Scholar]
- 33.Rastogi R. B., Singhal R. L. Evidence for the role of adrenocortical hormones in the regulation of noradrenaline and dopamine metabolism in certain brain areas. Br. J. Pharmacol. (1978);62:131–136. doi: 10.1111/j.1476-5381.1978.tb07015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., Weisstaub N., Lee J., Duman R., Arancio O., Belzung C., Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. (2003);301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 35.Shen C. P., Tsimberg Y., Salvadore C., Meller E. Activation of Erk and JNK MAPK pathways by acute swim stress in rat brain regions. BMC. Neurosci. (2004);5:36. doi: 10.1186/1471-2202-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stutzmann G. E., LeDoux J. E. GABAergic antagonists block the inhibitory effects of serotonin in the lateral amygdala: a mechanism for modulation of sensory inputs related to fear conditioning. J. Neurosci. (1999);19:RC8. doi: 10.1523/JNEUROSCI.19-11-j0005.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sweatt J. D. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J. Neurochem. (2001);76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- 38.Tiraboschi E, Tardito D, Kasahara J, Moraschi S, Pruneri P, Gennarelli M, Racagni G, Popoli M. Selective phosphorylation of nuclear CREB by fluoxetine is linked to activation of CaM kinase IV and MAP kinase cascades. Neuropsychopharmacology. (2004);29:1831–1840. doi: 10.1038/sj.npp.1300488. [DOI] [PubMed] [Google Scholar]
- 39.Van de Kar L. D., Piechowski R. A., Rittenhouse P. A., Gray T. S. Amygdaloid lesions: differential effect on conditioned stress and immobilization-induced increases in corticosterone and renin secretion. Neuroendocrinology. (1991);54:89–95. doi: 10.1159/000125856. [DOI] [PubMed] [Google Scholar]
- 40.Wong D. L. Epinephrine biosynthesis: hormonal and neural control during stress. Cell Mol. Neurobiol. (2006);26:891–900. doi: 10.1007/s10571-006-9056-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu S. L., Hsu L. S., Tu W. T., Wang W. F., Huang Y. T., Pawlak C. R., Ho Y. J. Effects of D-cycloserine on the behavior and ERK activity in the amygdala: role of individual anxiety levels. Behav. Brain Res. (2008);187:246–253. doi: 10.1016/j.bbr.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Yamano Y., Yoshioka M., Toda Y., Oshida Y., Chaki S., Hamamoto K., Morishima I. Regulation of CRF, POMC and MC4R gene expression after electrical foot shock stress in the rat amygdala and hypothalamus. J. Vet. Med. Sci. (2004);66:1323–1327. doi: 10.1292/jvms.66.1323. [DOI] [PubMed] [Google Scholar]
- 43.Yoshihara T., Yawaka Y. Repeated immobilization stress in the early postnatal period increases stress response in adult rats. Physiol. Behav. (2008);93:322–326. doi: 10.1016/j.physbeh.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Zhao Y., Ma R., Shen J., Su H., Xing D., Du L. A mouse model of depression induced by repeated corticosterone injections. Eur. J. Pharmacol. (2008);581:113–120. doi: 10.1016/j.ejphar.2007.12.005. [DOI] [PubMed] [Google Scholar]


