Abstract
Glucosamine (GS) is well known for the treatment of inflam-mation. However, the mechanism and efficacy of GS for skin inflammation are unclear. The aim of this study was to evaluate the effects and mechanism of GS in the mouse 12-O-tetradecanoyl 13-acetate (TPA)-induced ear edema model. TPA-induced ear edema was evoked in ICR or transglutaminase 2 (Tgase-2) (-/-) mice. GS was administered orally (10-100 mg/kg) or topically (0.5-2.0 w/v %) prior to TPA treatment. Orally administered GS at 10 mg/kg showed a 76 or 57% reduction in ear weight or myeloperoxidase, respectively, and a decreased expression of cyclooxy-genase-2 (COX-2), NF-κB and Tgase-2 in TPA-induced ear edema by western blot and immunohistochemistry. Role of Tgase-2 in TPA ear edema is examined using Tgase-2 (-/-) mice and TPA did not induce COX-2 expression in ear of Tgase-2 (-/-) mice. These observations suggested that Tgase-2 is involved in TPA-induced COX-2 expression in the inflamed ear of mice and anti-inflammatory effects of glucosamine is mediated through suppression of Tgase-2 in TPA ear edema.
Keywords: Glucosamine, TPA-induced ear edema, Transglutaminase-2, Cyclooxygenase-2, NF-κB, Tgase-2 (-/-) mice
INTRODUCTION
Glucosamine (GS), 2-amino-2-deoxy-D-glucose, is an ami-no monosaccharide that is one of the essential components of mucopolysaccharides and chitin. Glycosaminoglycans are components of connective tissue, skin, tendons, ligaments and cartilage. GS is readily synthesized in the body from glucose. Given the high concentration in joint tissues, the hy-pothesis that GS supplements would relieve the symptoms of osteoarthritis (OA) was developed more than 30 years ago (D'Ambrosio et al., 1981). These effects of GS were shown in carrageenan- and cotton pellet-induced acute and subacute inflammation in rats at 25 mg/kg dose (Kim et al., 2005). GS at 250 mg/kg showed a mild effect in carrageenan-induced edema and moderate inhibition of paw swelling against devel-oping arthritis (Singh et al., 2007).
Recently, glucosamine showed positive effects in atopic dermatitis-like skin lesions in NC/Nga mice via inhibition of Th2 cell development (Kim et al., 2011a). Combination treat-ments of glucosamine with FK-506 also produces a benefi cial effects in atopic dermatitis-like skin lesions in NC/Nga mice (Kim et al., 2012).
GS was also tested as a constituent of a new anti-inflam-matory formulation (SAG) in a 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced ear edema model. SAG dose-de-pendently inhibited the edematic responses of arachidonic acid (AA)- and TPA-induced ear edema in mice (Choi et al.,2005). But, in this case, efficacy of glucosamine by itself was not shown in TPA-induced ear edema and detailed histological studies on the effects of glucosamine were not done.
TPA promotes skin carcinogenesis via inflammatory re-sponses and TPA-induced inflammatory responses are re-lated with induction of pro-inflammatory cytokines, cyclooxy-genase-2, reactive oxygen species and NF-κB (Chung et al., 2007; Song et al., 2008).
Recently, glucosamine was reported to act as a chemo-sensitizer via inhibition of transglutaminase-2 (Tgase-2) in doxorubicin-resistant MCF7 cells (Kim et al., 2009; Jeong et al., 2010).
Tgase-2 is a multifunctional protein with both intracellular and extracellular functions. In addition to catalyzing Ca2+-de-pendent transamidation reactions (Lorand and Graham, 2003; Mehta, 2005; Lee and Kim, 2009), it can bind and hydrolyze GTP/GDP with a similar affinity and catalytic rate to the α sub-unit of large heterotrimeric G proteins and small Ras-type G proteins (Mhaouty-Kodja, 2004). Tgase-2 can activate NF-κB via polymerization of I-κB (Lee et al., 2004). But role of Tgase-2 is not known in TPA-induced skin inflammation.
Therefore, we were interested in the effect and related mechanism of glucosamine on Tgase-2 in TPA-induced ear edema. To our knowledge, the role of Tgase-2 in TPA ear ede-ma has not been reported yet.
In this report, we evaluated the efficacy of glucosamine in a TPA-induced dermatitis model and found that Tgase-2 expres-sion suppressed by glucosamine is involved in the anti-inflam-matory action of glucosamine by TPA-induced inflammation.
MATERIALS AND METHODS
Materials
Primary antibodies purchased: (1) rabbit polyclonal murine COX-2 antibody (Cayman Chemical, Ann Arbor, MI, USA), (2) rabbit polyclonal anti-NF-κB p65 antibody (Novus biological, Littleton, CO, USA), (3) polyclonal anti-actin antibody (Santa Cruz, CA, USA).
Animals
Male ICR mice (Orientbio, Seoul, Korea), 7 weeks old were used in this experiment. They were acclimatized in the animal room at least 1 week prior to use. Throughout the experimental period, animals had free access to water and a commercial diet. The mice were randomly assigned into groups consisting of five animals per group, and were fasted overnight prior to experimentation. Tgase-2 knockout mice (C57BL/6) used were established by Dr. Ho Lee (Kim et al., 2010). The ex-periments were conducted under the guidelines for the care and use of experimental animals of the Korea Association for Laboratory Animal Science.
12-O-tetradecanoylphorbol 13-acetate (TPA)-induced mouse ear edema
Edema was induced on the right ear by topical applica-tion of 20 μl of 12-O-tetradecanoylphorbol 13-acetate (TPA; Sigma, St. Louis, USA) in acetone (2.5 μg/ear) with a micro-pipette (De Young et al., 1989; Kim et al., 2011b). To evaluate the inflammatory effects of TPA, ear lobe samples were col-lected using a 6 mm biopsy punch, weighed and measured for MPO (myeloperoxidase) levels 24 h after TPA application. The percentage of inhibition (% I) was calculated according to the following formula:
% I = [1-Rt. Ear (Drug- No. treated) / Rt. Ear (TPA only- No.treated)]×100.
Myeloperoxidase (MPO) assay
The ear samples were placed in 0.5 ml of 80 mM sodium phosphate buffer, pH 5.4, containing 0.5% hexadecyltrimeth-ylammonium bromide (HTAB) (Sigma) (Bradley et al., 1982) and homogenized for 45 sec at 0℃, after which they were sonicated in an ice bath for 10 sec (Heat Systems-Ultrason-ics, Plainview, NY). The sonicated samples were centrifugated at 12,000X g at 4℃ for 15 min. Fifty μl of supernatant was added to a 96-well plate, and then 200 μl of a mixture con-taining 0.167 mg/ml o-dianisidine dihydrochloride (Sigma) and 0.0005% hydrogen peroxidase (Sigma) were added to each of the wells. The change of absorbance at 460 nm was mea-sured with a SPECTRAmax® 190 microplate spectrophotom-eter (Molecular Device Corporation, Calif., USA).
Evaluation of anti-inflammatory effects of glucosamine
Animals were orally administered 0.5 ml of D (+)-GS hydro-chloride (Sigma) both immediately and 6 h after TPA applica-tion at doses of 5 mg/kg, 10 mg/kg or 20 mg/kg, respectively. The vehicle control animals were administered sterilized sa-line, and the positive control animals, with 10 mg/kg of dexa-methasone. Also, the mice were topically administered 20 μl of D(+)-GS hydrochloride (Sigma) in vehicle, both immediately and 6 h after TPA application at concentrations of 0.5% (w/v), 1% (w/v) and 2% (w/v) for the three groups, respectively. GS hydrochloride (Sigma) was dissolved in a mixture of distilled water (one part) and acetone (nine parts) for topical applica-tion. The vehicle control animals were topically administered a DW:acetone solution (1:9) and the positive control animals, 0.5% hydrocortisone.
COX-2 and Tgase-2 western blot
Proteins from ear tissues were extracted in lysis buffer containing 20 mmol/L Tris-HCl (pH 7.6), 1 mmol/L EDTA, 140 mmol/L NaCl, 1% NP40, 1% protease inhibitor, 1 mmol/L phenylmethylsulfonyl fluoride, and 1 mmol/L sodium vana-date. Equivalent amounts of proteins from each treatment group were resolved on sodium dodecyl sulfate (SDS)-poly-acrylamide gel electrophoresis and immunoblotted with pri-mary antibodies.
Histological analysis
For histological analysis, ear tissue was fixed in 10% neu-tral buffered formaldehyde and embedded in paraffin wax according to standard methods. Sections were stained with hematoxylin and eosin.
Immunohistochemistry was performed by standard ABC technique. Tissue sections were deparaffinized in xylene and rehydrated in graded alcohols. Antigenic retrieval was per-formed by pressure cooking in 10 mM citric acid buffer (pH 6.0). Hydrogen peroxide (3%) was used to quench endoge-nous peroxidase activity for 10 min. For blocking buffer, 10% normal goat serum was used for 30 min. Sections were then incubated with primary antibody for 1 hour at room tempera-ture. Biotinylated goat anti-rabbit IgG antibody and ABC solu-tion (Vector Laboratories, Burlingame, CA, USA) were applied sequentially. Diaminobenzidine (DAB) was used to visualize a positive signal. Immunostained sections were lightly coun-terstained in hematoxylin according to the manufacturer’s instructions, dehydrated in graded ethanol, cleared in xylene and mounted with a coverslip using Canada balsam (Junsei Chemical, Tokyo, Japan).
Statistics
All data are presented as means and S.D. Statistical sig-nificance was analyzed using a student’s t-test; p<0.05 was considered significant when compared with the TPA-only con-trol group.
RESULTS
Effects of GS in TPA ear edema model
Twenty μl of D (+)-GS hydrochloride in vehicle were topi-cally administered to the mice both immediately and 6 h after TPA application at concentrations of 0.5% (w/v), 1% (w/v) and 2% (w/v), respectively. GS hydrochloride was dissolved in a mixture of distilled water (one part) and acetone (nine parts) for topical application. We measured the weights of punched ear as well as MPO activity. After topical administration, GS hydrochloride showed significant anti-inflammatory effects in the TPA-induced ear edema mouse model evaluated by ear weight or MPO activity (Fig. 1A, 1B). Hydrocortisone (0.5%) was used as positive control and showed 99% and 97% inhibi-tion in ear weight and MPO analysis, respectively.
Fig. 1. Effects of GS on TPA-induced ear edema. GS (0.5-2% (w/v)) was applied topically to the ear of mice at the same time as a TPA application (2.5 μg/ear; A B) or orally administered (5-20 mg/kg; C D). Ear edema was measured 4 h after application of TPA.Data are the means ± SD of 10 animals. *p<0.05 relative to the control group was considered to be signifi cant. Dexamethasone or hydrocortisone was used as a positive control.
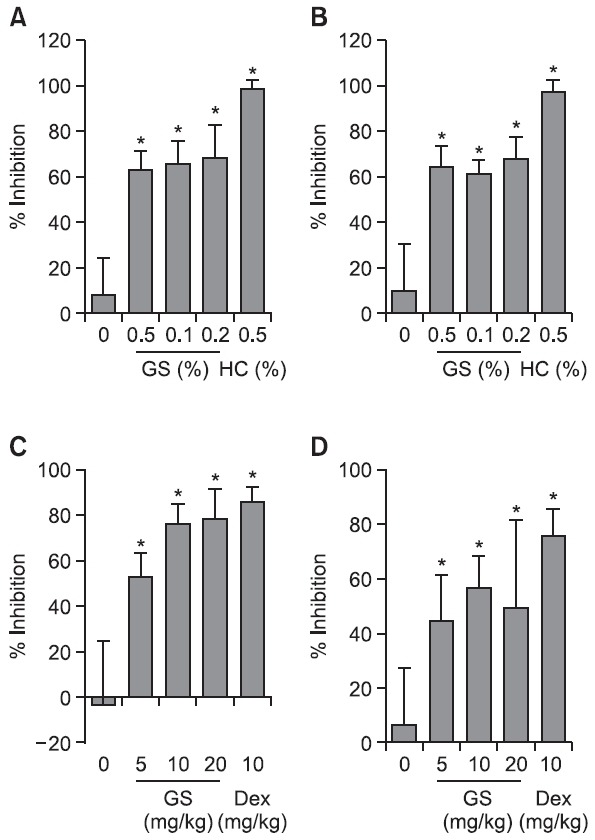
Then, the animals were orally administered 0.5 ml of D(+)-GS hydrochloride both immediately and 6 h after TPA applica-tion at doses of 5 mg/kg, 10 mg/kg and 20 mg/kg, respectively. Oral administration of the GS hydrochloride showed signifi-cant anti-inflammatory effects in the TPA-induced skin inflammation
model (Fig. 1C, 1D). Specifically, GS at the doses of 5 and 10 mg/kg showed the strongest anti-inflammatory effects, that is, about 76-78% inhibition according to ear weights; fur-ther, there was a 50-57% inhibition in the MPO analysis (Fig. 1C, 1D). Dexamethasone (10 mg/kg) was used as positive control and showed 86% and 75% inhibitions in ear weight and MPO activity, respectively.
Effects of orally administered GS on COX-2 expression and NF-κB activation in the TPA ear edema model
We examined the effects of GS on NF-κB target genes such as COX-2 in the TPA-ear edema model to confi rm the anti-inflammatory effects of GS in an in vivo model as others have reported in vitro (Largo et al., 2003).
In Fig. 2, it was confirmed by western blot that COX-2 was highly expressed in TPA-treated ears of mice compared with
Fig. 2. Effects of GS on COX-2 expression and NF-κB activation in the TPA-induced ear edema model. Three experimental groups were included three ICR mice/group. Group 1 received acetone group 2 received TPA group 3 received TPA and GS (10 mg/kg body weight). Edema was induced in both ears of the each mouse by topical application of 2.5 μg TPA dissolved in 25 μl of acetone to the inner ear surfaces. (A) Effect of GS on-TPA-induced changes of COX-2 protein in mouse ear skin. (B) For histological analysis ear skin was prepared and stained with hematoxylin and eosin. Control: Normal skin TPA: TPA-treated skin TPA+GS: TPA plus orally administered GS skin. (C) 25 μl of TPA (100 μg/ml in ac-etone) was applied to each ear of BALB/C mice and ear biopsy samples were taken at 6 h and subjected to immunostaining with anti-mouse COX-2 antibody (1:10 dilution ratio). (D) Immunostain-ing with anti-mouse p65 of NF-κB antibody (1:50 dilution ratio).
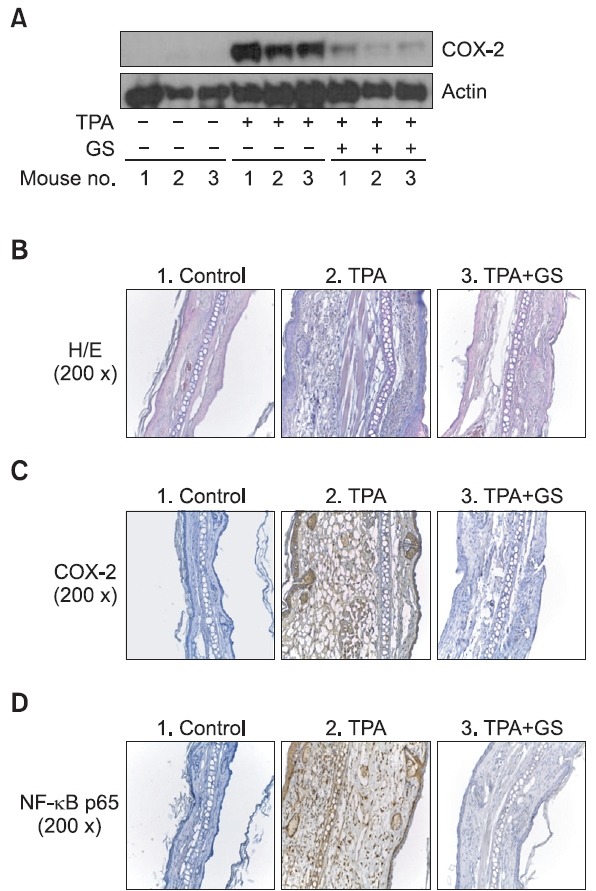
the vehicle treated ear group. Orally GS administration at 10 mg/kg showed suppressed expression of COX-2 (Fig. 2A).
In histological studies, TPA treatment produced a marked increase in ear thickness and an abundant infi ltration of in-flammatory cells in the epidermis and dermis whereas the ac-etone-treated vehicle control group did not show histological and ear thickness changes. In contrast, the GS-treated group showed a remarkable reduction in ear thickness and inflam-matory response in the epidermis and dermis (Fig. 2B).
Immunohistochemical analysis of COX-2 was performed.Strong positive signals for COX-2 were mostly found in epider-mis. In the TPA-treated group, epidermal hyperplasia and high levels of COX-2 expression were found. On the contrary, the TPA and GS-treated group showed mild to moderate epider-mal hyperplasia and dermal edema. The expression of COX-2 in the epidermus was remarkably suppressed in the TPA and GS-treated group; the acetone treated group was provided for comparison (Fig. 2C).
Immunolabelling of NF-κB p65 was found in epidermal ke-ratinocytes and inflammatory cells in the epidermis and der-mis. Cytoplasmic staining of NF-κB p65 was mostly found in epidermal keratinocytes, while nuclear staining of NF-κB p65 was found in inflammatory cells. Inflammatory cells within the lesion consisted of lymphocytes and a small number of neu-trophils. NF-κB p65 was strongly expressed in lymphocytes and inflammatory cells which were present in dermis and epi-dermis of the TPA-treated group as well as the TPA and GS co-treated group. However, the number of inflammatory cells in the TPA and GS co-treated group was much smaller than in TPA-treated group (Fig. 2D).
Effects of orally administered GS on Tgase-2 expression in the TPA ear edema model
Considering the observation that GS suppressed COX-2 expression and inhibited Tgase-2 (Kim et al., 2009), we examined
whether GS suppressed the expression of Tgase-2 in the TPA-ear edema model. At first, we examined the expression of Tgase-2 in TPA-treated ears of mice. As a result, the expres-sion of Tgase-2 was increased in the TPA-treated group com-pared with vehicle control groups (Fig. 3A). We tested whether GS infl uenced the expression of Tgase-2 since Tgase-2 is one of target genes in the activation of NF-κB. As a result, the 10 mg/kg GS treated mice group showed suppression of Tgase-2 expression compared with the TPA only treated group deter-mined by western blot (Fig. 3A).
Fig. 3. Effects of orally administered GS on Tgase-2 expression in the TPA ear edema model. Three experimental groups included three ICR mice/group. Group 1 received acetone group 2 received TPA group 3 received TPA and GS (10 mg/kg body weight). Ede-ma was induced in both ears of each mouse by topical application of 2.5 μg TPA dissolved in 25 μl of acetone to the inner ear sur-faces. (A) Effect of GS on TPA-induced changes in Tgase-2 protein in mouse ear skin. (B) 25 μl of TPA (100 μg/ml in acetone) was applied to each ear of BALB/c mice and ear biopsy samples were taken at 6 h and subjected to immunostaining with anti-mouse Tgase-2 antibody (1:50 dilution ratio).
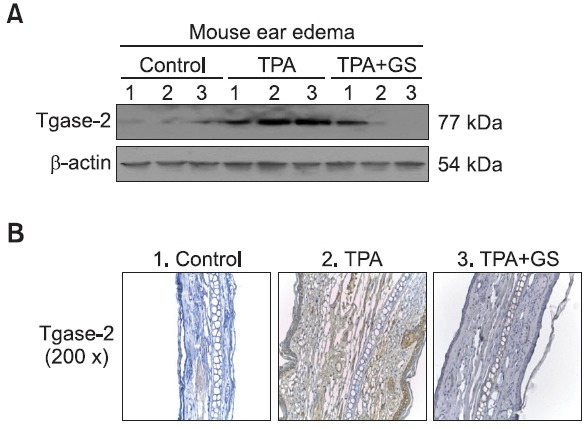
Using immunohistochemical analysis, the expression of Tgase-2 was found in epidermal keratinocytes and inflamma-tory cells (Fig. 3B). Immunostaining pattern for Tgase-2 was nuclear and cytoplasmic. In the TPA-treated group, a high level of Tgase-2 expression was found in hyperplastic epider-mal keratinocytes and lymphocytes in addition to inflamma-tory cells within the dermis and blood vessels. The expres-sion of Tgase-2 in the TPA and GS co-treated group was less than that of TPA-treated group. On the contrary, expression of Tgase-2 was not found in vehicle control group (Fig. 3B).
Tgase-2 involvement in COX-2 expression by TPA-induced ear edema
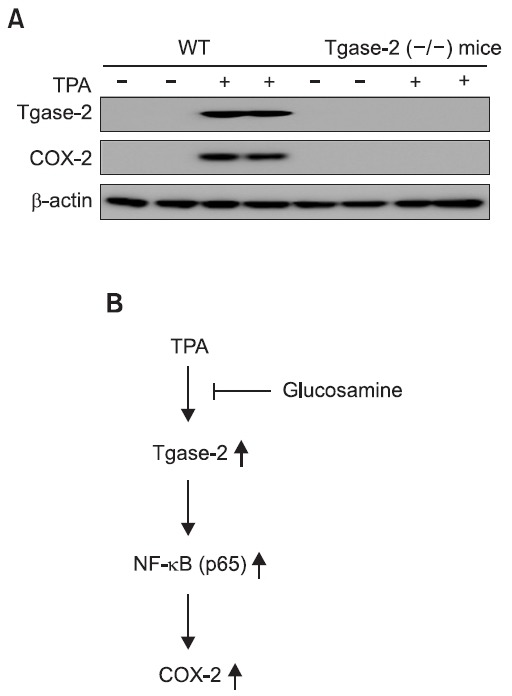
To examine the effect of Tgase-2 on COX-2 expression in TPA ear edema, we topically applied TPA to ears of wild-type and Tgase-2 (-/-) mice in the same manner described in the Methods and ear tissue of TPA-treated Tgase-2 (-/-) were ex-amined for COX-2 and Tgase-2 expression by western blot
analysis (Fig. 4A). Ears of TPA-applied mice showed induction of COX-2 and Tgase-2 but the increased expression of COX-2 was not observed in Tgase-2 (-/-) mice (Fig. 4A).
Fig. 4. Tgase-2 involvement in COX-2 expression after TPA-in-duced ear edema. Two experimental groups included three or two wild type mice or Tgase-2 (-/-) mice/group. Group 1 received ac-etone group 2 received TPA. Edema was induced in both ears of each mouse by topical application of 2.5 μg TPA dissolved in 25 μl of acetone to the inner ear surfaces. (A) Effects of TPA on Tgase-2 and COX-2 expression on ear skin of wild type mice or Tgase-2 (-/-) mice. (B) Scheme of involvement of Tgase-2 and effects of glucos-amine on TPA-induced COX-2 expression.
DISCUSSION
In this study, we found glucosamine has anti-inflammatory activity on TPA-induced skin inflammation. Glucosamine sup-pressed Tgase-2 expression in inflamed ear tissue by TPA. The notion that COX-2 expression induced by TPA is related to expression of Tgase-2 is confirmed using Tgase-2 (-/-) mice.
GS has long been used by patients with arthritis. Glucos-amine sulfate with potassium salt (GS-K) is effective in pain relief and is safe as glucosamine sulfate with sodium salt (GS-Na) for treatment of mild and moderate degrees of knee osteoarthritis (Wangroongsub et al., 2010). Recently, effica-cy of glucosamine was reported in a model of chronic skin inflammation including NC/Nga mice with or without FK-506 treatment (Kim et al., 2012). However, the detailed role of GS as related to Tgase-2 has not been reported in these dermal inflammation models.
GS can decrease nitric oxide production and even control neutrophil functions (i.e. expression of adhesion molecules, p38 phosphorylation and chemotaxis) through the inhibition of NF-κB-dependent gene expression (Shikhman et al., 2001; Hua et al., 2002). But, how GS affects the NF-κB pathway is not clear. Recently, our colleagues reported that GS can act as a Tgase-2 inhibitor modulating the actions of NF-κB (Kim et al., 2009).
As shown in Fig 1A and 1B, dose-dependency was diffi-cult to achieve with topical application of GS in suppressing TPA-induced ear edema in mice. This reflects some of the hy-drophilic characteristics of GS and the vehicle used (acetone-water). In Fig. 1C and 1D, GS demonstrated very good anti-in-flammatory activity in the TPA ear edema model (Fig. 1C, 1D). These results suggested that GS might be also useful in con-trolling acute inflammation such as TPA-induced dermatitis.
GS suppressed COX-2 expression in the TPA-ear edema model (Fig. 2A). These results are consistent with the effect of GS in several cell lines such as macrophages and human chondrocytes (Largo et al., 2003). GS inhibited significantly Tgase-2 at high concentrations (Kim et al., 2009; Jeong et al., 2010) and Tgase-2 activates NF-κB which is involved in TPA-induced ear edema models (Lee et al., 2004; Rafi et al., 2007). But, the role of Tgase-2 has not been studied in TPA-induced inflammation.
TPA-treated ears of mice showed increased expression of Tgase-2. TPA-induced Tgase-2 expression was also ob-served in HT-1080 cells (Park and Lee, 2011). The expres-sion of Tgase-2 was suppressed in the GS-treated group after TPA-induced inflammation (Fig. 3). These results showed the possibility that Tgase-2 is involved in TPA-induced ear edema and suggested that the beneficial effect of GS might be related with suppression of Tgase-2. The role of Tgase-2 was exam-ined in TPA-induced ear edema using Tgase-2 (-/-) and wild-type mice. We examined the expression of COX-2 which is a well-known target gene of NF-κB and one of the main targets for skin inflammation. The expression of COX-2 was not ob-served in TPA-induced ear edema of the TPA-treated Tgase-2(-/-) mice (Fig. 4A). These results suggested that Tgase-2 is involved in the expression of COX-2 in TPA-induced inflam-mation. The mechanism of involvement of Tgase-2 in COX-2 expression through NF-κB activation was suggested as po-lymerization of IκB in BV-1 microglia cells (Lee et al., 2004; Mehta et al., 2010).
Generally, several Tgases such as Tgase 1, 3, and 5, but not Tgase-2, are expressed in the epidermis and involved in terminal differentiation of keratinocytes (Eckert et al., 2005). Tgase-2 is induced in TPA-treated skin and suggests a spe-cial role in skin inflammation. Similarly, in other inflammatory conditions such as renal ischemic injury, Tgase-2 induction is also observed (Kim et al., 2010). But a more detailed role of Tgase-2 in skin requires further study.
Considering our results, Tgase-2 might be one of the key players of inflammation. The modulation of Tgase-2 expres-sion by GS and the suppression of COX-2 by Tgase-2 inhibi-tion suggests that Tgase-2 might be a new target for explain-ing the action of GS and Tgase-2 may be a new target for modulating skin inflammation (Fig. 4B). GS can also be used for skin inflammation if proved in clinical trials.
Acknowledgments
This study was supported by grants from the National Can-cer Center (NCC1110011-1), National Research Foundation grant (No. 2010-0029919, 2011-0022074), and a Research Program for New Drug Target Discovery grant (2011-0030173) from the Ministry of Education, Science & Technology, Korea.
References
- 1.Bradley P. P. Priebat D. A. Christensen R. D. Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. (1982);78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 2.Choi Y. H. Son K. H. Chang H. W. Bae K. Kang S. S. Kim H. P. New anti-inflammatory formulation containing Synurus deltoides extract. Arch. Pharm. Res. (2005);28:848–853. doi: 10.1007/BF02977352. [DOI] [PubMed] [Google Scholar]
- 3.Chung W. Y. Park J. H. Kim M. J. Kim H. O. Hwang J. K. Lee S. K. Park K. K. Xanthorrhizol inhibits 12-O-tetradec-anoylphorbol-13-acetate-induced acute inflammation and two-stage mouse skin carcinogenesis by blocking the expression of ornithine decarboxylase cyclooxygenase-2 and inducible nitric ox-ide synthase through mitogen-activated protein kinases and/or the nuclear factor-kappa B. Carcinogenesis. (2007);28:1224–1231. doi: 10.1093/carcin/bgm005. [DOI] [PubMed] [Google Scholar]
- 4.D'Ambrosio E. Casa B. Bompani R. Scali G. Scali M. Glucosamine sulphate: a controlled clinical investigation in arthro-sis. Pharmatherapeutica. (1981);2:504–508. [PubMed] [Google Scholar]
- 5.De Young L. M. Kheifets J. B. Ballaron S. J. Young J. M. Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions. (1989);26:335–341. doi: 10.1007/BF01967298. [DOI] [PubMed] [Google Scholar]
- 6.Eckert R. L. Sturniolo M. T. Broome A. M. Ruse M. Rorke E. A. Transglutaminase function in epidermis. J. Invest. Der-matol. (2005);124:481–492. doi: 10.1111/j.0022-202X.2005.23627.x. [DOI] [PubMed] [Google Scholar]
- 7.Hua J. Sakamoto K. Nagaoka I. Inhibitory actions of glu-cosamine a therapeutic agent for osteoarthritis on the functions of neutrophils. J. Leukoc. Biol. (2002);71:632–640. [PubMed] [Google Scholar]
- 8.Jeong K. C. Ahn K. O. Lee B. I. Lee C. H. Kim S. Y. The mechanism of transglutaminase 2 inhibition with glucosamine: implications of a possible anti-inflammatory effect through trans-glutaminase inhibition. J. Cancer Res. Clin. Oncol. (2010);136:143–150. doi: 10.1007/s00432-009-0645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim C. H. Cheong K. A. Park C. D. Lee A. Y. Glu-cosamine improved atopic dermatitis-like skin lesions in NC/Nga mice by inhibition of Th2 cell development. Scand. J. Immunol. (2011a);73:536–545. doi: 10.1111/j.1365-3083.2011.02526.x. [DOI] [PubMed] [Google Scholar]
- 10.Kim C. H. Cheong K. A. Park C. D. Lee A. Y. Thera-peutic effects of combination using glucosamine plus tacrolimus (FK-506) on the development of atopic dermatitis-like skin lesions in NC/Nga mice. Scand. J. Immunol. (2012);75:471–478. doi: 10.1111/j.1365-3083.2011.02659.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim D. S. Kim B. Tahk H. Kim D. H. Ahn E. R. Choi C. Jeon Y. Park S. Y. Lee H. Oh S. H. Kim S. Y. Transglu-taminase 2 gene ablation protects against renal ischemic injury by blocking constant NF-κB activation. Biochem. Biophys. Res. Com-mun. (2010);403:479–484. doi: 10.1016/j.bbrc.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 12.Kim D. S. Park K. S. Jeong K. C. Lee B. I. Lee C. H. Kim S. Y. Glucosamine is an effective chemo-sensitizer via trans-glutaminase 2 inhibition. Cancer Lett. (2009);273:243–249. doi: 10.1016/j.canlet.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Kim J. C. Shin J. Y. Shin D. H. Kim S. H. Park S. H. Park R. D. Park S. C. Kim Y. B. Shin Y. C. Synergistic antiinflam-matory effects of pinitol and glucosamine in rats. Phytother. Res. (2005);19:1048–1051. doi: 10.1002/ptr.1788. [DOI] [PubMed] [Google Scholar]
- 14.Kim M. H. Nugroho A. Choi J. Park J. H. Park H. J. Rhododendrin an analgesic/anti-inflammatory arylbutanoid glyco-side from the leaves of Rhododendron aureum. Arch. Pharm. Res. (2011b);34:971–978. doi: 10.1007/s12272-011-0614-1. [DOI] [PubMed] [Google Scholar]
- 15.Largo R. Alvarez-Soria M. A. Díez-Ortego I. Calvo E. Sánchez-Pernaute O. Egido J. Herrero-Beaumont G. Glucos-amine inhibits IL-1beta-induced NFkappaB activation in human osteoarthritic chondrocytes. Osteoarthritis Cartilage. (2003);11:290–298. doi: 10.1016/S1063-4584(03)00028-1. [DOI] [PubMed] [Google Scholar]
- 16.Lee C. H. Kim S. Y. NF-kappaB and therapeutic approach. Biomol. Ther. (2009);17:219–240. doi: 10.4062/biomolther.2009.17.3.219. [DOI] [Google Scholar]
- 17.Lee J. Kim Y. S. Choi D. H. Bang M. S. Han T. R. Joh T. H. Kim S. Y. Transglutaminase 2 induces nuclear factor-kappaB activation via a novel pathway in BV-2 microglia. J. Biol. Chem. (2004);279:53725–53735. doi: 10.1074/jbc.M407627200. [DOI] [PubMed] [Google Scholar]
- 18.Lorand L. Graham R. M. Transglutaminases: crosslink-ing enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Biol. (2003);4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- 19.Mehta K. Mammalian transglutaminases: a family portrait. Prog. Exp. Tumor Res. (2005);38:1–18. doi: 10.1159/000084229. [DOI] [PubMed] [Google Scholar]
- 20.Mehta K. Kumar A. Kim H. I. Transglutaminase 2: a multi-tasking protein in the complex circuitry of inflammation and cancer. Biochem. Pharmacol. (2010);80:1921–1929. doi: 10.1016/j.bcp.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Mhaouty-Kodja S. Ghalpha/tissue transglutaminase 2: an emerging G protein in signal transduction. Biol. Cell. (2004);96:363–367. doi: 10.1016/j.biolcel.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Park M. K. Lee C. H. Alpinia katsumadai suppresses mi-gration and 12-O-tetradecanoylphorbol-13-acetate-induced inva-sion of HT-1080 cells through suppression of transglutaminase-2 matrix metalloproteinase-2 and matrix metalloproteinase-9 expre-ssion. Cancer Prevention Research. (2011);16:326–332. [Google Scholar]
- 23.Rafi M. M. Yadav P. N. Rossi A. O. Glucosamine inhibits LPS-induced COX-2 and iNOS expression in mouse macrophage cells (RAW 264.7) by inhibition of p38-MAP kinase and transcrip-tion factor NF-kappaB. Mol. Nutr. Food Res. (2007);51:587–593. doi: 10.1002/mnfr.200600226. [DOI] [PubMed] [Google Scholar]
- 24.Shikhman A. R. Kuhn K. Alaaeddine N. Lotz M. N-acetylglucosamine prevents IL-1 beta-mediated activation of hu-man chondrocytes. J. Immunol. (2001);166:5155–5160. doi: 10.4049/jimmunol.166.8.5155. [DOI] [PubMed] [Google Scholar]
- 25.Singh S. Khajuria A. Taneja S. C. Khajuria R. K. Singh J. Qazi G. N. Boswellic acids and glucosamine show syner-gistic effect in preclinical anti-inflammatory study in rats. Bioorg. Med. Chem. Lett. (2007);17:3706–3711. doi: 10.1016/j.bmcl.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 26.Song H. Y. Lee J. A. Ju S. M. Yoo K. Y. Won M. H. Kwon H. J. Eum W. S. Jang S. H. Choi S. Y. Park J. Topical transduction of superoxide dismutase mediated by HIV-1 Tat pro-tein transduction domain ameliorates 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced inflammation in mice. Biochem. Pharma-col. (2008);75:1348–1357. doi: 10.1016/j.bcp.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Wangroongsub Y. Tanavalee A. Wilairatana V. Ngarmukos S. Comparable clinical outcomes between glucosamine sul-fate-potassium chloride and glucosamine sulfate sodium chloride in patients with mild and moderate knee osteoarthritis: a random-ized double-blind study. J. Med. Assoc. Thai. (2010);93:805–811. [PubMed] [Google Scholar]


