Abstract
Rumex acetosa is a perennial herb that is widely distributed across eastern Asia. Although the hot water extract of R. acetosa has been used to treat gastritis or gastric ulcers as a folk medicine, no scientific report exists for the use of this plant to treat gastric ulcers. Hence, the present study was undertaken to assess the anti-ulcer activity of water and 70% ethanol extracts obtained from R. acetosa, using an HCl/ethanol-induced gastric ulcer model in mice. Anti-inflammatory and free radical-scavenging activities of these two extracts were also evaluated and compared. As a result, the administration of R. acetosa extracts significantly reduced the occurrence of gastric ulcers. However, significant differences in protective activity against gastric ulcers were observed between the two samples. In the case of the group pretreated with an ethanol extract dosage of 100 mg/kg, the protective effect (90.9%) was higher than that of water extract (41.2%). Under histological evaluation, pretreatment with R. acetosa extracts reversed negative effects, such as inflammation, edema, moderate hemorrhaging and loss of epithelial cells, presented by HCl/ ethanol-treated stomachs. Meanwhile, R. acetosa extracts showed potent DPPH radical-scavenging activity and decreased NO production in a murine macrophage cell line, RAW 264.7, in a dose-dependent manner without affecting cellular viability. The greater anti-ulcer and NO production inhibitory activities exhibited by ethanol extracts compared to water extracts could be ascribed to the higher emodin levels, a major anthraquinone component of this plant.
Keywords: Rumex acetosa, Gastric ulcer, Antioxidant activity, Anti-inflammatory activity
INTRODUCTION
The therapeutic management of peptic ulcers includes several classes of drugs, such as proton pump inhibitors, histamine receptor blockers, drugs affecting the mucosal barrier, and prostaglandin analogs. However, there is still a need for the development of new anti-ulcer drugs because of the emergence of tolerance and side effects, which make the effi cacy of these treatments questionable (Mard et al., 2008).
Rumex acetosa L. (Polygonaceae) is a perennial herb that is widely distributed in eastern Asia (Anonymous, 1999; Lee, 2003). The leaf of this plant resembles that of spinach, and the plant is well known for strong acidity in Europe and America, where it is also known as sorrel. The extract of R. acetosa has been reported to have heat-cleaning, diuretic, insecticidal, antimicrobial, and anticancer activities (Lee et al., 2005; Gescher et al., 2011; Wegiera et al., 2011). Decoction of this plant has been used as a folk medicine in Korea to treat arthritis, gastritis and gastric ulcers, and as a substitute for rhubarb, which is an important crude drug for gastrointestinal problems. There are commercial herbal tonic products such as EssiacTM and Flor-EssenceTM which contain R. acetosa, that are traditionally prepared as cancer therapy for natives in North America (Tamayo et al., 2000; Tai et al., 2004; Leonard et al., 2006). Flavones including vitexin, rutin, and kaempferol were isolated from the aerial parts of this plant with another flavone C-glycosides and flavonol O-glycosides (Kato and Morita, 1990). From the roots of R. acetosa, chrysophanein, hyperin, proanthocyanidin and phloroglucinol derivatives were isolated with anthraquinones, such as emodin and chrysophanol (Bicker et al., 2009; Lim et al., 2011).
Although hot water extracts of R. acetosa have been used to treat gastritis or gastric ulcers, no scientific reports exist concerning the use of this plant this manner. Meanwhile, it has been reported that emodin displays anti-ulcerogenic and anti-inflammatory activities, and in our pre-study, the emodin content in 70% ethanol extract (EER) was higher than in water extracts (WER) (Goel et al., 1991). Hence, the present study was undertaken to assess and compare the anti-ulcer activity of EER and WER obtained from R. acetosa using an HCl/ ethanol-induced gastric ulcer model in mice. The free radicalscavenging and anti-inflammatory activities of these two extracts were also evaluated and compared.
MATERIALS AND METHODS
General
Sucralfate, 1,1-diphenyl-2-picrylhydrazyl (DPPH), lipopolysaccharide (LPS), phosphoric acid, N-(1-naphthyl) ethylenediamine dihydrochloride, sulfanilamide, sodium nitrite, butylated hydroxytoluene (BHT), L-ascorbic acid (LAA) and emodin were purchased from the Sigma Chemical Co. (St. Louis, MO). Dimethyl sulfoxide (DMSO) was purchased from the Merck Co. (Darmstadt, Germany). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS) and the antibiotic mixture (penicillin-streptomycin) were purchased from Hyclone (South Logan, UT, USA). All other chemicals were reagent-grade.
Plant material
The whole plant of Rumex acetosa L. (Polygonaceae) was collected from the fields of the Sancheong province in May 2011. After cleaning with water, samples were dried at room temperature in the shade. These plant materials were identified by Prof. J. H. Park of College of Pharmacy, Pusan National University, Korea. The voucher specimen (APG-1103) was deposited in the Herbarium of the College of Pharmacy, Gyeongsang National University.
Sample preparation and HPLC conditions
The dried plant material was ground into powder with a mill. The finely pulverized samples were weighed (5 g), and 500 ml of water or 70% ethanol solution in water was added, respectively. The mixtures were extracted with a soxhlet extractor for 3 hr at 100℃ and 80℃, respectively. The extract was later filtered using filter papers (Whatman No. 40), and centrifuged for 10 min at 4℃, 5,000 rpm. The filtrate was lyophilized, and the exact amount was weighed and suspended in water for the following bioassays. A portion of the filtrate was pre-treated with Sep-Pack catridge (C18, Waters, USA) prior to HPLC analysis.
The Agilent 1100 HPLC system used for analysis (Avondale, CA, USA) consisted of a temperature-controlled autosampler, column oven, and a binary pump. A 10 μl volume of sample solutions, or standard, was directly injected on a YMC J'sphere ODS-H80 column (4 μm, 4.6×150 mm; YMC, Kyoto, Japan) using a gradient acetonitrile-water solvent system. The step gradient elution was as follows: 10% acetonitrile for the first 5 min, 10% to 80% acetonitrile for a further 25 min, and then, 80% to 90% for the next 5 min, followed by maintaining the condition for another 5 min. A conditioning phase (40-45 min) was subsequently used to return the column to its initial state. The flow rate was 1.0 ml/min, and the column temperature was set at 35℃. The eluent was detected at 254 nm using a DAD detector. Chemstation software (Hewlett-Packard, Avondale, CA, USA) was used to operate this HPLC system. Emodin was used as a standard compound.
Animals
Male ICR mice (4 weeks old; weighing 24-28 g; Koatech, Korea) were used in the in vivo experiments. The animals were housed in an air-conditioned room (24 ± 2℃), under a 12 hr light/dark cycle. Each animal was used for one experiment only. All procedures relating to animal care and treatment conformed to the Animal Care Guidelines of the Animal Experiment Committee of Gyeongsang National University.
HCl/ethanol-induced gastric ulcer
The protective activity against gastric ulcer in mice was evaluated according to the method described previously with slight modifications (Ogawa et al., 2011). After 16 hr of fasting, the mice were divided randomly into five groups (5 mice per a group). Sham-operated and vehicle-treated groups were given 0.2 ml/25 g of water. Another group was treated with sucralfate (100 mg/kg, p.o.), that was suspended in 0.25% sodium carboxymethylcellulose solution (Samchun Chemicals, Korea) as a positive control. The other two groups were treated with WER and EER in water (100 mg/kg, p.o.). The administration dosage (100 mg/kg, p.o.) in mice has been decided according to the related published reports (Karimi et al., 2004; Ogawa et al., 2011). The mice, except for those belonging to the shamoperated group, were administered 150 mM HCl/ethanol (0.2 ml/25 g body weight) after 1 hr of sample treatment. The mice were euthanized using carbon dioxide after 1 hr of HCl/ethanol treatment, and their stomachs were removed and fixed with 10 ml of 1% formalin for 30 min. Next, the stomachs were opened, along the greater curvature, and were gently rinsed with saline. The gastric ulcers were evaluated according to the area of gastric lesion with specific image analysis software (Isolution Lite, IMT i-solution Inc., Vancouver, Canada).
Histological analysis
Histological evaluation was performed on the glandular stomach of the mice. The tissue samples were preserved in 10% neutral buffered formalin and processed for routine paraffin block preparation. Sections approximately 8 μm in thickness were cut and stained with hematoxylin and eosin. Mucosal injury evaluation was performed under light microscopy, and the histopathological alterations were assessed by comparing lesions.
DPPH radical scavenging activity
Based on the method introduced by Gordon et al. (2001), 200 μl of the 0.2 mM DPPH solution in methanol (w/v) was mixed with 800 μl sample solution of different concentrations (2.5, 5.0, 10, 25 and 50 μg/ml) in an Eppendorf tube and the solution was incubated at room temperature for 30 min. After incubation, absorbance of mixtures was measured at 517 nm against the blank, methanol. DPPH radical scavenging activity was calculated with the following formula: % activity = {(Ac- As)/Ac}×100, where Ac and As are absorbance of the control and sample, respectively. BHT and L-ascorbic acid were used as positive controls.
Cell culture
RAW 264.7 cells were obtained from the Korean Cell Line Bank (KCLB, Korea). The macrophage-like murine cell line RAW 264.7 was cultured at 37℃ and 5% CO2 in DMEM supplemented with 1% penicillin/streptomycin and 10% fetal calf serum.
Determination of intracellular NO production
RAW 264.7 cells were seeded at a density of 1×104 cells/ well in 96-well plates and were incubated for 24 hr. These cells were treated with each sample solutions at the final concentrations of 1, 10, 100 μg/ml for 1 hr before exposure to LPS (100 ng/ml) for stimulation of the cells. After 24 hr incubation, nitrite levels in culture media were measured by Griess reaction to assess NO production (Malyshev and Shnyra, 2003). Briefly, 50 μl of cell culture medium was mixed with 50 μl of Griess reagent [equal volumes of 1% w/v sulfanilamide in 5% v/v phosphoric acid and 0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride) in water] and incubated for 10 min at room temperature. Absorbance was later measured at 540 nm using a microplate reader. Fresh culture medium was used as a blank in all of the experiments. Nitrite levels in samples were determined from a standard sodium nitrite curve.
Cell viability assay
Cell viability was measured by EZ-Cytox cell viability assay kit (Daeillab Service, Korea). RAW 264.7 cells were plated into 96-well microtiter plates at a density of 1×104 cells/well. After 24 hr of incubation, the culture medium was replaced with fresh culture medium containing each sample solutions at the final concentrations of 1, 10, and 100 μg/ml, and subsequently cultured for 24 hr. For each treatment, 10 μl of assay reagent, WST solution including 4-[3-(4-iodophenyl)-2-(4-nitrophenyl)- 2H-5-tetrazolio]-1,3-benzene disulfonate was added to each well followed by a 1-hr incubation. Color development by water-soluble formazan formation was measured at 450 nm using a Victor X5 microplate reader (PerkinElmer, USA). Viability was calculated by subtraction of the mean values without assay reagent from those with WST substrate, and was expressed as percentage of control.
Statistical analysis
The data are the results obtained from more than three samples of two- or three-independent experiments, and are expressed as the mean ± S.E.M. The differences among samples were statistically evaluated via one-way analysis of variance (ANOVA). The values were considered statistically significant at probability (p) values equal to or less than 0.05.
RESULTS
HPLC profiles of WER and EER
A high performance liquid chromatographic method was applied to compare the chemical differences between hot water extracts (WER) of R. acetosa, generally used for herbal remedies, and 70% alcoholic extracts (EER), usually used for plant sample preparations in chemical analysis and bioassays. As shown in Fig. 1, the height of most peaks was much higher in EER than in WER. Particulary, more than eight times greater emodin content was determined in EER (2.09 ± 0.30 mg/g per extract) than in WER (0.24 ± 0.06 mg/g per extract).
Fig. 1. HPLC chromatograms of emodin and Rumex acetosa extracts. (A) Chromatogram of emodin (10 μg/ml); (B) and (C) chromatograms of WER and EER, respectively.
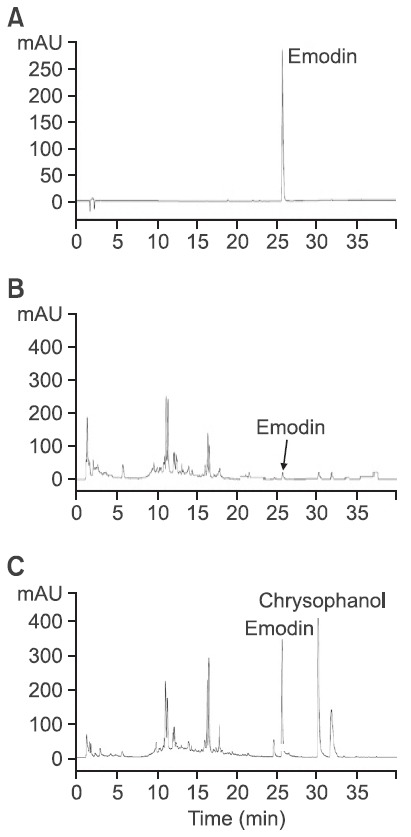
Another major peak observed at 30.4 min could be anticipated as chrysophanol, which has the same retention time and UV spectrum observed in previously published studies (Lim et al., 2011).
Anti-ulcer effect of WER and EER on HCl/ethanol-induced gastric ulcer model in mice
HCl and absolute ethanol, were used as necrotizing agents of gastric mucosa to produce gastric ulcers in mice. Gastric mucosa damage was evaluated based on the area of the gastric ulcer. The protection percentage was calculated with respect to the ulcer control group, considered as 100% damage. The administration of HCl/ethanol produced lesions on the gastric mucosa (73.3 ± 17.6 mm2), which were significantly reduced to 43.1 ± 12.1 and 6.7 ± 2.7 mm2, respectively in the animals pretreated with WER and EER at the dose of 100 mg/ kg (Fig. 2). In case of the group pretreated with EER, the protective effect (90.9%) was higher than that of WER (41.2%). Sucralfate (100 mg/kg) used as a reference drug also significantly reduced the gastric lesions by 84.4% (11.4 ± 6.5 mm2), compared to the vehicle group (Fig. 2D). Histological evaluation showed that HCl/ethanol treated stomachs presented inflammation, edema, moderate hemorrhaging, and a considerable loss of epithelial cells (Fig. 3B). Conversely, the pretreatment with R. acetosa extracts reversed these negative effects, and the histological aspect in the group pretreated with EER was similar to those observed in normal stomachs (Fig. 3A) and the pretreated group (Fig. 3C) with sucralfate used as
Fig. 2. The protective effects of WER and EER on HCl/ethanolinduced gastric ulcer model in mice. (A) Normal stomach, (B) glandular stomach treated with vehicle, (C) the stomach of positive control group treated with sucralfate, 100 mg/kg, (D) the stomach of test group treated with WER, 100 mg/kg, (E) the stomach of test group treated with EER, 100 mg/kg, p.o., 1 hr before administration of 150 mM HCl/ethanol, (F) determination of the gastric ulcer area (mm2). Data are presented as the mean ± S.E.M. *p<0.05 and **p<0.01, significantly different from gastric ulcer group (B).
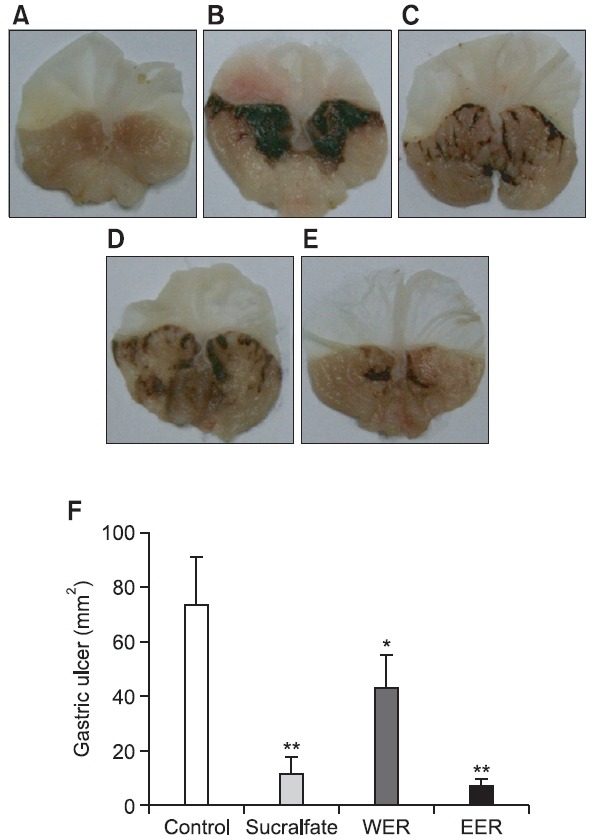
Fig. 3. Histological evaluation of the protective effects of WER and EER with hematoxylin and eosin (HE) stained longitudinal sections through gastric mucosa in an HCl/ethanol-induced gastric ulcer model. (A) Sham control group, (B) vehicle group, (C) positive control group treated with sucralfate, 100 mg/kg, (D, E) the stomach of test groups treated with WER and EER, 100 mg/kg, p.o., 1 hr before administration of 150 mM HCl/ethanol, respectively. A scale bar represents 150 μm.
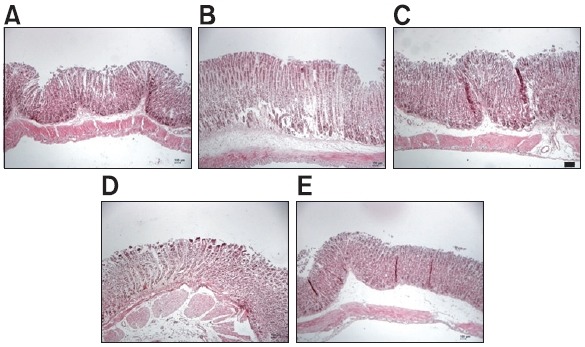
the reference drug.
Free radical-scavenging activity
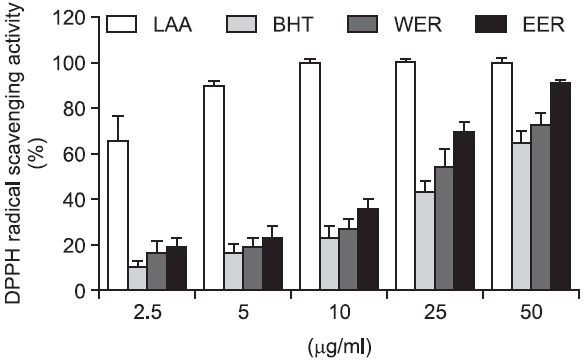
Because both ulcerous and inflammatory processes are related to an increase of free radicals (Pedernera et al., 2006), the free radical-scavenging activity of R. acetosa extract was also evaluated. As a result, R. acetosa extracts showed potent DPPH radical scavenging activity, which was greater than that of the reference compound, BHT (Fig. 4). Meanwhile, using IC50 values, EER showed more potent radical scavenging activity than that of WER (16.4 μg/ml versus 28.6 μg/ml, respectively).
Fig. 4. Differences in DPPH radical scavenging activity between WER and EER (2.5–50 μg/ml). Ascorbic acid and BHT were used as references. Absorbance of the reaction was measured at 517 nm. Values are the mean ± S.E.M.
Effect of WER and EER on intracellular NO production in LPS-stimulated RAW 264.7 cells
It is well-known that NO is involved in the modulation of the gastric mucosal integrity, and is important to the regulation of acid and alkaline secretions, mucus secretion, and gastric mucosal blood flow (Andreo et al., 2006). Therefore, the inhibitory activity of R. acetosa extracts against LPS-stimulated NO production was evaluated in RAW 264.7 cell line. Our results showed that R. acetosa extracts decreased NO production in
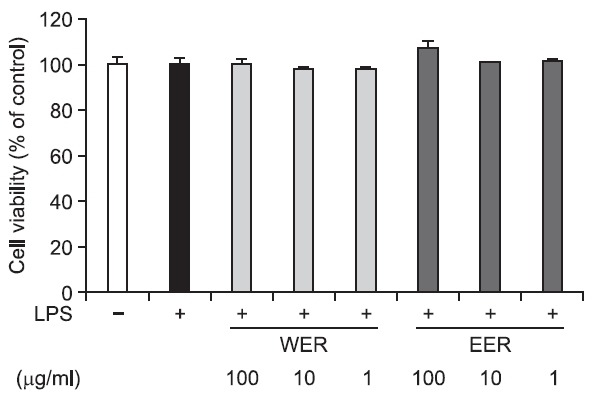
a dose-dependent manner at the concentrations of 1, 10 and 100 μg/ml, and the inhibitory activity was greater in EER than in WER (Fig. 5). In addition, both extracts did not affect cellular viability (Fig. 6).
Fig. 5. Effect of WER and EER on NO production in LPS-stimulated RAW 264.7 cells. **p<0.01, ***p<0.001 vs. LPS-treated control.
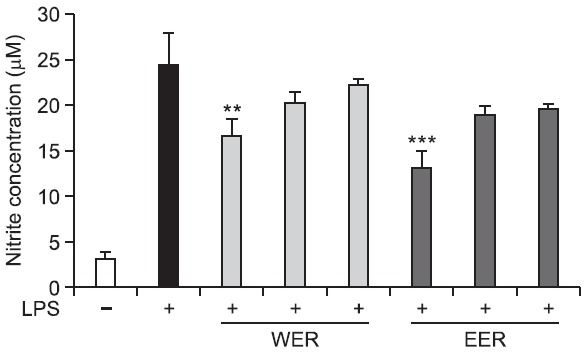
Fig. 6. Cytotoxic effect of WER and EER on LPS-stimulated RAW 264.7 cells. Cell viability was expressed relative to untreated control (100%).
DISCUSSION
Rumex acetosa is widely distributed in eastern Asia, America and Europe, and has strong vitality as regarded as a weed. In addition, sprouts and young leaves of this plant have been publically recognized as a possible material for functional foods in Korea. Therefore, if this material were to be developed as a plant source for production of health products or natural medicines, its simplicity of cultivation and safety as a food material would be immense merits. The aim of this study was the evaluation of the anti-ulcer activity of hot water extracts (WER) and 70% ethanol extracts (EER), obtained from the whole parts of R. acetosa against HCl/ethanol induced gastric model in mice. In general, hot water extract of R. acetosa has been used for herbal remedy using this plant, and 70% alcoholic extract has been used for chemical analysis
and bioassays in a laboratory setting. Meanwhile, it is known that gastric ulcers are also associated with inflammation processes in the gastric mucosa. NO is involved in the modulation of the gastric mucosal integrity, and is important to the regulation of acid and alkaline secretions, mucus secretion, and gastric mucosal blood flow (Andreo et al., 2006). Both ulcerous and infl ammatory processes are related to an increase of free radicals (Pedernera et al., 2006). Therefore, the NO production’s inhibitory and free radical-scavenging activities of the two extracts were also evaluated and compared.
The results of the present study demonstrated that both WER and EER showed significant gastro-protective effects on the HCl/ethanol-induced ulcer model. These gastro-protective effects were confi rmed by histological and intracellular NO production data. However, significant differences in protective activity against gastric ulcer in mice and NO production inhibitory activity in RAW 264.7 macrophages were observed between WER and EER. In case of the group pretreated with EER, the protective effect (90.9%) was higher than that of WER (41.2%). Because emodin has been reported to have an anti-ulcerogenic and anti-inflammatory effect, it is suggested that the greater anti-ulcer and NO production inhibitory activities of EER could be ascribed to its higher emodin content (Goel et al., 1991; Kumar et al., 1998; Lee et al., 2005; Lin et al., 2010; Ko et al., 2010; Ok et al., 2012). Meanwhile, there is a possibility that other components contribute to these activities, because WER also showed significant anti-ulcer and NO production inhibitory activities. Under histological evaluation, pre-treatment with R. acetosa extracts reversed the changes such as inflammation, edema, moderate hemorrhaging and considerable loss of epithelial cells, exhibited by HCl/ethanoltreated stomachs. Therefore, 70% ethanol is recommended as an effective solvent for the preparation of R. acetosa extract to treat gastritis or gastric ulcers.
Furthermore, these two samples presented potent antioxidant properties, indicating the involvement of protective mechanisms on the oxidative stress induced by ethanol in the gastric mucosa. In other words, the high free radical scavenging activity of R. acetosa extracts in the DPPH test suggests that the antioxidant activity may be one of the mechanisms of its anti-ulcerogenic and anti-inflammatory properties, because both ulcerous and inflammatory processes are related to an increase of free radicals (Pedernera et al., 2006). Emodin, a major anthraquinone component of this plant, failed to show DPPH free radical-scavenging activity in our experiment (data not shown), and the content was quite low in WER. These results suggest that the antioxidant activities of WER and EER resulted from the other constituents showing their peaks at retention times of less than 20 min (Fig. 1). In fact, phenolic compounds, such as the flavones, proanthocyanidin and phloroglucinol derivatives, isolated from this plant are well-known to have antioxidant activity (Kato and Morita, 1990; Cai et al., 2004; Bicker et al., 2009).
The present study demonstrates, for the first time, the antiulcer property of R. acetosa, on an experimental model of gastric ulceration in mice. These findings contribute to the pharmacological validation for the ethanol extract of R. acetosa as an anti-ulcerogenic agent with anti-inflammatory property. Nonethelss, phytochemical and pharmacological investigations are still needed to elucidate more fully the exact mechanism of action of the R. acetosa extract.
Acknowledgments
This work was supported by grants from the Next-Generation BioGreen 21 Program (SSAC, Grant # PJ008119), Rural Development Administration, Republic of Korea.
References
- 1.Andreo M. A., Ballesteros K. V., Hiruma-Lima C. A., Machado da Rocha L. R., Souza Brito A. R., Vilegas W. Effect of Mouriri pusa extracts on experimentally induced gastric lesions in rodents: role of endogenous sulfhydryls compounds and nitric oxide in gastroprotection. J. Ethnopharmacol. (2006);107:431–441. doi: 10.1016/j.jep.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Traditional Chinese Dictionary (Ch). Shanghai Scientific Press; Shanghai.: (1999). [Google Scholar]
- 3.Bicker J., Petereit F., Hensel A. Proanthocyanidins and a phloroglucinol derivative from Rumex acetosa L. Fitoterapia. (2009);80:483–495. doi: 10.1016/j.fitote.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Cai Y., Luo Q., Sun M., Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. (2004);74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gescher K., Hensel A., Hafezi W., Derksen A., Kühn J. Oligomeric proanthocyanidins from Rumex acetosa L. inhibit the attachment of herpes simplex virus type-1. Antiviral Res. (2011);89:9–18. doi: 10.1016/j.antiviral.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Goel R. K., Das Gupta G., Ram S. N., Pandey V. B. Antiulcerogenic and anti-inflammatory effects of emodin, isolated from Rhamnus triquerta wall. Indian J. Exp. Biol. (1991);29:230–232. [PubMed] [Google Scholar]
- 7.Gordon M. H., Paiva-Martins F., Almeida M. Antioxidant activity of hydroxytyrosol acetate compared with that of other olive oil polyphenols. J. Agric. Food Chem. (2001);49:2480–2485. doi: 10.1021/jf000537w. [DOI] [PubMed] [Google Scholar]
- 8.Karimi G., Hosseinzadeh H., Ettehad N. Evaluation of the gastric antiulcerogenic effects of Portulaca oleracea L. extracts in mice. Phytother. Res. (2004);18:484–487. doi: 10.1002/ptr.1463. [DOI] [PubMed] [Google Scholar]
- 9.Kato T., Morita Y. C-glycosylflavones with acetyl substitution from Rumex acetosa L. Chem. Pharm. Bull. (1990);38:2277. doi: 10.1248/cpb.38.2277. [DOI] [Google Scholar]
- 10.Ko J. C., Su Y. J., Lin S. T., Jhan J. Y., Ciou S. C., Cheng C. M., Lin Y. W. Suppression of ERCC1 and Rad51 expression through ERK1/2 inactivation is essential in emodin-mediated cytotoxicity in human non-small cell lung cancer cells. Biochem. Pharmacol. (2010);79:655–664. doi: 10.1016/j.bcp.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Kumar A., Dhawan S., Aggarwal B. B. Emodin (3-methyl- 1,6,8-trihydroxyanthraquinone) inhibits TNF-induced NF-kappaB activation, IkappaB degradation, and expression of cell surface adhesion proteins in human vascular endothelial cells. Oncogene. (1998);17:913–918. doi: 10.1038/sj.onc.1201998. [DOI] [PubMed] [Google Scholar]
- 12.Lee C. B. Coloured Flora of Korea I. Hyangmoonsa; Seoul: (2003). p. 246. [Google Scholar]
- 13.Lee N. J.,, Choi J. H.,, Koo B. S.,, Ryu S. Y.,, Han Y. H.,, Lee S. I., Lee D. U. Antimutagenicity and cytotoxicity of the constituents from the aerial parts of Rumex acetosa. Biol. Pharm. Bull. (2005);28:2158–2161. doi: 10.1248/bpb.28.2158. [DOI] [PubMed] [Google Scholar]
- 14.Leonard B. J., Kennedy D. A., Cheng F. C., Chang K. K., Seely D., Mills E. An in vivo analysis of the herbal compound essiac. Anticancer Res. (2006);26:3057–3063. [PubMed] [Google Scholar]
- 15.Lim J. P., Park Y. S., Hong M. W., Kim D. K. Quantitative analysis of anthraquinones from the roots of Korean Natural Rumex species plants. Kor. J. Pharmacogn. (2011);42:297–301. [Google Scholar]
- 16.Lin M. L., Lu Y. C., Chung J. G., Li Y. C., Wang S. G., N G S. H., Wu C. Y., Su H. L., Chen S. S. Aloe-emodin induces apoptosis of human nasopharyngeal carcinoma cells via caspase- 8-mediated activation of the mitochondrial death pathway. Cancer Lett. (2010);291:46–58. doi: 10.1016/j.canlet.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Malyshev I. Y., Shnyra A. Controlled modulation of inflammatory, stress and apoptotic responses in macrophages. Curr. Drug Targets Immune Endocr. Metabol. Disord. (2003);3:1–22. doi: 10.2174/1568008033340342. [DOI] [PubMed] [Google Scholar]
- 18.Mard S. A., Bahari Z., Eshaghi N., Farbood Y. Antiulcerogenic effect of Securigera securidaca L. seed extract on various experimental gastric ulcer models in rats. Pak. J. Biol. Sci. (2008);11:2619–2623. doi: 10.3923/pjbs.2008.2619.2623. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa K., Oyagi A., Tanaka J., Kobayashi S., Hara H. The protective effect and action mechanism of Vaccinium myrtillus L. on gastric ulcer in mice. Phytother. Res. (2011);25:1160–1165. doi: 10.1002/ptr.3413. [DOI] [PubMed] [Google Scholar]
- 20.Ok S., Kim S. M., Kim C., Nam D., Shim B. S., Kim S. H., Ahn K. S., Choi S. H., Ahn K. S. Emodin inhibits invasion and migration of prostate and lung cancer cells by downregulating the expression of chemokine receptor CXCR4. Immunopharmacol. Immunotoxicol. (2012) doi: 10.3109/08923973.2012.654494. in press. [DOI] [PubMed] [Google Scholar]
- 21.Pedernera A. M.,, Guardia T., Calderón C. G., Rotelli A. E., de la Rocha N. E., Genaro S. D., Pelzer L. E. Anti-ulcerogenic and anti-inflammatory activity of the methanolic extract of Larrea divaricata Cav. in rat. J. Ethnopharmacol. (2006);105:415–420. doi: 10.1016/j.jep.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Tai J., Cheung S., Wong S., Lowe C. In vitro comparison of Essiac and Flor-Essence on human tumor cell lines. Oncol. Rep. (2004);11:471–476. [PubMed] [Google Scholar]
- 23.Tamayo C., Richardson M. A., Diamond S., Skoda I. The chemistry and biological activity of herbs used in Flor-Essence herbal tonic and Essiac. Phytother. Res. (2000);14:1–14. doi: 10.1002/(SICI)1099-1573(200002)14:1<1::AID-PTR580>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 24.Wegiera M., Kosikowska U., Malm A., Smolarz H. D. Antimicrobial activity of the extracts from fruits of Rumex species. Cent. Eur. J. Biol. (2011);6:1036–1043. doi: 10.2478/s11535-011-0066-0. [DOI] [Google Scholar]


