Abstract
This study was performed to examine the hepatoprotective effect of isorhamnetin-3-O-galactoside, a flavonoid glycoside isolated from Artemisia capillaris Thunberg (Compositae), against carbon tetrachloride (CCl4)-induced hepatic injury. Mice were treated intraperitoneally with vehicle or isorhamnetin-3-O-galactoside (50, 100, and 200 mg/kg) 30 min before and 2 h after CCl4 (20 μl/kg) injection. Serum aminotransferase activities and hepatic level of malondialdehyde were significantly higher after CCl4 treatment, and these increases were attenuated by isorhamnetin-3-O-galactoside. CCl4 markedly increased serum tumor necrosis factor-α level, which was reduced by isorhamnetin-3-O-galactoside. The levels of inducible nitric oxide synthase (iNOS), cyclooxygenase- 2 (COX-2), and heme oxygenase-1 (HO-1) protein and their mRNA expression levels were significantly increased after CCl4 injection. The levels of HO-1 protein and mRNA expression levels were augmented by isorhamnetin-3-O-galactoside, while isorhamnetin- 3-O-galactoside attenuated the increases in iNOS and COX-2 protein and mRNA expression levels. CCl4 increased the level of phosphorylated c-Jun N-terminal kinase, extracellular signal-regulated kinase and p38, and isorhamnetin-3-O-galactoside reduced these increases. The nuclear translocation of nuclear factor kappa B (NF-κB), activating protein-1, and nuclear factor erythroid 2-related factor 2 (Nrf2) were signifi cantly increased after CCl4 administration. Isorhamnetin-3-O-galactoside attenuated the increases of NF-κB and c-Jun nuclear translocation, while it augmented the nuclear level of Nrf2. These results suggest that isorhamnetin-3-O-galactoside ameliorates CCl4-induced hepatic damage by enhancing the anti-oxidative defense system and reducing the inflammatory signaling pathways.
Keywords: Carbon tetrachloride, Heme oxygenase-1, Hepatotoxicity, Inflammation, Isorhamnetin-3-O-galactoside, Oxidative stress
INTRODUCTION
Acute and chronic liver diseases constitute a global concern and the concern is worsened by the lack of reliable liver protective drugs, despite the increasing need for agents to protect the liver from damage. Therefore, complementary and alternative medicines for the treatment of liver diseases have been receiving considerable interest (Seeff et al., 2001). Therapeutically effective agents from natural products may reduce the risk of clinical toxicity.
Carbon tetrachloride (CCl4) has long been known as a toxicant and has been widely used in many in vitro and in vivo toxicology studies. CCl4causes liver toxicity, resulting in cellular necrosis, fatty degeneration, fibrosis and cirrhosis (Taïeb et al., 2005). Administered CCl4 is metabolized by cytochrome P450 (CYP), primarily CYP2E1 and results in the formation of trichloromethyl radical ( · CCl3), which initiates lipid peroxidation and protein oxidation leading to hepatocellular damage (Manibusan et al., 2007).
Artemisia capillaris Thunb. (Compositae) is one of the oldest and most commonly prescribed herbs in Eastern traditional medicine, and has been used as an analgesic, antimicrobial agent and a remedy for the treatment of hepatitis and bilious disorders (Chang and But, 1987). An aqueous extract of Artemisia capillaris was shown to inhibit interleukin (IL)-1 receptor- and tumor necrosis factor (TNF) receptor-induced cytotoxicity and ethanol-induced apoptosis of HepG2 cells (Koo et al., 2002). A previous study reported that Artemisia capillaris reduced the lipopolysaccharide-induced inflammatory response in a human hepatoma cell line and in the rat liver (Hong et al., 2004), and prevented 2,2'-azobis(2-amidinopropane) dihydrochloride-induced liver damage in rats (Han et al., 2006).
Isorhamnetin-3-O-galactoside (Fig. 1), one of the flavonoid constituents from Artemisia capillaris, exerted antiinflammatory activity by inhibiting the production of 5-lipoxygenase- induced leukotriene (Kwon et al., 2011).
Fig. 1. The structure of isorhamnetin-3-O-galactoside.
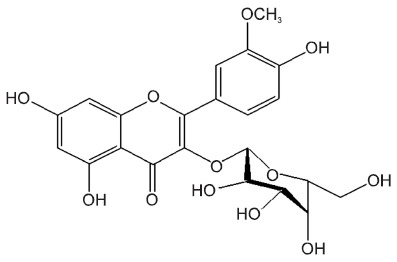
Therefore, this study was designed to investigate the protective effects of isorhamnetin-3-O-galactoside against CCl4- induced acute hepatic injury, with particular attention to the oxidative stress and inflammatory pathways.
MATERIALS AND METHODS
Isolation of isorhamnetin-3-O-galactoside from an Artemisia capillaris
The whole plant of Artemisia capillaris Thunb. was dried and grinded to powder. The dried powder (9.5 kg) was then extracted with hot MeOH (50.0 l×3 times) for 3 h. After filtration, total filtrate was concentrated to dryness in vacuo at 400℃ to obtain the MeOH extract (900 g). Following this, the MeOH extract was suspended in distilled water:MeOH (9:1) and successively partitioned with dichloromethane (CH2Cl2), ethyl acetate (EtOAc), n-butanol (n-BuOH) to yield CH2Cl2 (354 g), EtOAc (141 g), n-BuOH (196 g) fractions, respectively, as well as the H2O residue (218 g). n-BuOH fractions were chromatographed over HP-20 diaion using the H2O:MeOH; (1:0→6:4→4:6→0:1) solvent system to afford 4 major subfractions H2O (132 g), 40% MeOH (30.4 g), 60% MeOH (21 g) and MeOH (6.8 g), respectively. 60% MeOH subfraction was chromatographed in a silica gel column using CH2Cl2:MeOH (20:1) with a gradual increase in MeOH in order to obtain 17 subfractions (6F-1 to 6F-17). Decantation of subfraction 6F-3 yielded isorhamnetin-3-O-galactoside (270 mg).
1H-NMR (400 MHz, DMSO-d6) δ : 12.63 (1H, s, -OH), 10.91 (1H, brs, -OH ), 9.83 (1H, brs, -OH), 8.02 (1H, d, J = 2.0 Hz, H-2'), 7.49 (1H, dd, J = 2.0 & 8.0 Hz, H-6'), 6.90 (1H, d, J = 8.0 Hz, H-5'), 6.45 (1H, d, J = 2.0 Hz, H-8), 6.31 (1H, d, J = 2.0 Hz, H-6), 5.52 (1H, d, J = 8.0 Hz, H-1'), 5.20 (1H, brd), 4.91 (1H, brs, -OH), 4.55 (1H, brd, -OH), 4.50 (1H, brs, -OH), 3.85 (3H, s, -OCH3); 13C-NMR (100 MHz, DMSO-d6) δ : 177.4 (C-4), 164.2(C-7), 161.2 (C-5), 156.4 (C-9), 156.3 (C-2), 149.4 (C- 3'), 146.99 (C-4'), 133.1 (C-3), 121.9 (C-6'), 121.1 (C-1'), 115.1 (C-5'), 113.5 (C-2'), 104.0 (C-10), 101.6 (Gal-1), 98.7 (C-6), 93.7 (C-8), 75.9 (Gal-2), 73.1 (Gal-3), 71.3 (Gal-5), 67.96 (Gal-4), 60.3 (Gal-6), 55.99 (-OCH3).
Treatment of animals
Male ICR mice weighing 25-30 g (Daehan Biolink Co., Eum-seong, Korea) were fasted overnight but given tap water ad libitum. All animals were treated humanely under the Sung-kyunkwan University Animal Care Committee Guidelines. The animals were randomly assigned to 6 groups comprising 8-10 animals per group. The mice in group 1 (control) intraperitoneally received only olive oil (10 ml/kg). In groups 2 to 6, CCl4 dissolved in olive oil (1:499, v/v) was administered intraperitoneally (final concentration of 20 μl/kg). Groups 1 and 2 (vehicle) were treated intraperitoneally with Tween-80 in saline (1:9, v/v). The animals in groups 3 to 5 were treated intraperitoneally with isorhamnetin-3-O-galactoside (50, 100, and 200 mg/kg), and group 6 was intraperitoneally treated with silymarin (positive control, 800 mg/kg), 30 min before and 2 h after CCl4 injection. The timing of the isorhamnetin-3-O-galactoside treatment was selected based on the previous report (Kim et al., 2010). Blood was collected 24 h after CCl4 administration. Each liver was isolated and stored at -75℃ for analysis, except for the left lobe, which was used for histological studies.
Serum aminotransferases activities
Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were determined by standard spectrophotometric procedures using the ChemiLab ALT and AST assay kits (IVDLab Co., Uiwang, Korea), respectively.
Histological analysis
Liver tissues were removed from a portion of the left lobe, and fixed immediately in 10% neutral buffered formalin, embedded in paraffin, and then sectioned at 5 μm thickness. Serial sections were stained with hematoxylin and eosin for evaluation of portal inflammation, hepatocellular necrosis, and inflammatory cell infiltration. The sections were examined in a blind manner under an Olympus CKX 41 microscope (Olympus optical Co. Ltd., Tokyo, Japan).
Lipid peroxidation
The steady-state level of malondialdehyde (MDA), which is the end product of lipid peroxidation, was analyzed in liver homogenates by spectrophotometric measurement of the levels of thiobarbituric acid reactive substances at 535 nm, as described by Buege and Aust (1978), using 1,1,3,3-tetraethoxypropane (Sigma, St. Louis, MO, USA) as the standard.
Serum TNF-α level
Serum concentration of TNF-α was quantified using a commercial TNF-α enzyme-linked immunosorbent assay (ELISA) kit (BD Biosciences Co., CA, USA).
Western blot immunoassay
Freshly isolated liver tissue was homogenized in lysis buffer for the preparation of whole protein extracts. NE-PER® (Pierce Biotechnology, Rockford, IL, USA) was used for the extraction of nuclear proteins according to the manufacturer’s instructions. The BCA Protein Assay kit (Pierce Biotechnology) was used to determine protein concentrations. Protein samples were loaded on 10-15% polyacrylamide gels, separated by sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE) and then transferred to PVDF membranes (Millipore, Billerica, MA, USA) using the Semi-Dry Trans-Blot Cell (Bio-Rad Laboratories, Hercules, CA, USA). After the transfer, the membranes were washed with 0.1% Tween-20 in 1×Tris Buffered Saline (TBS/T) and blocked for 1 h at room temperature with 5% (w/v) skim milk powder in TBS/T. The blots were then incubated overnight at 4℃ with primary antibodies. The next day, the blots were incubated in appropriate secondary antibodies and were detected using an ECL detection system (iNtRON Biotechnology Co., Ltd., Korea), according to the manufacturer’s instructions. ImageQuantTMTL software (Amersham Biosciences/GE Healthcare, Piscataway, NJ, USA) was used for the densitometric evaluation of visualized immuno-reactive bands. Primary antibodies against inducible nitric oxide synthase (iNOS) (Transduction Laboratories, San Jose, CA, USA; 1:1,000 dilution), cyclooxygenase-2 (COX-2) (Cayman, Ann Arbor, MI, USA; 1:1,000 dilution), phoshoprylated (p)-p38, p-c-Jun N-terminal kinase (JNK), total p38, and total JNK (Cell Signaling Technology Inc., Beverly, MA, USA; 1:1,000), p-extracellular signal-regulated kinase (ERK) and total ERK (Cell Signaling Technology Inc.; 1:2,000), nuclear factor (NF)-κB/p65 (Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:1,000) and c-Jun p39 phosphorylated on serine- 63 (Santa Cruz Biotechnology; 1:500), heme oxygenase-1 (HO-1) (Stressgen Bioreagents Corp., Ann Arbor, MI, USA; 1:2,500) and finally nuclear factor erythroid 2-related factor 2 (Nrf2) (Santa Cruz Biotechnology; 1:1,000) were used and the signals were normalized to that of β-actin (Sigma; 1:2,500 dilution) or lamin B1 (Abcam, Cambridge, UK; 1:2,500).
Total RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted and the first strand of cDNA was synthesized by reverse transcription of total RNA using oligo(dT)12-18 primer and SuperScriptTM II RNase H-Reverse Transcriptase (Invitrogen Tech-LineTM, Carlsbad, CA, USA). PCR reaction was carried out in a 20 μl reaction volume with a diluted cDNA sample. Final reaction concentrations were as follows: sense and antisense primers, 10 pM; dNTP mix, 250 μM; ×10 PCR buffer, and Ex Taq DNA polymerase, 0.5 U/reaction. PCR was carried out with an initial denaturation step at 94℃ for 5 min, and a final extension step at 72℃ for 7 min in the GeneAmp 2700 thermocycler (Applied Biosystems, Foster City, CA, USA). The primers used in this study are demonstrated in Table 1. Amplification cycling conditions were as follows: 35 cycles at 94℃ for 30 s, 65℃ for 30 s, and 72℃ for 30 s for iNOS; 35 cycles at 94℃ for 30 s, 60℃ for 30 s, and 72℃ for 30 s for COX-2; 30 cycles of 94℃ (30 s), 56℃ (30 s), and 72℃ (30 s) for HO-1; 35 cycles of 94℃ (30 s), 62℃ (30 s) and 72℃ (60 s) for β-actin. Following RT-PCR, 10 μl samples of the PCR products were visualized by ultraviolet illumination after electrophoresis through 1.5% agarose gel and ethidium bromide staining. The intensity of each PCR product was analyzed semi-quantitatively using a digital camera (DC120, Eastman Kodak, New Haven, CT, USA).
Table 1.
PCR primers used in this study and the amplifi ed product length
| Gene (accession number) | Primer sequences (5`-3`) | Product length (bp) |
|---|---|---|
| TNF-α (M11731) | Sense: AGCCCACGTCGTAGCAAACCACCAA | 446 |
| Antisense: AACACCCATTCCCTTCACAGAGCAAT | ||
| iNOS (NM_010927) | Sense: AAGCTGCATGTGACATCGACCCGT | 598 |
| Antisense: GCATCTGGTAGCCAGCGTACCGG | ||
| COX-2 (NM_011198) | Sense: ACTCACTCAGTTTGTTGAGTCATTC | 582 |
| Antisense: TTTGATTAGTACTGTAGGGTTAATG | ||
| HO-1 (NM_010442) | Sense: AACAAGCAGAACCCAGTCT | 374 |
| Antisense: TGTCATCTCCAGAGTGTTC | ||
| β-Actin (X03672) | Sense: TGGAATCCTGTGGCATCCATGAAA | 348 |
| Antisense: TAAAACGCAGCTCAGTAACAGTCCG | ||
Statistical analysis
All results are presented as mean ± SEM. The overall significance of the data was examined by one-way analysis of variance. Differences between the groups were considered significant at p<0.05 with the appropriate Bonferroni correction made for multiple comparisons.
RESULTS
Effect of isorhamnetin-3-O-galactoside on hepatocellular damage
As shown in Table 2, serum ALT and AST activities were 40 ± 5 U/l and 32 ± 3 U/l in the control group. The vehicle-treated CCl4 group showed increases in serum ALT and AST activities at 24 h after CCl4 injection (13,001 ± 636 U/l and 10,090 ± 730 U/l, respectively). Isorhamnetin-3-O-galactoside at doses of 100 and 200 mg/kg significantly attenuated the increases in ALT activity to 9,501 ± 666 U/l and 9,362 ± 540 U/l, respectively. Consistent with the ALT data, the serum AST levels were significantly attenuated to 6,345 ± 669 U/l and 6,979 ± 755 U/l by 100 and 200 mg/kg of isorhamnetin-3-O-galactoside, respectively. Silymarin at 800 mg/kg also significantly attenuated the increases in ALT and AST activities to 9429 ± 607 U/l and 6,946 ± 765 U/l, respectively. However, 50 mg/kg of isorhamnetin-3-O-galactoside did not affect the serum ALT and AST activities.
Table 2.
Effect of isorhamnetin-3-O-galactoside on serum aminotransferase activities and lipid peroxidation
| Groups | ALT (U/l) | AST (U/l) | MDA (nmol/mg protein) | |
|---|---|---|---|---|
| Control | 40.0 ± 5.0 | 32.0 ± 3.3 | 0.5 ± 0.04 | |
| CCl4 | ||||
| Vehicle | 13,001 ± 636* | 10,090 ± 730* | 3.2 ± 0.5* | |
| Isorhamnetin-3-O-galactoside | 50 mg/kg | 12,769 ± 542* | 9,743 ± 430* | 2.6 ± 0.1* |
| 100 mg/kg | 9,501 ± 666*,# | 6,345 ± 669*,# | 1.5 ± 0.1*,# | |
| 200 mg/kg | 9,362 ± 540*,# | 6,979 ± 755*,# | 1.4 ± 0.1*,# | |
| Sylymarin 800 mg/kg | 9,429 ± 607*,# | 6,946 ± 765*,# | 1.5 ± 0.2*,# | |
Isorhamnetin-3-O-galactoside (50, 100, and 200 mg/kg) or silymarin (800 mg/kg) was administered intraperitoneally 30 min before and 2 h after CCl4 injection. Liver damage was assessed by measurement of circulating serum ALT and AST activities. The values are presented as mean ± SEM for 8-10 mice per group. *Significantly different (p<0.01) from the control group. #Significantly different (p<0.01) from the vehicle-treated CCl4 group.
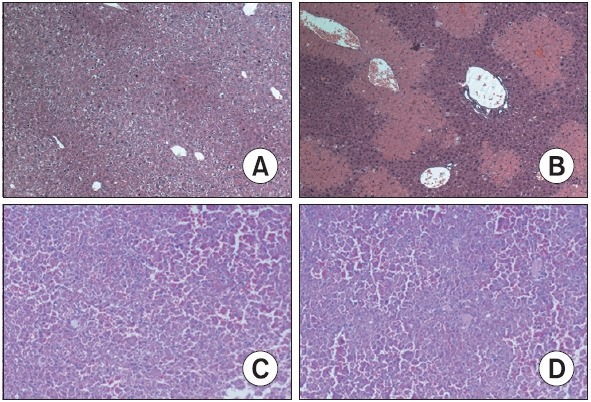
The histological features shown in Fig. 2A demonstrate a normal liver lobular architecture and cell structure in the control group. In contrast, hepatocyte ballooning and necrosis were observed in the vehicle-treated CCl4 group with multiple areas of portal inflammation as well as a moderate increase in inflammatory cell infiltration (Fig. 2B). These pathological changes were attenuated by 100 mg/kg of isorhamnetin-3-Ogalactoside (Fig. 2C) and 800 mg/kg of silymarin (Fig. 2D).
Fig. 2. Effect of isorhamnetin-3-O-galactoside on the histological changes in the liver (original magnification ×100). (A) Control group, showing normal lobular architecture and cell structure (B) vehicle-treated CCl4 group, showing extensive hepatocellular damage with the presence of portal inflammation, centrizonal necrosis, and Kupffer cell hyperplasia (C) isorhamnetin-3-O-galactoside (100 mg/kg)-treated CCl4 group, showing mild portal inflammation and minimal hepatocellular necrosis and Kupffer cell hyperplasia (D) silymarin (800 mg/kg)-treated CCl4 group, showing mild portal inflammation and minimal hepatocellular necrosis and Kupffer cell hyperplasia.
Isorhamnetin-3-O-galactoside alone did not affect serum aminotransferase activities (data not shown).
Effect of isorhamnetin-3-O-galactoside on lipid peroxidation
In the control group, the level of MDA was 0.5 ± 0.04 nmol/mg protein. Following CCl4 injection, the level of MDA increased to 3.2 ± 0.5 nmol/mg protein. 100 and 200 mg/kg of isorhamnetin-3-O-galactoside significantly attenuated this increase in the MDA level (1.5 ± 0.1 nmol/mg protein and 1.4 ± 0.1 nmol/mg protein, respectively), while 50 mg/kg of isorhamnetin-3-O-galactoside did not affect the level of MDA (Table 2).
Effect of isorhamnetin-3-O-galactoside on TNF-α, iNOS, COX-2, HO-1 and Nrf2 protein expression
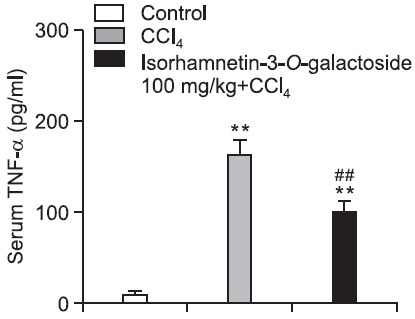
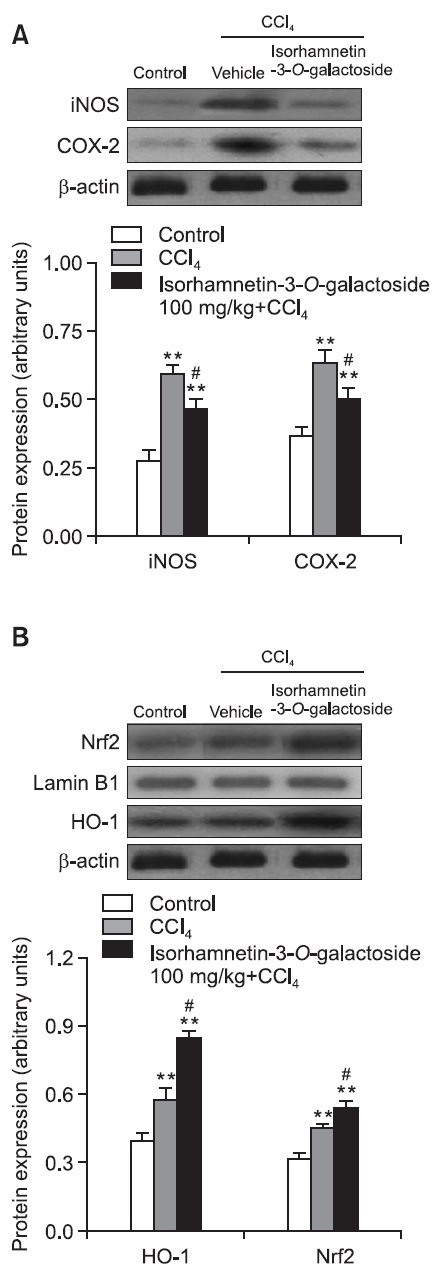
The serum level of TNF-α in the control group was 9.0 ± 2.6 pg/ml. After CCl4 treatment, the serum level of TNF-α increased to 162.6 ± 17.9 pg/ml and this increase was significantly attenuated (98.3 ± 14.0 pg/ml) by 100 mg/kg of isorhamnetin- 3-O-galactoside (Fig. 3). Compared with the control group, the levels of iNOS, COX-2 and HO-1 protein expression were 2.2-, 1.7- and 1.7-fold higher in the CCl4 group, respectively. 100 mg/kg of isorhamnetin-3-O-galactoside signifi cantly attenuated the increases in iNOS and COX-2 protein expression levels, while augmenting HO-1 protein expression.
Fig. 3. Effect of isorhamnetin-3-O-galactoside (100 mg/kg) on the serum TNF-α level after CCl4 administration. Serum level of TNF-α was measured using a commercially available ELISA kit. Values are presented as mean ± SEM of 8-10 mice per group. **Signifi - cantly different (p<0.01) from the control group. ##Signifi cantly different (p<0.01) from the vehicle-treated CCl4 group.
To investigate the mechanism of HO-1 upregulation by isorhamnetin- 3-O-galactoside, we assessed the nuclear translocation of Nrf2, a well-established transcription factor that regulates HO-1 expression. At 24 h after CCl4 injection, the level of nuclear Nrf2 protein expression was increased and 100 mg/ kg of isorhamnetin-3-O-galactoside was found to enhance this increase (Fig. 4).
Fig. 4. Effect of isorhamnetin-3-O-galactoside (100 mg/kg) on (A) iNOS and COX-2, and (B) HO-1 and Nrf2 protein expression after CCl4 administration. Western blot analysis was performed to measure iNOS, COX-2 and HO-1 protein expression levels in liver tissue. Nrf2 was measured by western blot analysis using nuclear extracts from the liver. Values are presented as mean ± SEM of 8-10 mice per group. **Significantly different (p<0.01) from the control group. #Significantly different (p<0.05) from the vehicle-treated CCl4 group.
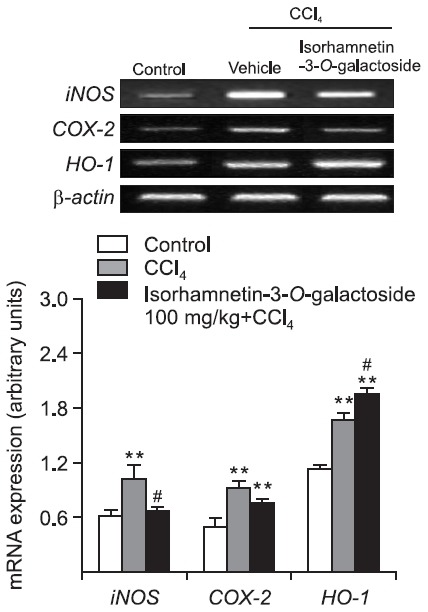
Effect of isorhamnetin-3-O-galactoside on iNOS, COX-2 and HO-1 mRNA expression
Compared with the control group, the levels of iNOS, COX- 2 and HO-1 mRNA expression increased to 1.7-, 1.9- and 1.5-fold in the CCl4 group, respectively. 100 mg/kg of isorhamnetin- 3-O-galactoside significantly attenuated the increase of iNOS mRNA expression, while augmenting HO-1 mRNA expression (Fig. 5).
Fig. 5. Effect of isorhamnetin-3-O-galactoside (100 mg/kg) on iNOS, COX-2 and HO-1 mRNA expression after CCl4 administration. RT-PCR was performed to measure iNOS, COX-2 and HO-1 mRNA expression levels in liver tissue. Values are presented as mean ± SEM of 8-10 mice per group. **Significantly different (p<0.01) from the control group. #Significantly different (p<0.05) from the vehicle-treated CCl4 group.
Effect of isorhamnetin-3-O-galactoside on phosphorylation of mitogen-activated protein kinases (MAPKs) and nuclear translocation of NF-κB and activating protein (AP)-1
After CCl4 injection, phosphorylation of JNK, ERK, and p38 increased to 2.6 times, 1.5 times, and 1.4 times the control value, respectively. 100 mg/kg of isorhamnetin-3-O-galactoside attenuated the phosphorylation of JNK, ERK and p38.
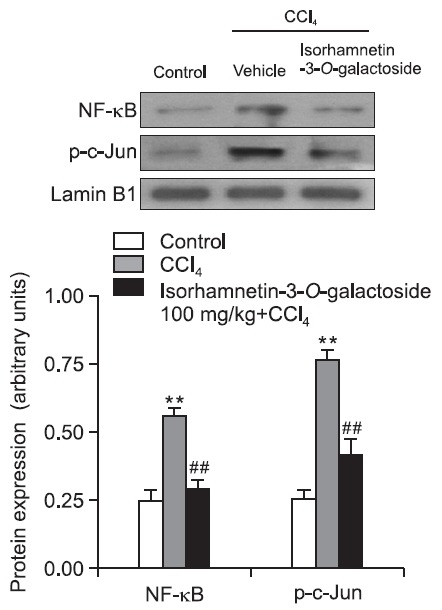
Moreover, nuclear levels of p 65, a subunit of NF-κB, and phosphorylated c-Jun (p-c-Jun) showed a marked increase in the CCl4 group compared with the control group, respectively. Isorhamnetin-3-O-galactoside at 100 mg/kg significantly attenuated these increases (Fig. 6,7).
Fig. 6. Effect of isorhamnetin-3-O-galactoside (100 mg/kg) on JNK, ERK and p38 protein expression after CCl4 administration. Western blot analysis was performed to measure the phosphorylated and total forms of JNK, ERK and p38 in liver tissue. Values are presented as mean SEM of 8-10 mice per group. *, **Significantly different (p<0.05, p<0.01) from the control group. #, ##Significantly different (p<0.05, p<0.01) from the vehicle-treated CCl4 group.
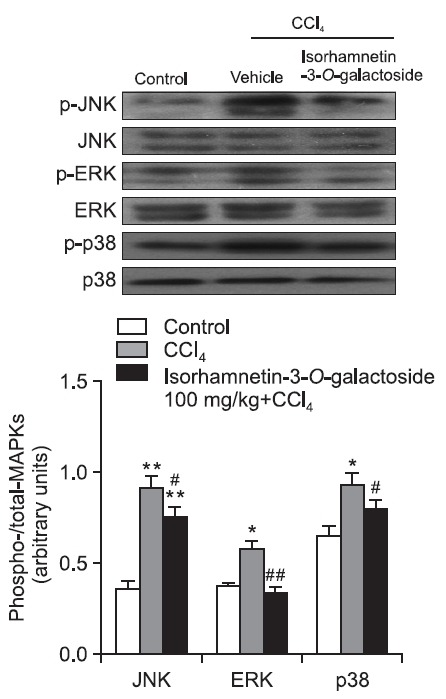
Fig. 7. Effect of isorhamnetin-3-O-galactoside (100 mg/kg) on NF- κB and p-c-Jun protein expression after CCl4 administration. NF-κB and p-c-Jun were measured by western blot analysis using nuclear extracts from liver. Values are presented as mean ± SEM of 8-10 mice per group. **Significantly different (p<0.01) from the control group. ##Significantly different (p<0.01) from the vehicle-treated CCl4 group.
DISCUSSION
CCl4-induced hepatic injury is a widely used experimental model for hepatoprotective drug screening. Recently, we screened the 70% ethanol and water extracts of Artemisia capillaris and its active components for hepatoprotective agents. Among them, hyperoside and isorhamnetin-3-O-galactoside were shown to inhibit CCl4-induced hepatotoxicity in primary hepatocyte cultures (data not shown). In this study, we investigated the hepatoprotective effects of isorhamnetin-3-Ogalactoside against CCl4-induced liver injury in vivo.
In the vehicle-treated CCl4 group, the ALT and AST activities were dramatically increased, indicating severe hepatocellular damage. In contrast, isorhamnetin-3-O-galactoside markedly decreased the release of ALT and AST. The hepatoprotective effect of isorhamnetin-3-O-galactoside appeared to be similar to that of silymarin, a potent hepatoprotective agent. The histological examination of the liver samples strongly supports the protective effect of isorhamnetin-3-O-galactoside. CCl4 caused centrizonal necrosis, portal inflammation, and Kupffer cell hyperplasia. These alterations were signifi cantly attenuated by isorhamnetin-3-O-galactoside. These results suggest that isorhamnetin-3-O-galactoside may be appropriate for clinical applications to treat liver disorders.
CCl4 is responsible for oxidative stress and lipid peroxidation
through the CYP2E1-mediated generation of the highly reactive · CCl3, leading to eventual cellular damage characterized by hepatocellular necrosis (Taïeb et al., 2005). In this study, isorhamnetin-3-O-galactoside exhibited protective effects by suppressing CCl4-mediated oxidative damage through decreased production of free radical derivatives, as evidenced by the decreased MDA level at doses of 100 mg/kg and 200 mg/kg. Stressors such as excessive oxidative stress readily induce upregulation of HO-1 (Otterbein and Choi, 2000). HO-1 is an endogenous and cytoprotective enzyme with the main activity catabolizing the oxidative degradation of heme into carbon monoxide (CO), free iron, and biliverdin. Bilirubin/biliverdin is a potent antioxidant that scavenges peroxyradicals, and CO exerts a powerful antiinflammatory effect (Ryter et al., 2007). Several dietary phytochemicals such as glycyrrhizin and hyperoside were shown to induce HO-1 protein and gene expression in our previous studies (Lee et al., 2007; Choi et al., 2011). Nrf2 is a transcription factor that induces expression of various cytoprotective enzymes possessing the antioxidant response element (ARE) in the promoter region and targets genes including glutathione peroxidase, glutathione S-transferase, glutamate cysteine ligase, and HO-1 (Kobayashi and Yamamoto, 2006). In our study, hepatic Nrf2 and HO-1 protein and gene expression levels were markedly increased after CCl4 treatment. Furthermore, treatment with isorhamnetin-3- O-galactoside markedly augmented Nrf2 translocation and HO-1 protein and mRNA expression, suggesting that a strong induction of HO-1 via Nrf2 by isorhamnetin-3-O-galactoside protects liver cells from CCl4-induced oxidative cellular injuries.
Kupffer cells release a number of proinflammatory mediators which are believed to aggravate CCl4-induced hepatic injury (Badger et al., 1996). TNF-α, a proinflammatory cytokine, is rapidly produced by macrophages in response to tissue damage (Brouckaert and Fiers, 1996). DeCicco et al., (1998) have reported the stimulation of TNF-α production in both serum and the liver following CCl4 administration, and suggested that · CCl3 activates Kupffer cells to release TNF- α. TNF-α also stimulates the release of cytokines from macrophages and induces the phagocytic oxidative metabolism and NO production (Morio et al., 2001). Nitric oxide (NO) is produced through the action of iNOS, and is involved in various processes including vasodilation and neurotransmission, and the nonspecific host defense system. NO can also react with reactive oxygen species to form cytotoxic oxidants such as peroxynitrite (Rodenas et al., 1995).
Previous studies reported that the induction of COX in the inflammatory response is the secondary effect of CCl4-induced hepatotoxicity (Basu, 1999). COX-2 is induced by proinflammatory stimuli to form prostaglandins from arachidonic acid (Planagumá et al., 2005). The results of this study showed a significant increase in the serum TNF-α level and iNOS and COX-2 protein and mRNA expression levels in the liver after CCl4 administration. These increases were attenuated by treatment with isorhamnetin-3-O-galactoside treatment, suggesting suppression of inflammatory responses.
The two transcription factors, NF-κB and AP-1, are sensitive to the redox status in abnormal physiological conditions such as CCl4-induced acute liver injury (Morio et al., 2001). NF-κB is an early response transcription factor and the nuclear translocation of NF-κB leads to gene expression of proinflammatory cytokines. AP-1 is a dimer consisting of JUN, FOS, ATF and MAF; and the combination in the AP-1 complex affects its DNA binding activity. The MAPK family plays important roles in the regulation of cell proliferation and death in response to various cellular stresses. During CCl4 challenge, oxidative stress and infl ammatory cytokines activate MAPK kinases, leading to phosphorylation of JNK and p38 (Iida et al., 2007). The major target of JNK and p38 is AP-1 and activation of AP-1 mediates ROS-induced hepatocellular death (Czaja, 2003). On the other hand, ERK is involved in survival signals by regulating cell proliferation after partial hepatectomy or CCl4 intoxication (Taniguchi et al., 2004). In this study, isorhamnetin-3-O-galactoside attenuated the increases in MAPK and the nuclear levels of NF-κB and AP-1.
These results provide evidence for the hepatoprotective effect of isorhamnetin-3-O-galactoside in CCl4-induced hepatotoxicity through enhancement of the antioxidative defense system and downregulation of the proinflammatory pathway.
Acknowledgments
This work was supported by a grant from the Korea Food and Drug Administration (Studies on the Identifi cation of Efficacy of Biologically Active Components from Oriental Herbal Medicines).
References
- 1.Badger D. A., Sauer J. M., Hoglen N. C., Jolley C. S., Sipes I. G. The role of inflammatory cells and cytochrome P450 in the potentiation of CCl4-induced liver injury by a single dose of retinol. Toxicol. Appl. Pharmacol. (1996);141:507–519. doi: 10.1006/taap.1996.0316. [DOI] [PubMed] [Google Scholar]
- 2.Basu S. Oxidative injury induced cyclooxygenase activation in experimental hepatotoxicity. Biochem. Biophys. Res. Commun. (1999);254:764–767. doi: 10.1006/bbrc.1998.9956. [DOI] [PubMed] [Google Scholar]
- 3.Brouckaert P., Fiers W. Tumor necrosis factor and the systemic inflammatory response syndrome. Curr. Top. Microbiol.Immunol. (1996);216:167–187. doi: 10.1007/978-3-642-80186-0_8. [DOI] [PubMed] [Google Scholar]
- 4.Buege J. A., Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. (1978);52:302–310. doi: 10.1016/S0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 5.Chang H. M., But P. P. H. Pharmacology and applications of chinese materia medica. World Scientific; Singapore: (1987). [Google Scholar]
- 6.Choi J. H., Kim D. W., Yun N., Choi J. S., Islam M. N., Kim Y.S., Lee S. M. Protective effects of hyperoside against carbon tetrachloride-induced liver damage in mice. J. Nat. Prod. (2011);74:1055–1060. doi: 10.1021/np200001x. [DOI] [PubMed] [Google Scholar]
- 7.Czaja M. J. The future of GI and liver research: editorial perspectives. III. JNK/AP-1 regulation of hepatocyte death. Am. J.Physiol. Gastrointest. Liver Physiol. (2003);284:G875–879. doi: 10.1152/ajpgi.00549.2002. [DOI] [PubMed] [Google Scholar]
- 8.DeCicco L. A., Rikans L. E., Tutor C. G., Hornbrook. K. R. Serum and liver concentrations of tumor necrosis factor alpha and interleukin-1beta following administration of carbon tetrachloride to male rats. Toxicol. Lett. . (1998);98:115–121. doi: 10.1016/S0378-4274(98)00110-6. [DOI] [PubMed] [Google Scholar]
- 9.Han K. H., Jeon Y. J., Athukorala Y., Choi K. D., Kim C. J., Cho J.K., Sekikawa M., Fukushima M., Lee C. H. A water extract of Artemisia capillaris prevents 2,2'-azobis(2-amidinopropane) dihydrochloride-induced liver damage in rats. J. Med. Food . (2006);9:342–347. doi: 10.1089/jmf.2006.9.342. [DOI] [PubMed] [Google Scholar]
- 10.Hong S. H., Seo S. H., Lee J. H., Choi B. T. The aqueous extract from Artemisia capillaris Thunb. inhibits lipopolysaccharideinduced infl ammatory response through preventing NF-kappaB activation in human hepatoma cell line and rat liver. Int. J. Mol. Med. . (2004);13:717–720. [PubMed] [Google Scholar]
- 11.Iida C., Fujii K., Kishioka T., Nagae R., Onishi Y., Ichi I., Kojo S. Activation of mitogen activated protein kinase (MAPK) during carbon tetrachloride intoxication in the rat liver. Arch. Toxicol. (2007);81:489–493. doi: 10.1007/s00204-007-0181-x. [DOI] [PubMed] [Google Scholar]
- 12.Kim H. Y., Kim J. K., Choi J. H., Jung J. Y., Oh W. Y., Kim D. C., Lee H. S., Kim Y. S., Kang S. S., Lee S. H., Lee S. M. Hepatoprotective effect of pinoresinol on carbon tetrachloride-induced hepatic damage in mice. J. Pharmacol. Sci. (2010);112:105–112. doi: 10.1254/jphs.09234FP. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi M., Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzyme. Regul. (2006);46:113–140. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Koo H. N., Hong S. H., Jeong H. J., Lee E. H., Kim N. G., Choi S.D., Ra K. W., Kim K. S., Kang B. K., Kim J. J., Oh J. G., Kim H. M. Inhibitory effect of Artemisia capillaris on ethanol-induced cytokines (TNF-alpha, IL-1alpha) secretion in Hep G2 cells. Immunopharmacol. Immunotoxicol. (2002);24:441–453. doi: 10.1081/IPH-120014728. [DOI] [PubMed] [Google Scholar]
- 15.Kwon O. S., Choi J. S., Islam M. N., Kim Y. S., Kim H. P. Inhibition of 5-lipoxygenase and skin infl ammation by the aerial parts of Artemisia capillaris and its constituents. Arch. Pharm. Res. (2011);34:1561–1569. doi: 10.1007/s12272-011-0919-0. [DOI] [PubMed] [Google Scholar]
- 16.Lee C. H., Park S. W., Kim Y. S., Kang S. S., Kim J. A., Lee S.H., Lee S. M. Protective mechanism of glycyrrhizin on acute liver injury induced by carbon tetrachloride in mice. Biol.Pharm. Bull. (2007);30:1898–1904. doi: 10.1248/bpb.30.1898. [DOI] [PubMed] [Google Scholar]
- 17.Manibusan M. K., Odin M., Eastmond D. A. Postulated carbon tetrachloride mode of action: a review. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. (2007);25:185–209. doi: 10.1080/10590500701569398. [DOI] [PubMed] [Google Scholar]
- 18.Morio L. A., Chiu H., Sprowles K. A., Zhou P., Heck D. E., Gordon M. K., Laskin D. L. Distinct roles of tumor necrosis factor-alpha and nitric oxide in acute liver injury induced by carbon tetrachloride in mice. Toxicol. Appl. Pharmacol. . (2001);172:44–51. doi: 10.1006/taap.2000.9133. [DOI] [PubMed] [Google Scholar]
- 19.Otterbein L. E., Choi A. M. Heme oxygenase: colors of defense against cellular stress. Am. J. Physiol. Lung Cell Mol. (2000);279:L1029–1037. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- 20.Planagum? A., Cl?ria J., Miquel R., L?pez-Parra M., Titos E., Masferrer J. L., Arroyo V., Rod?s J. The selective cyclooxygenase- 2 inhibitor SC-236 reduces liver fi brosis by mechanisms involving non-parenchymal cell apoptosis and PPARgamma activation. FASEB J. . (2005);19:1120–1122. doi: 10.1096/fj.04-2753fje. [DOI] [PubMed] [Google Scholar]
- 21.Ródenas J., Mitjavila M. T., Carbonell T. Simultaneous generation of nitric oxide and superoxide by infl ammatory cells in rats. Free Radic. Biol. Med. (1995);18:869–875. doi: 10.1016/0891-5849(94)00215-6. [DOI] [PubMed] [Google Scholar]
- 22.Ryter S. W., Morse D., Choi A. M. Carbon monoxide and bilirubin: potential therapies for pulmonary/vascular injury and disease. Am. J. Respir. Cell Mol. Biol. . (2007);36:175–182. doi: 10.1165/rcmb.2006-0333TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seeff L. B., Lindsay K. L., Bacon B. R., Kresina T. F., Hoofnagle J. H. Complementary and alternative medicine in chronic liver disease. Hepatology . (2001);34:595–603. doi: 10.1053/jhep.2001.27445. [DOI] [PubMed] [Google Scholar]
- 24.Taïeb D., Malicet C., Garcia S., Rocchi P., Arnaud C., Dagorn J. C., Iovanna J. L., Vasseur S. Inactivation of stress protein p8 increases murine carbon tetrachloride hepatotoxicity via preserved CYP2E1 activity. Hepatology . (2005);42:176–182. doi: 10.1002/hep.20759. [DOI] [PubMed] [Google Scholar]
- 25.Taniguchi M., Takeuchi T., Nakatsuka R., Watanabe T., Sato K. Molecular process in acute liver injury and regeneration induced by carbon tetrachloride. Life Sci. . (2004);75:1539–1549. doi: 10.1016/j.lfs.2004.02.030. [DOI] [PubMed] [Google Scholar]


