Abstract
Radiation therapy, the most commonly used for the treatment of brain tumors, has been shown to be of major significance in tu-mor control and survival rate of brain tumor patients. About 200,000 patients with brain tumor are treated with either partial large field or whole brain radiation every year in the United States. The use of radiation therapy for treatment of brain tumors, however, may lead to devastating functional deficits in brain several months to years after treatment. In particular, whole brain radiation therapy results in a significant reduction in learning and memory in brain tumor patients as long-term consequences of treatment. Although a number of in vitro and in vivo studies have demonstrated the pathogenesis of radiation-mediated brain injury, the cel-lular and molecular mechanisms by which radiation induces damage to normal tissue in brain remain largely unknown. Therefore, this review focuses on the pathophysiological mechanisms of whole brain radiation-induced cognitive impairment and the iden-tification of novel therapeutic targets. Specifically, we review the current knowledge about the effects of whole brain radiation on pro-oxidative and pro-inflammatory pathways, matrix metalloproteinases (MMPs)/tissue inhibitors of metalloproteinases (TIMPs) system and extracellular matrix (ECM), and physiological angiogenesis in brain. These studies may provide a foundation for defin-ing a new cellular and molecular basis related to the etiology of cognitive impairment that occurs among patients in response to whole brain radiation therapy. It may also lead to new opportunities for therapeutic interventions for brain tumor patients who are undergoing whole brain radiation therapy.
Keywords: Whole brain radiation, Cognitive impairment, Reactive oxygen species, Inflammation, Extracellular matrix, Physi-ological angiogenesis
BRAIN TUMORS AND RADIATION THERAPY
Brain tumors are one of the most aggressive and detrimental forms of cancer. Approximately 210,000 cases of primary and metastatic brain tumors are estimated to be diagnosed each year in the United States (American Brain Tumor Association, 2012; National Brain Tumor Society, 2012). Indeed, brain tu-mors are the most common of the solid tumors in children and the second leading cause of cancer-related deaths in children under the age of 20. Although the exact cause of brain tumors is still unknown, several risk factors such as certain genetic disorders, environmental factors, and electromagnetic fields have been identified (Chandana et al., 2008). Treatment op-tions for brain tumors are selected based on a number of dif-ferent factors including tumor type, location and size of tumor, tumor grade, and age and general health of the patient. It is generally accepted that standard treatments for brain tumors include surgery, chemotherapy, and radiation therapy. Table 1 summarizes the advantages and disadvantages of standard therapeutic approaches for patients with brain tumors.
Table 1.
Standard therapeutic options for brain tumor treatment
| Treatment | Pros | Cons | References |
|---|---|---|---|
| Surgery | • Reduction of elevated intracranial pressure by safely removing tumor for preserving neurological function | • Difficulty of achieving a complete resection without damaging cru-cial structures and normal brain function near tumor site | Cohadon (1990), Simpson et al. (1993), Hess (1999), Castro et al. (2003), Rampling et al. (2004), Koo et al. (2006), Mut (2012) |
| • Complete cure of symptoms in case of relatively benign tumors or low-grade brain tumors | • Presence of inoperable cases due to the inaccessible distribution | ||
| • Infection, bleeding, blood clots, blood pressure instability, neuro-logical deficits, coma, and death | |||
| Chemotherapy | • Availability of various drugs and drug combinations | • Restricted application due to insuf-ficient delivery of drugs across the blood-brain barrier | Graham and Cloughesy (2004), Rampling et al. (2004), Koo et al. (2006), Buckner et al. (2007) |
| • Improvement and enhancement in efficacy by bioengineering and advanced nanotechnology | • Development of multi-drug resis-tance by cancer cells as well as microvascular endothelial cells | ||
| • Immunosuppression, fatigue, bruises and bleeding, nausea, vomiting, diarrhea, and hair loss | |||
| Radiation therapy | • Ease of administration | • Cognitive impairment (learning and memory loss) | Sheline et al. (1980), New (2001), Denham and Hauer-Jensen (2002), Stone et al. (2003), Béhin and Delattre (2004), Moulder and Cohen (2007) |
| • Limited damage to surrounding healthy tissues/cells by localized treatment | • Hormonal alteration (growth hor-mone deficiency) | ||
| • Non-invasive approach | • Radiation-mediated necrosis (brain swelling) | ||
| • Treatment for inoperable and/or metastatic brain tumors | • Risk of secondary malignancy | ||
Surgery is usually the first step in treatment of patients with most benign and malignant brain tumors. It is generally recommended to remove as much tumor as possible when a tumor is accessible, provide a tumor tissue sample (biopsy) for an accurate diagnosis, remove at least part of the tumor to relieve intracranial pressure, and reduce the amount of tumor to be treated with chemotherapeutic drugs or radiation. Clini-cal data have shown that a near-total resection is important in improving survival in patients with high-grade gliomas (Simp-son et al., 1993; Hess, 1999). Even though surgical procedure serves as an initial treatment method and has curative effect for intracranial tumors that are located in the outer portion of
the brain, it may not be efficient for all malignant brain tumors. Deeply-seated tumors within the brain that are not accessible or tumors locating near critical or sensitive areas in the brain that control language, movement, vision, or other important functions cannot be surgically removed because of the exces-sive risk of neurological damage during the operation. Both general and specific risks to brain tumor surgery depend greatly on the extent of the procedure and include infection, bleeding, formation of blood clots, blood pressure instabil-ity, temporary or permanent neurological deficits, coma, and death (Cohadon, 1990; American Brain Tumor Association, 2012).
Chemotherapy uses one or more type of drug(s) to kill cancer cells. Even though chemotherapy alone gives mild ad-vantage to treat brain tumors, it usually provides an adjuvant outcome in combination with surgery and radiation therapy. In fact, the survival benefit in the patients with high-grade gliomas was observed when they were treated with a combination of chemotherapy and radiation therapy (Hegi et al., 2005; Stupp et al., 2005). Although there have been great improvements in the development of chemotherapeutic agents for the treatment of brain tumors, the clinical applications of currently available drugs for brain tumors are very limited due to significant side effects and insufficient delivery. The common clinical side ef-fects of chemotherapy for brain tumors include suppression of the immune system, fatigue, bruises, bleeding, nausea, vomit-ing, diarrhea, and hair loss. In addition, the presence of blood-brain barrier (BBB) has been identified as a major obstacle for chemotherapeutic treatment of brain tumors. While many efforts have been made to administer chemotherapy to brain tumors that circumvent the BBB in order to improve delivery of drugs, chemotherapy might not be suggested as an effec-tive treatment method for brain tumors (Buckner et al., 2007; American Brain Tumor Association, 2012).
Radiation therapy has been commonly used as the stan-dard treatment for brain tumors (Tsao et al., 2005; Khuntia et al., 2006; Kantor et al., 2008). It employs controlled high energy rays such as x-ray and γ-ray to either kill cancer cells directly or interfere with their ability to grow. Radiation can be given by either external or internal means; external radio-therapy is a critical component to treat brain tumors in many patients (Buckner et al., 2007). For example, stereotactic ra-diosurgery delivers a high dose of radiation during a single session from an external source, such as gamma knife and linear accelerator (LINAC), to treat brain tumors. Whole brain radiation therapy is another way of providing external radiation and is commonly used to treat various brain tumors by ad-ministering ionizing radiation to the entire brain. Whole brain radiation therapy may be given before, during, or after che-motherapy, or following partial or complete surgical removal of brain tumors. In addition, whole brain radiation therapy can be used to treat inoperable brain tumors and metastatic tu-mors that have spread to the brain from other part of the body. Walker et al. (1979) suggested dose-dependent effects of ra-diation on malignant gliomas by demonstrating the relation-ship between increased radiation therapy dose and increased survival. Other clinical trials demonstrated that post-operative radiation therapy provides significant survival benefits com-pared with surgery alone or chemotherapy (Andersen, 1978; Walker et al., 1978). Additionally, recent advances in neuro-imaging technologies with three-dimensional computerized treatment planning system and three-dimensional conformal radiotherapy (3D-CRT) have markedly enhanced efficacy and safety of radiation therapy (Bucci et al., 2005). Therefore, ra-diation therapy has been shown to be of major significance in tumor control and survival rate of brain tumor patients (Sheline et al., 1980). According to the Central Brain Tumor Registry of the United States (CBTRUS), about 200,000 patients with brain tumors are treated with either partial large-field or whole brain radiation every year in the United States (Stone et al., 2004; Moulder and Cohen, 2007).
RADIATION THERAPY AND BRAIN INJURY
The use of radiation therapy for treatment of brain tumors is limited by the risk of radiation-induced damage to the normal, healthy brain tissue that can subsequently lead to devastating functional deficits (Sheline et al., 1980; New, 2001; Denham and Hauer-Jensen, 2002; Stone et al., 2003; Béhin and De-lattre, 2004; Moulder and Cohen, 2007). Radiation-induced brain injury is classified as acute, early delayed (subacute),
and late delayed reactions based on the timing of onset of symptoms (Tofilon and Fike, 2000; Kim et al., 2008; Ramanan et al., 2010). Acute injury, occurring 48 hours to weeks after whole brain radiation therapy, is fairly mild to moderate in severity and is involved in fatigue, hair loss, skin erythema, headache, nausea, drowsiness, and emesis. Early delayed (subacute) injury is observed 1 to 6 months after whole brain radiation therapy and is associated with the clinical symptoms of fatigue, somnolence, short-term memory loss, and transient demyelination. Even though acute and early delayed injuries can lead to severe medical conditions, it is generally believed that most of the symptoms and signs of these injuries are re-versible. On the other hand, late delayed injury, occurring 6 months to several years after whole brain radiation therapy, is considered irreversible and progressive and is characterized by demyelination, vascular abnormalities, and ultimate white matter necrosis (Schultheiss and Stephens, 1992).
Previous studies have demonstrated that late delayed in-jury is largely responsible for cognitive impairment (DeAnge-lis et al., 1989; Roman and Sperduto, 1995; Akiyama et al., 2001; Johannesen et al., 2003; Bentzen, 2006; Shi et al., 2006; Welzel et al., 2008; Douw et al., 2009; Warrington et al., 2012). Indeed, progressive impairments in learning and memory were observed in 40-50% of brain tumor patients as long-term consequences of radiation therapy. Recent random-ized, prospective human clinical trials also provide evidence that the addition of whole brain radiation to stereotactic ra-diosurgery may cause a significant reduction in learning and memory in patients with brain tumors (Chang et al., 2009). Consistent with the human studies, a significant deterioration of memory function was observed in aged rats over a 7-month period post-radiation therapy (Lamproglou et al., 1995). Yo-neoka et al. (1999) found a similar, late onset of cognitive impairment in adult rats at 12 months following cranial irra-diation. Additionally, a fractionated dose of γ-ray irradiation to rats resulted in a significant increase in working memory errors primarily at 6 and 9 months (Brown et al., 2007). More-over, it was found that a clinically relevant regimen of frac-tionated whole brain radiation led to significant impairments in spatial learning and reference memory in rats (Shi et al., 2006). Furthermore, our most recent study demonstrated that a clinical fractionated series of whole brain radiation induces a transient deficit in contextual learning, disruption of work-ing memory, and progressive impairment of special learning in mice (Warrington et al., 2012). In addition to cognitive impair-ment, whole brain radiation causes other brain injuries includ-ing growth hormone deficiency and motor dysfunction (Table 2) (Darzy et al., 2005; Manda et al., 2007; Sara et al., 2011; Quik et al., 2012). Although there have been significant devel-opments in understanding pathophysiological mechanisms as summarized in Table 3, limited information on the etiology of radiation-induced damage to normal brain tissue is currently available. In particular, the cellular and molecular mechanisms responsible for whole brain radiation therapy-mediated cogni-tive impairment remain largely unknown. At present, there are no successful treatments or effective preventive strategies for radiation-induced brain injury. Therefore, the present review specifically focuses on three pathophysiological mechanisms by which whole brain radiation induces cognitive impairments; (1) effects of radiation therapy on oxidative stress and inflammation
Table 2.
Types of radiation-induced brain injury
| Type of injury | Test | Doses (Total/fractions) | Species | References |
|---|---|---|---|---|
| Cognitive impairment | • Morris water maze test | 25 Gy/single | Rat | Akiyama et al. (2001) |
| 10, 20, and 40 Gy/single | Rat | Liu et al. (2010b) | ||
| 20 Gy/4 and 40 Gy/8 | Rat | Zhou et al. (2011) | ||
| • Auditory verbal learning test, Medical College of Georgia Complex figures test, Attentional performance test, Multiple-choice test of vocabulary knowledge | 40 Gy/20 and 36 Gy/18 | Human | Welzel et al. (2008) | |
| • Letter-digit substitution test, Concept-shifting test, Stroop color-word test, Visual verbal learning test, Memory comparison test, Categoric word flu-ency | 56.6 ± 7.0 Gy/30.6 ± 3.9 | Human | Douw et al. (2009) | |
| • Behavior tests (IntelliCage) | 6 Gy/single | Mouse | Barlind et al. (2010) | |
| • Barnes maze test | 36 Gy/8 | Mouse | Warrington et al. (2012) | |
| Growth hormone deficiency | • Insulin tolerance test, Growth hormone-releasing hor-mone-arginine stimulation test | 53.5 ± 10.0 Gy (Biological effective dose) | Human | Darzy et al. (2005) |
| • Growth hormone-releasing hormone-arginine stimulation test | 59.4 Gy (50.1-60) /29.7 | Human | Sara et al. (2011) | |
| 55.1 ± 5.0 Gy/29.1 ± 1.5 | Human | Quik et al. (2012) | ||
| Motor dysfunction | • Spontaneous motor activity test | 6 Gy/single | Mouse | Manda et al. (2007) |
Table 3.
Pathophysiological mechanisms of whole brain radiation-induced cognitive impairment
| Mechanisms of action | Biomarker | Doses (Total/fractions) | Species | References |
|---|---|---|---|---|
| Oxidative stress | • MDA | 10 Gy (single) | M | Limoli et al. (2004) |
| • ROS, NF-κB, PAI-1, NOX4 | 1-10Gy (single) | R | Collins-Underwood et al. (2008) | |
| Inflammation | • COX-2, TNF-α, IL-1β, IL-6, iNOS, ICAM-1, MIP-2, MCP-1 | 5-35 Gy (single) | M | Kyrkanides et al. (2002) |
| • TNF-α, IL-1β, MCP-1 | 10 Gy (single) | R | Lee et al. (2010b) | |
| • c-Jun, TNF-α, IL-1β, IL-6, COX-2 | 10 Gy (single) | M | Deng et al. (2012) | |
| Extracellular matrix | • MMPs, TIMPs, Collagen type IV | 10 Gy (single), 40 Gy/8 | R, M | Lee et al. (2012) |
| • EMMPRIN | GKS (Max. 75 Gy) | R | Wei et al. (2012) | |
| Physiological angiogenesis | • VEGF, Ang-1, Ang-2, Tie-2 | 10 Gy (single) | R | Lee et al. (2011) |
| • VEGF | GKS (Max. 75 Gy) | R | Wei et al. (2012) | |
| Stem/progenitor cell death | • Caspase-3, p53, Nitrotyrosine, AIF | 8 Gy (single) | R | Fukuda et al. (2005) |
| • PARP, Annexin V, γ-HA2X | 1-5 Gy (single) | H | Acharya et al. (2010) | |
| Impaired neurogenesis | • NeuN, Tuj1, GFAP, NG2 | 10 Gy (single) | R | Monje et al. (2003) |
| • Ki-67, DCX, NeuN, GFAP, NG2, CD68 | 2-10 Gy (single) | M | Rola et al. (2004) | |
MDA: Malondialdehyde, ROS: Reactive oxygen species, NF-κB: Nuclear factor-κB, PAI: Plasminogen activator inhibitor, NOX: NADPH oxidase, COX: Cyclooxygenase, TNF: Tumor necrosis factor, IL: Interleukin, iNOS: Inducible nitric oxide synthase, ICAM: Intercellular ad-hesion molecule, MIP: Monocyte inflammatory protein, MCP: Monocyte chemoattractant protein, MMP: Matrix metalloproteinase, TIMP: Tissue inhibitor of metalloproteinases, EMMPRIN: Extracellular matrix metalloproteinase inducer, VEGF: Vascular endothelial growth fac-tor, Ang: Angiopoietin, Tie: Endothelial receptor tyrosine kinase, p53: Tumor suppressor protein 53, AIF: Apoptosis inducing factor, PARP: Poly (ADP-ribose) polymerase, γ-HA2X: Phosphorylated histone H2A, NeuN: Neuron-specific nuclear protein, Tuj1: Neuron-specific class III β-tubulin, GFAP: Glial fibrillary acidic protein, NG2: Chondroitin sulfate proteoglycan, DCX: Doublecortin, CD68: Cluster of differentiation 68, GKS: Gamma knife surgery, M: Mouse, R: Rat, H: Human.
in brain, (2) effects of radiation therapy on matrix metal-loproteinases and extracellular matrix in brain, and (3) effects of radiation therapy on physiological angiogenesis in brain. It will help identify therapeutic targets for novel preventive and treatment approaches for brain tumor patients who suffer from significant side effects after whole brain radiation therapy.
Radiation therapy and inflammation in brain
The pro-oxidative and pro-inflammatory environments have been implicated in the pathophysiological process of brain injury and subsequent development of various neurodegen-erative diseases (McGeer and McGeer, 1995; Dheen et al., 2007). Indeed, oxidative stress can induce expression of pro-inflammatory mediators, such as cytokines, chemokines, and adhesion molecules, via redox-responsive transcription fac-tor-mediated molecular signaling pathways. It is well known that expression of pro-inflammatory genes is up-regulated by increased oxidative stress through activation of a variety of transcription factors, such as activator protein-1 (AP-1), nu-clear factor-κB (NF-κB), cAMP responsive element-binding protein (CREB), specificity protein-1 (SP-1), and signal trans-ducers and activators of transcription (STATs) (Wung et al., 1997; Verhasselt et al., 1998; Lakshminarayanan et al., 1998; Simon et al., 1998; Bouloumie et al., 1999; Grösch and Kaina, 1999; Park et al., 2001; Lee et al., 2001a; Lee et al., 2001b; Lee et al., 2001c; Lee et al., 2001d; Flora et al., 2002; Lee et al., 2003; Lee et al., 2010b).
Evidence suggests that oxidative stress-mediated over-expression of pro-inflammatory mediators is associated with brain microvascular endothelial cell dysfunction and BBB disruption leading to the initiation and progression of neu-rodegenerative diseases. For example, amyloid β (Aβ) pep-tides contribute to pathogenesis in Alzheimer’s disease (AD) through pro-oxidative and pro-inflammatory mechanisms. Pre-vious studies have shown that Aβ-induced oxidative stress in brain can lead to an inflammatory cascade via secretion of interferon-γ (IFN-γ) and interleukin-1β (IL-1β), as well as ex-pression of CD40 in human brain microvascular endothelial cells (Suo et al., 1998; Akiyama et al., 2000). It was also demonstrated that Aβ increases the ability of monocytes/macro-phages to infiltrate into brain tissue across the BBB (Fiala et al., 1998; Giri et al., 2002). Additionally, oxidative stress and inflammation in brain have been suggested to actively partici-pate in the neurodegenerative process of Parkinson’s disease (PD) (McGeer et al., 2001; Schulz and Falkenburger, 2004). Degeneration of nigral dopaminergic neurons was observed in both an inflammation-mediated rat model and an in vitro cell culture model of PD (Liu and Hong, 2003). It was also found that cyclooxygenase-2 (COX-2) expression was induced spe-cifically within the substantia nigra pars compacta (SNpc) do-paminergic neurons in human postmortem PD specimens and in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD during the destruction of the nigrostriatal pathway (Teismann et al., 2003). Furthermore, treatment with antioxidant compounds or non-steroidal anti-inflammatory drugs (NSAIDs) exhibited beneficial effects such as delaying the onset or slowing the progression of neurodegenerative diseases including AD and PD (McGeer and McGeer, 1995; Akiyama et al., 2000). These findings provide compelling evi-dence that oxidative stress-mediated inflammatory responses in brain play a significant role in the pathogenesis of neurologi-cal disorders.
Recent evidence has identified oxidative stress and inflam-mation as important pathways leading to radiation-induced brain injury (Hong et al., 1995; Olschowka et al., 1997; Chi-ang et al., 1997; Kim et al., 2002; Denham and Hauer-Jensen, 2002; Gaber et al., 2003; Baluna et al., 2006). For example, a marked elevation of COX-1/-2 activity and subsequent production of prostaglandin E2 (PGE2) synthesis in brain fol-lowing ionizing radiation augments central nervous system (CNS) inflammation through up-regulation of a variety of pro-inflammatory mediators including tumor necrosis factor-α (TNF-α), IL-1β, IL-6, inducible nitric oxide synthase (iNOS), intercellular adhesion molecule-1 (ICAM-1), and matrix metal-loproteinase-9 (MMP-9) (Kyrkanides et al., 2002; Moore et al., 2005). Enhanced expression of adhesion molecules, such as ICAM-1, vascular cell adhesion molecule-1 (VCAM-1) and E-selectin, was also observed in irradiated brains (Hong et al., 1995; Olschowka et al., 1997; Gaber et al., 2003; Baluna et al., 2006).
Radiation has been reported to up-regulate expression of pro-inflammatory cytokines and chemokines in brain. A rapid induction of gene expressions of the pro-inflammatory cy-tokines, such as TNF-α and IL-1β, in response to radiation has been implicated in radiotherapy-associated damages to the brain (Hong et al., 1995; Gaber et al., 2003). Moreover, a significant and marked up-regulation of mRNA and protein expression of pro-inflammatory mediators, including TNF-α, IL-1β, and monocyte chemoattractant protein-1 (MCP-1), was observed in hippocampal and cortical regions isolated from ir-radiated brains. Interestingly, cytokine expression was region-ally specific since TNF-α levels were significantly elevated in cortex compared to hippocampus and IL-1β levels were ele-vated in hippocampus compared to cortical samples. A series of electrophoretic mobility shift assays (EMSA) also demonstrated that whole brain radiation significantly increased acti-vation of pro-oxidative and pro-inflammatory transcription fac-tors including AP-1, NF-κB, and CREB. (Raju et al., 2000; Lee et al., 2010b). Furthermore, both in vitro and in vivo studies showed that whole brain radiation-induced pro-inflammatory environments in the brain may be, at least in part, mediated through activation of microglia, suggesting the potential con-tribution of specific type of cells to the overexpression of pro-inflammatory mediators in the brain after radiation (Lee et al., 2010b; Conner et al., 2011). These data provide robust evidence indicating that oxidative stress-mediated inflamma-tion is one of the major consequences of whole brain radiation and plays a pivotal role in subsequent radiation-induced tis-sue injury to normal brain. These studies may contribute to a deeper understanding of the pathophysiological mechanisms responsible for radiation-induced brain injury at the cellular and molecular levels. More importantly, it may provide a foun-dation for the development of novel strategies for prevention and treatment of radiation-induced brain injury specifically tar-geted against pro-oxidative and pro-inflammatory pathways.
In contrast, acute immune responses in cancer patients un-dergoing radiation therapy may have positive effects. Sepah and Bower (2009) detected higher levels of pro-inflammatory cytokines, such as IL-1β and IL-6, in early-stage breast and prostate cancer patients after radiation treatment, suggesting that the acute inflammatory responses may facilitate normal tissue repair processes. It is well known that aging is an im-portant prognostic factor in determining the response of brain tumors to radiation therapy (Flowers, 2000; Schindler et al., 2008). Clinical studies have shown that the use of radiation therapy for treatment of malignant gliomas resulted in signifi-cantly lower survival rates for patients older than 70 years of age compared with those for patients aged 70 and younger (Peschel et al., 1993; Villà et al., 1998). In addition, patients aged 50 or under survived longer than patients over 50 after radiation therapy due to inherent differences in the sensitiv-ity of clonogenic cells to radiation (Rosenblum et al., 1982). These studies clearly indicate that aging exerts a profound effect on the efficacy of radiation therapy for treatment of brain tumors. Although it is generally believed that the immune re-sponses and the effectiveness of radiation therapy decline with age, the association among aging, inflammation, and ra-diation therapy remains to be further investigated. Our recent data demonstrated that radiation-induced acute inflamma-tory responses, such as overexpression of pro-inflammatory cytokines (e.g., TNF-α, IL-1β, and IL-6), adhesion molecules (e.g., ICAM-1, VCAM-1, and E-selectin), chemokine (e.g.,MCP-1), and matrix metalloproteinases (e.g., MMP-9), were significantly impaired in aged brain (Lee et al., 2010a). The impaired response to whole brain radiation with age appears to reveal a generalized attenuation of the cellular response to damage and a reduced capacity of aging tissues to induce essential repair systems necessary for cellular maintenance. Additionally, these data contribute to a better understanding of age-dependent changes in radiation-mediated immune and inflammatory responses in brain and may lead to the develop-ment of effective treatment strategies for brain tumor patients who are undergoing radiation therapy.
Since the induction of both pro-oxidative and pro-inflammatory
pathways in brain plays crucial roles in the pathophysi-ological mechanisms of radiation-mediated brain injury, thera-pies selectively targeting these pathways have shown great potentials in protecting the brain from damages (Table 4). Indeed, a variety of therapeutics with antioxidant activity has been identified as radioprotectors against brain injury. Human clinical study revealed significant improvements in global cog-nitive ability, memory, and executive function among patients with nasopharyngeal carcinoma who received α-tocopherol, the most biologically active form of vitamin E and a fat-sol-uble antioxidant, for 1 year after radiation therapy (Chan et al., 2004). Pre-treatment with α-lipoic acid, a widely available over-the-counter nutritional supplement in the United States, prior to whole body x-ray irradiation resulted in a significant neuroprotection by attenuating oxidative stress in cerebellum and recovering cognitive dysfunction in irradiated mice (Man-da et al., 2007). Additionally, radioprotective actions of melato-nin (N-acetyl-5-methoxytryptamine), a naturally occurring hor-mone with powerful antioxidant property, have demonstrated the potential clinical use for prevention of oxidative stress-me-diated brain damage induced by ionizing radiation (Erol et al., 2004; Undeger et al., 2004; Shirazi et al., 2007). Recent study also showed that pre-administration of the methanol extract of Vernonia amygdalina leaf, a well-known for its antioxidant ac-tivity, significantly mitigated the radiation-induced gross mor-phometry changes in rat brain, such as reduction of the relative weight of the whole brain, relative weight of the cerebellum, the maximum width, rostrocaudal dimension, and dorsoven-tral extent of the cerebellum (Owoeye et al., 2011). In addi-tion to antioxidants, anti-inflammatory approaches have been employed as therapeutic strategies to radiation-induced brain injury. Monje et al. (2003) showed that inflammatory blockade with indomethacin, one of the most common NSAIDs, restored the imapired neurogenesis caused by cranial irradiation. Ad-ministration of another anti-inflammatory drug pioglitazone, a peroxisomal proliferator-activated receptor (PPAR) agonist, prior to, during, and up to 4- or 54-weeks after fractionated whole brain radaition significantly recovered the radiation-induced cognitive impairment in rats (Zhao et al., 2007; Ra-manan et al., 2010). Moreover, treatment of rats with ramipril, one of the angiotensin-converting enzyme (ACE) inhibitors with anti-inflammatory activity, significantly ameriolated radia-tion-induced brain damage (Kim et al., 2004; Ryu et al., 2007; Jenrow et al., 2010). Furthermore, chronic administration of atorvastatin, a member of drug class known as statins which have also shown to possess antioxidant and anti-inflammatory properties (Kim et al., 2008), and ramipril exhibited combined protective effects against radiation-induced impairment of hip-pocampal neurogenesis in rats (Jenrow et al., 2011). These studies provide compelling evidence that pharmacological strategies designed to selectively target oxidative stress- and inflammation-dependent pathways in brain could reduce radi-ation-induced damages to normal brain tissue.
Table 4.
Therapeutic targets against whole brain radiation-induced cognitive impairment
| Target pathway | Therapeutics | Mechanisms of action | References |
|---|---|---|---|
| Oxidative stress | • α-Tocopherol, α-lipoic acid, melato-nin, bitter leaf extract | Antioxidant properties | Chan et al. (2004), Erol et al. (2004), Manda et al. (2007), Owoeye et al. (2011) |
| • Cu(II), Mn(IV), V(IV) 2-methyl-ami-nopyridine complexes | SOD mimetic activities | Abou-Seif et al. (2003) | |
| • EUK-207, EUK-451 | SOD/catalase mimetic activities | Vorotnikova et al. (2010) | |
| Inflammation | • Indomethacin | NSAIDs | Monje et al. (2003) |
| • Ramipril | Anti-inflammatory ACE inhibitor | Jenrow et al. (2010), Kim et al. (2004) | |
| • Pioglitazone | Anti-inflammatory PPARγ agonist | Zhao et al. (2007) | |
| • Fenofibrate | Anti-inflammatory PPARα agonist | Ramanan et al. (2009) | |
| • L-165041 | Anti-inflammatory PPARδ agonist | Schnegg et al. (2012) | |
| • Atorvastatin | Anti-inflammatory statins | Jenrow et al. (2011) | |
| • Tamoxifen | Anti-inflammatory activity | Liu et al. (2010a) | |
| Physiological angiogenesis | • Hypoxia | Recovery of vessel rarefaction | Warrington et al. (2012) |
| • Gammaphos | Prevention of endothelial cell loss | Lyubimova and Hopewell (2004) | |
| • Bevacizumab | Reduction of capillary leakage | Gonzalez et al. (2007) | |
| Neurogenesis | • Human embryonic stem cells | Delivery of stem/precursor cells | Acharya et al. (2009) |
| • Human neural stem cells | Replacement of neural stem cells | Acharya et al. (2011) | |
SOD: Superoxide dismutase, NSAIDs: Non-steroidal anti-inflammatory drugs, ACE: Angiotensin-converting enzyme, PPAR: Peroxisomal proliferator-activated receptor.
Radiation therapy and extracellular matrix in brain
The BBB is a complex neuroprotective system consist-ing of brain microvascular endothelial cells, astrocytes, peri-cytes, and basement membrane (Rubin and Staddon, 1999). It provides a highly selective barrier that tightly regulates the exchange of materials and cells between the circulation and brain tissue (Abbott et al., 2006). Under physiological condi-tions, the BBB restricts and controls the movement of various chemical substances and macromolecules to maintain the brain homeostasis that is essential for the normal operation of the nervous system (Banerjee and Bhat, 2007). In some cases, however, the BBB becomes disrupted or modified as a consequence of various pathological insults (Banerjee and Bhat, 2007). Indeed, alteration or disruption of the BBB is commonly found in patients with neurological disorders, such as stroke, traumatic brain injury (TBI), AD, PD, and HIV-1 de-mentia (Staddon et al., 1995; Rubin and Staddon, 1999; To-borek et al., 2005; Banerjee and Bhat, 2007).
Studies have shown that alterations in the BBB may be responsible for injury to the normal brain tissue after radia-tion therapy (Diserbo et al., 2002; Nordal and Wong, 2005). For example, radiation mediates disruption of the BBB by damaging the structural and functional integrity of the micro-vasculature in brain (Baker and Krochak, 1989; Rubin et al.,1994). In addition, Delattre et al. (1989) demonstrated that cranial irradiation (CRT) markedly increased regional capillary permeability and capillaries of normal brain tissue are more sensitive to the acute effects of CRT than capillaries found in brain tumors. It was also found that BBB permeability was significantly increased in rat brain after whole brain and whole body irradiation (d’Avella et al., 1992; Diserbo et al., 2002). Furthermore, evidence from other in vivo studies has revealed a rapid increase in BBB breakdown in response to interstitial brachytherapy (Fike et al., 1985; Groothuis et al., 1987; Ber-nstein et al., 1990). The cellular and molecular mechanisms by which radiation induces BBB disruption, however, remain unsolved.
The extracellular matrix (ECM) is a complex of various pro-teins and proteoglycans, including collagens, laminin, fibro-nectin, and tenascin (Paulsson, 1992). Besides acting as a physical barrier to the passage of macromolecules and cells, ECM separates adjacent tissues, provides mechanical sup-port for cell attachment, and serves as a substratum for cell migration and a medium of communication between cells (Rutka et al., 1988; Paulsson, 1992; Tilling et al., 2002). In par-ticular, since ECM proteins are major molecular constituents of the basement membrane and maintain the integrity of the BBB, degradation and consequent rearrangement of ECM are critically involved in the breakdown of the BBB. For example, the injection of bacterial collagenase to rat brain resulted in degradation of ECM, disruption of basement membrane, and an increase in BBB permeability (Rosenberg et al., 1993). In addition, an increased degradation of collagen type IV was found to be significantly associated with BBB disruption in a rat model of bacterial meningitis (Sellner and Leib, 2006) and a mouse model of herpessimplex virus (HSV) encephalitis (Sellner et al., 2006). Tilling et al. (1998) also reported that ECM constituents such as collagen type IV, fibronectin, and laminin significantly increased the transcellular electrical re-sistance of primary brain microvascular endothelial cells in an in vitro model of BBB, indicating that these proteins play an important role in enhancing barrier properties.
The matrix metalloproteinases (MMPs) are a large family of ECM-degrading enzymes and have been implicated in the pathophysiological processes of neurodegenerative diseases by causing BBB disruption (Mun-Bryce and Rosenberg, 1998; Romanic et al., 1998; Strup-Perrot et al., 2005). Indeed, in a variety of physiological and pathological conditions, MMPs be-come activated and play a key role in degradation of the ECM proteins (Planas et al., 2001; Kim and Joh, 2012). Depend-ing on substrate specificity and structural differences, MMPs are subdivided into gelatinases (MMP-2 and -9), collagenases (MMP-1, -8, -13, and -18), stromelysins (MMP-3, -10, and -11), matrilysins (MMP-7 and -26), metalloelastase (MMP-12), and membrane-type (MT) MMPs (MMP-14, -15, -16, -17, -24, and-25) (Romanic et al., 1998; Visse and Nagase, 2003; Strup-Perrot et al., 2005). In particular, the gelatinases MMP-2 and MMP-9, the most commonly investigated MMPs in the CNS, are able to degrade ECM components including collagen type IV which is essential for maintaining BBB integrity (Kim and Joh, 2012). The enzymatic activity of MMPs is regulated by tis-sue inhibitors of metalloproteinases (TIMPs), the endogenous inhibitors with a higher affinity for specific MMPs (Aoudjit et al., 1999; Lukes et al., 1999). For example, TIMP-1 inhibits MMP-9 activity by forming a specific complex with MMP-9, whereas MMP-2 is bound by TIMP-2 (Aoudjit et al., 1999; Wang et al., 2000; Giannelli et al., 2002; Sellner and Leib, 2006). There-fore, a favorable balance of MMPs/TIMPs system plays a piv-otal role in maintaining normal homeostasis in the CNS which is essential for preventing neurological disorders (Gardner and Ghorpade, 2003; Kim and Joh, 2012).
Evidence from in vivo and in vitro studies has demonstrated that MMPs and TIMPs are associated with radiation-induced damage to various tissues. For example, the overexpression of MMP-2 and MMP-9 was observed in lung after thoracic ir-radiation (Yang et al., 2006; Yang et al., 2007). Araya et al. (2001) have reported that radiation causes a significant eleva-tion of MMP-2 production but no effect on TIMP-2 in human airway epithelial cells after irradiation, indicating the balance between MMP-2 and TIMP-2 was in favor of MMP-2 promot-ing proteolysis. Additionally, the use of pelvic radiation therapy for prostate cancer patients resulted in significant increases in MMP-2 and MMP-9 activity in rectal mucosa (Hovdenak et al., 2002). It was also found that abdominal irradiation led to a significant elevation in MMP-2 and MMP-14 levels in rat ileum (Strup-Perrot et al., 2005). Moreover, radiation-mediated up-regulation of MMP-2 expression has been observed in various cell types, including astrocytes, endothelial cells, and epithe-lial cells (Sawaya et al., 1994; Nirmala et al., 2000; Zhao et al., 2004). Furthermore, recent study provides evidence that whole brain radiation differentially regulates MMPs/TIMPs system in brain and an imbalance between MMP-2 activity and TIMP-2 expression may have an important role in the pathogenesis of radiation-induced brain injury by degrading ECM components of the BBB basement membrane (Lee et al., 2012). These findings may contribute to defining a novel cellular and molecular basis for radiation-induced BBB disrup-tion and subsequent brain injury that will lead to new oppor-tunities for preventive and therapeutic interventions for brain tumor patients who are undergoing radiotherapy. Further studi-es, however, are necessary to elucidate the exact mechanis-tic links among MMPs/TIMPs system, ECM degradation, and BBB disruption in brain after whole brain radiation therapy.
Based on previous studies related to the pivotal role of ECM in normal homeostasis in brain, strategies aimed at blocking ECM degradation or modulating MMPs/TIMPs system in brain may be attractive for preventing and/or attenuating radiation-induced brain injury. One potential experimental approach is to administer a series of pharmacological agents that se-lectively inhibit MMPs by different mechanisms of action, including minocycline, simvastatin, AG3340, DPC-A37668, GM6001, PD166793, and Ro-31-9790 (Barnett et al., 2007; Garcia-Alloza et al., 2009; Krishnamurthy et al., 2009), to ani-mal models of whole brain radiation therapy which can lead to identification of novel drugs for prevention and/or treatment for radiation-induced brain injury. However, there are no re-ports demonstrating therapeutic approaches targeting ECM or MMPs/TIMPs system in irradiated brain.
Radiation therapy and physiological angiogenesis in brain
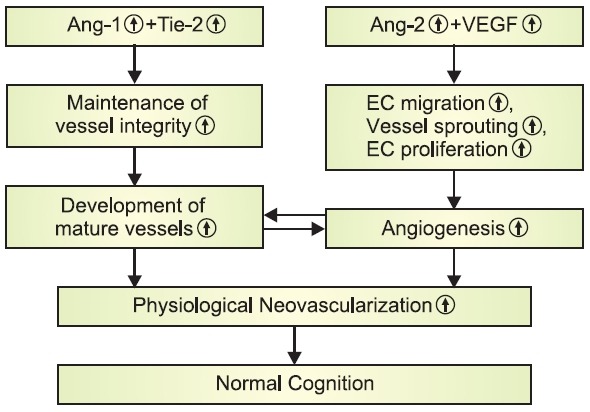
Angiogenesis is the process of developing new blood ves-sels from pre-existing vessels. It has been known to play criti-cal roles not only in many physiological processes such as embryonic development and wound healing, but also in the development of a number of pathological conditions includ-ing progression of tumors. These events are characterized by the dynamic, temporally and spatially coordinated interactions among endothelial cells, angiogenic factors, and ECM proteins (Miller et al., 1994; Hanahan, 1997). One of the most impor-tant and extensively studied angiogenic factors is vascular en-dothelial growth factor (VEGF) which has a potent and specific activity for the vascular endothelium (Ferrara, 1999; Ribatti, 2005; Tammela et al., 2005). VEGF and its receptors serve to initiate endothelial cell proliferation, endothelial cell migration, and production of new capillary sprouts, which promote vascu-logenesis and angiogenesis (Breier et al., 1992; Plate, 1999; Ferrara et al., 2003). VEGF is also considered as a survival factor for endothelial cells by protecting them from apoptosis (Ferrara, 1999; Alavi et al., 2003). In addition to VEGF, angio-poietins are a second family of vascular regulatory molecules that are also specific for the vascular endothelium involving in both physiological and pathological blood vessel genera-tion (Davis et al., 1996). Although angiopoietin-1 (Ang-1) is not directly associated with endothelial cell proliferation (Davis et al., 1996), it mediates interactions between the endothelium and the surrounding matrix, which leads to stimulation of EC migration (Witzenbichler et al., 1998), sprouting (Koblizek et al., 1998), and tubule formation (Hayes et al., 1999). Indeed, Ang-1 is necessary for subsequent vascular remodeling as well as vessel maturation and stabilization, while VEGF plays an active role during the early stages of vessel development (Sato et al., 1995). All angiopoietin families, such as Ang-1,-2, -3, and -4, bind to the endothelial receptor tyrosine kinase (Tie-2) which is typically expressed by vascular endothelial cells (Peters et al., 2004). The balance of Ang-1/Tie-2 system has been known to be necessary for vessel maturation and stabilization (Sato et al., 1995). Ang-2 serves as a functional antagonist of Ang-1. By blocking Tie-2 signaling, Ang-2 leads to a loosening of tight vascular structure (Maisonpierre et al., 1997; Mandriota and Pepper, 1998; Yancopoulos et al., 2000). This loosening of cell-matrix and cell-cell interactions allows the endothelial cells to become more sensitive and respon-sive toward the other angiogenic factors such as VEGF. For example, in the absence of the activating signal from VEGF, Ang-2 promotes endothelial cell death and subsequently leads to rarefaction of vessels. In the presence of high expres-sion levels of VEGF, however, the process of physiological angiogenesis is facilitated by Ang-2 (Mandriota and Pepper, 1998; Yancopoulos et al., 2000). These studies suggest that a dynamic interplay among angiogenic factors, such as Ang-1, Ang-2, Tie-2, and VEGF, plays a key role in regulating various aspects of physiological angiogenesis (Fig. 1).
Fig. 1. Dynamic interaction among Ang-1, Ang-2, Tie-2, and VEGFin the regulation of physiological angiogenesis and cognition.
It is widely believed that radiation-mediated injury to nor-mal tissues including brain is a consequence of acute and late damages to the microvascular endothelium (Dimitrievich et al., 1984;
Baker and Krochak, 1989; Ljubimova et al., 1991; Roth et al., 1999; Nguyen et al., 2000). Several studies have identi-fied microvascular networks as the most sensitive part in re-sponse to the radiation therapy and demonstrated the critical role of microvasculature in the pathogenesis of radiation-in-duced damages to normal tissues. For example, an increased permeability and an irregular proliferation of endothelial cells of microvasculature were observed in irradiated normal tis-sues (Baker and Krochak, 1989). Results from early and late effects of ionizing radiation on the normal tissue microvascu-lar networks showed adverse alterations in the structure and function of microvasculatures such as significant decreases in vessel diameter and capillary surface area, a significant increase in vessel hematocrit, and a significant reduction of blood flow in locally irradiated hamster cremaster muscles (Roth et al., 1999; Nguyen et al., 2000). A number of previ-ous studeis also suggest that radiation-induced early and per-sistent damages to the microvasculature may be responsible for cerebral vessel rarefaction leading to brain injury includ-ing cognitive impairments. Brown et al. (2005) revealed that fractionated whole brain radiation, a clinically relevant regi-men of radiation therapy for brain tumor patients, substantially decreased both vessel density and length in rat brains at 10 weeks post-irradiation. A significant decrease in vessel den-sity in rat brain with cognitive impairment was also observed from 10 weeks to 52 weeks after fractionated whole brain ra-diation, suggesting a potential role for loss of cerebral capillary in radiation-induced dementia (Brown et al., 2007). Recent studies further confirmed the whole brain radiation-induced cerebral microvascular rarefaction and cognitive impairments (Warrington et al., 2011; Warrington et al., 2012). It was also found that a single exposure of rat brain strongly decreased cerebral blood flow (CBF) at 12 and 18 months after radiation (Keyeux et al., 1997).
Recent evidence has demonstrated that the reduction of the number of endothelial cells may be responsible for the radiation-induced decrease in vessel density in brain. For ex-ample, the local irradiation of the rat brain caused a progres-sive and dose-related depletion in endothelial cells in the cho-roid plexus (Calvo et al., 1987). A dose-dependent decrease in endothelial cell number was also observed in rat brain within 24 hours and maintained for up to 1 month after irradiation (Ljubimova et al., 1991). In addition, Lyubimova and Hopewell (2004)
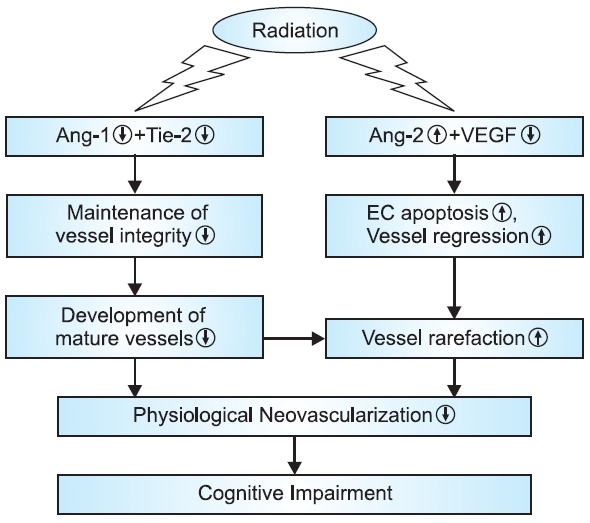
observed the time-dependent changes in endothelial cell number in rat brain for up to 65 weeks after irradiation. The initial marked loss of endothelial cells and the subsequent slow decline in endothelial cell density were detected at 24 hours and between 26 and 52 weeks after irradiation, respectively. These studies clearly indicate that cerebrovascular endothe-lial cells are the primary target cell population in the radiation-induced brain injury. Moreover, a radiation-mediated dose-and time-dependent induction of apoptosis of endothelial cells was observed in mouse central nervous system including spi-nal cord sections and multiple regions of the brain (medulla, pons, and hippocampus), suggesting that radiation-induced loss of endothelial cells in brain is mediated by apoptotic cell death (Peña et al., 2000). Results from our recent study fur-ther confirmed that whole brain radiation significantly reduced endothelial cell density in brain by increasing endothelial cell apoptosis and decreasing endothelial cell proliferation (Lee et al., 2011). A significant decrease in mRNA and protein expres-sion of Ang-1, Tie-2, and VEGF was also detected in irradi-ated rat brains compared with sham-irradiated controls, while whole brain radiation significantly up-regulated Ang-2 mRNA and protein expression (Lee et al., 2011). This study provides evidence for the first time that radiation-mediated differential regulation of various angiogenic factors may be responsible for attenuating physiological angiogenesis resulting in vessel rarefaction in irradiated brain (Fig. 2)
Fig. 2. Effects of whole brain radiation on physiological angiogen-esis and cognition.
Although more detailed mechanisms of radiation-induced vessel rarefaction in brain remain to be further investigated, re-covering cerebrovascular rarefaction by facilitating physiologi-cal angiogenesis in brain sounds a reasonable approach as therapeutic intervention strategy for treatment of radiation-induced brain injury (Table 4). Warrington et al. (2011) assessed the effects of hypoxia as a potential mechanism to reverse the radiation-in-duced microvascular rarefaction and found out that chronic systemic hypoxia was capable of completely restor-ing cerebrovascular density in irradiated animal brain. More importantly, treatment of animals with systemic hypoxia com-pletely reversed whole brain radiation-induced impairments in learning and memory (Warrington et al., 2012). In addition, the radioprotective drug gammaphos (S-2[3-amino propylamino] ethylphosphorothioate) exerted protective effects on cerebro-vascular system though effective prevention of endothelial cell loss in brain (Plotnikova et al., 1984; Plotnikova et al., 1988; Lyubimova and Hopewell, 2004). It was also found that less than 10% of animals receiving gammaphos showed brain in-jury such as necrosis, while approximately 50% of the animals that had not received gammaphos exhibited brain injury by 65 weeks after irradiation (Lyubimova and Hopewell, 2004).
CONCLUSIONS
Whole brain radiation therapy continues to be a main treat-ment modality in the therapeutic management of brain tumors.The clinical use of radiotherapy, however, has been limited by the risk of radiation-mediated damages to normal brain tissue that can eventually cause serious brain injury including cogni-tive impairment. At present, the cellular and molecular mecha-nisms related to the etiology of cognitive impairment that oc-curs among brain tumor patients in response to whole brain radiation therapy remain largely unknown. In this review, we described three pathophysiological mechanisms that whole brain radiation leads to cognitive impairment by (1) triggering induction of pro-oxidative and pro-inflammatory environments in brain, (2) causing imbalance MMPs/TIMPs system and deg-radation of ECM in brain, and (3) alerting physiological angio-genesis through differential regulation of angiogenic factors in brain. These findings may contribute to defining a cellular and molecular basis for radiation-induced cognitive impairment. It will also help identify therapeutic targets for novel preventive and/or treatment strategies for brain tumor patients who suffer from significant clinical side effects after whole brain radiation therapy.
Acknowledgments
This work was supported by Grant Number R01NS056218 from the National Institute of Neurological Disorders and Stroke (NINDS).
References
- 1.Abbott N. J., Rönnbäck L., Hansson E. Astrocyte-endo-thelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. (2006);7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 2.Abou-Seif M. A., El-Naggar M. M., El-Far M., Ramadan M., Salah N. Amelioration of radiation-induced oxidative stress and biochemical alteration by SOD model compounds in pre-treat-ed gamma-irradiated rats. Clin. Chim. Acta. (2003);337:23–33. doi: 10.1016/S0009-8981(03)00192-X. [DOI] [PubMed] [Google Scholar]
- 3.Acharya M. M., Christie L. A., Lan M. L., Donovan P. J., Cotman C. W., Fike J. R., Limoli C. L. Rescue of radiation-induced cognitive impairment through cranial transplantation of human em-bryonic stem cells. Proc. Natl. Acad. Sci. USA. (2009);106:19150–19155. doi: 10.1073/pnas.0909293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acharya M. M., Christie L. A., Lan M. L., Giedzinski E., Fike J. R., Rosi S. L., Imoli C. L. Human neural stem cell trans-plantation ameliorates radiation-induced cognitive dysfunction. Cancer Res. (2011);71:4834–4845. doi: 10.1158/0008-5472.CAN-11-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acharya M. M., Lan M. L., Kan V. H., Patel N. H., Giedzinski E., Tseng B. P., Limoli C. L. Consequences of ionizing radiation-induced damage in human neural stem cells. Free Radic. Biol. Med. (2010);49:1846–1855. doi: 10.1016/j.freeradbiomed.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Akiyama H. Barger S. Barnum S. Bradt B. Bauer J. Cole G. M. Cooper N. R. Eikelenboom P. Emmerling M. Fiebich B. L. Finch C. E. Frautschy S. Griffin W. S. Hampel H. Hull M. Landreth G. Lue L. Mrak R. Mackenzie I. R. McGeer P. L. O'Banion M. K. Pachter J. Pasinetti G. Plata-Salaman C. Rog-ers J. Rydel R. Shen Y. Streit W. Strohmeyer R. Tooyoma I. Van Muiswinkel F. L. Veerhuis R. Walker D. Webster S. We-grzyniak B. Wenk G. Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol. Aging. (2000);21:383–421. doi: 10.1016/S0197-4580(00)00124-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akiyama K., Tanaka R., Sato M., Takeda N. Cognitive dysfunction and histological findings in adult rats one year after whole brain irradiation. Neurol. Med. Chir (Tokyo). (2001);41:590–598. doi: 10.2176/nmc.41.590. [DOI] [PubMed] [Google Scholar]
- 8.Alavi A., Hood J. D., Frausto R., Stupack D. G., Cheresh D. A. Role of Raf in vascular protection from distinct apoptotic stimuli. Science. (2003);301:94–96. doi: 10.1126/science.1082015. [DOI] [PubMed] [Google Scholar]
- 9.American Brain Tumor Association. http://www.abta.org/news/brain-tumor-fact-sheets/ Brain Tumor Facts. (2012)
- 10.Andersen A. P. Postoperative irradiation of glioblastomas. Re-sults in a randomized series. Acta. Radiol. Oncol. Radiat. Phys. Biol. (1978);17:475–484. doi: 10.3109/02841867809128178. [DOI] [PubMed] [Google Scholar]
- 11.Aoudjit F., Masure S., Opdenakker G., Potworowski E. F., St-Pierre Y. Gelatinase B (MMP-9), but not its inhibitor (TIMP-1), dictates the growth rate of experimental thymic lymphoma. Int. J. Cancer. (1999);82:743–747. doi: 10.1002/(SICI)1097-0215(19990827)82:5<743::AID-IJC19>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Araya J., Maruyama M., Sassa K., Fujita T., Hayashi R., Matsui S., Kashii T., Yamashita N., Sugiyama E., Kobayashi M. Ionizing radiation enhances matrix metalloproteinase-2 produc-tion in human lung epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. (2001);280:L30–38. doi: 10.1152/ajplung.2001.280.1.L30. [DOI] [PubMed] [Google Scholar]
- 13.Baker D. G., Krochak R. J. The response of the micro-vascular system to radiation: a review. Cancer Invest. (1989);7:287–294. doi: 10.3109/07357908909039849. [DOI] [PubMed] [Google Scholar]
- 14.Baluna R. G., Eng T. Y., Thomas C. R. Adhesion mol-ecules in radiotherapy. Radiat. Res. (2006);166:819–831. doi: 10.1667/RR0380.1. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee S., Bhat M. A. Neuron-glial interactions in blood-brain barrier formation. Annu. Rev. Neurosci. (2007);30:235–258. doi: 10.1146/annurev.neuro.30.051606.094345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barlind A., Karlsson N., Björk-Eriksson T., Isgaard J., Blomgren K. Decreased cytogenesis in the granule cell layer of the hippocampus and impaired place learning after irradiation of the young mouse brain evaluated using the IntelliCage platform. Exp. Brain Res. (2010);201:781–787. doi: 10.1007/s00221-009-2095-8. [DOI] [PubMed] [Google Scholar]
- 17.Barnett J. M., McCollum G. W., Fowler J. A., Duan J. J., Kay J. D., Liu R. Q., Bingaman D. P., Penn J. S. Pharmacologic and genetic manipulation of MMP-2 and -9 affects retinal neovas-cularization in rodent models of OIR. Invest. Ophthalmol. Vis. Sci. (2007);48:907–915. doi: 10.1167/iovs.06-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Béhin A., Delattre J. Y. Complications of radiation therapy on the brain and spinal cord. Semin. Neurol. (2004);24:405–417. doi: 10.1055/s-2004-861535. [DOI] [PubMed] [Google Scholar]
- 19.Bentzen S. M. Preventing or reducing late side effects of ra-diation therapy: radiobiology meets molecular pathology. Nat. Rev. Cancer. (2006);6:702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 20.Bernstein M., Marotta T., Stewart P., Glen J., Resch L., Henkel-man M. Brain damage from 125I brachytherapy evaluated by MR imaging, a blood-brain barrier tracer, and light and electron microscopy in a rat model. J. Neurosurg. (1990);73:585–593. doi: 10.3171/jns.1990.73.4.0585. [DOI] [PubMed] [Google Scholar]
- 21.Bouloumie A., Marumo T., Lafontan M., Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J. (1999);13:1231–1238. [PubMed] [Google Scholar]
- 22.Breier G., Albrecht U., Sterrer S., Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. (1992);114:521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- 23.Brown W. R., Blair R. M., Moody D. M., Thore C. R., Ahmed S., Robbins M. E., Wheeler K. T. Capillary loss precedes the cognitive impairment induced by fractionated whole-brain irra-diation: a potential rat model of vascular dementia. J. Neurol. Sci. (2007);257:67–71. doi: 10.1016/j.jns.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Brown W. R., Thore C. R., Moody D. M., Robbins M. E., Wheel-er K. T. Vascular damage after fractionated whole-brain ir-radiation in rats. Radiat. Res. (2005);164:662–668. doi: 10.1667/RR3453.1. [DOI] [PubMed] [Google Scholar]
- 25.Bucci M. K., Bevan A., Roach M. 3rd. Advances in radia-tion therapy: conventional to 3D, to IMRT, to 4D, and beyond. CA. Cancer J. Clin. (2005);55:117–134. doi: 10.3322/canjclin.55.2.117. [DOI] [PubMed] [Google Scholar]
- 26.Buckner J. C., Brown P. D., O'Neill B. P., Meyer F. B., Wetmore C. J., Uhm J. H. Central nervous system tumors. Mayo. Clin. Proc. (2007);82:1271–1286. doi: 10.4065/82.10.1271. [DOI] [PubMed] [Google Scholar]
- 27.Calvo W., Hopewell J. W., Reinhold H. S., van den Berg A. P., Yeung T. K. Dose-dependent and time-dependent changes in the choroid plexus of the irradiated rat brain. Br. J. Radiol. (1987);60:1109–1117. doi: 10.1259/0007-1285-60-719-1109. [DOI] [PubMed] [Google Scholar]
- 28.Castro M. G., Cowen R., Williamson I. K., David A., Jimenez-Dalmaroni M. J., Yuan X., Bigliari A., Williams J. C., Hu J., Lowen-stein P. R. Current and future strategies for the treatment of malignant brain tumors. Pharmacol. Ther. (2003);98:71–108. doi: 10.1016/S0163-7258(03)00014-7. [DOI] [PubMed] [Google Scholar]
- 29.Chan A. S., Cheung M. C., Law S. C., Chan J. H. Phase II study of alpha-tocopherol in improving the cognitive function of patients with temporal lobe radionecrosis. Cancer. (2004);100:398–404. doi: 10.1002/cncr.11885. [DOI] [PubMed] [Google Scholar]
- 30.Chandana S. R., Movva S., Arora M., Singh T. Primary brain tumors in adults. Am. Fam. Physician. (2008);77:1423–1430. [PubMed] [Google Scholar]
- 31.Chang E. L., Wefel J. S., Hess K. R., Allen P. K., Lang F. F., Ko-rnguth D. G., Arbuckle R. B., Swint J. M., Shiu A. S., Maor M. H., Meyers C. A. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. (2009);10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 32.Chiang C. S., Hong J. H., Stalder A., Sun J. R., Withers H. R., McBride W. H. Delayed molecular responses to brain ir-radiation. Int. J. Radiat. Biol. (1997);72:45–53. doi: 10.1080/095530097143527. [DOI] [PubMed] [Google Scholar]
- 33.Cohadon F. Indications for surgery in the management of glio-mas. Adv. Tech. Stand. Neurosurg. (1990);17:189–234. doi: 10.1007/978-3-7091-6925-4_6. [DOI] [PubMed] [Google Scholar]
- 34.Collins-Underwood J. R., Zhao W., Sharpe J. G., Robbins M. E. NADPH oxidase mediates radiation-induced oxidative stress in rat brain microvascular endothelial cells. Free Radic. Biol. Med. (2008);45:929–938. doi: 10.1016/j.freeradbiomed.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conner K. R., Forbes M. E., Lee W. H., Lee Y. W., Riddle D. R. AT1 receptor antagonism does not influence early radiation-induced changes in microglial activation or neurogenesis in the normal rat brain. Radiat. Res. (2011);176:71–83. doi: 10.1667/RR2560.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darzy K. H., Pezzoli S. S., Thorner M. O., Shalet S. M. The dynamics of growth hormone (GH) secretion in adult cancer survivors with severe GH defi ciency acquired after brain irradiation in childhood for nonpituitary brain tumors: evidence for preserved pulsatility and diurnal variation with increased secretory disorderli-ness. J. Clin. Endocrinol. Metab. (2005);90:2794–2803. doi: 10.1210/jc.2004-2002. [DOI] [PubMed] [Google Scholar]
- 37.d'Avella D., Cicciarello R., Albiero F., Mesiti M., Gagliardi M. E., Russi E., d'Aquino A., Tomasello F., d'Aquino S. Quantitative study of blood-brain barrier permeability changes after experimental whole-brain radiation. Neurosurgery. (1992);30:30–34. doi: 10.1227/00006123-199201000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Davis S., Aldrich T. H., Jones P. F., Acheson A., Compton D. L., Jain V., Ryan T. E., Bruno J., Radziejewski C., Maisonpierre P. C., Yancopoulos G. D. Isolation of angiopoietin-1, a li-gand for the TIE2 receptor, by secretion-trap expression cloning. Cell. (1996);87:1161–1169. doi: 10.1016/S0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 39.DeAngelis L. M., Delattre J. Y., Posner J. B. Radiation-induced dementia in patients cured of brain metastases. Neurology. (1989);39:789–796. doi: 10.1212/WNL.39.6.789. [DOI] [PubMed] [Google Scholar]
- 40.Delattre J. Y., Shapiro W. R., Posner J. B. Acute effects of low-dose cranial irradiation on regional capillary permeability in experimental brain tumors. J. Neurol. Sci. (1989);90:147–153. doi: 10.1016/0022-510X(89)90097-X. [DOI] [PubMed] [Google Scholar]
- 41.Deng Z., Sui G., Rosa P. M., Zhao W. Radiation-induced c-Jun activation depends on MEK1-ERK1/2 signaling pathway in microglial cells. PLoS. One. (2012);7:e36739. doi: 10.1371/journal.pone.0036739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Denham J. W., Hauer-Jensen M. The radiotherapeutic injury--a complex 'wound'. Radiother. Oncol. (2002);63:129–145. doi: 10.1016/S0167-8140(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 43.Dheen S. T., Kaur C., Ling E. A. Microglial activation and its implications in the brain diseases. Curr. Med. Chem. (2007);14:1189–1197. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- 44.Dimitrievich G. S., Fischer-Dzoga K., Griem M. L. Radio-sensitivity of vascular tissue. I. Differential radiosensitivity of capil-laries: a quantitative in vivo study. Radiat. Res. (1984);99:511–535. doi: 10.2307/3576327. [DOI] [PubMed] [Google Scholar]
- 45.Diserbo M., Agin A., Lamproglou I., Mauris J., Staali F., Multon E., Amourette C. Blood-brain barrier permeability after gamma whole-body irradiation: an in vivo microdialysis study. Can. J. Physiol. Pharmacol. (2002);80:670–678. doi: 10.1139/y02-070. [DOI] [PubMed] [Google Scholar]
- 46.Douw L., Klein M., Fagel S. S., van den Heuvel J., Taphoorn M. J., Aaronson N. K., Postma T. J., Vandertop W. P., Mooij J. J., Boer-man R. H., Beute G. N., Sluimer J. D., Slotman B. J., Reijneveld J. C., Heimans J. J. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. (2009);8:810–818. doi: 10.1016/S1474-4422(09)70204-2. [DOI] [PubMed] [Google Scholar]
- 47.Erol F. S., Topsakal C., Ozveren M. F., Kaplan M., Ilhan N., Ozer-can I. H., Yildiz O. G. Protective effects of melatonin and vitamin E in brain damage due to gamma radiation: an experi-mental study. Neurosurg. Rev. (2004);27:65–69. doi: 10.1007/s10143-003-0291-8. [DOI] [PubMed] [Google Scholar]
- 48.Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr. Top. Microbiol. Immunol. (1999);237:1–30. doi: 10.1007/978-3-642-59953-8_1. [DOI] [PubMed] [Google Scholar]
- 49.Ferrara N., Gerber H. P., LeCouter J. The biology of VEGF and its receptors. Nat. Med. (2003);9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 50.Fiala M., Zhang L., Gan X., Sherry B., Taub D., Graves M. C., Hama S., Way D., Weinand M., Witte M., Lorton D., Kuo Y. M., Roher A. E. Amyloid-beta induces chemokine secre-tion and monocyte migration across a human blood--brain barrier model. Mol. Med. (1998);4:480–489. [PMC free article] [PubMed] [Google Scholar]
- 51.Fike J. R., Cann C. E., Phillips T. L., Bernstein M., Gutin P. H., Turowski K., Weaver K. A., Davis R. L., Higgins R. J., DaSil-va V. Radiation brain damage induced by interstitial 125I sources: a canine model evaluated by quantitative computed tomography. Neurosurgery. (1985);16:530–537. doi: 10.1227/00006123-198504000-00015. [DOI] [PubMed] [Google Scholar]
- 52.Flora G., Lee Y. W., Nath A., Maragos W., Hennig B., Toborek M. Methamphetamine-induced TNF-alpha gene expression and activation of AP-1 in discrete regions of mouse brain: potential role of reactive oxygen intermediates and lipid peroxidation. Neu-romolecular Med. (2002);2:71–85. doi: 10.1385/NMM:2:1:71. [DOI] [PubMed] [Google Scholar]
- 53.Flowers A. Brain tumors in the older person. Cancer Control. (2000);7:523–538. doi: 10.1177/107327480000700604. [DOI] [PubMed] [Google Scholar]
- 54.Fukuda A., Fukuda H., Jönsson M., Swanpalmer J., Hertzman S., Lannering B., Björk-Eriksson T., Màrky I., Blomgren K. Progenitor cell injury after irradiation to the developing brain can be modulated by mild hypothermia or hyperthermia. J. Neurochem. (2005);94:1604–1619. doi: 10.1111/j.1471-4159.2005.03313.x. [DOI] [PubMed] [Google Scholar]
- 55.Gaber M. W., Sabek O. M., Fukatsu K., Wilcox H. G., Kiani M. F., Merchant T. E. Differences in ICAM-1 and TNF-alpha expression between large single fraction and fractionated irradia-tion in mouse brain. Int. J. Radiat. Biol. (2003);79:359–366. doi: 10.1080/0955300031000114738. [DOI] [PubMed] [Google Scholar]
- 56.Garcia-Alloza M., Prada C., Lattarulo C., Fine S., Borrelli L. A., Betensky R., Greenberg S. M., Frosch M. P., Bacskai B. J. Matrix metalloproteinase inhibition reduces oxidative stress associated with cerebral amyloid angiopathy in vivo in transgenic mice. J. Neurochem. (2009);109:1636–1647. doi: 10.1111/j.1471-4159.2009.06096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gardner J., Ghorpade A. Tissue inhibitor of metalloprotein-ase (TIMP)-1: the TIMPed balance of matrix metalloproteinases in the central nervous system. J. Neurosci. Res. (2003);74:801–806. doi: 10.1002/jnr.10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giannelli G., Bergamini C., Marinosci F., Fransvea E., Quaranta M., Lupo L., Schiraldi O., Antonaci S. Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular carcinoma. Int. J. Can-cer. (2002);97:425–431. doi: 10.1002/ijc.1635. [DOI] [PubMed] [Google Scholar]
- 59.Giri R., Selvaraj S., Miller C. A., Hofman F., Yan S. D., Stern D., Zlokovic B. V., Kalra V. K. Effect of endothelial cell polarity on beta-amyloid-induced migration of monocytes across normal and AD endothelium. Am. J. Physiol. Cell Physiol. (2002);283:895–904. doi: 10.1152/ajpcell.00293.2001. [DOI] [PubMed] [Google Scholar]
- 60.Gonzalez J., Kumar A. J., Conrad C. A., Levin V. A. Effect of bevacizumab on radiation necrosis of the brain. Int. J. Radiat. Oncol. Biol. Phys. (2007);67:323–326. doi: 10.1016/j.ijrobp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 61.Graham C. A., Cloughesy T. F. Brain tumor treatment: che-motherapy and other new developments. Semin. Oncol. Nurs. (2004);20:260–272. [PubMed] [Google Scholar]
- 62.Groothuis D. R., Wright D. C., Ostertag C. B. The effect of 125I interstitial radiotherapy on blood-brain barrier function in normal canine brain. J. Neurosurg. (1987);67:895–902. doi: 10.3171/jns.1987.67.6.0895. [DOI] [PubMed] [Google Scholar]
- 63.Grösch S., Kaina B. Transcriptional activation of apurinic/apyrimidinic endonuclease (Ape, Ref-1) by oxidative stress re-quires CREB. Biochem. Biophys. Res. Commun. (1999);261:859–863. doi: 10.1006/bbrc.1999.1125. [DOI] [PubMed] [Google Scholar]
- 64.Hanahan D. Signaling vascular morphogenesis and mainte-nance. Science. (1997);277:48–50. doi: 10.1126/science.277.5322.48. [DOI] [PubMed] [Google Scholar]
- 65.Hayes A. J., Huang W. Q., Mallah J., Yang D., Lippman M. E., Li L. Y. Angiopoietin-1 and its receptor Tie-2 participate in the regulation of capillary-like tubule formation and survival of en-dothelial cells. Microvasc. Res. (1999);58:224–237. doi: 10.1006/mvre.1999.2179. [DOI] [PubMed] [Google Scholar]
- 66.Hegi M. E., Diserens A. C., Gorlia T., Hamou M. F., de Tribolet N., Weller M., Kros J. M., Hainfellner J. A., Mason W., Mariani L., Bromberg J. E., Hau P., Mirimanoff R. O., Cairncross J. G., Jan-zer R. C., Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. (2005);352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 67.Hess K. R. Extent of resection as a prognostic variable in the treatment of gliomas. J. Neurooncol. (1999);42:227–231. doi: 10.1023/A:1006118018770. [DOI] [PubMed] [Google Scholar]
- 68.Hong J. H., Chiang C. S., Campbell I. L., Sun J. R., Withers H. R., McBride W. H. Induction of acute phase gene ex-pression by brain irradiation. Int. J. Radiat. Oncol. Biol. Phys. (1995);33:619–626. doi: 10.1016/0360-3016(95)00279-8. [DOI] [PubMed] [Google Scholar]
- 69.Hovdenak N., Wang J., Sung C. C., Kelly T., Fajardo L. F., Hau-er-Jensen M. Clinical significance of increased gelatinolytic activity in the rectal mucosa during external beam radiation therapy of prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. (2002);53:919–927. doi: 10.1016/S0360-3016(02)02808-0. [DOI] [PubMed] [Google Scholar]
- 70.Jenrow K. A., Brown S. L., Liu J., Kolozsvary A., Lapanowski K., Kim J. H. Ramipril mitigates radiation-induced impairment of neurogenesis in the rat dentate gyrus. Radiat. Oncol. (2010);5:6. doi: 10.1186/1748-717X-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jenrow K. A., Liu J., Brown S. L., Kolozsvary A., Lapanowski K., Kim J. H. Combined atorvastatin and ramipril mitigate radiation-induced impairment of dentate gyrus neurogenesis. J. Neurooncol. (2011);101:449–456. doi: 10.1007/s11060-010-0282-x. [DOI] [PubMed] [Google Scholar]
- 72.Johannesen T. B., Lien H. H., Hole K. H., Lote K. Radio-logical and clinical assessment of long-term brain tumour survivors after radiotherapy. Radiother. Oncol. (2003);69:169–176. doi: 10.1016/S0167-8140(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 73.Kantor G., Laprie A., Huchet A., Loiseau H., Dejean C., Maze-ron J. J. Radiation therapy for glial tumors: technical as-pects and clinical indications. Cancer Radiother. (2008);12:687–694. doi: 10.1016/j.canrad.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 74.Keyeux A., Brucher J. M., Ochrymowicz-Bemelmans D., Charlier A. A. Late effects of X irradiation on regulation of cerebral blood flow after whole-brain exposure in rats. Radiat. Res. (1997);147:621–630. doi: 10.2307/3579629. [DOI] [PubMed] [Google Scholar]
- 75.Khuntia D., Brown P., Li J., Mehta M. P. Whole-brain ra-diotherapy in the management of brain metastasis. J. Clin. Oncol. (2006);24:1295–1304. doi: 10.1200/JCO.2005.04.6185. [DOI] [PubMed] [Google Scholar]
- 76.Kim J. H., Brown S. L., Jenrow K. A., Ryu S. Mechanisms of radiation-induced brain toxicity and implications for future clinical trials. J. Neurooncol. (2008);87:279–286. doi: 10.1007/s11060-008-9520-x. [DOI] [PubMed] [Google Scholar]
- 77.Kim J. H., Brown S. L., Kolozsvary A., Jenrow K. A., Ryu S., Rosen-blum M. L., Carretero O. A. Modification of radiation injury by ramipril, inhibitor of angiotensin-converting enzyme, on optic neuropathy in the rat. Radiat. Res. (2004);161:137–142. doi: 10.1667/RR3124. [DOI] [PubMed] [Google Scholar]
- 78.Kim S. H., Lim D. J., Chung Y. G., Cho T. H., Lim S. J., Kim W. J., Suh J. K. Expression of TNF-alpha and TGF-beta 1 in the rat brain after a single high-dose irradiation. J. Korean Med. Sci. (2002);17:242–248. doi: 10.3346/jkms.2002.17.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim Y. S., Joh T. H. Matrix metalloproteinases, new in-sights into the understanding of neurodegenerative disorders. Bio-mol. Ther. (2012);20:133–143. doi: 10.4062/biomolther.2012.20.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koblize T. I., Weiss C., Yancopoulos G. D., Deutsch U., Risau W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr. Biol. (1998);8:529–532. doi: 10.1016/S0960-9822(98)70205-2. [DOI] [PubMed] [Google Scholar]
- 81.Koo Y. E., Reddy G. R., Bhojani M., Schneider R., Philbert M. A., Rehemtulla A., Ross B. D., Kopelman R. Brain cancer diagnosis and therapy with nanoplatforms. Adv. Drug Deliv. Rev. (2006);58:1556–1577. doi: 10.1016/j.addr.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 82.Krishnamurthy P., Peterson J. T., Subramanian V., Singh M., Singh K. Inhibition of matrix metalloproteinases improves left ventricular function in mice lacking osteopontin after myocardial infarction. Mol. Cell Biochem. (2009);322:53–62. doi: 10.1007/s11010-008-9939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kyrkanides S., Moore A. H., Olschowka J. A., Daeschner J. C., Wil-liams J. P., Hansen J. T., Kerry O'Banion M. Cyclooxy-genase-2 modulates brain inflammation-related gene expression in central nervous system radiation injury. Brain Res. Mol. Brain Res. (2002);104:159–169. doi: 10.1016/s0169-328x(02)00353-4. [DOI] [PubMed] [Google Scholar]
- 84.Lakshminarayanan V., Drab-Weiss E. A., Roebuck K. A. H2O2 and tumor necrosis factor-alpha induce differential binding of the redox-responsive transcription factors AP-1 and NF-kappaB to the interleukin-8 promoter in endothelial and epithelial cells. J. Biol. Chem. (1998);273:32670–32678. doi: 10.1074/jbc.273.49.32670. [DOI] [PubMed] [Google Scholar]
- 85.Lamproglou I., Chen Q. M., Boisserie G., Mazeron J. J., Poisson M., Baillet F., Le Poncin M., Delattre. J. Y. Radiation-induced cognitive dysfunction: an experimental model in the old rat. Int. J. Radiat. Oncol. Biol. Phys. (1995);31:65–70. doi: 10.1016/0360-3016(94)00332-F. [DOI] [PubMed] [Google Scholar]
- 86.Lee W. H., Cho H. J., Sonntag W. E., Lee Y. W. Radiation attenuates physiological angiogenesis by differential expression of VEGF, Ang-1, tie-2 and Ang-2 in rat brain. Radiat. Res. (2011);176:753–760. doi: 10.1667/RR2647.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee W. H., Sonntag W. E., Lee Y. W. Aging attenuates radiation-induced expression of pro-inflammatory mediators in rat brain. Neurosci. Lett. (2010a);476:89–93. doi: 10.1016/j.neulet.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee W. H., Sonntag W. E., Mitschelen M., Yan H, Lee Y. W. Irradiation induces regionally specific alterations in pro-inflammatory environments in rat brain. Int. J. Radiat. Biol. (2010b);86:132–144. doi: 10.3109/09553000903419346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee W. H., Warrington J. P., Sonntag W. E., Lee Y. W. Irradiation alters MMP-2/TIMP-2 system and collagen type IV deg-radation in brain. Int. J. Radiat. Oncol. Biol. Phys. (2012);82:1559–1566. doi: 10.1016/j.ijrobp.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee Y. W., Hennig B., Fiala M., Kim K. S., Toborek M. Cocaine activates redox-regulated transcription factors and induc-es TNF-alpha expression in human brain endothelial cells. Brain Res. (2001a);920:125–133. doi: 10.1016/S0006-8993(01)03047-5. [DOI] [PubMed] [Google Scholar]
- 91.Lee Y. W., Hennig B., Toborek M. Redox-regulated mech-anisms of IL-4-induced MCP-1 expression in human vascular en-dothelial cells. Am. J. Physiol. Heart Circ. Physiol. (2003);284:H185–192. doi: 10.1152/ajpheart.00524.2002. [DOI] [PubMed] [Google Scholar]
- 92.Lee Y. W., Hennig B., Yao J., Toborek M. Methamphet-amine induces AP-1 and NF-kappaB binding and transactivation in human brain endothelial cells. J. Neurosci. Res. (2001b);66:583–591. doi: 10.1002/jnr.1248. [DOI] [PubMed] [Google Scholar]
- 93.Lee Y. W., Kühn H., Hennig B., Neish A. S., Toborek M. IL-4-induced oxidative stress upregulates VCAM-1 gene expres-sion in human endothelial cells. J. Mol. Cell Cardiol. (2001c);33:83–94. doi: 10.1006/jmcc.2000.1278. [DOI] [PubMed] [Google Scholar]
- 94.Lee Y. W., Park H. J., Hennig B., Toborek M. Linoleic acid induces MCP-1 gene expression in human microvascular en-dothelial cells through an oxidative mechanism. J. Nutr. Biochem. (2001d);12:648–654. doi: 10.1016/S0955-2863(01)00186-3. [DOI] [PubMed] [Google Scholar]
- 95.Limoli C. L., Giedzinski E., Rola R., Otsuka S., Palmer T. D., Fike J. R. Radiation response of neural precursor cells: linking cellular sensitivity to cell cycle checkpoints, apoptosis and oxidative stress. Radiat. Res. (2004);161:17–27. doi: 10.1667/RR3112. [DOI] [PubMed] [Google Scholar]
- 96.Liu B., Hong J. S. Role of microglia in inflammation-medi-ated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J. Pharmacol. Exp. Ther. (2003);304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- 97.Liu J. L., Tian D. S., Li Z. W., Qu W. S., Zhan Y., Xie M. J., Yu Z. Y., Wang W., Wu G. Tamoxifen alleviates irradiation-in-duced brain injury by attenuating microglial inflammatory response in vitro and in vivo. Brain Res. (2010a);1316:101–111. doi: 10.1016/j.brainres.2009.12.055. [DOI] [PubMed] [Google Scholar]
- 98.Liu Y., Xiao S., Liu J., Zhou H., Liu Z., Xin Y., Suo W. Z. An experimental study of acute radiation-induced cognitive dys-function in a young rat model. AJNR. Am. J. Neuroradiol. (2010b);31:383–387. doi: 10.3174/ajnr.A1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ljubimova N. V., Levitman M. K., Plotnikova E. D., Eidus LKh. Endothelial cell population dynamics in rat brain after local irradiation. Br. J. Radiol. (1991);64:934–940. doi: 10.1259/0007-1285-64-766-934. [DOI] [PubMed] [Google Scholar]
- 100.Lukes A., Mun-Bryce S., Lukes M., Rosenberg G. A. Ex-tracellular matrix degradation by metalloproteinases and central nervous system diseases. Mol. Neurobiol. (1999);19:267–284. doi: 10.1007/BF02821717. [DOI] [PubMed] [Google Scholar]
- 101.Lyubimova N., Hopewell J. W. Experimental evidence to support the hypothesis that damage to vascular endothelium plays the primary role in the development of late radiation-induced CNS injury. Br. J. Radiol. (2004);77:488–492. doi: 10.1259/bjr/15169876. [DOI] [PubMed] [Google Scholar]
- 102.Maisonpierre P. C., Suri C., Jones P. F., Bartunkova S., Wiegand S. J., Radziejewski C., Compton D., McClain J., Aldrich T. H., Papa-dopoulos N., Daly T. J., Davis S., Sato T. N., Yancopoulos G. D. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. (1997);277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 103.Manda K., Ueno M., Moritake T., Anzai K. Radiationin-duced cognitive dysfunction and cerebellar oxidative stress in mice: protective effect of alpha-lipoic acid. Behav. Brain Res. (2007);177:7–14. doi: 10.1016/j.bbr.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 104.Mandriota S. J., Pepper M. S. Regulation of angiopoietin-2 mRNA levels in bovine microvascular endothelial cells by cytokines and hypoxia. Circ. Res. (1998);83:852–859. doi: 10.1161/01.RES.83.8.852. [DOI] [PubMed] [Google Scholar]
- 105.McGeer P. L., McGeer E. G. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res. Brain Res. Rev. (1995);21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 106.McGeer P. L., Yasojima K., McGeer E. G. Inflammation in Parkinson's disease. Adv. Neurol. (2001);86:83–89. [PubMed] [Google Scholar]
- 107.Miller J. W., Adamis A. P., Shima D. T., D'Amore P. A., Moulton R. S., O'Reilly M. S., Folkman J., Dvorak H. F., Brown L. F., Berse B., et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am. J. Pathol. (1994);145:574–584. [PMC free article] [PubMed] [Google Scholar]
- 108.Monje M. L., Toda H., Palmer T. D. Inflammatory block-ade restores adult hippocampal neurogenesis. Science. (2003);302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 109.Moore A. H., Olschowka J. A., Williams J. P., Okunieff P., O'Banion M. K. Regulation of prostaglandin E2 synthesis after brain irradiation. Int. J. Radiat. Oncol. Biol. Phys. (2005);62:267–272. doi: 10.1016/j.ijrobp.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 110.Moulder J. E., Cohen E. P. Future strategies for mitiga-tion and treatment of chronic radiation-induced normal tissue injury. Semin. Radiat. Oncol. (2007);17:141–148. doi: 10.1016/j.semradonc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 111.Mun-Bryce S., Rosenberg G. A. Matrix metalloproteinases in cerebrovascular disease. J. Cereb. Blood Flow. Metab. (1998);18:1163–1172. doi: 10.1097/00004647-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 112.Mut M. Surgical treatment of brain metastasis: a review. Clin. Neurol. Neurosurg. (2012);114:1–8. doi: 10.1016/j.clineuro.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 113.National Brain Tumor Society. http://www.braintumor.org/news/press-kit/brain-tumor-facts.html Brain Tumor Quick Facts. (2012)
- 114.New P. Radiation injury to the nervous system. Curr. Opin. Neurol. (2001);14:725–734. doi: 10.1097/00019052-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 115.Nguyen V., Gaber M. W., Sontag M. R., Kiani M. F. Late effects of ionizing radiation on the microvascular networks in nor-mal tissue. Radiat. Res. (2000);154:531–536. doi: 10.1667/0033-7587(2000)154[0531:LEOIRO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 116.Nirmala C., Jasti S. L., Sawaya R., Kyritsis A. P., Konduri S. D., Ali-Osman F., Rao J. S., Mohanam S. Effects of radiation on the levels of MMP-2, MMP-9 and TIMP-1 during morphogenic glial-endothelial cell interactions. Int. J. Cancer. (2000);88:766–771. doi: 10.1002/1097-0215(20001201)88:5<766::AID-IJC13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 117.Nordal R. A., Wong C. S. Molecular targets in radiation-induced blood-brain barrier disruption. Int. J. Radiat. Oncol. Biol. Phys. (2005);62:279–287. doi: 10.1016/j.ijrobp.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 118.Olschowka J. A., Kyrkanides S., Harvey B. K., O'Banion M. K., Williams J. P., Rubin P., Hansen J. T. ICAM-1 induction in the mouse CNS following irradiation. Brain Behav. Immun. (1997);11:273–285. doi: 10.1006/brbi.1997.0506. [DOI] [PubMed] [Google Scholar]
- 119.Owoeye O., Farombi E. O., Onwuka S. K. Gross morphometric reduction of rats' cerebellum by gamma irradiation was mitigated by pretreatment with Vernonia amygdalina leaf extract. Rom. J. Morphol. Embryol. (2011);52:81–88. [PubMed] [Google Scholar]
- 120.Park H. J., Lee Y. W., Hennig B., Toborek M. Linoleic acid-induced VCAM-1 expression in human microvascular endo-thelial cells is mediated by the NF-kappa B-dependent pathway. Nutr. Cancer. (2001);41:126–134. doi: 10.1080/01635581.2001.9680623. [DOI] [PubMed] [Google Scholar]
- 121.Paulsson M. Basement membrane proteins: structure, ass-embly, and cellular interactions. Crit. Rev. Biochem. Mol. Biol. (1992);27:93–127. doi: 10.3109/10409239209082560. [DOI] [PubMed] [Google Scholar]
- 122.Peña L. A., Fuks Z., Kolesnick R. N. Radiation-induced apoptosis of endothelial cells in the murine central nervous sys-tem: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res. (2000);60:321–327. [PubMed] [Google Scholar]
- 123.Peschel R. E., Wilson L., Haffty B., Papadopoulos D., Rosenzweig K., Feltes M. The effect of advanced age on the ef-ficacy of radiation therapy for early breast cancer, local prostate cancer and grade III-IV gliomas. Int. J. Radiat. Oncol. Biol. Phys. (1993);26:539–544. doi: 10.1016/0360-3016(93)90973-Y. [DOI] [PubMed] [Google Scholar]
- 124.Peters K. G., Kontos C. D., Lin P. C., Wong A. L., Rao P., Huang L., Dewhirst M. W., Sankar S. Functional significance of Tie2 signaling in the adult vasculature. Recent. Prog. Horm. Res. (2004);59:51–71. doi: 10.1210/rp.59.1.51. [DOI] [PubMed] [Google Scholar]
- 125.Planas A. M., Solé S., Justicia C. Expression and activa-tion of matrix metalloproteinase-2 and -9 in rat brain after transient focal cerebral ischemia. Neurobiol. Dis. (2001);8:834–846. doi: 10.1006/nbdi.2001.0435. [DOI] [PubMed] [Google Scholar]
- 126.Plate K. H. Mechanisms of angiogenesis in the brain. J. Neuropathol. Exp. Neurol. (1999);58:313–320. doi: 10.1097/00005072-199904000-00001. [DOI] [PubMed] [Google Scholar]
- 127.Plotnikova E. D., Levitman M. K., Shaposhnikova V. V., Koshevoy J. V., Eidus L. K. Protection of microcirculation in rat brain against late radiation injury by gammaphos. Int. J. Radiat. Oncol.Biol. Phys. (1984);10:365–368. doi: 10.1016/0360-3016(84)90055-5. [DOI] [PubMed] [Google Scholar]
- 128.Plotnikova E. D., Levitman M. K., Shaposhnikova V. V., Koshevoj J. V., Eidus L. K. Protection of microvasculature in rat brain against late radiation injury by gammaphos. Int. J. Radiat. Oncol. Biol. Phys. (1988);15:1197–1201. doi: 10.1016/0360-3016(88)90204-0. [DOI] [PubMed] [Google Scholar]
- 129.Quik E. H., Valk G. D., Drent M. L., Stalpers L. J., Kenemans J. L., Koppeschaar H. P., Dam P. S. Reduced growth hor-mone secretion after cranial irradiation contributes to neurocogni-tive dysfunction. Growth Horm. IGF. Res. (2012);22:42–47. doi: 10.1016/j.ghir.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 130.Raju U., Gumin G. J., Tofilon P. J. Radiation-induced tran-scription factor activation in the rat cerebral cortex. Int. J. Radiat. Biol. (2000);76:1045–1053. doi: 10.1080/09553000050111514. [DOI] [PubMed] [Google Scholar]
- 131.Ramanan S., Kooshki M., Zhao W., Hsu F. C., Riddle D. R., Robbins M. E. The PPARalpha agonist fenofibrate pre-serves hippocampal neurogenesis and inhibits microglial activation after whole-brain irradiation. Int. J. Radiat. Oncol. Biol. Phys. (2009);75:870–877. doi: 10.1016/j.ijrobp.2009.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ramanan S., Zhao W., Riddle D. R., Robbins M. E. Role of PPARs in Radiation-Induced Brain Injury. PPAR. Res. (2010);2010:234975. doi: 10.1155/2010/234975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rampling R., James A., Papanastassiou V. The present and future management of malignant brain tumours: surgery, radio-therapy, chemotherapy. J. Neurol. Neurosurg. Psychiatry. (2004);75(Suppl 2):24–30. doi: 10.1136/jnnp.2004.040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ribatti D. The crucial role of vascular permeability factor/vas-cular endothelial growth factor in angiogenesis: a historical review. Br. J. Haematol. (2005);128:303–309. doi: 10.1111/j.1365-2141.2004.05291.x. [DOI] [PubMed] [Google Scholar]
- 135.Rola R., Raber J., Rizk A., Otsuka S., VandenBerg S. R., Morhardt D. R., Fike J. R. Radiation-induced impairment of hip-pocampal neurogenesis is associated with cognitive deficits in young mice. Exp. Neurol. (2004);188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 136.Roman D. D., Sperduto P. W. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int. J. Radiat. Oncol. Biol. Phys. (1995);31:983–998. doi: 10.1016/0360-3016(94)00550-8. [DOI] [PubMed] [Google Scholar]
- 137.Romanic A. M., White R. F., Arleth A. J., Ohlstein E. H., Barone F. C. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloprotein-ase-9 reduces infarct size. Stroke. (1998);29:1020–1030. doi: 10.1161/01.STR.29.5.1020. [DOI] [PubMed] [Google Scholar]
- 138.Rosenberg G. A., Estrada E., Kelley R. O., Kornfeld M. Bacterial collagenase disrupts extracellular matrix and opens blood-brain barrier in rat. Neurosci. Lett. (1993);160:117–119. doi: 10.1016/0304-3940(93)90927-D. [DOI] [PubMed] [Google Scholar]
- 139.Rosenblum M. L., Gerosa M., Dougherty D. V., Reese C., Barger G. R., Davis R. L., Levin V. A., Wilson C. B. Age-re-lated chemosensitivity of stem cells from human malignant brain tumours. Lancet. (1982);1:885–887. doi: 10.1016/s0140-6736(82)92154-7. [DOI] [PubMed] [Google Scholar]
- 140.Roth N. M., Sontag M. R., Kiani M. F. Early effects of ionizing radiation on the microvascular networks in normal tissue. Radiat. Res. (1999);151:270–277. doi: 10.2307/3579938. [DOI] [PubMed] [Google Scholar]
- 141.Rubin L. L., Staddon J. M. The cell biology of the blood-brain barrier. Annu. Rev. Neurosci. (1999);22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- 142.Rubin P., Gash D. M., Hansen J. T., Nelson D. F., Williams J. P. Disruption of the blood-brain barrier as the primary effect of CNS irradiation. Radiother. Oncol. (1994);31:51–60. doi: 10.1016/0167-8140(94)90413-8. [DOI] [PubMed] [Google Scholar]
- 143.Rutka J. T., Apodaca G., Stern R., Rosenblum M. The extracellular matrix of the central and peripheral nervous systems: structure and function. J. Neurosurg. (1988);69:155–170. doi: 10.3171/jns.1988.69.2.0155. [DOI] [PubMed] [Google Scholar]
- 144.Ryu S., Kolozsvary A., Jenrow K. A., Brown S. L., Kim J. H. Mitigation of radiation-induced optic neuropathy in rats by ACE inhibitor ramipril: importance of ramipril dose and treatment time. J. Neurooncol. (2007);82:119–124. doi: 10.1007/s11060-006-9256-4. [DOI] [PubMed] [Google Scholar]
- 145.Sara M., Claudio F., Marco L., Roberto L., Elena M., Micaela M., Lucia P., Elena B., Marina S., Michele R. Time course of hypothalamic-pituitary deficiency in adults receiving cranial ra-diotherapy for primary extrasellar brain tumors. Radiother. Oncol. (2011);99:23–28. doi: 10.1016/j.radonc.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 146.Sato T. N., Tozawa Y., Deutsch U., Wolburg-Buchholz K., Fujiwara Y., Gendron-Maguire M., Gridley T., Wolburg H.,, Risau W., Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. (1995);376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 147.Sawaya R., Tofilon P. J., Mohanam S., Ali-Osman F., Liotta L. A., Stetler-Stevenson W. G., Rao J. S. Induction of tissue-type plasminogen activator and 72-kDa type-IV collagenase by ion-izing radiation in rat astrocytes. Int. J. Cancer. (1994);56:214–218. doi: 10.1002/ijc.2910560212. [DOI] [PubMed] [Google Scholar]
- 148.Schindler M. K., Forbes M. E., Robbins M. E., Riddle. D. R. Aging-dependent changes in the radiation response of the adult rat brain. Int. J. Radiat. Oncol. Biol. Phys. (2008);70:826–834. doi: 10.1016/j.ijrobp.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schnegg C. I., Kooshki M., Hsu F. C., Sui G., Robbins M. E. PPARδ prevents radiation-induced proinflammatory re-sponses in microglia via transrepression of NF-κB and inhibition of the PKCα/MEK1/2/ERK1/2/AP-1 pathway. Free Radic. Biol. Med. (2012);52:1734–1743. doi: 10.1016/j.freeradbiomed.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schultheiss T. E., Stephens L. C. Invited review: perma-nent radiation myelopathy. Br. J. Radiol. (1992);65:737–753. doi: 10.1259/0007-1285-65-777-737. [DOI] [PubMed] [Google Scholar]
- 151.Schulz J. B., Falkenburger B. H. Neuronal pathology in Parkinson's disease. Cell Tissue Res. (2004);318:135–147. doi: 10.1007/s00441-004-0954-y. [DOI] [PubMed] [Google Scholar]
- 152.Sellner J., Leib S. L. In bacterial meningitis cortical brain damage is associated with changes in parenchymal MMP-9/TIMP-1 ratio and increased collagen type IV degradation. Neurobiol. Dis. (2006);21:647–656. doi: 10.1016/j.nbd.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 153.Sellner J., Simon F., Meyding-Lamade U., Leib S. L. Her-pes-simplex virus encephalitis is characterized by an early MMP-9 increase and collagen type IV degradation. Brain Res. (2006);1125:155–162. doi: 10.1016/j.brainres.2006.09.093. [DOI] [PubMed] [Google Scholar]
- 154.Sepah S. C., Bower J. E. Positive affect and inflammation during radiation treatment for breast and prostate cancer. Brain Be-hav.Immun. (2009);23:1068–1072. doi: 10.1016/j.bbi.2009.06.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sheline G. E., Wara W. M., Smith V. Therapeutic irradia-tion and brain injury. Int. J. Radiat. Oncol. Biol. Phys. (1980);6:1215–1228. doi: 10.1016/0360-3016(80)90175-3. [DOI] [PubMed] [Google Scholar]
- 156.Shi L., Adams M. M., Long A., Carter C. C., Bennett C., Sonntag W. E., Nicolle M. M., Robbins M., D'Agostino R., Brunso-Bechtold J. K. Spatial learning and memory deficits after whole-brain irradiation are associated with changes in NMDA re-ceptor subunits in the hippocampus. Radiat. Res. (2006);166:892–899. doi: 10.1667/RR0588.1. [DOI] [PubMed] [Google Scholar]
- 157.Shirazi A., Ghobadi G., Ghazi-Khansari M. A radiobiologi-cal review on melatonin: a novel radioprotector. J. Radiat. Res. (2007);48:263–272. doi: 10.1269/jrr.06070. [DOI] [PubMed] [Google Scholar]
- 158.Simon A. R., Rai U., Fanburg B. L., Cochran B. H. Activa-tion of the JAK-STAT pathway by reactive oxygen species. Am. J. Physiol. (1998);275:1640–1652. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- 159.Simpson J. R., Horton J., Scott C., Curran W. J., Rubin P., Fisch-bach J., Isaacson S., Rotman M., Asbell S. O., Nelson J. S. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int. J. Radiat. Oncol. Biol. Phys. (1993);26:239–244. doi: 10.1016/0360-3016(93)90203-8. [DOI] [PubMed] [Google Scholar]
- 160.Staddon J. M., Herrenknecht K., Schulze C., Smales C., Ru-bin L. L. Signal transduction at the blood-brain barrier. Biochem. Soc. Trans. (1995);23:475–479. doi: 10.1042/bst0230475. [DOI] [PubMed] [Google Scholar]
- 161.Stone H. B., Coleman C. N., Anscher M. S., McBride W. H. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. (2003);4:529–536. doi: 10.1016/S1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 162.Stone H. B., Moulder J. E., Coleman C. N., Ang K. K., Anscher M. S., Barcellos-Hoff M. H., Dynan W. S., Fike J. R., Grdina D. J., Greenberger J. S., Hauer-Jensen M., Hill R. P., Kolesnick R. N., Macvittie T. J., Marks C., McBride W. H., Metting N., Pellmar T., Purucker M., Robbins M. E., Schiestl R. H., Seed T. M., To-maszewski J. E., Travis E. L., Wallner P. E., Wolpert M., Za-harevitz D. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop, December 3-4, 2003. Radiat. Res. (2004);162:711–728. doi: 10.1667/RR3276. [DOI] [PubMed] [Google Scholar]
- 163.Strup-Perrot C., Vozenin-Brotons M. C., Vandamme M., Linard C., Mathé D. Expression of matrix metalloproteinases and tissue inhibitor metalloproteinases increases in X-irradiated rat il-eum despite the disappearance of CD8a T cells. World J. Gastro-enterol. (2005);11:6312–6321. doi: 10.3748/wjg.v11.i40.6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups, National Cancer Institute of Canada Clinical Trials Group. Stupp R., Mason W. P., van den Bent M. J., Weller M., Fisher B., Taphoorn M. J., Belanger K., Brandes A. A., Marosi C., Bogdahn U., Curschmann J., Janzer R. C., Ludwin S. K., Gorlia T., All-geier A., Lacombe D., Cairncross J. G., Eisenhauer E., Miri-manoff R. O. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. (2005);352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 165.Suo Z., Tan J., Placzek A., Crawford F., Fang C., Mullan M. Alzheimer's beta-amyloid peptides induce inflammatory cascade in human vascular cells: the roles of cytokines and CD40. Brain Res. (1998);807:110–117. doi: 10.1016/S0006-8993(98)00780-X. [DOI] [PubMed] [Google Scholar]
- 166.Tammela T., Enholm B., Alitalo K., Paavonen K. The biol-ogy of vascular endothelial growth factors. Cardiovasc. Res. (2005);65:550–563. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 167.Teismann P., Tieu K., Choi D. K., Wu D. C., Naini A., Hunot S., Vila M., Jackson-Lewis V., Przedborski S. Cyclooxygen-ase-2 is instrumental in Parkinson's disease neurodegeneration. Proc. Natl. Acad. Sci. USA. (2003);100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tilling T., Engelbertz C., Decker S., Korte D., Hüwel S., Galla H. J. Expression and adhesive properties of basement mem-brane proteins in cerebral capillary endothelial cell cultures. Cell Tissue Res. (2002);310:19–29. doi: 10.1007/s00441-002-0604-1. [DOI] [PubMed] [Google Scholar]
- 169.Tilling T., Korte D., Hoheisel D., Galla H. J. Basement membrane proteins influence brain capillary endothelial barrier function in vitro. J. Neurochem. (1998);71:1151–1157. doi: 10.1046/j.1471-4159.1998.71031151.x. [DOI] [PubMed] [Google Scholar]
- 170.Toborek M., Lee Y. W., Flora G., Pu H., András I. E., Wylegala E., Hennig B., Nath A. Mechanisms of the blood-brain bar-rier disruption in HIV-1 infection. Cell Mol. Neurobiol. (2005);25:181–199. doi: 10.1007/s10571-004-1383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tofilon P. J., Fike J. R. The radioresponse of the central nervous system: a dynamic process. Radiat. Res. (2000);153:357–370. doi: 10.1667/0033-7587(2000)153[0357:TROTCN]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 172.Supportive Care Guidelines Group of Cancer Care Ontario|s Program in Evidence-based Care. Tsao M. N., Lloyd N. S., Wong R. K., Rakovitch E., Chow E., Laperriere N. Radiothera-peutic management of brain metastases: a systematic review and meta-analysis. Cancer Treat. Rev. (2005);31:256–273. doi: 10.1016/j.ctrv.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 173.Undeger U., Giray B., Zorlu A. F., Oge K., Baçaran N. Protective effects of melatonin on the ionizing radiation induced DNA damage in the rat brain. Exp. Toxicol. Pathol. (2004);55:379–384. doi: 10.1078/0940-2993-00332. [DOI] [PubMed] [Google Scholar]
- 174.Verhasselt V., Goldman M., Willems F. Oxidative stress up-regulates IL-8 and TNF-alpha synthesis by human dendritic cells. Eur. J. Immunol. (1998);28:3886–3890. doi: 10.1002/(SICI)1521-4141(199811)28:11<3886::AID-IMMU3886>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 175.Villà S., Viñolas N., Verger E., Yaya R., Martínez A., Gil M., More-no V., Caral L., Graus F. Efficacy of radiotherapy for malignant gliomas in elderly patients. Int. J. Radiat. Oncol. Biol. Phys. (1998);42:977–980. doi: 10.1016/S0360-3016(98)00356-3. [DOI] [PubMed] [Google Scholar]
- 176.Visse R., Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochem-istry. Circ. Res. (2003);92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 177.Vorotnikova E., Rosenthal R. A., Tries M., Doctrow S. R., Braun-hut S. J. Novel synthetic SOD/catalase mimetics can miti-gate capillary endothelial cell apoptosis caused by ionizing radia-tion. Radiat. Res. (2010);173:748–759. doi: 10.1667/RR1948.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Walker M. D., Alexander E. Jr., Hunt W. E., MacCarty C. S., Mahaley M. S. Jr., Mealey J. Jr., Norrell H. A., Owens G., Ransohoff J., Wilson C. B., Gehan E. A., Strike T. A. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J. Neurosurg. (1978);49:333–343. doi: 10.3171/jns.1978.49.3.0333. [DOI] [PubMed] [Google Scholar]
- 179.Walker M. D., Strike T. A., Sheline G. E. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int. J. Radiat. Oncol. Biol. Phys. (1979);5:1725–1731. doi: 10.1016/0360-3016(79)90553-4. [DOI] [PubMed] [Google Scholar]
- 180.Wang Z., Juttermann R., Soloway P. D. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J. Biol. Chem. (2000);275:26411–26415. doi: 10.1074/jbc.M001270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Warrington J. P., Csiszar A., Johnson D. A., Herman T. S., Ahmad S., Lee Y. W., Sonntag W. E. Cerebral microvascular rarefaction induced by whole brain radiation is reversible by sys-temic hypoxia in mice. Am. J. Physiol. Heart Circ. Physiol. (2011);300:H736–744. doi: 10.1152/ajpheart.01024.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Warrington J. P., Csiszar A., Mitschelen M., Lee Y. W., Sonntag W. E. Whole brain radiation-induced impairments in learn-ing and memory are time-sensitive and reversible by systemic hy-poxia. PLoS. One. (2012);7:e30444. doi: 10.1371/journal.pone.0030444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wei M., Li H., Huang H., Xu D., Zhi D., Liu D., Zhang Y. Increased expression of EMMPRIN and VEGF in the rat brain after gamma irradiation. J. Korean Med. Sci. (2012);27:291–299. doi: 10.3346/jkms.2012.27.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Welzel G., Fleckenstein K., Schaefer J., Hermann B., Kraus-Tiefen-bacher U., Mai S. K., Wenz F. Memory function be-fore and after whole brain radiotherapy in patients with and without brain metastases. Int. J. Radiat. Oncol. Biol. Phys. (2008);72:1311–1318. doi: 10.1016/j.ijrobp.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 185.Witzenbichler B., Maisonpierre P. C., Jones P., Yancopoulos G. D., Isner J. M. Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase Tie2. J. Biol. Chem. (1998);273:18514–185121. doi: 10.1074/jbc.273.29.18514. [DOI] [PubMed] [Google Scholar]
- 186.Wung B. S., Cheng J. J., Hsieh H. J., Shyy Y. J., Wang D. L. Cyclic strain-induced monocyte chemotactic protein-1 gene expression in endothelial cells involves reactive oxygen species activation of activator protein 1. Circ. Res. (1997);81:1–7. doi: 10.1161/01.RES.81.1.1. [DOI] [PubMed] [Google Scholar]
- 187.Yancopoulos G. D., Davis S., Gale N. W., Rudge J. S., Wiegand S. J., Holash J. Vascular-specific growth factors and blood vessel formation. Nature. (2000);407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 188.Yang K., Liu L., Zhang T., Wu G., Ruebe C., Ruebe C., Hu Y. TGF-betal transgenic mouse model of thoracic irradiation: modulation of MMP-2 and MMP-9 in the lung tissue. J. Huazhong Univ. Sci. Technolog. Med. Sci. (2006);26:301–304. doi: 10.1007/BF02829557. [DOI] [PubMed] [Google Scholar]
- 189.Yang K., Palm J., König J., Seeland U., Rosenkranz S., Feiden W., Rübe C., Rübe C. E. Matrix-Metallo-Proteinases and their tissue inhibitors in radiation-induced lung injury. Int. J. Radiat. Biol. (2007);83:665–676. doi: 10.1080/09553000701558977. [DOI] [PubMed] [Google Scholar]
- 190.Yoneoka Y., Satoh M., Akiyama K., Sano K., Fujii Y., Tanaka R. An experimental study of radiation-induced cognitive dys-function in an adult rat model. Br. J. Radiol. (1999);72:1196–1201. doi: 10.1259/bjr.72.864.10703477. [DOI] [PubMed] [Google Scholar]
- 191.Zhao W., Goswami P. C., Robbins M. E. Radiation-in-duced up-regulation of Mmp2 involves increased mRNA stability, redox modulation, and MAPK activation. Radiat. Res. (2004);161:418–429. doi: 10.1667/3155. [DOI] [PubMed] [Google Scholar]
- 192.Zhao W., Payne V., Tommasi E., Diz D. I., Hsu F. C., Robbins M. E. Administration of the peroxisomal proliferator-activat-ed receptor gamma agonist pioglitazone during fractionated brain irradiation prevents radiation-induced cognitive impairment. Int. J. Radiat. Oncol. Biol. Phys. (2007);67:6–9. doi: 10.1016/j.ijrobp.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 193.Zhou H., Liu Z., Liu J., Wang J., Zhou D., Zhao Z., Xiao S., Tao E., Suo W. Z. Fractionated radiation-induced acute en-cephalopathy in a young rat model: cognitive dysfunction and his-tologic findings. Am. J. Neuroradiol. (2011);32:1795–1800. doi: 10.3174/ajnr.A2643. [DOI] [PMC free article] [PubMed] [Google Scholar]


