Abstract
During the course of evolution, animals encountered the harmful effects of fungi, which are strong pathogens. Therefore, they have developed powerful mechanisms to protect themselves against these fungal invaders. β-Glucans are glucose polymers of a linear β(1,3)-glucan backbone with β(1,6)-linked side chains. The immunostimulatory and antitumor activities of β-glucans have been reported; however, their mechanisms have only begun to be elucidated. Fungal and particulate β-glucans, despite their large size, can be taken up by the M cells of Peyer's patches, and interact with macrophages or dendritic cells (DCs) and activate systemic immune responses to overcome the fungal infection. The sampled β-glucans function as pathogen-associated molecular patterns (PAMPs) and are recognized by pattern recognition receptors (PRRs) on innate immune cells. Dectin-1 receptor systems have been incorporated as the PRRs of β-glucans in the innate immune cells of higher animal systems, which function on the front line against fungal infection, and have been exploited in cancer treatments to enhance systemic immune function. Dectin-1 on macrophages and DCs performs dual functions: internalization of β-glucan-containing particles and transmittance of its signals into the nucleus. This review will depict in detail how the physicochemical nature of β-glucan contributes to its immunostimulating effect in hosts and the potential uses of β-glucan by elucidating the dectin-1 signal transduction pathway. The elucidation of β-glucan and its signaling pathway will undoubtedly open a new research area on its potential therapeutic applications, including as immunostimulants for antifungal and anti-cancer regimens.
Keywords: β-Glucan, Triple helix, Dectin-1, Toll-like receptor (TLR), Peyer's patch, Signal transduction
INTRODUCTION
Since ancient times, many higher fungi of the Basidiomycetes have been used as folk medicines. The major effects of medicinal mushrooms, immunity potentiation and antitumor activities, are attributed to β-glucans. Because α-glucans are eukaryotic nutrient components, they are easily degraded by mammalian enzymes and do not have immunostimulatory activity. In contrast, β-glucans from various fungi are not digested by human enzymes when orally administered, but instead are taken up in the small intestine, which stimulates mucosal and systemic immunity (Vos et al., 2007). Uptaken β-glucans stimulates antitumor activities as well as protective activities against fungal and bacterial infections in animals and human. Despite their high molecular weight, β-glucans, when orally administered, are absorbed in the intestine and activate innate and adaptive immunities.
β-Glucans are glucose polymers that have a backbone of linear β(1,3)-linked D-glucose molecules with β(1,6)-linked side chains of diverse sizes that occur at different intervals along the backbone. Among the various β(1,3), β(1,4), and β(1,6) β-glucan linkages, only β(1,3) stimulates immunity and shows antitumor activities. The first reported major function of β-glucans was antitumor activity (Chihara et al., 1970); since then, many other biological activities have been reported, including antifungal, anti-infection (Onderdonk et al., 1992), radioprotective (Gu et al., 2005), cholesterol reduction (Wolever et al., 2011), and postprandial glucose metabolic activities (Battilana et al., 2001). β-Glucans have been used in prophylactic applications for their immunopotentiation activity as vaccine or adjuvant candidates against Aspergillus (Torosantucci et al., 2005) and Candida vaginal (Pietrella et al., 2010) infections in animals, and against Vibrio infection in marine fish (Zhu et al., 2006). The β-glucans in plants such as oat and barley have primarily β(1,4) linkages, whereas the β-glucans of mushrooms and fungi have a β(1,3) backbone branched with short β(1,6)-linked side chains (Yan et al., 2005). These differences in the structure, conformation, and source of these glucans might affect their biological activities (Brown and Gordon, 2001).
Research on β-glucans has been focused on two areas. The most recent major research area is the stimulation of innate immunity by β-glucan by binding to the β-glucan receptor dectin-1 and TLRs, which has been investigated by employing zymosan β-glucan from Saccharomyces cerevisiae. The other major research area is the antitumor activity of fungal β-glucans, which is mediated by stimulation of macrophages and DCs. Some β-glucans of mushrooms, such as lentinan, schizophyllan and PSK have already been commercialized and are used clinically for the treatment of various cancers (Sullivan et al., 2006). The major well-characterized and functional β-glucans are listed in Table 1.
Table 1.
Biologically active fungal β-glucans and their chain linkages and clinical trials
| β-Glucan (Fungi source) | Glucan chain linkage | Clinical trial |
|---|---|---|
| Pleuran (Pleurotus ostreatus) | β(1,4)- or β(1,6)-branched for every fourth β(1,3)-glucan backbone (Karacsonyi and Kuniak, 1994) | Respiratory infection (Bergendiova et al., 2011) NK cell activation (Bobovčák et al., 2010) |
| Lentinan (Lentinus edodes) | One β(1,6) branched residue for every three β(1,3) glucose residues with MW 400-1,000 kDa (Sasaki and Takasuka, 1976) | Gastric cancer (Nakano et al., 1999) Colon carcinoma (Ng and Yap, 2002) Pancreatic cancer (Shimizu et al., 2009) |
| Schizophyllan (SPG) (Schizophyllum commune) | One β(1,6) branched residue for every three β(1,3) glucose residues with MW 450 kDa (Bae et al., 2004) | Gastric cancer (Fujimoto et al., 1984) Cervical cancer (Okamura et al., 1986) |
| Krestin (PSK) (Trametes versicolor) | Protein-bound with β(1,6) side chain, and β(1,3)-branched β(1,4) main chain glucan with MW 94-100 kDa (Kurashige et al., 1997) | Stomach cancer (Nakazato et al., 1994) Colorectal cancer (Torisu et al., 1990) Breast cancer (Yokoe et al., 1997) Lung cancer (Hayakawa et al., 1993) |
| Maitake D-Fraction (Grifola frondosa) | Mixture of β(1,6) main chain with β(1,4)-branched and β(1,3)main chain with β(1,6)-branched (Kidd, 2000) | Lung and breast cancer (Kodama et al., 2002) HIV infection (Nanba et al., 2000) |
| Scleroglucan (SSG) (Sclerotinia sclerotiorum) | One β(1,6) branched glucose residue for every three β(1,3) glucose residues (Pretus et al., 1991; Palleschi et al., 2005) | NA* |
| Zymosan (Saccharomyces cerevisiae) | Crude cell wall extract of genetically engineering yeast containing a mixture of β(1,3)(1,6) glucans and mannose (Di Carlo and Fiore, 1958) | NA* |
| Yeast whole glucan (Saccharomyces cerevisiae) | Highly purified spherical β(1,3) glucan with β(1,6)-linkage of yeast cell wall with 2-4 mm diameter by alkaline extraction (Yan et al., 2005) | NA* |
*NA: not available.
STRUCTURE-ACTIVITY RELATIONSHIP OF β-GLUCANS
Biological activities of helical β-glucans
β-Glucans adopt one of the three typical conformations in an aqueous environment: a triple helix, a single helix, or a random coil. A triple helix can be converted into a random coil by treatment with NaOH, and the random coil can then be converted into a single helix by neutralization with HCl. Finally, the single helical conformation can be renatured to the original triple helix by heating or dialysis (Zhang et al., 2004). Contrary to this, Young et al. reported quite different conformational
states for the triple helices and single helices. They showed that a closed triple helix can be converted into single strand by partially opening one end of the triple helix, and then neutralization can return it to the original closed triple helix (Young et al., 2000). This partially open hypothesis is more persuasive than the complete dissociated strand hypothesis because returning to the original triple helix from single helix may not be easy without a complementary strand. A cross-sectional view of the triple helix of β-glucan in water showed that it has four water boundaries, rendering it difficult to dissolve in water. The approximate diameters of the innermost core and outermost shell of the helix are 1.68 and 2.79 nm, respectively (Yoshiba et al., 2003). X-ray crystallographic studies showed that the triple helix formation of schizophyllan has a diameter of 2.6 nm and a 1.8-nm pitch. Scanning electron microscopy revealed that the triple helix is always right-handed and that the inclination of the angle is 20-25° (Bae et al., 2004).
The physicochemical properties of high molecular weight β-glucans are closely correlated to biological activity potency. However, the potency of immunostimulation of the triple and single helix conformations is still controversial. In some reports, the triple helix conformation of β-glucan has been shown to play an important role in enhancing biological activities, such as cytokine secretion and antitumor activity (Zhang et al., 2005). Wang and Zhang investigated the in vitro inhibitory activity of β-glucan against the proliferation of sarcoma-180 tumor cells, and demonstrated that denatured, single-stranded β-glucan lentinan had weaker inhibitory activity than the triple helix conformation. Furthermore, introduction of a sulfate group on the single chain lentinan increased antitumor activity due to increased binding of the β-glucan to receptors on immune cells via electrostatic interactions that activated the immune response. They also found that the antitumor activity of the triple helix was higher than that of the sulfated or single-stranded conformation due to the greater stiffness of the triple helix structure (Wang and Zhang, 2009). In contrast, the single helix conformation has also been reported to have stronger activity than the triple helix (Ohno et al., 1996). In another report, both the single and triple helices had strong TNF-a and IL-6 cytokine releasing activity in a macrophage cell line (Okazaki et al., 1995). In addition, a partially opened single helix induced more nitric oxide than did the original triple helix (Young et al., 2000). Activation of innate immunity by β-glucan is initiated by its binding to the specific β-glucan receptor dectin-1 on macrophages (Batbayar et al., 2011) and DCs (Kim et al., 2010) or to CR3 on granulocytes and natural killer (NK) cells (Thornton et al., 1996). β-Glucans also directly activate adaptive immune cells such as B cells (Dong et al., 2007), T cells (Tian et al., 2011), NK cells (Kobayashi et al., 2012), eosinophils (Mahauthaman et al., 1988), and neutrophils (van Bruggen et al., 2009).
Biological activities of highly complex β-glucans
It has been reported that a higher degree of structural complexity in β-glucans is associated with more potent immunomodulatory and anti-cancer effects. Additionally, β(1,3)-glucans with a higher molecular weight or a greater degree of β(1,6)-linkages tend to have stronger stimulatory effects on macrophages in mice (Cleary et al., 1999). In a report on TNF-α secretion induced by Grifola β-glucan, higher molecular weight (over than 450 kDa) glucan was more potent than lower molecular weight (below 450 kDa) glucan (Okazaki et al., 1995). Ishibashi et al. showed that heat-treated grifolan reduced the secretion of TNF-α as well as molecular weight. The precipitated, insoluble fraction of the heat-treated β-glucan, recovered by centrifugation, but not the soluble fraction, retained TNF-α secretion inducing ability. These studies suggested that both the insoluble and the soluble high molecular weight form of grifolan were required for induction of TNF-α secretion by macrophages (Ishibashi et al., 2001).
Biological activities of particulate β-glucans
The potency of the biological activities of water-soluble and particulate β-glucans is still controversial. It was recently noted that particulate b-glucans have stronger immunostimulating activities than soluble ones. Qi et al. reported that orally administered, yeast-derived, particulate β-glucan activated DCs and macrophages via the C-type lectin (CTL) receptor dectin-1 pathway in mice. They also reported that although water-soluble β-glucan binds to DCs and macrophages, it does not activate DCs (Qi et al., 2011). In a report that compared particulate and soluble β-glucans, only particulate β-glucans, such as whole glucan particles (WGP) and zymosan, induced dectin-1-dependent activation of innate immune cells, including phagocytosis and induction of TNF-α, IL-6, and reactive oxygen species (ROS) from bone marrow-derived macrophage and DCs (Goodridge et al., 2011). In addition to these, β(1,3)-glucan particles from Saccharomyces cerevisiae have been reported to elicit strong humoral and cellular responses to antigens entrapped in glucan particles in mice, demonstrating their possible utility as a vaccine delivery tool (Huang et al., 2012). Particulate β-glucan isolated from S. cerevisiae also induced TNF-α in macrophages isolated from murine wounds (Roy et al., 2011). Hino et al. reported that macrophages released soluble glucans into the medium after phagocytizing insoluble β-glucan particles. They found that these particles were fragmented by ROS produced by macrophages, and that the released soluble β-glucan was reactive to dectin-1 and biologically active in terms of macrophage activation (Hino et al., 2012). Recently, a new mechanism was reported for how immune cells distinguish and respond to invading fungal pathogens, which includes particulate and soluble β-glucans. According to the model mechanism of “phagocytic synapse” formation between dectin-1 on bone marrow-derived macrophages and β-glucans proposed by Goodridge et al., the CD45 and CD148 tyrosine phosphatases are excluded from the clustered sites of dectin-1 induced by particulate β-glucan, thereby enabling downstream signaling through hemITAM and the cytosolic domain of dectin-1 and other enzymes involved in signal transduction. In contrast, soluble β-glucans bind to dectin-1 and cause dectin-1 clustering. However, they cannot exclude CD45 and CD148 around dectin-1, and thus signal propagation fails. Therefore, they proposed that dectin-1 signaling is activated only by particulate β-glucans, not by soluble β-glucans. This model could explain how innate immune receptors distinguish direct fungal contact with β-glucans from soluble β-glucans shed by fungi in remote sites of the body (Goodridge et al., 2011).
Biological activities of soluble β-glucans
In contrast, there have also been many reports on immunopotentiation by water-soluble β-glucans. Fang et al. showed that water-soluble β-glucan from Grifola frondosa, with a molecular weight of 300 kDa, strongly induced TNF-α and IL-6 production, activation of Syk and NF-κB signaling in peritoneal macrophages, and inhibition of sarcoma-180 growth in mice (Fang et al., 2012). Gaullier et al. reported that soluble β-glucan from Lentinus edodes increased the number of B cells, but did not change the number of NK cells, when orally administered for 6 weeks in healthy elderly people (Gaullier et al., 2011). Masuda et al. showed that highly purified, soluble β(1,3)(1,6)-glucan obtained from Grifola frondosa rapidly induced GM-CSF production through dectin-1-independent ERK and p38 MAPK activation. Subsequently, β-glucan induced GM-CSF-enhanced proliferation of resident macrophages and dectin-1 expression, which permitted dectin-1-mediated TNF-α induction through the Syk pathway (Masuda et al., 2012). In addition, soluble yeast β(1,3)-glucan itself was reported to induce the production of significant amounts of IL-8 and tissue factor. This glucan had a strong synergistic effect on LPS-induced secretion of IL-8 and IL-10, but not on TNF-α or IL-6 production (Engstad et al., 2002).
MUCOSAL UPTAKE OF β-GLUCAN
Intestinal uptake of β-glucans
Orally administered, natural β-glucans, such as lentinan and schizophyllan, are known for showing their immunopotentiating effects, and have been used in tumor immunotherapy for more than 30 years. However, despite their excellent antitumor activities induced by dietary consumption, the mechanism underlying the uptake of high-molecular weight β-glucan in the lumen is not clear. The absorption mechanisms of α-glucans and β-glucans in the intestine are quite different. α-Glucans, when orally delivered, are easily degraded by digestive enzymes. In contrast, β-glucans are non-digestible due to the absence of the appropriate enzyme; therefore, they could reach the intestine intact.
The effects of orally inoculated β-glucan have been reported in experimental animals. Sakurai et al. reported that SSG β-glucan from Sclerotinia sclerotiorum augmented the phagocytic activity and IL-1 production of alveolar macrophages in mice. They also found that supernatant of Peyer's patch cells from mice that were orally administered SSG stimulated the lysosomal enzyme activity of alveolar macrophages in vitro and enhanced colony stimulating activity (Sakurai et al., 1992). Data from the oral delivery of fluorescently labeled, water-soluble β-glucans in rats showed that the maximum plasma glucan concentration occurred at approximately 4 h and by 24 h, more than 73% was eliminated from the plasma, depending on the β-glucan source. Orally administered glucans translocate from the gastrointestinal (GI) tract into the systemic circulation by binding to both the GI epithelium and gut-associated lymphoid tissue (GALT) cells, and this uptake process is not dectin-dependent (Rice et al., 2005). Sandvik et al. reported successful detection of plasma β-glucan in rats after oral administration of soluble Saccharomyces cerevisiae-derived β-(1,3)(1,6)-glucan for 14 days. The total amount detected in plasma was approximately 30 ng following 14 consecutive days of oral administration of 5-6 mg per day. Thus, only a minute fraction of a single oral dose was translocated to the plasma. They speculated that the mucosal DCs sample or interact with soluble β-glucans locally via projections across the epithelium, and then migrate via afferent lymphatics to the mesenteric lymph nodes, where immune modulation is initiated (Sandvik et al., 2007). It has been suggested that dietary administration of β-glucans is as effective as parenteral administration for potentiation of systemic immunity and protection against pathogens (Volman et al., 2008).
Uptake by microfold (M) cells in Peyer's patches
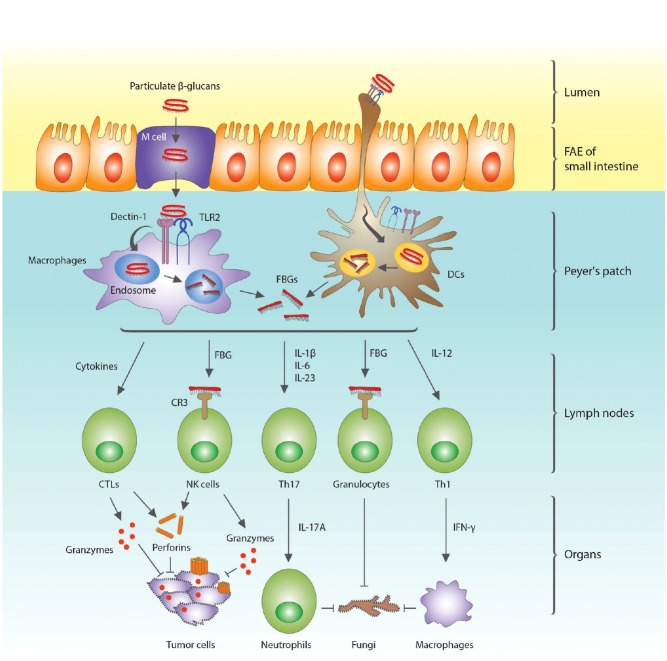
One possible mechanism for the uptake of β-glucan in the lumen could be passage through microfold cells (M cells) in the Peyer's patches of the small intestinal lumen. The luminal surface of the intestine limits the access of pathogens and antigens to underlying host tissues, and is protected by a single layer of epithelial cells bound by tight junctions. Located within the follicle-associated epithelium (FAE) of Peyer's patches, and occasionally within the villus epithelia, are M cells, a unique subset of specialized epithelial cells for transepithelial transport of macromolecules and particulate antigens (Donaldson et al., 2012).
As a mucosal particle portal site, M cells are sampling sites for macromolecules and microorganisms (Kernéis et al., 1997). M cells translocate luminal immunogens to Peyer's patches, where the innate immune systems begin to work. M cells are considered the initiation sites of mucosal immunity against immunogens and pathogens that invade epithelial barriers. Therefore, uptake and translocation of luminal immunogens to the innate immune cells in Peyer's patches appears to be a key trigger of systemic and mucosal immunities (Brayden et al., 2005). Since M cells enable the host's immune system to randomly sample intestinal pathogens and high-molecular weight particles, β-glucan might enter the matrix of Peyer's patches through M cells (Volman et al., 2008), which, by avoiding the phagolysosome during passage, rarely degrade particles (Owen, 1999). Therefore, the protective effects of orally administered β-glucans might be mediated by receptor-mediated interaction with M cells in Peyer's patches, where resident macrophages and DCs encounter β-glucans and increase cytokine production (Volman et al., 2008).
Uptake by DC projections
M cells are not the only cells that sample immunogens and pathogens from the intestinal lumen, and additional antigen sampling routes exist in the intestinal mucosal layer. As an alternative mechanism, the DCs of the FAE extend projections into the lumen to capture antigens for presentation to intraepithelial lymphocytes (Rescigno et al., 2001; Brayden et al., 2005). With the projected tips of the DCs, which extend through the apical epithelium, DCs can capture β-glucan by binding to various receptors, such as dectin-1, TLR2, TLR6, CR3, scavenger receptors, or lactosylceramide (Brown, 2006). When DCs capture infectious bacteria, they up-regulate the expression of occludin, which in turn allows the DCs to compete for epithelial occludin and open up the tight junctions like a zipper. Infiltrating DCs then face the gut lumen and can directly sample the luminal bacteria (Rescigno et al., 2001).
Receptors for β-glucan recognition
Innate immune cells in the intestine can recognize a pathogen's PAMP through PRRs and initiate the innate and adaptive immune responses. In response to invading fungi, the innate immune cells of the Peyer's patches recognize fungal membrane components such as mannan and β-glucan through members of the lectin receptor family. From inside to outside, fungal cell wall PAMPs are composed of chitin, β(1,3)(1,6)-glucan, and mannan. These PAMPs can be recognized by PRRs on monocytes, macrophages, and DCs. The β(1,3)(1,6)-glucans are recognized by dectin-1, TLR2, and TLR6, whereas mannans are recognized by dectin-2, mannan receptor (MR), TLR4, DC-SIGN, galectin 3, and FcRγ (Perez-Garcia et al., 2011). Macrophages and DCs engulf and fragmentize the β-glucans that are bound to dectin-1 and TLR2. Fragmented β-glucans (FBGs) bind to dectin-1 on macrophages and DCs, but fail to activate them, due to their inability to cluster dectin-1 on the membrane (Goodridge et al., 2011). The FBGs are released from the cells and activate NK cells and granulocytes by binding to CR3 (Chan et al., 2009). These activated NK cells release perforins and granzymes, which make pores and disintegrate the DNA of tumor cells, respectively (Zhu and Lin, 2006). Macrophages and neutrophils are activated by IFN-γ and IL-17A secreted by Th1 and Th17 lymphocytes, respectively, and provide protection against the infecting fungi (Fig. 1). Adaptive immune cells such as B and T cells can also be activated by TNF-α, IL-2, IL-10, and IL-12 secreted by macrophages and DCs (Chan et al., 2009). Since DCs and macrophages reside in Peyer's patches, β-glucans that are taken up bind and activate them. Since commensal bacteria, such as Escherichia coli, can penetrate M cells (Macpherson and Harris, 2004), LPS binding to TLR4 can also activate DCs and macrophages more vigorously in the Peyer's patches (Kim et al., 2009).
Fig. 1. Uptake of β-glucan in the small intestine and activation of innate and adaptive immune cells of Peyer's patches, lymph nodes, and systemic organs. Orally administered β-glucans can be either absorbed through M cells or through binding to the projected tips of dendritic cells (DCs) in the follicle-associated epithelium (FAE) of Peyer's patches, and subsequently bind to dectin-1 and TLR2. The macrophages or DCs engulf β-glucans and fragmented β-glucans (FBGs) are secreted in the lymph nodes. FBGs, like soluble β-glucans, bind to dectin-1, but are unable to activate macrophages and DCs. However, FGBs can activate NK cells and granulocytes by binding to complement receptor 3 (CR3) on these cells. The NK cells and cytokine-stimulated cytotoxic T lymphocytes (CTLs) secrete perforins and granzymes, which make pores and fragmenting the DNA in tumor cells, respectively. The FBG-bound granulocytes together with activated neutrophils and macrophages then remove the infecting fungi.
ANTI-FUNGAL INFECTION BY β-GLUCAN THROUGH DECTIN-1
In mammals, β-glucans have been shown to induce diverse biological activities against fungal infections and tumors. However, much criticism has been leveled against their actual physicochemical efficacy. β-Glucans are too large to be absorbed in the small intestines. Several attempts have been made on an industrial scale to enzymatically digest the large molecules into smaller ones for better absorption.
Dectin-1 was discovered and originally cloned as the β-glucan receptor in the last decade (Ilev et al., 2012). This discovery confirmed that β-glucans modulate the immune systems via dectin-1 (Drummond and Brown, 2011). As a C-type lectin, dectin-1 recognizes β-glucans with β(1,3)- and β(1,6)-linkages and subsequently internalizes them (Kerrigan and Brown, 2010). In addition, dectin-1 transmits its signal in DCs and macrophages through a cytoplasmic domain (Brown and Gordon, 2001; Taylor et al., 2007). During the immunostimulation of innate immune cells, dectin-1 functions as PRR that recognizes the PAMP of β-glucan. Dectin-1 also plays an important role in immunity against Pneumocystis carinii by inducing ROS (Brown, 2006). The anti-cancer effects of β-glucan have been verified; however, the major anti-cancer mechanisms remain to be elucidated. β-Glucans may act against cancer development by inducing anti-cancer molecules via the dectin-1 signaling pathway as shown in Fig. 1.
Lower animals also have developed defense mechanisms against fungal infection; however, no membrane spanning receptors are involved. Invertebrates utilize a unique PAMP recognition mechanism through PRRs before initiating immunity (Reid et al., 2009). In horseshoe crab, a unique serine protease, zymogen factor G, is used to recognize LPS and β-glucans (Muta, 2006). Target structures are also recognized by other auxiliary binding factors to ensure steady and detailed recognition of PAMPs. After the polysaccharides on the pathogenic agents are recognized, they are engulfed through a hemolymph coagulation cascade reaction.
Molecular structure of dectin-1
Dectin-1 is the first CLR that was discovered and is the best characterized CLR in this category to date (Drummond and Brown, 2011). Human dectin-1 is 247 amino acids in length and consists of three major regions, a C-type lectin-like carbohydrate recognition domain (CRD), a type II transmembrane region, and a cytoplasmic domain that contains an immunoreceptor tyrosinase-based activation motif (ITAM). When the CRD recognizes β(1,3)-glucan as the primary ligand, the hemITAM of dectin-1 is phosphorylated by Src, a non-receptor tyrosine kinase.
Dectin-1 receptors recognize only the specific configurations of β-glucans found in fungal agents (Ferwerda et al., 2010; Brown, 2011). When alternate structures are found in the cell wall, dectin-1 receptors often fail to recognize them as a ligand. The masked β-glucans of pathogens are a greater problem since they effectively escape host immune surveillance. When β-glucan in the cell wall is insufficiently exposed, dectin-1 no longer detects the masked form of β-glucan. A variant dectin-1 structure was discovered in a human pedigree with a higher risk of mucocutaneous fungal infections. This dectin-1 variant has a shortened CRD structure, and fails to recognize β-glucan as a ligand (Drummond and Brown, 2011).
A recent study indicated that significant cooperation exists between dectin-1 and galectin-3, which is considered an activator of angiogenesis and apoptosis in T cells. In dectin-1 signal transduction, the level of signaling can be enhanced through association with galectins. In galectin-3 deficient cells, β-glucan significantly loses its immune-activating functions, including TNF-α induction (Esteban et al., 2011). Several studies have indicated that β-glucan may provide immunity against Mycobacterium tuberculosis, which does not have β-glucan in their cell wall structure. This observation strongly suggests that dectin-1 may recognize various ligand forms in addition to β-(1,3)-D-glucans (Lee et al., 2009). Dissection of the molecular structure of dectin-1 will make it possible to acquire a more detailed understanding of the association between the receptor and ligand. Based on this structural information, better biomolecules can be generated to treat fungal infections, and possibly cancer.
Genetic annotation of dectin-1
Dectin-1 is encoded by CLEC7A, which refers to member A of CTL domain (CTLD) superfamily 7. The CLEC7A gene encompasses more than 10 Mbps of human chromosome 12, and is composed of 6 exons, and different combinations of these exons produce various alternative spliced isoforms. In 12p13, the natural killer gene complex region, CLEC7A is closely linked to other CTLD superfamily members (Sobanov et al., 2001). Dectin-1 genes are highly conserved across mammals, including human, mice, and yaks, which strongly implies that it has played an important role in the defense against fungal infections during the course of evolution.
Dectin-1 mutations
Sequence variants are found in the dectin-1 gene CLECA7. Numerous variants have been detected in exon 4 (Heinsbroek et al., 2012), and one significant variant has been found in exon 6. A recessive mutation in dectin-1 was found in a family with mucocutaneous fungal infections. The mutation caused an amino acid change, Y238X, which resulted in premature translation termination at the tyrosine residue of the dectin-1 receptor (Cunha et al., 2010; Chai et al., 2011). This mutation is associated with susceptibility to fungal infections, even though fungal killing and phagocytosis occur normally (del Pilar Jimenez-A et al., 2008; Plantinga et al., 2009; van der Velden et al., 2010). A single nucleotide change in this gene caused a nonsense mutation, which results in the deletion of the last 9 amino acids of the CRD. The mutation shows typical loss-of-function and reduced cytokine response upon fungal infection or challenge with β-glucan. Homozygotes for this mutation have lowered human antifungal defense, especially against mucocutaneous infections, which result in vaginal candidiasis and onychomycosis. These observations underscore the importance of dectin-1 as a prime source of antifungal action.
Regulation of dectin-1 expression
The regulation of dectin-1 transcription is not yet well understood. Dectin-1 is normally expressed on myeloid DCs, macrophages/monocytes, and B cells (Rand et al., 2010). Although it is also expressed in splenic T cells, its expression is low (Rivera et al., 2011). Dectin-1 transcription is greatly up-regulated by granulocyte-macrophage colony-stimulating factor (GM-CSF) (Serezani et al., 2012). In addition, enhanced expression of dectin-1 is observed in the presence of the cytokines that induce macrophage activation such as IL-4, IL-13, and IL-23. In contrast, dectin-1 expression is significantly down-regulated by IL-10, LPS, and corticosteroids. In airway epithelial cells, mycobacterium induces the expression of dectin-1 for stimulation of the innate immune response (Lee et al., 2009).
The involvement of G-protein coupled receptors (GPCRs) is a major research question related to dectin-1 function. However, no definite answer is currently available. Recent genetic and pharmacological studies indicated that dectin-1 expression is regulated by the levels of leukotriene B4 (LTB4) through its high affinity GPCR leukotriene B4 receptor (BLT1) in macrophage (Serezani et al., 2012). LTB4-BLT1 signaling modulates the expression of dectin-1 to ensure protective host responses against fungal infection. Dectin-1 expression
is negatively affected by peroxisome proliferator-activated receptor-γ (PPARγ) (Gales et al., 2010). Troglitazone, an agonist of PPARγ, decreases dectin-1-mediated DC activation and the expression of cytokines such as IL-1, IL-6, TNF-α, MIP-3α, and RANTES. The effects are believed to result from interference with MAPK, NF-κB, and caspase recruitment domain 9 (CARD9), which is one of the most important molecules in non-TLR signaling (Kock et al., 2011). This observation indicates that dectin-1 expression is considerably reduced by the presence of PPARγ.
SIGNAL TRANSDUCTION BY DECTIN-1
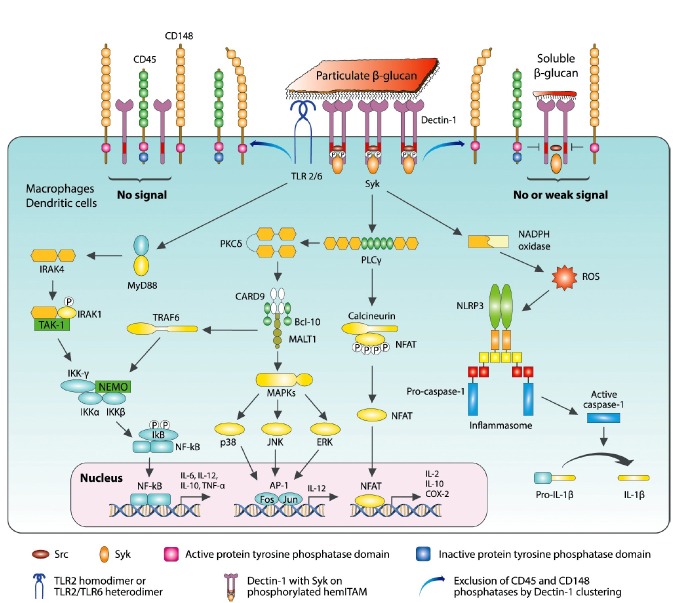
When the dectin-1 CRD recognizes particulate β(1,3)-glucans as the primary ligand, it assembles a multimeric complex which disperses CD45 and CD148 from their original locations as shown in Fig. 2. This unlocks the space needed for the ligand to associate with the receptor (Goodridge et al., 2011). Upon binding to particulate β-glucan, the hemITAM of dectin-1 is phosphorylated by Src, a non-receptor tyrosine kinase. Then, spleen tyrosine kinase (Syk) is attracted into its cytoplasmic domain and Syk-mediated signal transduction occurs. Syk also initiates the so-called integrated transcriptional response (ITR) by activating phospholipase Cγ (PLCγ) and PKCδ which start two key pathways in the ITR. Activated PKCδ then induces either NFAT or AP-1 gene, which are both involved in the expression of interleukins (Kerrigan and Brown, 2010; Xu et al., 2009b). Syk also triggers PLCγ, which subsequently induces calcineurin, and then turns on NFAT. NF-κB factor is also induced independent of Syk. In contrast, when dectin-1 is unbound or bound to small soluble β-glucans, it cannot exclude CD45 and CD148 phosphatases, which remove the phosphate from the hemITAM domain; therefore, no signal or only weak signals are generated. There are two major dectin-1 signaling pathways that depend on whether the signal is propagated through Syk-dependent or Syk-independent modes.
Fig. 2. Recognition of particulate and soluble β-glucans by dectin-1 and signal transduction in macrophages and DCs. When particulate β-glucan binds, dectin-1 molecules cluster, which excludes CD45 and CD148 and enables Src to associate and phosphorylate the hemITAM motif of dectin-1. Syk then binds to the phosphorylated hemITAM domain. In contrast, when dectin-1 is empty or is bound to small soluble β-glucan, it cannot exclude the CD45 and CD148 phosphatases, which remove the phosphate from the hemITAM domain. Therefore, no signal or only a weak signal is generated. Syk-mediated phagocytosis occurs instantly while the so-called integrated transcriptional response (ITR) begins with PLCγ activation. Three major pathways exist in the ITR: 1) CBM complex modulates the signaling for NF-κB via NEMO complex and, in parallel, activates MAPK to activate AP-1 expression after PKCδ activation; 2) Syk also triggers PLCγ, and subsequently calcineurin, which eventually turns on NFAT; 3) following Syk-dependent ROS production by NADPH oxidase, NLRP3 initiates IL-1 processing. In addition to ITR, β-glucans trigger the TLR2/6 dimeric receptor to attract MyD88 protein, which activates a series of kinases to activate on the NEMO/IKK complex, which finally induces NF-κB, which is also activated independent of Syk. In this Syk-independent pathway, Raf-1 induces p60 and this nuclear factor subsequently induces NF-κB. BCL-10: B cell lymphoma 10, CARD9: Caspase-recruitment domain 9, CRD: Carbohydrate recognition domain, IRAK: IL-1 receptor-associated kinase, MALT1: Mucosa-associated lymphoid tissue lymphoma translocation protein 1, MAPK: Mitogen-activated protein kinase, NEMO: NF-κB essential modulator, NLRP3: Nucleotide-binding domain and leucine-rich repeat containing protein 3, TAK: TGF-β-activated kinase, TLR: Toll-like receptor, TRAF: TNF receptor associated factor.
Syk-dependent signaling
Syk initially phosphorylates PLCγ, which utilizes two independent activation pathways depending on the secondary messengers. PLCγ synthesizes DAG and IP3 by digesting PIP3, which is predominantly found as bound to the plasma membrane (Xu et al., 2009a; Alvarez et al., 2010). DAG and IP3 dictate which process will occur in the dual dectin-1 pathway. When DAG is the messenger, PKC is immediately activated and induces CARD9-Bcl10-Malt1 (CBM) trimers in the cytosol (Saijo and Iwakura, 2011). The CBM complex has a caspase recruitment domain, which, if activated, induces various pro-inflammatory cytokine genes. This trimer participates in two different pathways by activating either MAP kinase or the NEMO-IKK complex. In the former pathway, MAP kinases phosphorylate ERK, JNK, and p38, which move into the nucleus and binds to an AP-1 transcriptional element. The latter pathway is significant since the NEMO complex the association of p50 and p60 with NF-κB for immunologic activation. Another route is modulated by PLC. Coincident with DAG production, IP3 is generated and causes the release of calcium ions from its cytosolic reservoir. The increased calcium induces the functional combination of calcineurin with phosphorylated NFAT, which eventually binds to its corresponding cis-elements in the nucleus after undergoing dephosphorylation (Greenblatt et al., 2010).
In macrophages, dectin-1 attracts Syk, which is important for dectin-1-stimulated ROS production. Under certain circumstances, Syk is activated in only a small population of macrophages, and this activation appears to be under tight regulation to avoid ROS over-production. In this Syk-mediated pathway, NLRP3 initiates interleukin-1β (IL-1β) processing following Syk-dependent ROS production (Said-Sadier et al., 2010). ROS is also induced by NADPH ox?dase in the presence of Syk. ROS is recruited into the inflammasome complex along with NRLP3, to destroy fungal invaders during caspase-1 activation, which converts pro-IL-1β into IL-1β (Underhill et al., 2005; Gross et al., 2009; Kumar et al., 2009; Kankkunen et al., 2010). ROS is produced during dectin-1 signaling; however, excessive generation of ROS may result in tissue damage and chronic inflammation. A recent study showed that PKC controls the production of ROS by modulating monocyte NADPH oxidase. The level of Syk may dictate the magnitude of PKCδ binding with dectin-1 (Hughes et al., 2010; Elsori et al., 2011). Through IL-1 and IL-23 production, β-glucan speeds up the differentiation of Th17 cells. IL-17A from Th17 cells attracts neutrophils to inflammatory sites and activates immune cells to achieve immunological coordination in the host when exposed to pathogens.
Syk-independent pathway (or Raf-1 pathway)
In addition to the pathways in Fig. 2, signals from dectin-1 may follow another Syk-independent route. Dectin-1 could trigger a Raf1-mediated pathway that modulates NF-κB activation (Gringhuis et al., 2011). In this case, Raf-1 binds to the cytoplasmic domain of dectin-1 and increases p60 phosphorylation independent of Syk, which also occurs in the Syk-dependent pathway. This pathway exerts a synergistic effect along with β-glucan signaling. In addition, this pathway may serve as an alternative signal transduction pathway when the Syk-dependent pathway is blocked. Along with dectin-1, dimeric TLR2/6 might be activated by β-glucan, and the activated TLR might utilize MyD88 to induce NF-κB, which is promptly expressed in both the Syk-dependent and Syk-independent pathways as previously described. TLR signaling is depicted in detail in the following section.
Signaling pathways of other than dectin-1 receptor
Another class of dectin receptor was recently discovered. This receptor, which was named as dectin-2, recognizes high mannose and α-mannan structures. Mannan is a polymer of mannose that is usually found in yeast, bacteria and plants. α-Mannan is mainly found in yeast cell walls, while β-forms are found in the cell walls of higher plants. In contrast to dectin-1, dectin-2 lacks an ITAM motif in the cytoplasmic domain. Dectin-2 consequently employs the Fc receptor γ recep (FcRγ), which contains an ITAM motif (Gringhuis et al., 2011; Goodridge et al., 2012). When FcRγ associates with dectin-2, Syk is recruited to the ITAM domain. The rest of the dectin-1 and dectin-2 signal transduction pathways are believed to be identical. Similar to dectin-1, dectin-2 plays important roles in defense against C. albicans by preferentially inducing Th17 cell differentiation. Mincle, a multi-tasking danger receptor, exclusively recognizes α-mannose and cord factor from the cell wall debris of fungi, yeasts, and mycobacteria (Dan et al., 2008). Following recognition of these polysaccharides as ligands, the Mincle receptor shares a signaling pathway similar to the dectin receptors, in particular, the same pathway as dectin-2.
Signaling by heterodimeric TLR2/TLR6 is primarily triggered by peptidoglycans (PGs), which are predominantly found in the bacterial cell wall. However, β-glucan is also recognized by the TLR complex (Gersuk et al., 2006). When TLR2 associates with PGs or β-glucans, TLR2 cooperates with dectin-1 in producing interleukins, TNF-α and ROS to induce a proinflammatory response (Gantner et al., 2003; Viriyakosol et al., 2005; Dennehy et al., 2009). Upon recognition of fungal agents by macrophages, TLRs recruit MyD88 to their cytoplasmic domain. Unlike dectin-1, TLRs lack an ITAM-motif, instead, MyD88 serves as adaptor molecule that attracts a kinase named IL-1 receptor-associated kinase (IRAK). Upon binding to the TLR cytoplasmic domain, MyD88 recruits IRAK-4, which then recruits IRAK-1 molecules in the cytosol, and then the IRAK complex attracts TRAF6. IRAK-4 then phosphorylates IRAK-1 and TRAF6 dissociates from the IRAK complex. This evidence strongly indicates that IRAK-1 actually binds to TRAF6 in the intermolecular association of IRAKs. The freed TRAF6 attracts a variety of component molecules, including TAK-1, TAB-1/-2, Uev1A, and Ubc13. Among these, activated TAK-1 is the actual functional molecule that phosphorylates the downstream NEMO-IKK complex during β-glucan-TLR signaling. From this point on, the TLR and dectin-1 signaling pathways share the NEMO-IKK complex to initiate NF-κB transcription. Indeed, synergistic effects might be implicated between these two signaling pathways. Although the exact pathway is not fully understood, MyD88 activates MAPK, which phosphorylates the AP-1 transcriptional factor that induces IL-12. This cytokine is believed to trigger Th1 cells to differentiate and release IFN-γ which stimulates macrophages (Saijo et al., 2010).
Influence of dectin-1 on acquired immunity
Currently, it is not known whether dectin-1 induces pathogen-specific adaptive immunity. However, dectin-1 contributes to the adaptive immune responses as well as autoimmune diseases and immune tolerance (Sun and Zhao, 2007). Activated dectin-1 is involved in acquired immunity through enhanced expression of various cytokines, which subsequently promote the differentiation of Th17 cells. Dectin-1 may help modulate Th17 differentiation by promoting IL-6 and IL-23 via TLR2 along with concurrent inhibition of IL-12 by Th1. In addition, dectin-1 may play a role in acquired immunity via rapid antigen presentation through enhanced phagocytosis and ROS production.
FUTURE PERSPECTIVES
Additional studies are required to elucidate the detailed association between β-glucan and its receptors, focusing on how these molecules prompt the innate immune system to protect the body. Their size and molecular structure are significant for dectin-1 binding and the ensuing innate immune cell response; only specific β-glucan structures are recognized as ligands.
Notable progress has been made in a variety of pharmacological applications since β-glucans were first proposed as a biological response modifier. β-Glucan has been used with anti-infective, anti-neoplastic, topical agents and even radiotherapy. When β-glucan is supplemented with ascorbate, it exerts a stronger effect. Ascorbate content is significantly decreased in activated macrophages. This reduced vitamin C content decreases anti-oxidant capacity, thus weakening motility and enzymatic production in macrophages. With the addition of ascorbate, immune function in the body is greatly boosted. When β-glucans are incorporated into various treatments, synergistic effects can be expected to maximize each remedy.
Glycobiology is one of the most studied areas in recent years. A variety of glycoproteins affect the immune system by binding to their specific receptors. Research on β-glucan and its derivatives are expected to aid in the discovery of specific receptors that lead to immune responses and inflammation. Using polysaccharides that are exclusively found in plants, bacteria and fungi, novel receptors can be found in organisms ranging from lower animals to mammals. Branched β-glucans, especially those from fungi, lead to hyperinflammation and necrosis. The extent of the branched structures of β-glucans may affect the degree of inflammation and host defense against fungi and tumors. Future characterization of the cause and effect of the branching in fungal polysaccharides may open a new field in glycobiology (Schäppi et al., 2008). Since the structure and binding characteristics of dectin-1 have been extensively studied, new studies may be directed at developing a potent new ligand that mimics β-glucan (Huang et al., 2010). Many carbohydrate receptors remain to be discovered, and advances in glycobiology might help biologists discover or re-discover the importance of receptors that recognize specific carbohydrates as functional ligands. In particular, water-insoluble β-glucan should be the major research focus in upcoming years. From studies focusing on zymosan, high-molecular weight glucans were shown to directly augment the immune response, most likely through specific receptors (Gitik et al., 2010; Lamkanfi et al., 2009).
During the course of mammalian evolution, fungal infection might have served as a selection pressure. Variation in the sequence of dectins and other polysaccharide receptors could provide another valuable window for understanding the genetic relatedness among mammals. A recent study reported that β-glucans markedly stimulate the immune response in vertebrates, including fleshy shrimp (Fenneropenaeus chinensis) and horseshoe crab (Bae et al., 2012). Although they do not have dectin-1 receptors, a significant anti-fungal effect can be obtained via dectin-1 independent signaling. This report also strongly suggests that immunostimulation effects can also be achieved in economically important invertebrates using β-glucans. These dectin-free immune responses should be studied in parallel with dectin-1 signal transduction.
To date, studies on β-glucans have been focused predominantly on their immunostimulating effects. As a source of soluble fiber, β-glucan may lessen the risk of heart-related diseases by lowering total cholesterol and LDL cholesterol. Since β-glucan and dectin-1 utilize the Syk signaling cascade, future studies should focus on potential pathogenesis caused by Syk-mediated cellular procedures. Indeed, over-expression of Syk results in hematological malignancies, allergy, autoimmunity, activation of viral oncogenes, and other non-hematopoietic tumors (Mócsai et al., 2010). The combined effects of β-glucan and anti-Syk agents should be pursued to understand the efficacy of β-glucans that utilize the dectin-1 receptor signaling pathway and, most importantly, to obtain balanced information on dectin-1-mediated signaling in terms of the immunomodulating effect of β-glucans.
CONCLUDING REMARKS
It has been reported that β-glucans with a higher degree of structural complexity, such as those that are triple helixes or particulate, are associated with potent immunopotentiating, antifungal and anticancer effects. Despite their high-molecular weight, β-glucans can be absorbed via M cells of the intestinal membrane and are detected by macrophages or DCs residing in Peyer’s patches. β-Glucans play a crucial role in activating the host immune response against fungal invasion. In the absence of its receptor dectin-1, β-glucan may not initiate an anti-fungal defense in host as an antigen for an acquired immune response. Indeed, in most cases, the level of defense is minimal without assistance from dectin-1. The importance of dectin-1 is dramatically demonstrated in the case of the dectin-1 Y238X mutation, in which CRD is not functional. In heterozygous carriers of this mutation, antifungal immunity is incomplete, and the level of immunity is not low enough to threaten normal life. However, homozygotes of this mutation are greatly affected and always experience a severe infection especially of the mucocutaneous membranes. Still, the dectin-1 free anti-fungal defense might function effectively and, theoretically, acquired immunity against β-glucan might be enhanced by consistent use of β-glucan. Significant cooperation exists between dectin-1 and other receptors against fungal polysaccharides. Future studies are needed to probe the interactions among these receptors. Needless to say, further elucidation of dectin-1 function and its potential ligands will aid in the development of biomolecules and therapeutics to reinforce the remedy of β-glucan against anti-fungal agents.
Acknowledgments
This work was supported by a research grant (2010-2011) from the Ministry for Food, Agriculture, Forestry and Fisheries (MIFAFF). The authors appreciate this financial support.
References
- 1.Alvarez Y., Valera I., Municio C., Hugo E., Padrón F., Blanco L., Rodríguez M., Fernandez N., Crespo A. S. Eicosanoids in the innate immune response, TLR and non-TLR routes. Mediator Inflamm. . (2010) doi: 10.1155/2010/201929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae A. H., Lee S. W., Ikeda M., Sano M., Shinkai S., Sakurai K. Rod-like architecture and helicity of the poly(C)/schizophyllan complex observed by AFM and SEM. Carbohyd. Res. . (2004);339:251–258. doi: 10.1016/j.carres.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 3.Bae S. H., Kim B. R., Kang B. J., Tsutsui N., Okutsu T., Shinji J., Jang I. K., Han C. H., Wilder M. N. Molecular coloning of prophenoloxidase and the effects of dietary β-glucan and rutin on immune response in hemocytes of the fleshy shrimp, Fenneropenaeus chinensis. Fish Shellfish Immunol. . (2012);33:597–604. doi: 10.1016/j.fsi.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 4.Batbayar S., Kim M. J., Kim H. W. Medicinal mushroom Lingzhi or Reishi, Ganoderma lucidum (W.Curt.,Fr.) P. Karst., β-glucan induces toll-like receptors and fails to induce inflammatory cytokines in NF-κB inhibitor-treated macrophages. Int. J. Med. Mushrooms . (2011);13:213–225. doi: 10.1615/IntJMedMushr.v13.i3.10. [DOI] [PubMed] [Google Scholar]
- 5.Battilana P., Ornstein K., Minehira K., Schwarz J. M., Acheson K., Schneiter P., Burri J., Jéquier E., Tappy L. Mechanisms of action of β-glucan in postprandial glucose metabolism in healthy men. Eur. J. Clin. Nutr. (2001);55:327–333. doi: 10.1038/sj.ejcn.1601160. [DOI] [PubMed] [Google Scholar]
- 6.Bergendiova K., Tibenska E., Majtan J. Pleuran (β-glucan from Pleurotus ostreatus) supplementation, cellular immune response and respiratory tract infections in athletes. Eur. J. Appl. Physiol. . (2011);111:2033–2040. doi: 10.1007/s00421-011-1837-z. [DOI] [PubMed] [Google Scholar]
- 7.Bobovčák M., Kuniaková R., Gabriž J., Majtán J. Effect of pleuran (β-glucan from Pleurotus ostreatus) supplementation on cellular immune response after intensive exercise in elite athletes. Appl. Physiol. Nutr. Metab. (2010);35:755–762. doi: 10.1139/H10-070. [DOI] [PubMed] [Google Scholar]
- 8.Brayden D. J., Jepson M. A., Baird A. W. Keynote review, intestinal Peyer's patch M cells and oral vaccine targeting. Drug Discov. Today . (2005);10:1145–1157. doi: 10.1016/S1359-6446(05)03536-1. [DOI] [PubMed] [Google Scholar]
- 9.Brown G. D., Gordon S. Immune recognition. A new receptor for β-glucans. Nature . (2001);413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 10.Brown G. D. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. . (2006);6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 11.Brown G. D. Innate antifungal immunity, the key role of phagocytes. Annu. Rev. Immunol. (2011);29:1–21. doi: 10.1146/annurev-immunol-030409-101229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chai L. Y., de Boer M. G., van der Velden W. J., Plantinga T. S., van Spriel A. B., Jacobs C., Halkes C. J., Vonk A. G., Blijlevens N. M., van Dissel J. T., Donnelly P. J., Kullberg B. J., Maertens J., Netea M. G. The Y238X stop codon polymorphism in the human β-glucan receptor dectin-1 and susceptibility to invasive aspergillosis. J. Infect. Dis. . (2011);203:736–743. doi: 10.1093/infdis/jiq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan G. C., Chan W. K., Sze D. M. The effects of β-glucan on human immune and cancer cells. J. Hematol. Oncol. . (2009);2:25. doi: 10.1186/1756-8722-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chihara G., Hamuro J., Maeda Y., Arai Y., Fukuoka F. Antitumour polysaccharide derived chemically from natural glucan (Pachyman). Nature . (1970);225:943–944. doi: 10.1038/225943a0. [DOI] [PubMed] [Google Scholar]
- 15.Cleary J. A., Kelly G. E., Husband A. J. The effect of molecular weight and β-1,6-linkages on priming of macrophage function in mice by (1,3)-β-D-glucan. Immunol. Cell Biol. . (1999);77:395–403. doi: 10.1046/j.1440-1711.1999.00848.x. [DOI] [PubMed] [Google Scholar]
- 16.Cunha C., Di Ianni M., Bozza S., Giovannini G., Zagarella S., Zelante T., D’Angelo C., Pierini A., Pitzurra L., Falzetti F., Carotti A., Perruccio K., Latgé J. P., Rodrigues F., Velardi A., Aversa F., Romani L., Carvalho A. Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity. Blood. (2010);116:5394–5402. doi: 10.1182/blood-2010-04-279307. [DOI] [PubMed] [Google Scholar]
- 17.Dan J. M., Kelly R. M., Lee C. K., Levitz S. M. Role of the mannose receptor in a murine model of Cryptococcus neoformans infection. Infect. Immun. (2008);76:2362–2367. doi: 10.1128/IAI.00095-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.del Pilar Jimenéz-A M., Viriyakosol S., Walls L., Datta S. K., Kirkland T., Heinsbroek S. E. M., Brown G., Fierer J. Susceptibility to Coccidioides species in C57BL/6 mice is associated with expression of a truncated splice variant of dectin-1 (Clec7a). Genes Immun. (2008);9:338–348. doi: 10.1038/gene.2008.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dennehy K. M., Willment J. A., Williams D. L., Brown G. D. Reciprocal regulation of IL-23 and IL-12 following co-activation of dectin-1 and TLR signaling pathways. Eur. J. Immunol. . (2009);39:1379–1386. doi: 10.1002/eji.200838543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Carlo F. J., Fiore J. V. On the composition of zymosan. Science . (1958);127:756–757. doi: 10.1126/science.127.3301.756-a. [DOI] [PubMed] [Google Scholar]
- 21.Donaldson D. S., Kobayashi A., Ohno H., Yagita H., Williams I. R., Mabbott N. A. M cell-depletion blocks oral prion disease pathogenesis. Mucosal Immunol. (2012);5:216–225. doi: 10.1038/mi.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong S. F., Chen J. M., Zhang W., Sun S. H., Wang J., Gu J. X., Boraschi D., Qu D. Specific immune response to HBsAg is enhanced by β-glucan oligosaccharide containing an α-(1→3)-linked bond and biased towards M2/Th2. Int. Immunopharmacol. (2007);7:725–733. doi: 10.1016/j.intimp.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Drummond R. A., Brown G. D. The role of dectin-1 in the host defense against fungal infections. Curr. Opin. Microbiol. (2011);14:392–399. doi: 10.1016/j.mib.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Elsori D. H., Yakubenko V. P., Roome T., Thiagarajan P. S., Bhattacharjee A., Yadav S. P., Cathcart M. K. Protein kinase Cδ is a critical component of dectin-1 signaling in primary human monocytes. J. Leukoc. Biol. (2011);90:599–611. doi: 10.1189/jlb.0610376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engstad C. S., Engstad R. E., Olsen J. O., Osterud B. The effect of soluble β-1,3-glucan and lipopolysaccharide on cytokine production and coagulation activation in whole blood. Int. Immunopharmacol. . (2002);2:1585–1597. doi: 10.1016/S1567-5769(02)00134-0. [DOI] [PubMed] [Google Scholar]
- 26.Esteban A., Popp M. W., Vyas V. K., Strijbis K., Ploegh H. L., Fink G. R. Fungal recognition is mediated by the association of dectin-1 and galectin-3 in macrophages. Proc. Natl. Acad. Sci. U. S. A. . (2011);108:14270–14275. doi: 10.1073/pnas.1111415108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang J., Wang Y., Lv X., Shen X., Ni X., Ding K. Structure of a β-glucan from Grifola frondosa and its antitumor effect by activating dectin-1/Syk/NF-κB signaling. Glycoconj. J. (2012);29:365–377. doi: 10.1007/s10719-012-9416-z. [DOI] [PubMed] [Google Scholar]
- 28.Ferwerda G., Netea M. G., Joosten L. A., van der Meer J. W. M., Romani L., Kullberg B. J. The role of toll-like receptors and C-type lectins for vaccination against Candida albicans. Vaccine . (2010);28:614–622. doi: 10.1016/j.vaccine.2009.10.082. [DOI] [PubMed] [Google Scholar]
- 29.Fujimoto S., Furue H., Kimura T., Kondo T., Orita K., Taguchi T., Yoshida K., Ogawa N. Clinical evaluation of schizophyllan adjuvant immunochemotherapy for patients with resectable gastric cancer-a randomized controlled trial. Jpn. J. Surg. (1984);14:286–292. doi: 10.1007/BF02469643. [DOI] [PubMed] [Google Scholar]
- 30.Galés A., Conduch? A., Bérnad J., Lefevre L., Olagnier D., Beraud M., Martin-Blondel G., Linas M. D., Auwerx J., Coste A., Pipy B. PPARγ controls dectin-1 expression required for host antifungal defense against Candida albicans. PLoS Pathog. (2010);6:e1000714. doi: 10.1371/journal.ppat.1000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gantner B. N., Simmons R. M., Canavera S. J., Akira S., Underhill D. M. Collaborative induction of inflammatory responses by dectin-1 and toll-like receptor 2. J. Exp. Med. . (2003);197:1107–1117. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaullier J. M., Sleboda J., Øfjord E. S., Ulvestad E., Nurminiemi M., Moe C., Tor A., Gudmundsen O. Supplementation with a soluble β-glucan exported from shiitake medicinal mushroom, Lentinus edodes (Berk.) Singer mycelium: a crossover, placebo-controlled study in healthy elderly. Int. J. Med. Mushrooms . (2011);13:319–326. doi: 10.1615/intjmedmushr.v13.i4.10. [DOI] [PubMed] [Google Scholar]
- 33.Gersuk G. M., Underhill D. M., Zhu L., Marr K. A. Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J. Immunol. (2006);176:3717–3724. doi: 10.4049/jimmunol.176.6.3717. [DOI] [PubMed] [Google Scholar]
- 34.Gitik M, Reichert F., Rotshenker S. Cytoskeleton plays a dual role of activation and inhibition in myelin and zymosan phagocytosis by microglia. FASEB J. (2010);24:2211–2221. doi: 10.1096/fj.09-146118. [DOI] [PubMed] [Google Scholar]
- 35.Goodridge H. S., Reyes C. N., Becker C. A., Katsumoto T. R., Ma J., Wolf A. J., Bose N., Chan A. S., Magee A. S., Danielson M. E., Weiss A., Vasilakos J. P., Underhill D. M. Activation of the innate immune receptor dectin-1 upon formation of a 'phagocytic synapse'. Nature . (2011);472:471–475. doi: 10.1038/nature10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodridge H. S., Underhill D. M., Touret N. Mechanisms of Fc receptor and dectin-1 activation for phagocytosis. Traffic. (2012);13:1062–1071. doi: 10.1111/j.1600-0854.2012.01382.x. [DOI] [PubMed] [Google Scholar]
- 37.Greenblatt M. B., Aliprantis A., Hu B., Glimcher L. H. Calcineurin regulates innate antifungal immunity in neutrophils. J. Exp. Med. (2010);207:923–931. doi: 10.1084/jem.20092531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gringhuis S. I., Wevers B. A., Kaptein T. M., van Capel T. M. M., Theelen B., Boekhout T., de Jong E. C., Geijtenbeek T. B. Selective C-Rel activation via Malt1 controls anti-fungal TH-17 immunity by dectin-1 and dectin-2. PLoS Pathog. (2011);7:e1001259. doi: 10.1371/journal.ppat.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gross O., Poeck H., Bscheider M., Dostert C., H?nnesschlager N., Endres S., Hartmann G., Tardivel A., Schweighoffer E., Tybulewicz V., Mocsai A., Tschopp J., Ruland J. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. (2009);459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 40.Gu Y. H., Takagi Y., Nakamura T., Hasegawa T., Suzuki I., Oshima M., Tawaraya H., Niwano Y. Enhancement of radioprotection and anti-tumor immunity by yeast-derived β-glucan in mice. J. Med. Food. (2005);8:154–158. doi: 10.1089/jmf.2005.8.154. [DOI] [PubMed] [Google Scholar]
- 41.Hayakawa K., Mitsuhashi N., Saito Y., Takahashi M., Katano S., Shiojima K., Furuta M., Niibe H. Effect of Krestin (PSK) as adjuvant treatment on the prognosis after radical radiotherapy in patients with non-small cell lung cancer. Anticancer Res. (1993);13:1815–1820. [PubMed] [Google Scholar]
- 42.Heinsbroek S. E., Oei A., Roelofs J. J., Dhawan S., te Velde A., Gordon S., de Jonge W. J. Genetic deletion of dectin-1 does not affect the course of murine experimental colitis. BMC Gastroenterol. (2012);12:33. doi: 10.1186/1471-230X-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hino S., Kito A., Yokoshima R., Sugino R., Oshima K., Morita T., Okajima T., Nadano D., Uchida K., Matsuda T. Discharge of solubilized and dectin-1-reactive β-glucan from macrophage cells phagocytizing insoluble β-glucan particles: involvement of reactive oxygen species (ROS)-driven degradation. Biochem. Biophys. Res. Commun. (2012);421:329–334. doi: 10.1016/j.bbrc.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Huang H., Ostroff G. R., Lee C. K., Specht C. A., Levitz S. M. Robust stimulation of humoral and cellular immune responses following vaccination with antigen-loaded β-glucan particles. MBio. (2010);1:e00164–10. doi: 10.1128/mBio.00164-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang H., Ostroff G. R., Lee C. K., Agarwal S., Ram S., Rice P. A., Specht C. A., Levitz S. M. Relative contributions of dectin-1 and complement to immune responses to particulate β-glucans. J. Immunol. . (2012);189:312–317. doi: 10.4049/jimmunol.1200603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hughes C. E., Pollitt A. Y., Mori J., Eble J. A., Tomlinson M. G., Hartwig J. H., O’Callaghan C. A., Futterer K., Watson S. P. CLEC-2 activates Syk through dimerization. Blood . (2010);115:2947–2955. doi: 10.1182/blood-2009-08-237834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iliev I. D., Funari V. A., Taylor K. D., Nguyen Q., Reyes C. N., Strom S. P., Brown J., Becker C. A., Fleshner P. R., Dubinsky M., Rotter J. I., Wang H. L., McGovern D. P., Brown G. D., Underhill D. M. Interactions between commensal fungi and the C-type lectin receptor dectin-1 influence colitis. Science . (2012);336:1314–1317. doi: 10.1126/science.1221789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishibashi K., Miura N. N., Adachi Y., Ohno N., Yadomae T. Relationship between solubility of grifolan, a fungal 1,3-β-D-glucan, and production of tumor necrosis factor by macrophages in vitro. Biosci. Biotechnol. Biochem. (2001);65:1993–2000. doi: 10.1271/bbb.65.1993. [DOI] [PubMed] [Google Scholar]
- 49.Kankkunen P., Teiril? L., Rintahaka J., Alenius H., Wolff H., Matikainen S. (1,3) β-Glucans activate both dectin-1 and NLRP3 inflammasome in human macrophages. J. Immunol. . (2010);184:6335–6342. doi: 10.4049/jimmunol.0903019. [DOI] [PubMed] [Google Scholar]
- 50.Karácsonyi S., Kuniak L. Polysaccharides of Pleurotus ostreatus: isolation and structure of pleuran, an alkali-insoluble β-D-glucan. Carbohydr. Polym. (1994);24:107–111. doi: 10.1016/0144-8617(94)90019-1. [DOI] [Google Scholar]
- 51.Kern?is S., Bogdanova A., Kraehenbuhl J. P., Pringault E. Conversion by Peyer's patch lymphocytes of human enterocytes into M cells that transport bacteria. Science . (1997);277:949–952. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- 52.Kerrigan A. M., Brown G. D. Syk-coupled C-type lectin receptors that mediate cellular activation via single tyrosine based activation motifs. Immunol. Rev. (2010);234:335–352. doi: 10.1111/j.0105-2896.2009.00882.x. [DOI] [PubMed] [Google Scholar]
- 53.Kidd P. M. The use of mushroom glucans and proteoglycans in cancer treatment. Altern. Med. Rev. (2000);5:4–27. [PubMed] [Google Scholar]
- 54.Kim S. I., Park H. G., Cho G. H., Ko I. S., Kim H. W. Cooperative effect of the lipopolysaccharide and culinary-medicinal cauliflower mushroom Sparassis crispa (Wulf.) Fr. (Aphyllophoromycetideae)-derived β-glucan on inflammatory cytokine secretion by the murine macrophage cell line. Int. J. Med. Mushrooms . (2009);11:9–20. doi: 10.1615/IntJMedMushr.v11.i1.20. [DOI] [Google Scholar]
- 55.Kim H. S., Kim J. Y., Lee H. K., Kim M. S., Lee S. R., Kang J. S., Kim H. M., Lee K. A., Hong J. T., Kim Y., Han S. B. Dendritic cell activation by glucan isolated from Umbilicaria esculenta. Immune Netw. (2010);10:188–197. doi: 10.4110/in.2010.10.6.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kobayashi T., Kawamura H., Kanda Y., Matsumoto H., Saito S., Takeda K., Kawamura T., Abo T. Natural killer T cells suppress zymosan A-mediated granuloma formation in the liver by modulating interferon-γ and interleukin-10. Immunol. (2012);136:86–95. doi: 10.1111/j.1365-2567.2012.03562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kock G., Bringmann A., Held S. A., Daecke S., Heine A., Brossart P. Regulation of dectin-1-mediated dendritic cell activation by peroxisome proliferator-activated receptor-gamma ligand troglitazone. Blood . (2011);117:3569–3574. doi: 10.1182/blood-2010-08-302224. [DOI] [PubMed] [Google Scholar]
- 58.Kodama N, Komuta K., Nanba H. Can maitake MD-fraction aid cancer patients? Altern. Med. Rev. (2002);7:236–239. [PubMed] [Google Scholar]
- 59.Kumar H., Kumagai Y., Tsuchida T., Koenig P. A., Satoh T., Guo Z., Jang M. H., Saitoh T., Akira S., Kawai T. Involvement of the NLRP3 inflammasome in innate and humoral adaptive immune responses to fungal β-glucan. J. Immunol. (2009);183:8061–8067. doi: 10.4049/jimmunol.0902477. [DOI] [PubMed] [Google Scholar]
- 60.Kurashige S., Akuzawa Y., Endo F. Effects of Lentinus edodes, Grifola frondosa and Pleurotus ostreatus administration on cancer outbreak, and activities of macrophages and lymphocytes in mice treated with a carcinogen, N-butyl-N-butanolnitrosoamine. Immunopharmacol. Immunotoxicol. (1997);19:175–183. doi: 10.3109/08923979709007657. [DOI] [PubMed] [Google Scholar]
- 61.Lamkanfi M., Malireddi R. K., Kanneganti T. D. Fungal zymosan and mannan activate the cryopyrin inflammasome. J. Biol. Chem. (2009);284:20574–20581. doi: 10.1074/jbc.M109.023689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee H. M., Yuk J. M., Shin D. M., Jo E. K. Dectin-1 is inducible and plays an essential role for Mycobacteria-induced innate immune responses in airway epithelial cells. J. Clin. Immunol. (2009);29:795–805. doi: 10.1007/s10875-009-9319-3. [DOI] [PubMed] [Google Scholar]
- 63.Macpherson A. J., Harris N. L. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. (2004);4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 64.Mahauthaman R., Howell C. J., Spur B. W., Youlten L. J., Clark T. J., Lessof M. H., Lee T. H. The generation and cellular distribution of leukotriene C4 in human eosinophils stimulated by unopsonized zymosan and glucan particles. J. Allergy Clin. Immunol. (1988);81:696–705. doi: 10.1016/0091-6749(88)91041-X. [DOI] [PubMed] [Google Scholar]
- 65.Masuda Y., Togo T., Mizuno S., Konishi M., Nanba H. Soluble β-glucan from Grifola frondosa induces proliferation and dectin-1/Syk signaling in resident macrophages via the GM-CSF autocrine pathway. J. Leukoc. Biol. (2012);91:547–556. doi: 10.1189/jlb.0711386. [DOI] [PubMed] [Google Scholar]
- 66.Mócsai A., Ruland J., Tybulewicz V. L. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat. Rev. Immunol. (2010);10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muta T. Molecular basis for invertebrate innate immune recognition of (1→3)-β-D-glucan as a pathogen-associated molecular pattern. Curr. Pharm. Des. (2006);12:4155–4161. doi: 10.2174/138161206778743529. [DOI] [PubMed] [Google Scholar]
- 68.Nakano H., Namatame K., Nemoto H., Motohashi H., Nishiyama K., Kumada K. A multi-institutional prospective study of lentinan in advanced gastric cancer patients with unresectable and recurrent diseases: effect on prolongation of survival and improvement of quality of life. Kanagawa Lentinan Research Group. Hepatogastroenterol. (1999);46:2662–2668. [PubMed] [Google Scholar]
- 69.Nakazato H., Koike A., Saji S., Ogawa N., Sakamoto J. Efficacy of immunochemotherapy as adjuvant treatment after curative resection of gastric cancer. Lancet . (1994);343:1122–1126. doi: 10.1016/S0140-6736(94)90233-X. [DOI] [PubMed] [Google Scholar]
- 70.Nanba H., Kodama N., Schar D., Turner D. Effects of maitake (Grifola frondosa) glucan in HIV-infected patients. Mycoscience . (2000);41:293–295. doi: 10.1007/BF02463941. [DOI] [Google Scholar]
- 71.Ng M. L., Yap A. T. Inhibition of human colon carcinoma development by lentinan from shiitake mushrooms (Lentinus edodes). J. Altern. Complement. Med. (2002);8:581–589. doi: 10.1089/107555302320825093. [DOI] [PubMed] [Google Scholar]
- 72.Ohno N., Hashimoto T, Adachi Y., Yadomae T. Conformation dependency of nitric oxide synthesis of murine peritoneal macrophages by β-glucans in vitro. Immunol. Lett. (1996);52:157–163. doi: 10.1016/0165-2478(96)02604-1. [DOI] [PubMed] [Google Scholar]
- 73.Okamura K., Suzuki M., Chihara T., Fujiwara A., Fukuda T., Goto S., Ichinohe K., Jimi S., Kasamatsu T., Kawai N., Mizuguchi K., Mori S., Nakano H., Noda K., Sekiba K., Suzuki K, Suzuki T, Takahashi K, Takeuchi K, Takeuchi S., Ogawa N. Clinical evaluation of schizophyllan combined with irradiation in patients with cervical cancer. A randomized controlled study. Cancer. (1986);58:865–872. doi: 10.1002/1097-0142(19860815)58:4<865::AID-CNCR2820580411>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 74.Okazaki M., Adachi Y., Ohno N., Yadomae T. Structure-activity relationship of (1→3)-β-D-glucans in the induction of cytokine production from macrophages, in vitro. Biol. Pharm. Bull. . (1995);18:1320–1327. doi: 10.1248/bpb.18.1320. [DOI] [PubMed] [Google Scholar]
- 75.Onderdonk A. B., Cisneros R. L., Hinkson P., Ostroff G. Anti-infective effect of poly-β 1-6-glucotriosyl-β 1-3-glucopyranose glucan in vivo. Infect. Immun. (1992);60:1642–1647. doi: 10.1128/iai.60.4.1642-1647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Owen R. L. Uptake and transport of intestinal macromolecules and microorganisms by M cells in Peyer’s patches - a personal and historical perspective. Semin. Immunol. (1999);11:157–163. doi: 10.1006/smim.1999.0171. [DOI] [PubMed] [Google Scholar]
- 77.Palleschi A., Bocchinfuso G., Coviello T., Alhaique F. Molecular dynamics investigations of the polysaccharide scleroglucan: first study on the triple helix structure. Carbohydr. Res. (2005);340:2154–2162. doi: 10.1016/j.carres.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 78.Perez-Garcia L. A., Diaz-Jimenez D. F., Lopez-Esparza A., Mora-Montes H. M. Role of cell wall polysaccharides during recognition of Candida albicans by the innate immune system. J. Glycobiol. (2011);1:102. [Google Scholar]
- 79.Pietrella D., Rachini A., Torosantucci A., Chiani P, Brown A. J., Bistoni F., Costantino P., Mosci P., d'Enfert C., Rappuoli R., Cassone A., Vecchiarelli A. A β-glucan-conjugate vaccine and anti-β-glucan antibodies are effective against murine vaginal candidiasis as assessed by a novel in vivo imaging technique. Vaccine. (2010);28:1717–1725. doi: 10.1016/j.vaccine.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 80.Plantinga T. S., van der Velden W. J., Ferwerda B., van Spriel A. B., Adema G., Feuth T., Donnelly J. P., Brown G. D., Kullberg B. J., Blijlevens N. M., Netea M. G. Early stop polymorphism in human DECTIN-1 is associated with increased candida colonization in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. (2009);49:724–732. doi: 10.1086/604714. [DOI] [PubMed] [Google Scholar]
- 81.Pretus H. A., Ensley H. E., McNamee R. B., Jones E. L., Browder I. W., Williams D. L. Isolation, physicochemical characterization and preclinical efficacy evaluation of soluble scleroglucan. J. Pharmacol. Exp. Ther. (1991);257:500–510. [PubMed] [Google Scholar]
- 82.Qi C., Cai Y., Gunn L., Ding C., Li B., Kloecker G., Qian K., Vasilakos J., Saijo S., Iwakura Y., Yannelli J. R., Yan J. Differential pathways regulating innate and adaptive antitumor immune responses by particulate and soluble yeast-derived β-glucans. Blood. (2011);117:6825–6836. doi: 10.1182/blood-2011-02-339812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rand T. G., Sun M., Gilyan A., Downey J., Miller J. D. Dectin-1 and inflammation-associated gene transcription and expression in mouse lungs by a toxic (1,3)-β-D glucan. Arch. Toxicol. (2010);84:205–220. doi: 10.1007/s00204-009-0481-4. [DOI] [PubMed] [Google Scholar]
- 84.Reid D. M., Gow N. A., Brown G. D. Pattern recognition: recent insights from dectin-1. Curr. Opin. Immunol. (2009);21:30–37. doi: 10.1016/j.coi.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rescigno M., Urbano M., Valzasina B., Francolini M., Rotta G., Bonasio R., Granucci F., Kraehenbuhl J. P., Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. (2001);2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 86.Rice P. J., Adams E. L., Ozment-Skelton T., Gonzalez A. J., Goldman M. P., Lockhart B. E., Barker L. A., Breuel K. F., Deponti W. K., Kalbfleisch J. H., Ensley H. E., Brown G. D., Gordon S., Williams D. L. Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J. Pharmacol. Exp. Ther. (2005);314:1079–1086. doi: 10.1124/jpet.105.085415. [DOI] [PubMed] [Google Scholar]
- 87.Rivera A., Hohl T. M., Collins N., Leiner I., Gallegos A., Saijo S., Coward J. W., Iwakura Y., Pamer E. G. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J. Exp. Med. . (2011);208:369–381. doi: 10.1084/jem.20100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roy S., Dickerson R., Khanna S., Collard E., Gnyawali U., Gordillo G. M., Sen C. K. Particulate β-glucan induces TNF-α production in wound macrophages via a redox-sensitive NF-κB-dependent pathway. Wound Repair Regen. (2011);19:411–419. doi: 10.1111/j.1524-475X.2011.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saïd-Sadier N., Padilla E., Langsley G., Ojcius D. M. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS One. (2010);5:e10008. doi: 10.1371/journal.pone.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saijo S., Ikeda S., Yamabe K., Kakuta S., Ishigame H., Akitsu A., Fujikado N., Kusaka T., Kubo S., Chung S. H., Komatsu R., Miura N., Adachi Y., Ohno N., Shibuya K., Yamamoto N., Kawakami K., Yamasaki S., Saito T., Akira S., Iwakura Y. Dectin-2 recognition of α-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity . (2010);32:681–691. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 91.Saijo S., Iwakura Y. Dectin-1 and dectin-2 in innate immunity against fungi. Int. Immunol. (2011);23:467–472. doi: 10.1093/intimm/dxr046. [DOI] [PubMed] [Google Scholar]
- 92.Sakurai T., Hashimoto K., Suzuki I., Ohno N., Oikawa S., Masuda A., Yadomae T. Enhancement of murine alveolar macrophage functions by orally administered β-glucan. Int. J. Immunopharmacol. (1992);14:821–830. doi: 10.1016/0192-0561(92)90080-5. [DOI] [PubMed] [Google Scholar]
- 93.Sandvik A., Wang Y. Y., Morton H. C., Aasen A. O., Wang J. E., Johansen F. E. Oral and systemic administration of β-glucan protects against lipopolysaccharide-induced shock and organ injury in rats. Clin. Exp. Immunol. (2007);148:168–177. doi: 10.1111/j.1365-2249.2006.03320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sasaki T., Takasuka N. Further study of the structure of lentinan, an anti-tumor polysaccharide from Lentinus edodes. Carbohydr. Res. (1976);47:99–104. doi: 10.1016/S0008-6215(00)83552-1. [DOI] [PubMed] [Google Scholar]
- 95.Sch?ppi M., Deffert C., Fiette L., Gavazzi G., Herrmann F., Belli D., Krause K. H. Branched fungal β-glucans causes hyperinflammation and necrosis in phagocyte NADPH oxidase-deficient mice. J. Pathol. (2008);214:434–444. doi: 10.1002/path.2298. [DOI] [PubMed] [Google Scholar]
- 96.Serezani C. H., Kane S., Collins L., Morato-Marques M., Osterholzer J. J., Peters-Golden M. Macrophage dectin-1 expression is controlled by leukotriene B4 via a GM-CSF/PU.1 axis. J. Immunol. (2012);189:906–915. doi: 10.4049/jimmunol.1200257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shimizu K., Watanabe S., Watanabe S., Matsuda K., Suga T., Nakazawa S., Shiratori K. Efficacy of oral administered superfine dispersed lentinan for advanced pancreatic cancer. Hepatogastroenterol. (2009);56:240–244. [PubMed] [Google Scholar]
- 98.Sobanov Y., Bernreiter A., Derdak S., Mechtcheriakova D., Schweighofer B., D?chler M., Kalthoff F., Hofer E. A novel cluster of lectin-like receptor genes expressed in monocytic, dendritic and endothelial cells maps close to the NK receptor genes in the human NK gene complex. Eur. J. Immunol. (2001);31:3493–3503. doi: 10.1002/1521-4141(200112)31:12<3493::AID-IMMU3493>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 99.Sullivan R., Smith J. E., Rowan N. J. Medicinal mushrooms and cancer therapy: translating a traditional practice into western medicine. Perspect. Biol. Med. (2006);49:159–170. doi: 10.1353/pbm.2006.0034. [DOI] [PubMed] [Google Scholar]
- 100.Sun L., Zhao Y. The biological role of dectin-1 in immune response. Int. Rev. Immunol. (2007);26:349–364. doi: 10.1080/08830180701690793. [DOI] [PubMed] [Google Scholar]
- 101.Taylor P. R., Tsoni S. V., Willment J. A., Dennehy K. M., Rosas M., Findon H., Haynes K., Steele C., Botto M., Gordon S., Brown G. D. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nat. Immunol. (2007);8:31–38. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thornton B. P., V?tvicka V., Pitman M., Goldman R. C., Ross G. D. Analysis of the sugar specificity and molecular location of the β-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18). J. Immunol. (1996);156:1235–1246. [PubMed] [Google Scholar]
- 103.Tian J., Ma J., Wang S., Yan J., Chen J., Tong J., Wu C., Liu Y., Ma B., Mao C., Jiao Z., Shao Q., Lu L., Xu H. Increased expression of mGITRL on D2SC/1 cells by particulate β-glucan impairs the suppressive effect of CD4+CD25+ regulatory T cells and enhances the effector T cell proliferation. Cell. Immunol. (2011);270:183–187. doi: 10.1016/j.cellimm.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 104.Torisu M., Hayashi Y., Ishimitsu T., Fujimura T., Iwasaki K., Katano M., Yamamoto H., Kimura Y., Takesue M., Kondo M., Nomoto K. Significant prolongation of disease-free period gained by oral polysaccharide K (PSK) administration after curative surgical operation of colorectal cancer. Cancer Immunol. Immunother. (1990);31:261–268. doi: 10.1007/BF01740932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Torosantucci A., Bromuro C., Chiani P., De Bernardis F., Berti F., Galli C., Norelli F., Bellucci C., Polonelli L., Costantino P., Rappuoli R., Cassone A. A novel glyco-conjugate vaccine against fungal pathogens. J. Exp. Med. (2005);202:597–606. doi: 10.1084/jem.20050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Underhill D. M., Rossnagle E., Lowell C. A., Simmons R. M. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood . (2005);106:2543–2550. doi: 10.1182/blood-2005-03-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.van Bruggen R., Drewniak A., Jansen M., van Houdt M., Roos D., Chapel H., Verhoeven A. J., Kuijpers T. W. Complement receptor 3, not dectin-1, is the major receptor on human neutrophils for β-glucan-bearing particles. Mol. Immunol. (2009);47:575–581. doi: 10.1016/j.molimm.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 108.van der Velden W. J., Plantinga T. S., Feuth T., Donnelly J. P., Netea M. G., Blijlevens N. M. The incidence of acute graft versus-host disease increases with Candida colonization depending the dectin-1 gene status. Clin. Immunol. (2010);136:302–306. doi: 10.1016/j.clim.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 109.Viriyakosol S., Fierer J., Brown G. D., Kirkland T. N. Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on toll-like receptor 2 and dectin-1. Infect. Immun. (2005);73:1553–1560. doi: 10.1128/IAI.73.3.1553-1560.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Volman J. J, Ramakers J. D., Plat J. Dietary modulation of immune function by β-glucans. Physiol. Behav. (2008);94:276–284. doi: 10.1016/j.physbeh.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 111.Vos A. P., M'Rabet L., Stahl B., Boehm G., Garssen J. Immune-modulatory effects and potential working mechanisms of orally applied nondigestible carbohydrates. Crit. Rev. Immunol. (2007);27:97–140. doi: 10.1615/CritRevImmunol.v27.i2.10. [DOI] [PubMed] [Google Scholar]
- 112.Wang X., Zhang L. Physicochemical properties and antitumor activities for sulfated derivatives of lentinan. Carbohydr. Res. (2009);344:2209–2216. doi: 10.1016/j.carres.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 113.Wolever T. M., Gibbs A. L., Brand-Miller J., Duncan A. M., Hart V., Lamarche B., Tosh S. M., Duss R. Bioactive oat β-glucan reduces LDL cholesterol in Caucasians and non-Caucasians. Nutr. J. (2011);10:130. doi: 10.1186/1475-2891-10-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xu S., Huo J., Lee K. G., Kurosaki T., Lam K. P. Phospholipase Cγ2 is critical for dectin-1-mediated Ca2+ flux and cytokine production in dendritic cells. J. Biol. Chem. (2009a);284:7038–7046. doi: 10.1074/jbc.M806650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu S. L., Huo J., Gunawan M., Su I. H., Lam K. P. Activated dectin-1 localizes to lipid raft microdomains for signaling and activation of phagocytosis and cytokine production in dendritic cells. J. Biol. Chem. (2009b);284:22005–22011. doi: 10.1074/jbc.M109.009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yan J., Allendorf D. J., Brandley B. Yeast whole glucan particle (WGP) β-glucan in conjunction with antitumor monoclonal antibodies to treat cancer. Expert Opin. Biol. Ther. (2005);5:691–702. doi: 10.1517/14712598.5.5.691. [DOI] [PubMed] [Google Scholar]
- 117.Yokoe T., Iino Y., Takei H., Horiguchi J., Koibuchi Y., Maemura M., Ohwada S., Morishita Y. HLA antigen as predictive index for the outcome of breast cancer patients with adjuvant immunochemotherapy with PSK. Anticancer Res. . (1997);17:2815–2818. [PubMed] [Google Scholar]
- 118.Yoshiba K., Teramoto A., Nakamura N., Kikuchi K., Miyazaki Y., Sorai M. Static water structure detected by heat capacity measurements on aqueous solutions of a triple-helical polysaccharide schizophyllan. Biomacromolecules . (2003);4:1348–1356. doi: 10.1021/bm0300251. [DOI] [PubMed] [Google Scholar]
- 119.Young S. H., Dong W. J., Jacobs R. R. Observation of a partially opened triple-helix conformation in 1→3-β-glucan by fluorescence resonance energy transfer spectroscopy. J. Biol. Chem. . (2000);275:11874–11879. doi: 10.1074/jbc.275.16.11874. [DOI] [PubMed] [Google Scholar]
- 120.Zhang X., Zhang L., Xu X. Morphologies and conformation transition of lentinan in aqueous NaOH solution. Biopolymers . (2004);75:187–195. doi: 10.1002/bip.20112. [DOI] [PubMed] [Google Scholar]
- 121.Zhang L., Li X., Xu X., Zeng F. Correlation between antitumor activity, molecular weight, and conformation of lentinan. Carbohydr. Res. (2005);340:1515–1521. doi: 10.1016/j.carres.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 122.Zhu K., Chi Z., Li J., Zhang F., Li M., Yasoda H. N., Wu L. The surface display of haemolysin from Vibrio harveyi on yeast cells and their potential applications as live vaccine in marine fish. Vaccine . (2006);24:6046–6052. doi: 10.1016/j.vaccine.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 123.Zhu X., Lin Z. Modulation of cytokines production, granzyme B and perforin in murine CIK cells by Ganoderma lucidum polysaccharides. Carbohydr. Polym. (2006);63:188–197. doi: 10.1016/j.carbpol.2005.08.002. [DOI] [Google Scholar]