Abstract
Resveratrol, a chemopreventive agent, is rapidly metabolized in the intestine and liver via glucuronidation. Thus, the pharmacokinetics of resveratrol limits its efficacy. To improve efficacy, the activity of resveratrol was investigated in the context of sphingolipid metabolism in human gastric cancer cells. Diverse sphingolipid metabolites, including dihydroceramides (DHCer), were tested for their ability to induce resveratrol cytotoxicity. Exposure to resveratrol (100 μM) for 24 hr induced cell death and cell cycle arrest in gastric cancer cells. Exposure to the combination of resveratrol and dimethylsphingosine (DMS) increased cytotoxicity, demonstrating that sphingolipid metabolites intensify resveratrol activity. Specifically, DHCer accumulated in a resveratrol concentration-dependent manner in SNU-1 and HT-29 cells, but not in SNU-668 cells. LC-MS/MS analysis showed that specific DHCer species containing C24:0, C16:0, C24:1, and C22:0 fatty acids chain were increased by up to 30-fold by resveratrol, indicating that resveratrol may partially inhibit DHCer desaturase. Indeed, resveratrol mildly inhibited DHCer desaturase activity compared to the specific inhibitor GT-11 or to retinamide (4-HPR); however, in SNU-1 cells resveratrol alone exhibited a typical cell cycle arrest pattern, which GT-11 did not alter, indicating that inhibition of DHCer desaturase is not essential to the cytotoxicity induced by the combination of resveratrol and sphingolipid metabolites. Resveratrol-induced p53 expression strongly correlated with the enhancement of cytotoxicity observed upon combination of resveratrol with DMS or 4-HPR. Taken together, these results show that DHCer accumulation is a novel lipid biomarker of resveratrol-induced cytotoxicity in human gastric cancer cells.
Keywords: Resveratrol, Dihydroceramide desaturase, Gastric cancer, 4-HPR, Sphingosine kinase
INTRODUCTION
Resveratrol dietary intake is associated with a decreased risk for cardiovascular disease. The epidemiologic finding of an inverse relationship between consumption of red wine and incidence of cardiovascular disease has been called the "French paradox" (Dudley et al., 2008). The effects of resveratrol include suppression of lipid peroxidation, eicosanoid synthesis, and platelet aggregation; thus, resveratrol shows anti-oxidant, anti-inflammatory, and vasorelaxant activity (Gorelik et al., 2008).
Resveratrol has been shown to exert an anti-proliferative activity in several mouse and human cancer cell lines, and anti-cancer activity in in vivo and in vitro models of skin, colon, and breast cancer (Aziz et al., 2005; Fukui et al., 2010; Nutakul et al., 2011). Recent studies suggest that the regulation of sphingolipid metabolism, especially the enhanced ceramide accumulation by resveratrol, may explain its anti-cancer properties (Scarlatti et al., 2003). The accumulation of ceramide by a variety of stress events induces cellular apoptosis, which further leads to a transient increase in ceramide. This upregulation of ceramide levels is derived from the activation of sphingomyelinases or de novo ceramide biosynthesis.
In the de novo sphingolipid synthetic pathway, ceramide is synthesized from the initial condensation of L-serine and palmitoyl-CoA via four distinct steps, the last one being the desaturation of dihydroceramide by dihydroceramide desaturase (Ulrich et al., 2007). Therefore, dihydroceramide desaturase is an essential enzyme in the synthesis of new ceramide. Intriguingly, resveratrol also inhibits dihydroceramide desaturase, leading to dihydroceramide accumulation and ceramide synthesis inhibition (Signorelli et al., 2009).
Since exogenous dihydroceramides with short N-acyl chains fail to mimic the anti-mitogenic effects of their respective ceramides in cultured cancer cells, for years, dihydroceramides have been regarded as innocuous lipids; however, recent reports indicate that dihydroceramides are in fact bioactive lipids, although their effects may differ from those elicited by ceramides (Idkowiak-Baldys et al., 2010). Dihydroceramide desaturase is part of the multienzyme complex, consisting of NADH-cytochrome b5 reductase, cytochrome b5, and desaturase (Schulze et al., 2000). The use of genetic animal models and pharmacological inhibitors to decrease dihydroceramide desaturase activity has conclusively demonstrated the biological properties of dihydroceramides.
We studied the pharmacological activity of resveratrol in gastric cancer cells, mainly focusing on the synthesis of ceramides and dihydroceramides during cell death induction. The present study demonstrates, for the first time, that resveratrol not only leads to the accumulation of endogenous ceramides, but it also significantly increases dihydroceramides. In addition, resveratrol specifically increases the amount of dihydroceramide containing lignoceric acid (DHCer-C24:0) in SNU-1 gastric cancer cells and HT-29 colon cancer cells.
MATERIALS AND METHODS
Materials
SNU-1, SNU-668, and HT-29 cells were purchased from the Korean Cell Line Bank (Seoul, Korea).
Resveratrol (trans) and 4-HPR (Fenretinide, N-(4-hydroxyphenyl) retinamide) were purchased from Sigma Chemical Co. (St. Louis, MO, USA), and RPMI 1640 medium, fetal bovine serum (FBS) was purchased from Gibco-BRL (Gland Island, NY, USA). The WST-1 assay kit was purchased from Roche (Basel, Switzerland). The dihydroceramide desaturase inhibitor GT-11 (N-C8:0-cyclopropenylceramide) was purchased from Matreya LLC (Pleasant Gap, PA, USA). Dimethylsphingosine (DMS) was purchased from Enzo Life Sciences, Inc. (Ann Arbor, MI, USA). The anti-human p53 monoclonal antibody was purchased from BD PharmingenTM (San Diego, CA, USA), and anti-β-actin was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Chemical standards of ceramides and dihydroceramides were obtained from Avanti Polar Lipids, Inc. (Alabaster, AL, USA). The liquid chromatography electrospray ionization tandem mass LTQ-XL ESI-ION TRAP system (LC-MS/MS) (Thermo Scientific; West Palm Beach, FL, USA) was used for ceramide analysis. Organic solvents for ceramide extraction and other reagents were of the highest purity available.
Cell culture
Human gastric cancer SNU-1 cells and other cells were routinely grown in RPMI 1640 medium supplemented with 10% FBS and 10 U/ml penicillin/streptomycin at 37℃ in 5% CO2. Cells were allowed to equilibrate for 2-3 hr prior to adding resveratrol. Resveratrol was dissolved in HPLC-grade ethanol and the concentration of ethanol was always maintained at 0.1% in both treated and untreated cells. The resveratrol stock solution was stored in darkness at -70℃.
WST-1 proliferation assay
Cells were seeded in 96-well culture plates at 3×104 cells per well, and were incubated for 3-4 hr. Next, the cells were incubated with resveratrol (0-100 μM) for 24 hr at 37℃ in 5%
Fig. 1. Chemical structure of resveratrol.
CO2. The cells in each well were then exposed to 10 μl WST-1 for 2 hr, after which absorbance was measured at 462 nm in a spectrophotometer.
Cytotoxicity and cell cycle analysis by flow cytometry
Cells were seeded at 2×106 cells per well in a 6-well plate. Exposure to resveratrol was conducted as described above. The cells were collected, gently resuspended into a single cell suspension in phosphate-buffered saline (PBS), and fixed overnight with 70% ice-cold ethanol at 4℃. Cell pellets were harvested and resuspended for 2 hr in 500 ml propidium iodide (PI) staining solution at 4℃. The apoptotic cell population was measured as the number of cells in the sub G0-G1 phase of the cell cycle, using the FACS Calibur instrument and associated software (Beckton & Dickinson Co., Palo Alto, CA, USA).
The assessment of apoptosis and necrosis was performed using the Annexin-V-FLOUS staining kit according to the manufacturer’s instructions (Roche, Mannheim, Germany). The cells were seeded in 6-well plates and allowed to grow for 24 hr. Cells were pretreated with 10, 50, and 100 μM resveratrol for 24 hr, and then exposed to a combination of 100 μM resveratrol and 5 μM GT-11 for 24 hr. The cells were harvested, resuspended in 200 μl binding buffer, and incubated with 2 μl FITC-labeled annexin-V and 2 μl PI (50 μg/ml stock solution) for 20-30 min in darkness at room temperature. Cells were analyzed in a FACS Calibur. After identifying negative and positive control populations, the percentages of annexin-V-negative/PI-negative (Santandreu et al., 2011), annexin-V-positive/PI-negative (apoptotic), and annexin-V-positive/PI-positive (necrotic) cells were calculated.
LC-MS/MS analysis of ceramide and DHCer
SNU-1 cells were seeded at 1×106 cells per dish in 100 mm dishes. After exposure to resveratrol (100 μM) as indicated, cell pellets were collected. Sphingolipids were extracted with a solution comprising 50 μl cell lysis buffer, 50 μl internal standard (C17-S1P), and 2 ml chloroform. The supernatant was collected after repeated extraction (final volume: 4 ml) and dried in a vacuum.
The dried residue was redissolved in 50 μl MeOH and, subsequently, injected (5 μl) into the LC-MS/MS system. The samples were separated on a reverse phase Luna C18 (2.0× 150 mm, ID: 5 μm) column, by a linear gradient between eluent A (1 mM ammonium formate in methanol containing 0.2% formic acid) and eluent B (2 mM ammonium formate in water containing 0.2% formic acid). The LC system was connected to the mass spectrometry system (LC-MS/MS) for further analysis.
Each sample was detected by product ion scans for m/z 264.4 for ceramide (Cer) and m/z 266.4 for DHCer. Based on this information, a multiple reaction monitoring (MRM) method was developed for the detection of fatty acyl chain length variants where each transition has a dwell time of ~25 ms. After establishing the MRM protocol, the intensity of the signals for the different sphingolipid subspecies was compared at each estimated mass using internal standards.
DHCer desaturase assay
The DHCer desaturase enzymatic reaction was optimized, as previously described (Spassieva et al., 2012). Briefly, the DHCer substrate was added to an Eppendorf tube and dried in a vacuum concentrator. If necessary, inhibitors were added at this point. The DHCer substrate/inhibitor mixtures were dissolved in 10 ml ethanol and supplemented with 80 ml of a 10 mg/ml bovine serum albumin (BSA)/PBS solution. A fraction of 0.3 mg protein from a rat microsomal suspension dissolved in 33 mM NADH/PBS solution was added. The final volume was then adjusted to 300 ml and samples were incubated at 37℃ for 1 hr. To stop the DHCer desaturase enzyme reaction, 800 ml methanol was added to each tube, and the resulting supernatant was kept at 4℃ for 10 min. An internal standard (C17-ceramide, 1 μM) was added to each tube. Finally, the samples were centrifuged in a tabletop centrifuge at 11,000× g for 3 min. LC-MS/MS was used to measure Cer and DHCer.
Immunoblotting analysis
Cells were washed with ice-cold PBS and lysed in buffer containing 62.25 mM Tris-HCl (pH 6.8), 2% sodium dodecyl sulfate (SDS), 10% glycerol, 50 mM dithiothreitol (DTT), and a protease inhibitor cocktail tablet (Roche, Mannheim, Germany). The lysates were centrifuged and the supernatants collected. The protein content of the supernatant was quantified using the BCA protein assay (Pierce, Rockford, IL) using BSA as a standard, according to the manufacturer’s manual. Proteins were separated in 10-15% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE). Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Amersham Pharmacia Biotech, Buckinghamshire, UK) at 250 mA in buffer containing 25 mM Tris-HCl, 192 mM glycine, and 20% methanol (pH 8.3). The PVDF membrane was then blocked in TBS-T (50 mM Tris, 150 mM NaCl, 0.05% Tween 20; adjusted pH with HCl to pH 7.6) with 5% nonfat dry milk at 4℃ overnight. The membrane was washed and incubated with the appropriate primary antibodies, including p53 and β-actin, at 4℃ overnight. After washing, the membrane was incubated with anti-mouse IgG secondary antibody (Cell signaling Technology Inc., Beverly, MA, USA) at 4℃ overnight. After washing, the band was visualized by luminescence and recorded using the LAS 3000 instrument (Fuji Photo Film Co., Tokyo, Japan). The proteins of interest were normalized to β-actin. Band intensity was quantified using Scion Image for Windows (Scion Corporation, Frederick, MD, USA).
BCA protein assay
The lysate from the solubilized tissue was mixed with the BCA reagents (Pierce, Rockford, IL., USA) and incubated for 30 min. The protein content was quantified using a Molecular Devices spectrophotometer (Sunnyvale, CA, USA) at 562 nm and a BSA standard curve.
Statistical analysis
Each experiment was performed at least three times. All quantitative data were expressed as means ± SD. Statistical differences were analyzed by Student’s t-test (*p<0.01).
RESULTS
Enhanced cytotoxicity of resveratrol combined with DMS
Resveratrol-induced cytotoxicity was assessed in SNU-1 cells. SNU-1 cells were exposed to 0, 10, 50, and 100 μM resveratrol for 24 hr. Cytotoxicity was greatly increased when cells were exposed to 50 μM resveratrol. Approximately 70% of the cells died in 100 μM resveratrol (Fig. 2A). Resveratrol was less cytotoxic in HT-29 cells than in SNU-1 cells.
Fig. 2. Resveratrol-induced cytotoxicity in SNU-1 cells (A) and HT-29 cells (B). (A) Cytotoxicity in SNU-1 cells was determined upon exposure to 10, 50, and 100 μM resveratrol for 24 hr. (B) Resveratrol and DMS concentration response in HT-29 cells. Control cells were exposed to ethanol. The cytotoxicity of resveratrol (50 and 100 μM) combined with DMS (5 and 10 μM) was measured at 24 hr using WST-1 or flow cytometry.
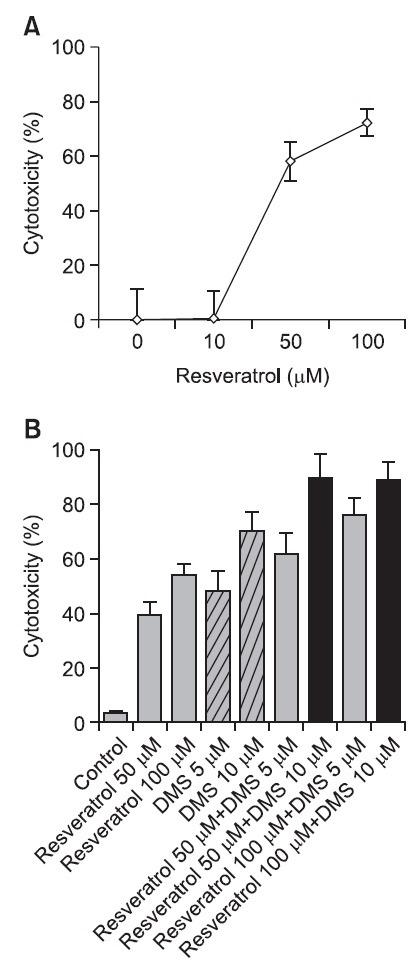
Dimethylsphingosine (DMS) has long been used as a pharmacological inhibitor of sphingosine kinase (Sphk), which synthesizes an anti-apoptotic lipid mediator, sphingosine 1-phosphate (S1P) (Yatomi et al., 1996). In this experimental system, exposure to DMS also triggered cytotoxicity in a concentration-dependent manner. Moreover, resveratrol combined with 5 or 10 μM DMS strongly increased cytotoxicity in HT29 cells (Fig. 2B). The enhancement of resveratrol activity by DMS suggests that resveratrol and DMS may be part of a common apoptotic signaling pathway. Alternatively, resveratrol may inhibit the specific step of sphingolipid metabolism
that regulates the biosynthesis of S1P, or that of other bioactive sphingolipids, such as ceramides.
Effect of resveratrol on SNU-668 cells
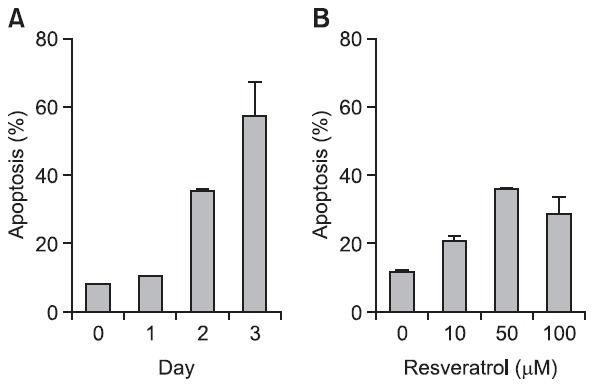
Cytotoxicity was further measured by PI and annexin-V staining in SNU-668 cells. Resveratrol-induced cytotoxicity was lower in these cells than in SNU-1 or HT-29 cells (Fig. 3A), in which exposure to 100 μM resveratrol for 3 days led to approximately 60% cytotoxicity. Furthermore, exposure of SNU-668 cells to 0-100 μM resveratrol for 48 hr showed a lack of concentration response (Fig. 3B), and cytotoxicity did not exceed 40%. The mechanisms underlying the different sensitivity of cell types to resveratrol remain to be understood.
Fig. 3. SNU-668 cells are resistant to resveratrol. (A) Apoptosis was determined upon exposure to 100 μM resveratrol for 3 days. (B) Prolonged exposure to 10, 50, and 100 μM resveratrol for 48 hr.
Analysis of Cer and DHCer induction by resveratrol
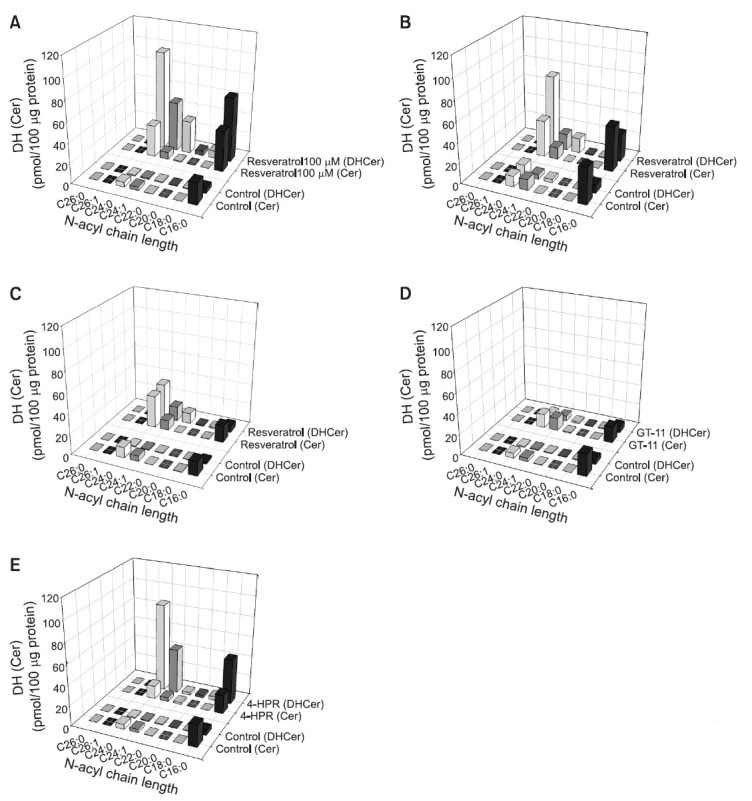
To determine which factors in sphingolipid metabolism correlate with cytotoxicity, metabolic intermediates, including Cer and DHCer, were analyzed by LC-MS/MS. SNU-1, HT-29, and SNU-668 cells were exposed to 100 μM resveratrol for 24 hr.
As expected from the fact that resveratrol is a partial inhibitor of DHCer desaturase, DHCer accumulation increased, except in SNU-668 cells (Fig. 4A-C). Unexpectedly, the concentration of Cer, the final product of DHCer desaturase, was significantly increased, although DHCer accumulation was high (Fig. 4A-C). The increased DHCer levels in SNU-1 cells were as follows: DHCer containing fatty acid chain C24:0 > C16:0 > C24:1 > C22:0. Specifically, C24:0 DHCer was increased 97-fold by 100 μM resveratrol in SNU-1 cells. Similarly, C24:0 DHCer was increased 30-fold in HT-29 cells, and 10-fold in SNU-668 cells, indicating that C24:0 DHCer correlated with cytotoxicity and may be a novel cytotoxic biomarker. The C24:0 Cer levels were also increased by 5-10 times in the cancer cells tested. Next, to assess resveratrol-induced cytotoxicity in relation to sphingolipid metabolism, changes in Cer and DHCer were investigated in the presence of the pharmacological DHCer desaturase inhibitors GT-11 and 4-HPR. GT-11 was first synthesized as a specific inhibitor of DHCer desaturase (Triola et al., 2004). A therapeutic agent in clinical evaluation in a variety of cancers, 4-HPR, was recently reported to inhibit DHCer desaturase activity (Apraiz et al., 2012). Interestingly, 5 μM GT-11 failed to accumulate C24:0 DHCer and C24:0 Cer in SNU-1 cells (Fig. 4D). By contrast, 10 μM 4-HPR effectively accumulated C24:0 DHCer, as did resveratrol in SNU-1 cells (Fig. 4E).
Fig. 4. Accumulation of Cer and DHCer by resveratrol. SNU-1 cells were exposed to 100 μM resveratrol (A) in combination with the indicated agents (GT-11: 5 μM (D); and 4-HRP: 10 μM (E)) for 24 hr. HT-29 cells (B) and SNU-668 cells (C) were exposed to 100 μM resveratrol for 24 hr. Cer and DHCer were analyzed by LC-MS/MS.
Inhibition of DHCer desaturase activity by resveratrol
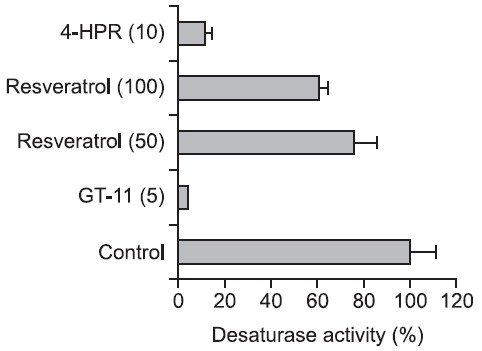
LC-MS/MS was used to measure DHCer desaturase activity and determine the amount of newly synthesized Cer from DHCer substrate. GT-11 and 4-HPR strongly inhibited DHCer desaturase activity: 10 μM 4-HPR and 5 μM GT-11 yielded 85% and 92% inhibition, respectively. Resveratrol also reduced DHCer desaturase activity in a concentration-dependent manner between 50 and 100 μM, which was less effective (Fig. 5). These results suggest that the resveratrol induced cytotoxicity is not simply explained by specific inhibition of DHCer desaturase activity; however, the accumulation of DHCer correlated strongly with resveratrol sensitivity.
Fig. 5. Inhibition of DHCer desaturase activity by resveratrol, GT-11, and 4-HPR. Rat liver microsome was prepared and incubated with resveratrol, GT-11, and 4-HPR, by mixing with an NADPH-regenerating system for 1 hr. Concentrations (μM) are given in parentheses.
Effect of resveratrol on the cell cycle
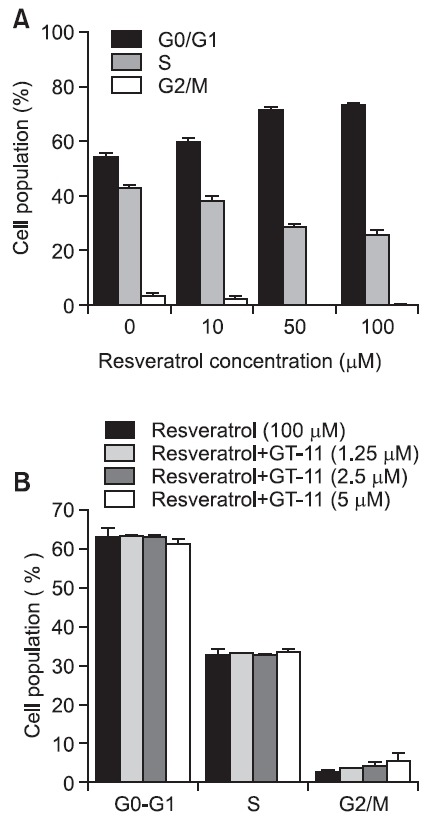
The effect of resveratrol on DNA synthesis was assessed by analyzing the cell cycle in SNU-1 cells. SNU-1 cells were exposed to 0, 10, 50, and 100 μM resveratrol for 24 hr. The
cell distribution in the G0/G1 phase increased in a concentration-dependent manner, while that in the S and G2/M phases decreased (Fig. 6A). The combination of resveratrol with 1.25, 2.5, or 5 μM GT-11 for 24 hr in SNU-1 cells was not additive (Fig. 6B). Additional incubation for 48 and 72 hr also failed to alter the cell cycle (data not shown). Therefore, the regulation of the cell cycle is also associated with DHCer concentration, not simply DHCer desaturase activity.
Fig. 6. Resveratrol decreased the number of SNU-1 cells in S and G2/M phase. (A) SNU-1 cells were incubated with 10, 50, and 100 μM resveratrol for 24 hr. (B) GT-11 (5 μM) did not influence the cell cycle distribution induced by resveratrol (100 μM).
p53 expression by resveratrol
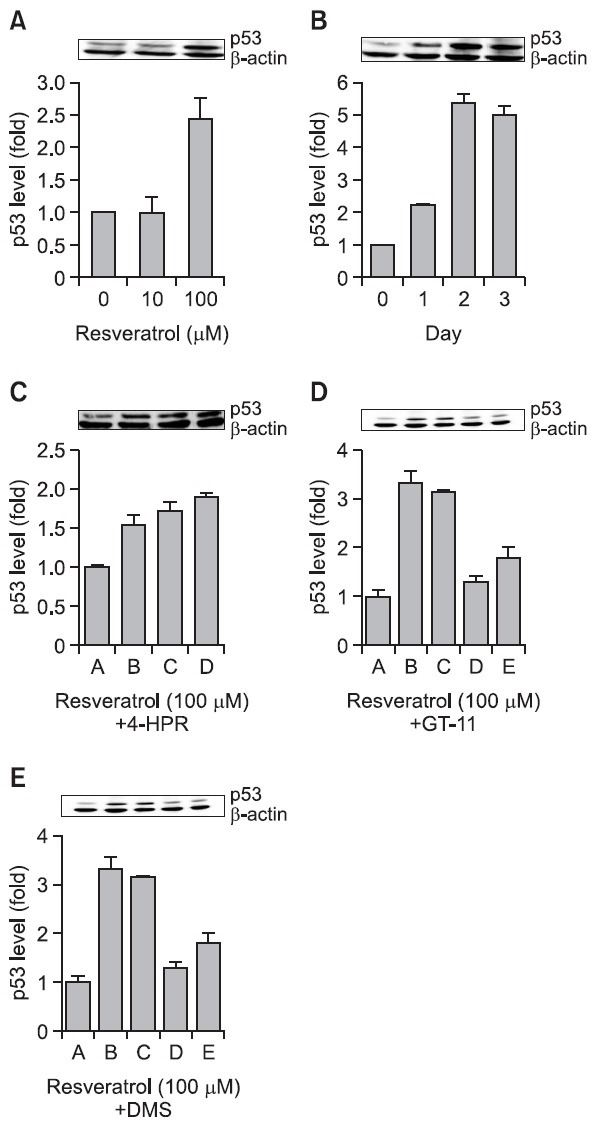
In cancer cells, resveratrol lowers Bcl-2 expression, activates proapoptotic Bax in a p53-dependent manner, and promotes cytochrome c release from mitochondria (She et al., 2001). p53 is a tumor suppressor protein that modulates both the cell cycle and apoptosis in a variety of cells (Santoro and Blandino, 2010). The maximum increase in p53 protein expression in SNU-1 cells was achieved upon exposure to 100 mM resveratrol (Fig. 7A) for 2 days (Fig. 7B). GT-11 combined with 100 μM resveratrol did not enhance the p53 expression level induced by 100 μM resveratrol, although GT-11 itself showed a little enhancement of p53 protein expression (Fig. 7C); however, 4-HPR enhanced the p53 expression initially activated by 100 μM resveratrol (Fig. 7D). Interestingly, the Sphk inhibitor DMS, in combination with 50 μM resveratrol, also activated p53 (Fig. 7E). These results demonstrate that DMS and 4-HPR can effectively target the enzymes involved in sphingolipid metabolism.
Fig. 7. Resveratrol increased the expression of the tumor-suppressor p53 protein in SNU-1 cells. (A) Cells were exposed to 0, 10, 100, and 200 μM resveratrol for 24 hr. (B) Cells were exposed to 100 μM resveratrol for 1-3 days. (C) SNU-1 cells were exposed to 100 μM resveratrol [B], 100 μM resveratrol with 1 μM 4-HPR [C], 100 μM resveratrol with 3 μM 4-HPR [D], 100 μM resveratrol with 5 μM 4-HPR [E] for 24 hr. (D) SNU-1cells were exposed to 100 μM resveratrol [B], 100 μM resveratrol with 1 μM GT-11 [C], 100 μM resveratrol with GT-11 [D], 100 μM resveratrol with 5 μM GT-11 [E] for 24hr. (E) SNU-1 cells were exposed to 100 μM resveratrol [B], 100 μM resveratrol with 1.25 μM DMS [C], 100 μM resveratrol with 2.5 μM DMS [D], and 100 μM resveratrol with 5.0 μM DMS [E] for 24 hr. The proteins were visualized by 10% SDS-PAGE and immunoblotting. The expression level of p53 was normalized to β-actin. The densitometry quantification data are expressed as the mean ± SD of three different experiments.
DISCUSSION
Resveratrol (3,5,4'-trihydroxy-trans stilbene) has emerged as a promising chemopreventive agent, although its pharmacokinetics in humans, even at high dose, do not permit the concentrations required for systemic prevention of cancer (Maier-Salamon et al., 2011). This report suggests that the efficacy of resveratrol may be enhanced upon combination with agents that increase DHCer levels in human gastric cancer SNU-1 cells and colon adenocarcinoma HT-29 cells. Indeed, cytotoxicity increased when resveratrol was combined with the Sphk inhibitor DMS (Fig. 2B). DMS blocks sphingosine 1-phosphate (S1P) synthesis, which exhibits an anti-apoptotic function and increases sphingosine and Cer, which act as pro-apoptotic lipids on the cell surface (Miyaji et al., 2005). Therefore, Spiegel group suggested that the balance of anti-apoptotic and pro-apoptotic lipids was an important regulator of cell fate (Edsall et al., 1998); however, our results indicate that resveratrol-induced cytotoxicity was cell-specific. For example, in SNU-668 gastric carcinoma cells, resveratrol did not show the strong cytotoxic activity observed in SNU-1 and HT-29 cells (Fig. 3A). The too much disintegrated cell debris by 100 μM resveratrol may not be counted as cell number in flow cytometric analysis (Fig. 3B).
Previously, resveratrol was thought to activate de novo sphingolipid biosynthesis and increase Cer production, thereby contributing to growth arrest and apoptosis induction (Scarlatti et al., 2003). Here, results suggest that the different sensitivity of cancer cells to resveratrol might be deeply related to sphingolipid, especially DHCer, distribution patterns. The LC-MS/MS analytical profile of various classes of Cer and DHCer altered by resveratrol suggests that the accumulation of DHCer, especially C24:0 DHCer, may be a reliable novel biomarker of cytotoxicity (Fig. 4A-C). Our experimental data show that the amounts of C24:0 DHCer were increased by as much as 97-fold, 30-fold, and 10-fold in SNU-1, HT-29, and SNU-668 cells, respectively. The order of resveratrol sensitivity was exactly correlated to the accumulation of DHCer. We do not exclude the possibility of the involvement of Cer accumulation in resveratrol sensitivity because an approximate 10-fold Cer increase was also observed in resveratrol-sensitive cells (SNU-1 and HT-29 cells); however, the accumulation of various types of DHCer (containing fatty acid chain C24:0, C16:0, C24:1, and C22:0) was clearly correlated with cytotoxicity in SNU-1 and HT-29 cells.
We further investigated whether the inhibition of DHCer desaturase may be a direct factor in cytotoxicity. Unexpectedly, GT-11, a specific lipid inhibitor of DHCer desaturase, did not lead to DHCer accumulation and thus no cytotoxicity was detected in SNU-1 cells, although GT-11 strongly inhibited DHCer desaturase activity (Fig. 4D and Fig. 5). In comparing GT-11 activity to that of 4-HPR, another DHCer desaturase inhibitor, strong accumulation of DHCer and greater cytotoxicity were observed in SNU-1 cells with 4-HPR (Fig. 4E and Fig. 5). These results suggest that the specific inhibition of DHCer desaturase is not a critical mechanism, but that the accumulation of DHCer is an essential marker of cytotoxicity. Actually, even though 100 μM resveratrol mildly inhibited DHCer desaturase, the accumulation of DHCer was nearly equal to that generated by 10 μM 4-HPR (Fig. 4A, B, E). Therefore, resveratrol-induced DHCer accumulation partially involves DHCer desaturase inhibition, but DHCer desaturase inhibition is not an essential factor in cytotoxicity.
A number of cancer cells respond to resveratrol by cell cycle arrest. Resveratrol decreased the S and G2/M phases and increased the G0/G1 phase at 0, 20, 50, and 100 μM for 24 hr (Fig. 6A). This inhibition pattern is a typical cell cycle arrest pattern in response to anti-cancer agents, and is also observed upon exposure of LNCaP prostate cancer cells to resveratrol (Narayanan et al., 2003). The specific DHCer desaturase inhibitor GT-11 did not further enhance the cell cycle changes, indicating that DHCer desaturase is not involved in the cell cycle arrest (Fig. 6B).
The tumor-suppressor protein, p53, is a significant indicator of response to resveratrol (Hsieh et al., 1999). Resveratrol increased p53 protein expression in a time- and concentration-dependent manner (Fig. 7A and B). GT-11 failed to increase resveratrol-induced p53 expression, indicating that DHCer inhibition is not directly related to p53 expression, while GT-11 mildly increased p53 expression (Fig. 7C). By contrast, 4-HPR or DMS in combination with resveratrol clearly increased p53 expression (Fig. 7D and E).
In this report, the accumulation of DHCer with different fatty acid chain lengths was strongly associated with resveratrol-induced cell cytotoxicity. The effect of resveratrol was significantly increased by DMS, a nonspecific Sphk inhibitor, and by 4-HPR, a nonspecific DHCer desaturase inhibitor, but not by GT-11, a specific DHCer desaturase. We further found that cytotoxicity, cell cycle arrest, and the expression of p53 by resveratrol were enhanced by DMS or 4-HPR. Other resveratrol properties, such as its anti-oxidant, anti-angiogenic, and calorie restriction activity, should be further investigated.
Acknowledgments
This work was supported by the research grant of the Chungbuk National University 2009.
References
- 1.Apraiz A., Idkowiak-Baldys J., Nieto-Rementeria N., Boyano M. D., Hannun Y. A., Asumendi A. Dihydroceramide accumulation and reactive oxygen species are distinct and nonessential events in 4-HPR-mediated leukemia cell death. Biochem. Cell Biol. (2012);90:209–223. doi: 10.1139/o2012-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aziz M. H., Reagan-Shaw S., Wu J., Longley B. J., Ahmad N. Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease? FASEB J. (2005);19:1193–1195. doi: 10.1096/fj.04-3582fje. [DOI] [PubMed] [Google Scholar]
- 3.Dudley J. I., Lekli I., Mukherjee S., Das M., Bertelli A. A., Das D. K. Does white wine qualify for French paradox? Comparison of the cardioprotective effects of red and white wines and their constituents: resveratrol, tyrosol, and hydroxytyrosol. J. Agric. Food Chem. (2008);56:9362–9373. doi: 10.1021/jf801791d. [DOI] [PubMed] [Google Scholar]
- 4.Edsall L. C., Van Brocklyn J. R., Cuvillier O., Kleuser B., Spiegel S. N,N-Dimethylsphingosine is a potent competitive inhibitor of sphingosine kinase but not of protein kinase C: modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochemistry. (1998);37:12892–12898. doi: 10.1021/bi980744d. [DOI] [PubMed] [Google Scholar]
- 5.Fukui M., Yamabe N., Zhu B. T. Resveratrol attenuates the anticancer efficacy of paclitaxel in human breast cancer cells in vitro and in vivo. Eur. J. Cancer. (2010);46:1882–1891. doi: 10.1016/j.ejca.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorelik S., Ligumsky M., Kohen R., Kanner J. A novel function of red wine polyphenols in humans: prevention of absorption of cytotoxic lipid peroxidation products. FASEB J. (2008);22:41–46. doi: 10.1096/fj.07-9041com. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh T. C., Juan G., Darzynkiewicz Z., Wu J. M. Resveratrol increases nitric oxide synthase, induces accumulation of p53 and p21(WAF1/CIP1), and suppresses cultured bovine pulmonary artery endothelial cell proliferation by perturbing progression through S and G2. Cancer Res. (1999);59:2596–2601. [PubMed] [Google Scholar]
- 8.Idkowiak-Baldys J., Apraiz A., Li L., Rahmaniyan M., Clarke C. J., Kraveka J. M., Asumendi A., Hannun Y. A. Dihydroceramide desaturase activity is modulated by oxidative stress. Biochem. J. (2010);427:265–274. doi: 10.1042/BJ20091589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maier-Salamon A., Böhmdorfer M., Thalhammer T., Szekeres T., Jaeger W. Hepatic glucuronidation of resveratrol: interspecies comparison of enzyme kinetic profiles in human, mouse, rat, and dog. Drug Metab. Pharmacokinet. (2011);26:364–373. doi: 10.2133/dmpk.DMPK-11-RG-006. [DOI] [PubMed] [Google Scholar]
- 10.Miyaji M., Jin Z. X., Yamaoka S., Amakawa R., Fukuhara S., Sato S. B., Kobayashi T., Domae N., Mimori T., Bloom E. T., Okazaki T., Umehara H. Role of membrane sphingomyelin and ceramide in platform formation for Fas-mediated apoptosis. J. Exp. Med. (2005);202:249–259. doi: 10.1084/jem.20041685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narayanan B. A., Narayanan N. K., Re G. G., Nixon D. W. Differential expression of genes induced by resveratrol in LNCaP cells: P53-mediated molecular targets. Int. J. Cancer. (2003);104:204–212. doi: 10.1002/ijc.10932. [DOI] [PubMed] [Google Scholar]
- 12.Nutakul W., Sobers H. S., Qiu P., Dong P., Decker E. A., McClements D. J., Xiao H. Inhibitory effects of resveratrol and pterostilbene on human colon cancer cells: a side-by-side comparison. J. Agric. Food Chem. (2011);59:10964–10970. doi: 10.1021/jf202846b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santandreu F. M., Valle A., Oliver J., Roca P. Resveratrol potentiates the cytotoxic oxidative stress induced by chemotherapy in human colon cancer cells. Cell Physiol. Biochem. (2011);28:219–228. doi: 10.1159/000331733. [DOI] [PubMed] [Google Scholar]
- 14.Santoro R., Blandino G. p53: The pivot between cell cycle arrest and senescence. Cell Cycle. (2010);9:4262–4263. doi: 10.4161/cc.9.21.13853. [DOI] [PubMed] [Google Scholar]
- 15.Scarlatti F., Sala G., Somenzi G., Signorelli P., Sacchi N., Ghidoni R. Resveratrol induces growth inhibition and apoptosis in metastatic breast cancer cells via de novo ceramide signaling. FASEB J. (2003);17:2339–2341. doi: 10.1096/fj.03-0292fje. [DOI] [PubMed] [Google Scholar]
- 16.Schulze H., Michel C., van Echten-Deckert G. Dihydroceramide desaturase. Methods Enzymol. (2000);311:22–30. doi: 10.1016/S0076-6879(00)11063-8. [DOI] [PubMed] [Google Scholar]
- 17.She Q. B., Bode A. M., Ma W. Y., Chen N. Y., Dong Z. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res. (2001);61:1604–1610. [PubMed] [Google Scholar]
- 18.Signorelli P., Munoz-Olaya J. M., Gagliostro V., Casas J., Ghidoni R., Fabriàs G. Dihydroceramide intracellular increase in response to resveratrol treatment mediates autophagy in gastric cancer cells. Cancer Lett. (2009);282:238–243. doi: 10.1016/j.canlet.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Spassieva S. D., Rahmaniyan M., Bielawski J., Clarke C. J., Kraveka J. M., Obeid L. M. Cell density-dependent reduction of dihydroceramide desaturase activity in neuroblastoma cells. J. Lipid. Res. (2012);53:918–928. doi: 10.1194/jlr.M019075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Triola G., Fabrias G., Dragusin M., Niederhausen L., Broere R., Llebaria A., van Echten-Deckert G. Specificity of the dihydroceramide desaturase inhibitor N-[(1R,2S)-2-hydroxy-1-hydroxymethyl-2-(2-tridecyl-1-cyclopropenyl)ethyl]octanami de (GT11) in primary cultured cerebellar neurons. Mol. Pharmacol. (2004);66:1671–1678. doi: 10.1124/mol.104.003681. [DOI] [PubMed] [Google Scholar]
- 21.Ulrich S., Huwiler A., Loitsch S., Schmidt H., Stein J. M. De novo ceramide biosynthesis is associated with resveratrol-induced inhibition of ornithine decarboxylase activity. Biochem. Pharmacol. (2007);74:281–289. doi: 10.1016/j.bcp.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Yatomi Y., Ruan F., Megidish T., Toyokuni T., Hakomori S., Igarashi Y. N,N-dimethylsphingosine inhibition of sphingosine kinase and sphingosine 1-phosphate activity in human platelets. Biochemistry. (1996);35:626–633. doi: 10.1021/bi9515533. [DOI] [PubMed] [Google Scholar]


