Abstract
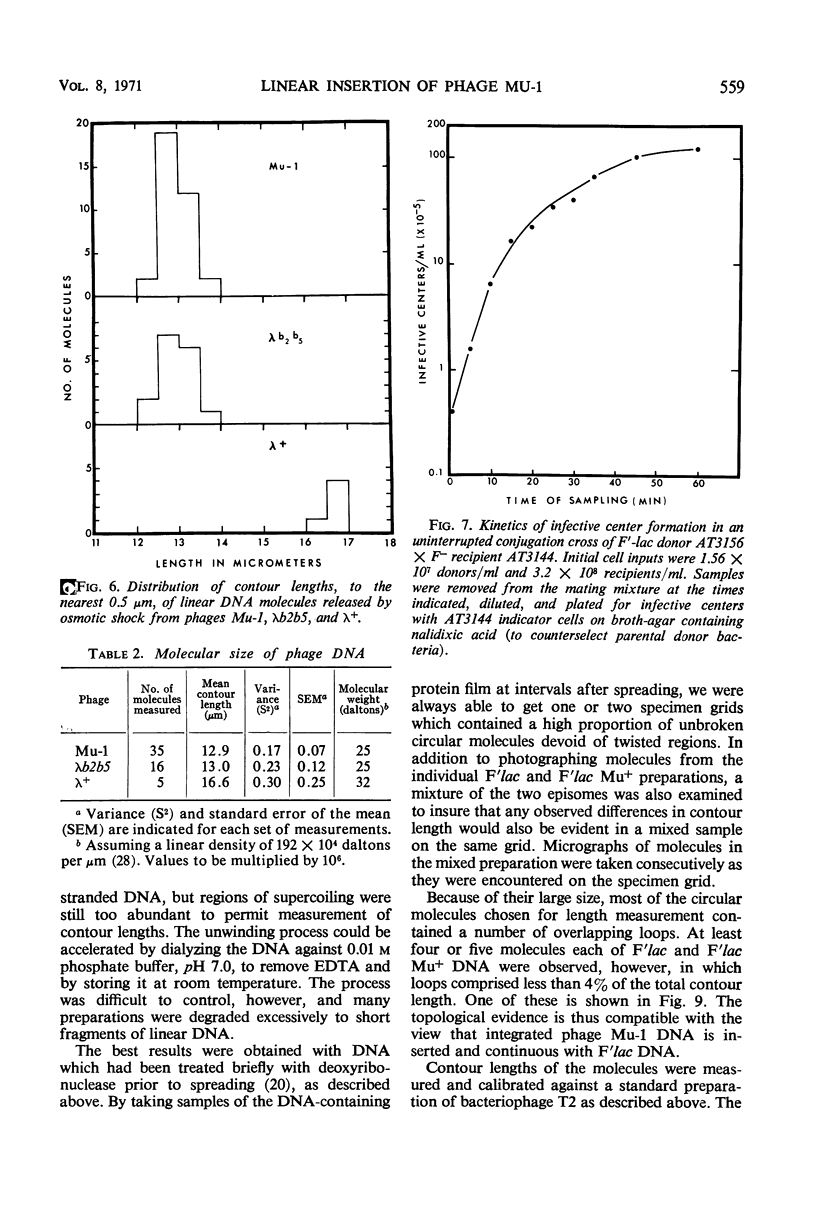
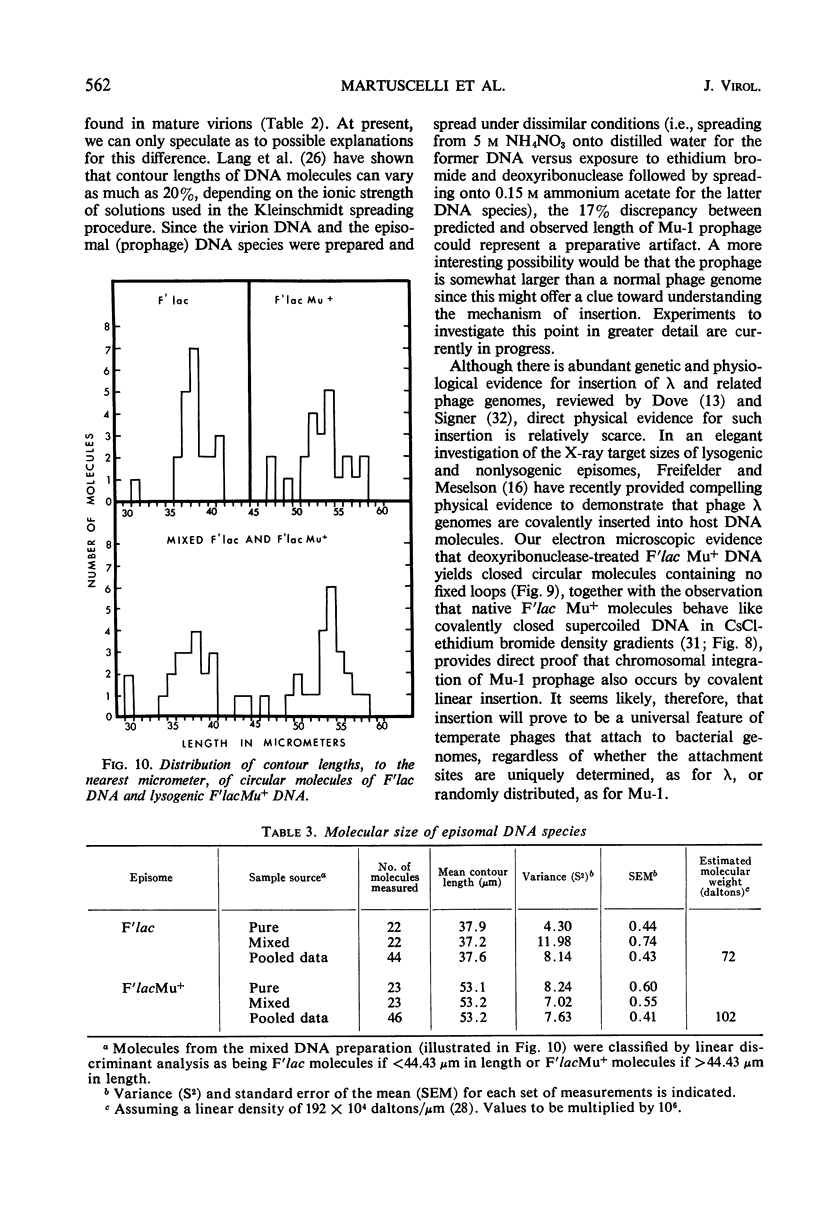
Temperate bacteriophage Mu-1 was used to generate a lysogenic derivative of the F′lac episome of Escherichia coli. Intact, covalently circular molecules of F′lac and lysogenic F′lac Mu+ deoxyribonucleic acid (DNA) were isolated and examined by electron microscopy. The mean contour lengths of F′lac and F′lac Mu+ molecules were 37.6 ± 0.4 μm and 53.2 ± 0.4 μm, respectively. The mean difference, 15.6 μm, is similar to the mean contour length of 12.9 ± 0.1 μm obtained for linear DNA molecules released by osmotic shock from mature phage Mu-1 virions. These results provide direct physical evidence that phage Mu-1 integrates by linear insertion of its genome into the DNA of lysogenic host bacteria. Chemical and physical analyses of phage Mu-1 DNA indicate that it is similar to E. coli DNA in respect of gross base composition, buoyant density, and melting temperature.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., BURNS S. N. Genetic variation in the sex factor of Escherichia coli. J Bacteriol. 1960 Mar;79:321–330. doi: 10.1128/jb.79.3.321-330.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S. I., Pritchard R. H. A map of four genes specifying enzymes involved in catabolism of nucleosides and deoxynucleosides in Escherichia coli. Mol Gen Genet. 1969 Aug 15;104(4):351–359. doi: 10.1007/BF00334234. [DOI] [PubMed] [Google Scholar]
- Ansz H. S., Zandberg J., van de Pol J. H., van Bruggen E. F. Circular DNA from Shigella paradysenteriae. Eur J Biochem. 1969 Jun;9(2):156–159. doi: 10.1111/j.1432-1033.1969.tb00589.x. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- CARO L. G. THE MOLECULAR WEIGHT OF LAMBDA DNA. Virology. 1965 Feb;25:226–236. doi: 10.1016/0042-6822(65)90201-1. [DOI] [PubMed] [Google Scholar]
- CHARGAFF E., LIPSHITZ R., GREEN C., HODES M. E. The composition of the deoxyribonucleic acid of salmon sperm. J Biol Chem. 1951 Sep;192(1):223–230. [PubMed] [Google Scholar]
- Cummings D. J., Kusy A. R., Chapman V. A., DeLong S. S., Stone K. R. Characterization of T-even bacteriophage substructures. I. Tail fibers and tail tubes. J Virol. 1970 Oct;6(4):534–544. doi: 10.1128/jvi.6.4.534-544.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Mondale L. Density-gradient banding of denatured deoxyribonucleic acid in cesium sulphate. Biochim Biophys Acta. 1966 Jul 13;120(3):448–453. doi: 10.1016/0926-6585(66)90311-6. [DOI] [PubMed] [Google Scholar]
- Freifelder D., Meselson M. Topological relationship of prophage lambda to the bacterial chromosome in lysogenic cells. Proc Natl Acad Sci U S A. 1970 Jan;65(1):200–205. doi: 10.1073/pnas.65.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Studies with Escherichia coli sex factors. Cold Spring Harb Symp Quant Biol. 1968;33:425–434. doi: 10.1101/sqb.1968.033.01.049. [DOI] [PubMed] [Google Scholar]
- Hane M. W., Wood T. H. Escherichia coli K-12 mutants resistant to nalidixic acid: genetic mapping and dominance studies. J Bacteriol. 1969 Jul;99(1):238–241. doi: 10.1128/jb.99.1.238-241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan E., Saedler H., Starlinger P. O0 and strong-polar mutations in the gal operon are insertions. Mol Gen Genet. 1968;102(4):353–363. doi: 10.1007/BF00433726. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER G., ZICHICHI M. L., WEIGLE J. J. Exchange of DNA in the recombination of bacteriophage lambda. Proc Natl Acad Sci U S A. 1961 Jun 15;47:869–878. doi: 10.1073/pnas.47.6.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEINSCHMIDT A. K., LANG D., JACHERTS D., ZAHN R. K. [Preparation and length measurements of the total desoxyribonucleic acid content of T2 bacteriophages]. Biochim Biophys Acta. 1962 Dec 31;61:857–864. [PubMed] [Google Scholar]
- Kemp C. L., Howatson A. F., Siminovitch L. Electron microscopy studies of mutants of lambada bacteriophage. I. General description and quantitation of viral products. Virology. 1968 Nov;36(3):490–502. doi: 10.1016/0042-6822(68)90174-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lang D., Bujard H., Wolff B., Russell D. Electron microscopy of size and shape of viral DNA in solutions of different ionic strengths. J Mol Biol. 1967 Jan 28;23(2):163–181. doi: 10.1016/s0022-2836(67)80024-x. [DOI] [PubMed] [Google Scholar]
- MACHATTIE L. A., THOMAS C. A., Jr DNA FROM BACTERIOPHAGE LAMBDA: MOLECULAR LENGTH AND CONFORMATION. Science. 1964 May 29;144(3622):1142–1144. doi: 10.1126/science.144.3622.1142. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Signer E. R. Lysogeny: the integration problem. Annu Rev Microbiol. 1968;22:451–488. doi: 10.1146/annurev.mi.22.100168.002315. [DOI] [PubMed] [Google Scholar]
- TAYLOR A. L. BACTERIOPHAGE-INDUCED MUTATION IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Dec;50:1043–1051. doi: 10.1073/pnas.50.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To C. M., Eisenstark A., Töreci H. Structure of mutator phage Mu1 of Escherichia coli. J Ultrastruct Res. 1966 Mar;14(5):441–448. doi: 10.1016/s0022-5320(66)80074-6. [DOI] [PubMed] [Google Scholar]
- Torti F., Barksdale C., Abelson J. Mu-1 bacteriophage DNA. Virology. 1970 Jul;41(3):567–568. doi: 10.1016/0042-6822(70)90179-0. [DOI] [PubMed] [Google Scholar]
- Toussaint A. Insertion of phage Mu. 1 within prophage lambda. A new approach for studying the control of the late functions in bacteriophage lambda. Mol Gen Genet. 1969;106(1):89–92. doi: 10.1007/BF00332824. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., BRUNER R., KENT R., WEIGLE J. Band-centrifugation of macromolecules and viruses in self-generating density gradients. Proc Natl Acad Sci U S A. 1963 Jun;49:902–910. doi: 10.1073/pnas.49.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLSON C., PERRIN D., COHN M., JACOB F., MONOD J. NON-INDUCIBLE MUTANTS OF THE REGULATOR GENE IN THE "LACTOSE" SYSTEM OF ESCHERICHIA COLI. J Mol Biol. 1964 Apr;8:582–592. doi: 10.1016/s0022-2836(64)80013-9. [DOI] [PubMed] [Google Scholar]
- WYATT G. R. The purine and pyrimidine composition of deoxypentose nucleic acids. Biochem J. 1951 May;48(5):584–590. doi: 10.1042/bj0480584. [DOI] [PMC free article] [PubMed] [Google Scholar]






