Abstract
Actoprotectors are preparations that enhance body stability against physical loads without increasing oxygen consumption or heat production. Or, in short, actoprotectors are synthetic adaptogens with a significant capacity to improve physical performance. This paper explores the history of actoprotectors’development, their pharmacological properties, mechanism of action, and practical application to the improvement of mental and physical performance. A brief summary of the clinico-pharmacological characteristics of the main representatives of this class (bemitil and bromantane) is provided. Some other synthesized compounds, and even natural ones such as ginseng, also are regarded as potential actoprotectors, and these are treated herein as well. Actoprotectors, owing to their wide-ranging pharmacological activities, high efficiency and safety, can be applied under either normal or extreme conditions.
Keywords: Actoprotector, Bemitil, Bromantane, Mental work capacity, Asthenia
INTRODUCTION
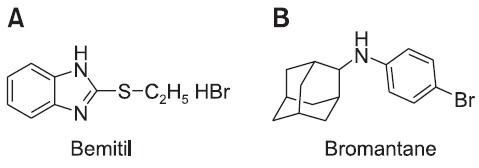
Investigations into a new class of pharmacologically active substances for improvement of physical and mental efficiency in humans, namely actoprotectors, were carried out under Professor Vladimir Vinogradov at the Military Medical Academy (then Leningrad, USSR; now, St. Petersburg, Russia)’s Department of Pharmacology throughout the 1970s. This work resulted in the development of the first and most commonly used actoprotector, bemitil (chemical structure: 2-ethylbenzimidazole hydrobromide, (Fig. 1A); English-language literature: “bemithil”, “bemithyl” or “bemethyl”; also known as “bemactor” and “metaprot” in later publications). This achievement earned Professor Vinogradov and his research team the State Prize of the USSR. Other actoprotectors subsequently were formulated as well, the most important of which, from the practical point of view, being bromantane (Fig. 1B).
Fig. 1. Chemical structures of most common actoprotectors, (A) bemitil (2-ethylthiobenzimidazole hydrobromide), (B) bromantane (N-(2-adamantil)-N-(para-bromophenyl)-amine).
The first recipients of bemitil were Soviet cosmonaughts. Bemitil also was successfully employed in preparing the athletes of the USSR’s national team for the 1980 Olympic Games held in Moscow. Later, throughout the 1990s, it was used as a basic medicinal agent in almost all of the corps of the Soviet and then Russian armies. Notably, its administration made it possible to increase soldiers’ endurance over long marches; in the Air Forces, Missile Troops, and Army Air Defense, it enhanced work capacity and stability to hypoxia; and in the Navy, it reinforced stability to hypoxia and, where applicable, high temperatures. The latter property, in fact, had determined its wide use by the “limited contingent” of Soviet troops in Afghanistan. Bemitil enabled soldiers, including Special Forces, to effectively perform combat missions under both hypoxic and high-temperature conditions. Bemitil’s effectiveness for various types of activities was shown also in its enhancement of the physical and mental capacities of rescue and other workers deployed in the wakes of the Chernobyl catastrophe (1986), the earthquakes in Armenia (1988), and the railway accidents in Bashkiria (1989) (Shabanov, 2009a). Bromantane also was employed in the Soviet and Russian armies, to shorten recovery times after strong physical exertion, though not as widely as bemitil.
After the disintegration of the USSR in 1991, the official manufacture and clinical use of bemitil was discontinued. However, owing to its wide-ranging pharmacological activity, high efficiency, and safety, its initial sports and military medicine applications have been extended widely to other branches of practical medicine. As for bromantane, its production continued after 1991, though its applications were limited, primarily, to sports medicine. Nowadays, bemitil is manufactured in Ukraine (commercial name: Antihot) and is widely used in preparing Ukrainian national sport teams for international competitions. Bromantane is manufactured in Russia (commercial name: Ladasten); since 1997, anti-doping regulations have prohibited its use in sports, though it has recently been utilized in the treatment of patients with asthenic and restless-asthenic
frustration (Akilov et al., 2007).
DEFINITION AND CLASSIFICATION OF ACTOPROTECTORS
Actoprotectors are preparations that enhance body stability against physical loads without increasing oxygen consumption or heat production. Actoprotectors comprehend metabolic drugs of a non-consumptive class of action, which to greater or lesser extents can possess antihypoxic activity. They differ from antihypoxants, however, in that they primarily (directly) stimulate protein synthesis and increase working capacity. Moreover, under hypoxic conditions, they exert an antihypoxic influence that can become stronger as a result of mitochondrion-decreased ability to oxidize substrates under higher physical loads, but they do not function in this way in other etiologies.
The principal difference between actoprotectors and psychostimulants (e.g. caffeine, sydnocarb, phenamine, methylphenidate, modafinil, adrafinil, armodafinil) is that actoprotectors are agents of non-exhaustive action. With actoprotectors, there is no increase in oxygen consumption or heat production; and, unlike nootropic agents – actoprotectors increase not only mental (intellectual) but also, and primarily, physical work capacity.
The distinction between actoprotectors and adaptogens is not straightforward. Their respective characteristics show many similarities and even identities. It was contended that the separation of actoprotectors as a new class of pharmacological compound was not justified theoretically, that is, that this classification was the result of the practical requirements of military medicine. Agents in the actoprotector class can reasonably be referred to as synthetic adaptogens, and their strong actoprotective effect can be regarded as a component of their adaptogenic action.
Our own opinion is that actoprotectors, most logically, should be regarded as synthetic adaptogens having a strong positive influence on physical work capacity. This means that for pharmacological classification convenience, some synthetic adaptogens that significantly enhance physical performance
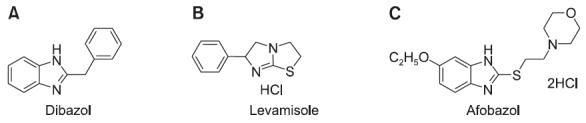
can be termed “actoprotectors” but that other synthetic adaptogens cannot. For example, benzimidazole derivatives dibazol (bendazol), levamisole and afobazol (Fig. 2), in the literature, have been considered to be adaptogens. Dibazol’s adaptogenic action was first realized in the context of immune-mechanism-enabled adaptation to difficult environmental conditions (Rusin, 1962a, 1962b, 1963a, 1963b, 1963c, 1967; Ratnikov and Nesterenko, 1984; Novikov and Bortnovskii, 1985; Udintsev et al., 1991; Sidorova and Kiikova, 2000); levamisole’s adaptogenic activity also is connected primarily with adaptive immune system changes (Voskanian et al., 1986; Alvarez-Pellitero et al., 2006; Chen et al., 2008; Fabrizi et al., 2010); afobazol has neuroprotective properties established, in vitro, by survival of HT-22 neurons in a model of oxidative stress and glutamate toxicity (Zenina et al., 2005), and its adaptogenic action is realized through central nervous system adaptation (Uyanaev and Fisenko, 2006; Litvintsev et al., 2007; Bogdan et al., 2011). As reflects their adaptogenic properties, benzimidazole derivatives are related to bemitil, but their influence on physical work capacity is either absent or minimal. In light of this fact, they cannot be referenced as actoprotectors. A concise definition of actoprotectors, in our opinion, is as follows: “synthetic adaptogens with a significant capacity to increase physical performance”.
Fig. 2. Chemical structures of three synthetic benzimidazole-derivative adaptogens: (A) dibazol (2-benzyl-benzimidazole), (B) levamisole ((-)-2,3,5,6-tetrahydro-6-phenylimidazo[2,1-b]thiazole hydrochloride), (C) afobazol (5-ethoxy-2-[2-(morpholino)-ethylthio]benzimidazole).
Further characteristics of actoprotector action provieded are as follows:
1. These agents have minimal pharmacological activity, which explains why the mechanism of their action is difficult to correlate with their influence on some concrete types of pharmacological receptors;
2. The efficacy of these drugs for rapid recovery is maximal only when they are administered immediately after exposure to extreme conditions;
3. The strongest effect of actoprotectors is observed in persons with low or middle resistance to extreme conditions, and they are almost absent in persons with high resistance;
4. The phenomena of resistance to extreme conditions are determined not by one concrete biochemical process, but by a complex of them, primarily the speed of their changes in the body as a response to extreme conditions;
5. The most optimal agents for resistance enhancement are agents that decrease entropy by transferring to a lower functional level the “fastest” parameters of reactivity: oxygen consumption, body temperature, heart rate;
6. Actoprotectors’ principal efficacy is independent of extreme conditions (physical load, stress, hypoxia, ischemia, hyperemia, gravitation overload etc.), which fact suggests their influence on the basic mechanisms of resistance;
7. Administration of actoprotectors (for example bemitil) can modify specific effects of many pathogenic chemotherapeutic and somatic direction’s drugs. This fact is a strong theoretical basis for co-administration of actoprotectors and pathogenic
therapy agents.
Actoprotectors can be classified into three groups based on their chemical compositions (Fig. 3):
Fig. 3. Current classification of actoprotectors.
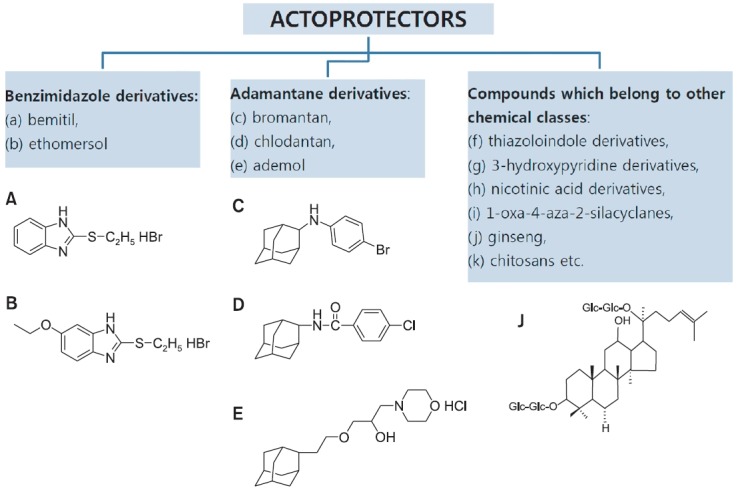
1. Benzimidazole derivatives (bemitil, ethomersol, etc.);
2. Adamantane derivatives (bromantane, chlodantan, ademol);
3. Compounds that belong to other chemical classes (thiazoloindole derivatives, 3-hydroxypyridine derivatives, nicotinic acid derivatives, 1-oxa-4-aza-2-silacyclanes, ginseng, chitosans, etc.).
The classic reference actoprotector is bemitil, the chemical structure of which is 2-ethylthiobenzimidazole hydrobromide (see Fig. 1A). Nowadays, just two compounds among all actoprotectors are permitted for medical administration: bemitil (commercial name: Antihot; certified in Ukraine as a dietary supplement) and bromantane (commercial name: Ladasten; certified in Russia as a drug).
The clinical uses of benzimidazole and adamantane actoprotectors are similar, but their pharmacokinetics and mechanisms of action are different. Actoprotectors can be widely used for recovery of work capacity, not only by healthy people but also by asthenic patients afflicted with various diseases.
BEMITIL AS REFERENCE ACTOPROTECTOR
As noted above, bemitil, the main representative of the benzimidazole class of derivatives, is the classic reference actoprotector. This substance, as are most of the other derivatives of imidazole, is fully absorbed in the alimentary tube, where absorption is accelerated by carbohydrate-saturated food. Bemitil penetrates the blood-brain barrier. A polymodal characteristic of the distribution of pharmacokinetic parameters has been revealed in studies on healthy volunteers. After biotransformation of the agent and its metabolites in the liver, they are removed by urine (Boĭko et al., 1986, 1987a, 1987b, 1991; Sergeeva and Gulyaeva, 2006; Kibal'chich et al., 2011). In course administration, the effect of bemitil increases in the first 3-5 days, and thereafter is maintained at the attained level.
Experimental research has established that single and course administration of bemitil effectively increases the physical work capacity of animals and accelerates rehabilitation after exertion under exhaustive loads (Dubovik and Bogomazov, 1987; Syrov et al., 2008). Analyses of such data demonstrates the essential differences between the actions of actoprotectors and psychostimulants: the psychostimulant sydnocarb increases work capacity according to all criteria (+10)-(+20%); the same time bemitil decreases start intensity of work, does not change volume of the work until the moment of intensity's decreasing for 50% of control level, but significantly increases maximal volume of the performed work (+33%) and resistance of mice for tiredness (+60%). Thus, the maximum stimulative effect of bemitil is observed in the considerable tiredness phase (Dubovik and Bogomazov, 1987). Significantly, the increase of physical work capacity and acceleration of rehabilitation has been observed not only under normal conditions, but also under extreme ones (hypoxia, over-heating, etc.; Spasov et al., 1990). The high efficacy of bemitil under the indicated conditions differs substantially from psychostimulants (phenamine, sydnocarb): in the latter case, the positive influence on work capacity (under normal conditions) diminishes or even becomes a negative effect under hypoxia or over-heating, due to increases in heat production, heat exhaustion, and oxygen consumption.
Numerous clinical studies have confirmed bemitil's positive
normal-conditions influence on the physical work capacity of healthy people and patients with asthenic disorders (Boĭko et al., 1986; Aleksandrovskiĭ et al., 1988; Makarov et al., 1997); its influence on helathy people under extreme conditions also has been documented: for example, high altitudes (Shahnazarov and Makhnovskii, 1991; Oliynyk and Shevchenko, 2009), heating microclimate (Pastushenkov and Badyshtov, 1995) and its combination with hypoxia (Sedov et al., 1991, 1993), and various factors related to long-duration voyages (Novikov, 1991) and space flight (Bobkov and Epishkin, 1988). Our study on high-performance athletes confirmed the positive effects of bemitil on the process and results of training (Oliynyk, 2009).
In summary, the positive influence of bemitil on mental as well as physical work capacity under both normal and extreme conditions (hypoxia, hot or cold temperatures) has been established. Specifically, bemitil improves reaction time, the capacity for instruction, and all intellectual processes. It also has an expressed antiasthenic effect, accelerating recovery processes after episodes of high exertion. However, bemitil does not cause psychomotor agitation (Bobkov and Epishkin, 1988).
A primary analysis of bemitil's effect under heavy physical loads demonstrated its influence on carbohydrates and energy metabolism: slight decreases of glycogen and creatine phosphate content in the liver and muscles and of glucose in the blood, lower accumulation of lactates in the tissues and blood, and lower increases in heat production and oxygen consumption were observed. After the period of exertion ended, rehabilitation of the factors under study was accelerated, and indeed, some of them showed super compensation.
It has been established that the therapeutic effect of bemitil is a function of its complex mechanism entailing cell genome activation, optimization of mitochondrial oxidation, oxidative
stress reduction, and stimulation of cellular immune response (Shabanov, 2009b).
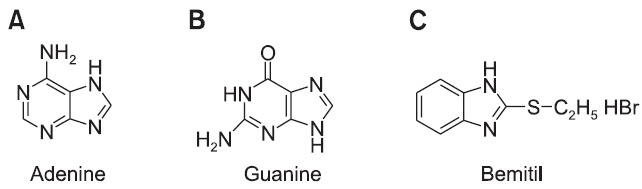
Basically, bemitil (and other benzimidazole actoprotectors) is similar to purine bases in its chemical structure (Fig. 4). It was supposed that this structural similarity explains the influence of bemitil on the cell genome, the amplifying expression of RNA and proteins, particularly enzymes of gluconeogenesis and oxidative phosphorylation, as a central link in bemitil’s mechanism of action. This activation is first expressed in organs (i.e. the liver, kidneys, and alimentary tube) having short-lived, renewable proteins. Administration of protein synthesis inhibitor actinomycin D eliminates the protective effect of bemitil under normal and hypoxic (Zarubina and Mironova, 2002) conditions. Probably bemitil does not induce synthesis of RNA and proteins by itself, but causes a positive modulating action on naturally developing processes of protein synthesis. In any case, the concrete mechanisms of bemitil-induced RNA and protein expression remain unknown.
Fig. 4. Chemical structures of the purine bases adenine (A) and guanine (B) in comparison with bemitil (C).
Bemitil primarily encourages anaerobic energy production, ATP formation, and resynthesis of glucoses from the products of carbohydrates decay (lactate and pyruvate) and from glycerol and amino acids, which mostly occurs in the liver and kidneys. Bemitil promotes the utilization of lactates (one of the main factors in work capacity reduction) under excessive physical loads which process is conjugated with the Cori and glucose-alanine cycles (Fig. 5). In these cycles, bemitil neutralizes and eliminates not only lactate but also the nitrogeneous products of decay (ammonia, etc.).
Fig. 5. Common circuitry of Cori cycle and glucose-alanine cycles.
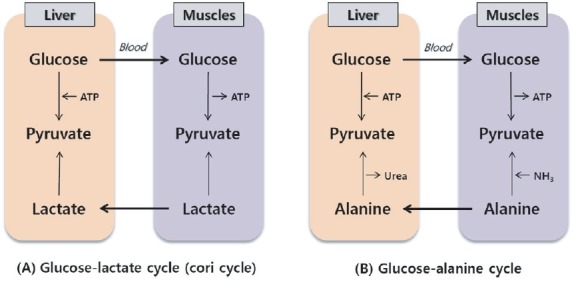
As was noted above, the mechanisms of glucose resynthesis from catabolism products are realized through gluconeogenesis processes; in this regard, the fact that the most articulated protein synthesis' activating effect of bemitil in organs with short-lived proteins permits the assumption that the influence on these organs (liver and kidneys) is the key link in the mechanism by which physical work capacity is increased under bemitil administration. The gluconeogenesis process, which takes place mainly in the liver and to a lesser extent in the cortex of the kidneys, consists of glucose resynthesis from the products of its disintegration (lactate and pyruvate) as well as from amino acids and the products of their degradation. The importance of gluconeogenesis processes under physical loads is that they entail utilization of lactate and resynthesis of consumed carbohydrates as necessary sources of energy (Rennie and Tipton, 2000; Fournier et al., 2002; De Feo et al., 2003). The importance of gluconeogenesis as a key link in the mechansm of action of bemitil was confirmed by experiments involving administration of the gluconeogenesis inhibitor tryptophan.
Enhancement of the synthesis of mitochondrial enzymes and the structural proteins of mitochondria effects an increase in energy production and maintenance of a high degree of contingency between oxidation and phosphorylation. Maintenance of a high level of ATP synthesis under oxygen deficiency conditions promotes apparent antihypoxic and antiischemic activity.
Another important aspect of the action of bemitil is its positive influence on oxidative balance. Bemitil has no direct antiradical properties (Plotnikov et al., 1992); its antioxidant action is known through induction of protein synthesis, including antioxidant enzymes (SOD, catalase, glutathione metabolism enzymes, etc). The utility of bemitil and ethomersol in inhibiting of free radical accumulation has been demonstrated in numerous experimental and clinical studies under various physiological and pathological conditions (Lobzin et al., 1992; Oliynyk et al., 2009). Evidence of this is the diminishment of the preventive effect of bemitil on the glutathione system under hypoxic conditions and actinomicyn D administration (Zarubina and Mironova, 2002).
The antioxidant properties of bemitil (and other actoprotectors) were instrumental to the discovery of their pharmacological activity. Numerous studies conducted in laboratories worldwide have ascertained that both long- and short-intensive physical loads cause oxygen stress (Marzatico et al., 1997; Kostka, 1999; Selamoglu et al., 2000; Bloomer and Goldfarb, 2004; Bloomer et al., 2005; Bloomer and Smith, 2009; Fisher-Wellman and Bloomer, 2009). The increase in the level of free radical processes under maximal and submaximal physical loads is explained by the activation of the sympathoadrenal system in response to muscular work. The essence of this mechanism lies in the following: the active forms of oxygen capable of initiating free radical responses can be generated both in catecholamine biosynthesis and in their decay, that is, when adrenaline oxidizes to adrenochrome (Bors et al., 1978; Diliberto and Allen, 1981). Additionally, the inflow through afferent and efferent nerves increases physical loads, and nerve pulse advancement is attended by free radical formation (Kol's et al., 1966). However, the mechanisms of oxidative stress have some peculiar features that accord with the nature of physical loads. Specifically, intensive physical loading in the aerobic energy-supply zone supports a peak tension in the work of muscular and cardiac mitochondria, and meanwhile, the danger of the “outflow” of oxygen-active forms from the electrical transporting link grows. The significant role in the mechanisms of oxidative stress development is played by acidosis, which is caused by an excess level of lactate under physical loads of submaximal power in the anaerobic energy supply zone.
In this respect, the concept of the importance of antioxidant properties to the realization of the total pharmacological activity of benzimidazole actoprotectors (bemitil, ethomersol) has a sound and seemingly very logical theoretical basis. This cencept, furthermore, accords with well known data on the properties of reference antioxidants ionol (dibunol) and vitamin E that increase tolerance for physical loads (Dillard et al., 1978; Krasikov, 1988). Indeed, bemitil (as well as other actoprotectors – ethomersol, bromantan etc.) can decrease oxidative stress, which can be especially important as one of the mechanisms that protects against oxidative damage to cells and tissues under physical loads, and, accordingly, promotes acceleration of recovery processes.
Bemitil's positive influence on the immune system is also related to expression of immune system proteins. Contrastingly, adaption to high altitudes and hypoxia as well as memory improvement, by bemitil, are related mainly to protein synthesis activation in the brain. Obviously, reinforcement of protein synthesis is one of the primary aspects of the mechanism of adaptive action as it affects the central nervous system, which effects includemental and physical work capacity enhancement under extreme conditions. However, it is necessary to admit, again, that the specific mechanisms of bemitil-induced protein expression remain unknown.
Bemitil also has antimutagenic properties, closely related to its antioxidant activity, that have been determined in numerous experimental studies. Thorough research on the genetic activity of bemitil has shown that it fails to induce recessive, age-related lethal mutations in drosophila, dominant lethal mutations in germ mammalian cells, and chromosomal damage in murine bone marrow cells and human peripheral blood cell cultures. Experiments on mice have demonstrated that therapeutic doses of bemitil cause a two-fold decrease in the level of aberrant cells induced by alkylating agents photrin and phopurin (Seredenin et al., 1986). In cultures of lymphocytes from 12 healthy donors and 12 patients with nettle-rash, the anticlastogenic effect of bemitil on the induction of chromosomal aberrations by photrin and dioxidine was investigated. Statistically significant protective effects of bemitil were demonstrated in cells of healthy donors after treatment with photrin or dioxidine, and after modification of the clastogenic action of photrin in lymphocytes of nettle-rash patients (Arutyunyan et al., 1994). In another study, intraperitoneal administration of the dust of chrysotile-asbestos and zeolites to C57BL/6 mice, in doses of 50 mg/kg, was found to elevate cell counts and to effect chromosomal aberrations in peritoneal fluid and bone marrow cells, as dependent on dust-exposure time. It was revealed that ascorbic acid, rutin, chemically modified flavonoid of Scutellaria baicalensis Georgy, and drugs such as bemitil and ethomersol over a broad concentration range (10−7-10−3 M) decreased or completely reduced the clustogenic action of zeolites and chrysotile-asbestos on cultured human whole blood. The ability of bemitil (1.8-19 mg/kg), unlike the others, to prevent the mutagenic effect of chrysotile-asbestos was confirmed by the recorded chromosomal aberrations in the cells of peritoneal fluid and bone marrow in mice (Daugel'-Dauge et al., 1995).
Bemitil, as well as the other well-studied actoprotectors, has no serious side effects. Bemitil and the other imidazole derivatives can cause dyspeptic disturbances (nausea, particularly on an empty stomach, though seldomly; vomiting; a general sense of discomfort in the region of the stomach and/or liver), psychoactivation effects (affective irritability, shortening of sleep quality and length), headache, and hyperemia of the face. Allergic reactions connected with the presence of bromide cannot be excluded. Bemitil is contraindicated under hypoglycemia and barbiturate administration conditions.
Thus, bemitil is a pharmacological agent of metabolic, non-exhaustive action, which comprises cell genome activation and expression of RNA and proteins, including enzymes and other proteins associated with the immune system. Also occurring
is expression of gluconeogenesis enzymes synthesis, which facilitates lactate utilization and carbohydrate resynthesis, which leads in turn to increased physical working capacity. The enhancement of the synthesis of the mitochondrial enzymes and structural proteins of the mitochondria makes possible an increase in energy production and the maintenance of a high degree contingency between oxidation and phosphorylation. Maintenance of high-level ATP synthesis under oxygen deficiency promotes apparent antihypoxic and antiischemic activity. As an indirect-action antioxidant, bemitil enhances biosynthesis of antioxidant enzymes. The antimutagenic properties of bemitil might be important to genomic protection against mutagenic factors of chemical, physical or biological origin. Finally, bemitil increases an organism's stability against the influence of extreme conditions (heavy physical loads, stresses, hypoxia, hyper- and hypothermia, etc.).
OTHER ACTOPROTECTORS
As was mentioned above, among the actoprotectors, and excluding bemitil, recently only bromantane has been permitted for practical administration; the other compounds are or were at the stage of clinical (ethomersol, chlodantan, ademol) or pre-clinical (all other compounds) study.
Bromantane
Morozov and Ivanova supposed that benzoylaminoadamantanes, adamantane derivatives of para-chlorophenoxyacetic acid, and other structurally close compounds increase the resistance of the human body with respect to extreme environmental factors rather than act as direct stimulants of the physical working capacity under normal conditions. Nonetheless, several compounds, including N-(2-adamantyl)-N-(para-bromophenyl)-amine (bromantane) (Fig. 1B) and N-(2-adamantyl)-N-(para-chlorobenzoyl) amine (ADK-910, chlodantane) (Fig. 6A), can increase physical performance; accordingly, in the literature, they are regarded as actoprotectors (Morozov and Ivanova, 2001).
Fig. 6. Chemical structures of three compounds with actoprotector activity, (A) chlodantane (N-(2-adamantyl)-N-(para-chlorobenzoyl) amine); (B) ademol (1-adamantylaethyloxy-3-morpholino-2-propanol hydrochloride); (C) ethomersol (5-ethoxy-2ethylthiobenzimidazole hydrochloride).
Bromantane, upon oral induction, is quickly but not fully absorbed from the gastrointestinal tract into the blood (bioavailability: 42%). It is quickly, and in large quantities, distributed over the tissues and organs, and is slowly eliminated from the body. Bromantane is highly lipophilic, is distributed into the lipids of brain and fat tissue and, finally, is deposited in adipose tissue. The speed of bromantane absorption from the gastrointestinal tract is much higher in women, so the half-life is respectively lower than in men. The time to achievement of the maximum concentration of blood bromantane is 2.75 hours in women, and 4.0 hours in men. The drug is metabolized in the liver, but its elimination occurs mostly through the adrenal gland. Bromantane metabolism is characterized mainly by hydroxylation in the 6th position of the adamantan cycle. All of the determined metabolites can be found in urine, even in two weeks after administration of bromantane (this last fact is important for doping control) (Burnat et al., 1997).
In terms of its pharmacological action, bromantane shows an antiasthenic effect, increases resistance to overheating, and, thereby, contributes to the restoration of working capacity after physical loads. This compound, which possesses combined stimulative and anxiolytic effects, increases physical and intellectual working capacity; inhibits the development of fatigue processes; accelerates restoration under common conditions and conditions complicated by hypoxia and hyperthermia; promotes improvement of mnemic processes (learning); improves the coordination of movements; increases body temperature; has a neuropsychoactivation effect (therefore it is sometimes referred to as a psychomotor stimulator); reveals antagonism to the sedative action of tranquilizers; displays a positive inotropic action without affecting the heart chronotropic function or systemic arterial pressure, and produces immunomodulation activity (Sedov et al., 1999; Morozov et al., 1999).
Whereas bromantane lacks hypno-sedative and neuromuscular relaxant properties, it does not possess any addictive potential. At its application, it does not, unlike typical psychostimulants, develop the phenomena of hyperstimulation.
It was determined that bromantane has a positive influence on the indices of psychophysiological conditions: range and stability of attention, complex sensomotor reaction, and the parameters of successful operator activity (Viatleva et al., 2000).
The mechanism of bromantane action is based on the facility to increase the activity of the lower centers of the central nervous system (the hypothalamus nuclei, the reticular nuclei of the operculum, the hippocampus). It does not exert any expressed action on noradrenergic mediators, but implements the activation properties through the dopaminergic system. Bromantane strengthens GABA-ergic mediation, reducing gene expression, supervising synthesis of GABA-transporters, and functioning as a return capture mediator. A potentiality for central serotonin holding effects is also assumed.
A definite role in the implementation of the bromantane pharmacological effect is played by its antiradical and membrane protective properties: bromantane increases immunity even after a single dose (increases the level of B-cells and circulating immune complex in the blood-stream), and it is more powerful than another synthetic adaptogen, levamizol, in terms of its effect on immunity (Morozov et al., 1999).
Bromantane stimulates synthesis of cytochrome P-450 and thus facilitates detoxifying liver functions and reduces the hypnotic action of thiopental sodium (but at the same time, does not weaken the anxiolytic effect of benzodiazepines).
Bromantane administration in therapeutic doses is characterized by the almost full absence of side effects including manifestations of withdrawal syndrome and hyperstimulation (Morozov et al., 1999). In animal experiments, toxic reactions were observed only after high doses of bromantane administration (>600 mg/kg). Lower doses (30-300 mg/kg) stimulated, and higher doses (600-9,600 mg/kg) suppressed behavioral activity. Spontaneous motor activity was increased after single treatment of bromantane in doses of 30-300 mg/kg, was not changed after treatment in doses of 600 mg/kg, but was inhibited after treatment in doses above 600 mg/kg. The drug reduced the pain sensitivity threshold in doses of 300-600 mg/kg, and elevated it, along with tactile sensitivity and reaction to knock, in doses above 600 mg/kg (Iezhitsa et al., 2002).
Additional and more detailed information on the pharmacological properties of bromantane is available in a foundational book, some review articles (Morozov et al., 1999; Morozov and Ivanova, 2001), and numerous clinical and experimental studies first and foremost from the laboratories of I.S. Morozov and S.B. Seredenin.
Chlodantane
This compound, also known as ADK-910, can be characterized as an adaptogen of the estrogen activity type. Chlodantane, exhibiting a broader activity spectrum than bromantan, is an adaptogen that is capable of protecting the organism against hypoxia, low and high temperatures, toxic chemicals, and other extreme factors. Its effect, in contrast to the action of well-known adaptogens, is manifested after only a single administration.
Although the mechanisms of chlodantane’s adaptogen activity have not been studied in detail, one of the most important contributions is the increase in the stability of cell membranes with respect to unfavorable factors. This is achieved, in particular, by decreasing the rate of overactivating lipid peroxidation (LPO) processes. Chlodantane also produces an immunostimulant action, which is more pronounced than the analogous effect of bromantane (Morozov and Ivanova, 2001). All of the data on chlodantane’s pharmacological properties have been derived from animal or cell culture experiments; no clinical research has been conducted.
Ademol
Another adamantan-derivative actoprotector, ademol (1-adamantylaethyloxy-3-morpholino-2-propanol hydrochloride) (Fig. 6B), was developed in Ukraine at the Institute of Organic Chemistry of National Academy of Sciences in the 1990s. It is the only actoprotector not primarily developed in Russia or connected to Soviet military or space programs. Ademol has been studied clinically, though not as an actoprotector; rather, the focus has been its uterotonic properties. Subsequently, ademol’s capacity to enhance memorization was established, and it was studied as an actoprotector and, later, as a nootropic agent at the Department of Pharmacology of Vinnitsa National Medical University. Ademol is now registered in Ukraine as a uterotonic drug; however, its manufacture has ceased, and it is no longer on the market.
Experimental studies on the actoprotective activity of ademol have shown that it is more effective than bemitil in terms of increasing the endurance of experimental animals (in swimming tests) under normal conditions; under extreme conditions (hypo- and hyperthermia, hypoxia), it is comparable to bemitil. The mechanism of ademol’s actoprotective action is related to the stimulation of DNA and RNA synthesis in the liver as well as normalization of ATP content in the muscles and its antioxidant properties; indeed, in many ways, this mechanism is similar to bemitil’s.
Ethomersol
Like bemitil, ethomersol (in the English-language literature: “ethomerzol”, “tomerzol” or “tomersol”), according to its chemical structure, is a benzimidazole derivative (5-ethoxy-2ethylthiobenzimidazole hydrochloride) (Fig. 6C) and has similar pharmacological properties. Like bemitil too, ethomersol was formulated in the former USSR for military medicine purposes. Bemitil, despite its numerous positive characteristics, carries some drawbacks, among which is low water solubility. Only in its parenteral forms can it be truly effective for extreme-condition treatment in military medicine contexts such as military toxicology, military surgery, and resuscitation practice. Of course, in these cases, the focus was not on bemitil’s actoprotective activity but rather on its properties as an effective regeneration and reparation aid.
Owing to that low water solubility drawback of bemitil, it could not be developed in injectable (intravenous or intramuscular) forms. Thus, in the latter half of the 1980s, ethomersol was developed as a water-soluble analog in order to enable further development in forms suitable for parenteral administration. The manufacturing technology necessary for those purposes was pioneered by Ukrainian pharmaceutics in Kharkov.
Clinical studies on ethomersol were conducted in 1990-91 at the toxicological clinic of the Military Medical Academy, specifically on patients suffering intoxication by phosphoorganic compounds (insecticides). Notwithstanding their generally positive results, these clinical studies uncovered new problems: the ethomersol solutions elicited a strong acid reaction (pH ~3), and caused many post-injection complications. The most common of these complications after intravenous administration were phlebitis and acute pain; after intramuscular administration, the reactions were aseptic inflammation, sometimes with subsequent necrosis, and acute pain. After the fall of the Soviet Union in 1991, clinical studies on ethomersol were interrupted; over the following 20 years, only a few animal experiments were completed.
As was already emphasized, ethomersol shares many commonalities with bemitil. Its positive influence on the energy production processes was shown in experimental studies on brain ischemia and craniocerebral trauma models (Kosolapov et al., 1996; Zarubina and Shabanov, 2005). Ethomersol interrupts the decrease of NAD-dependent breathing and the uncoupling of oxidative phosphorylation. It also shows antioxidant properties (it decreases lipid peroxidation products in tissues) in brain ischemia (Vaizova et al., 1994; Mironova et al., 2003).
Ethomersol shows properties of a central action vasodilator in blocking the potential-dependent and particularly the receptor-dependent calcium channels. The positive influence of ethomersol on the rheological properties of blood also has been established. It blocks the calcium channels of thrombocyte membranes and interrupts their activation under throm-bocyte aggregation inductor action, which consequently limits the development of thrombosis processes (Plotnikova et al., 1992b).
Ethomersol prevents the decrease of the erythrocyte capacity for deformation, possibly because of the improvement of the microviscosity of erythrocyte membranes. It induces the reduction of haemoglobin's affinity to oxygen and, consequently, increases the quantity of oxygen delivery to tissues (Plotnikova et al., 1991a, 1991b, 1992a).
Animal experiments have established that ethomersol also has hepatoprotective properties, which are indirectly related to its immunomodulant activity (Okovityi and Gaivoronskaia, 2002). Ethomersol is demonstrably capable of accelerating the process of liver regeneration following partial hepatectomy. The drug produces a rapid gain in liver mass, increases nucleic acid and glycogen contents, and improves the functional state, as manifested by a decrease in the blood bilirubin and a reduction in hexenal sleep duration. Ethomersole, moreover, produces a positive effect on liver morphology and the intracellular regeneration process (Gaivoronskaia et al., 2000).
Thiazoloindole derivatives
Most of the thiazoloindole-derivative compounds were synthesized and studied by Professor Vera Marysheva at the Military Medical Academy in post-USSR Russia. A series of 12 such compounds belonging to this class possessing antihypoxic activity were studied in order to reveal their actoprotective properties under normal and extreme conditions in rats and mice. Five of the compounds were shown to protect animals from exhaustive loads 1 hour and 24 hours after administration; four produced the same effect under acute hypercapnic hypoxia conditions. By contrast, under the conditions of acute hemic hypoxia, none of the compounds affected physical endurance (Gavreev et al., 2010).
3-Hydroxypyridine derivatives
Experiments with mice have shown that most of the 15 new 3-hydroxypyridine-derivative compounds possess antioxidant, actoprotective, and antihypoxic properties. Based on the results of treadmill and swimming tests, the actoprotective action of IBKhF-1, 11 and 14 greatly surpassed those of bemitil and bromantane under ordinary conditions. The inhibitor of gluconeogenesis tryptophan cancelled the stimulative action of IBKhF-1, 2 and 11 in the treadmill exercise. Gluconeogenesis activation, accordingly, can be considered to be one of the major components of the actoprotective action of 15 compounds. IBKhF-1, 11 and 14 also bested bemytil and bromantane under the extreme conditions of hyperthermic running and of swimming with acute hypoxia combined with hypercapnia. IBKhF-2 and 14, additionally, were better than amtisol (the standard antihypoxic agent) and bemitil against acute hypoxia in a pressure chamber, and IBKhF-4 and 14 excelled against them in a thermal chamber (Iasnetsov et al., 2011).
Six- and seven-membered 1-oxa-4-aza-2-silacyclanes
This is a new class of compounds synthesized and studied at Russian State Medical University. Generally, the biological activity of eight 1-oxa-4-aza-2-silacyclanes with the OSiCH(2)N fragment, including 6-membered 2-sila-5-morpholinones (1-3), 4-acyl-2-silamorpholines (4-6), and 7-membered derivatives of salicylic acid (7, 8), were studied. Compounds 1 and 3-6 showed a certain antihypoxic action. Compounds 2 (40 mg/kg), 4 (20 mg/kg), 6 (40 mg/kg), 7 (20 mg/kg) and 8 (40 mg/kg) reduced the physical performance of intact animals. Compound 1 (20 mg/kg) influenced physical performance in a moderate-positive way under the condition of chlorophos poisoning. Compounds 5-8 displayed protective properties against chlorophos poisoning at the dose of LD50, and compounds 2, 4, 5, 7, at the LD100 dose. The influence of compounds 1 and 2 on the emotional behavior of mice also was studied (Kurochka et al., 1998).
Chitosan
This compound differs from the other natural-origin adatogens in its strong actoprotective action established in animal experiments (Khasina et al., 2005). However, we consider those results to be only preliminary ones, due to the fact that the actoprotective activity of chitosan was not compared with a reference actoprotector bemitil (or, at least, bromantane).
Panax ginseng
Panax ginseng C.A. Meyer is a perennial plant grown in China, Korea, Japan, and Russia. The dried roots and extract of this plant are used in some traditional medicines to treat a variety of conditions, including asthenia and neuroses of varied genesis. The main active ingredients of ginseng are ginsenosides. Ginsenosides are a special group of triterpenoid saponins that can be classified into two groups according to the skeleton of their aglycones, namely, the dammarane- and oleanane types: the Rb1 group (characterized by the presence of protopanaxadiol: Rb1, Rb2, Rc and Rd) and the Rg1 group (protopanaxatriol: Rg1, Re, Rf, and Rg2). Ginsenosides are found almost exclusively in Panax species (ginseng), and to the present date, more than 150 naturally occurring ginsenosides have been isolated from the roots, leaves/stems, fruits, and/or flower heads of ginseng. Ginsenosides have been the focus of a great deal of research as, indeed, they are believed to be the main forces behind the many and glowing claims of ginseng’s efficacy. These steroid-like phytochemicals are beneficial in countering the negative influences of stress. The glycosides act on the adrenal glands, helping to prevent adrenal hypertrophy and excess corticosteroid production in response to stress. Ginsenosides increase protein synthesis and the activity of neurotransmitters in the brain; they stimulate the formation of blood vessels and improve blood circulation in the brain, thereby improving memory and cognitive abilities. Ginseng also is used in the treatment of diabetes, migraine, infections, and cancer, as well as for radiation and chemotherapy protection, to aid in sleep, and to stimulate the appetite (Attele et al., 1999; Choi, 2008; Christensen, 2009).
The results of several animal experiments (Grandhi et al., 1994; Wang and Lee, 1998) and human studies (Voces et al., 1999; Ziemba et al., 1999; Kim et al., 2005; Liang et al., 2005; Yeh et al., 2011) attest to the fact that ginseng (administrated as an extract) can significantly increase physical work capacity; such data, in fact, allow ginseng to be referred to as an actoprotector of natural origin. Nonetheless, the results of other studies (Morris et al., 1996; Engels and Wirth, 1997; Allen et al., 1998; Engels et al., 2001; Jung et al., 2004; Kulaputana et al., 2007; Ping et al., 2011) show no significant influence on physical performance, effectively refuting the more positive conclusions on its actoprotective properties.
It seems logical that such controversies are connected to the relevant studies’ different doses and course durations, as well as the varying qualities and compositions of the ginseng supplements utilized therein. Such a conclusion permits continuance of the idea that ginseng is a potential actoprotector, at least until further investigation of its influence on physical work capacity, endurance and restoration under exhaustive physical loads (compared with the reference actoprotector bemitil) has been conducted. So, as we see, perhaps the benzimidazole and adamantane derivatives are not alone in possessing actoprotective properties. The recently undertaken pharmacological studies on other perspective compounds are only the beginning.
CONCLUSIONS
Actoprotectors are preparations that enhance body stability against physical loads without increasing oxygen consumption or heat production. Or, in short, actoprotectors can be considered to be synthetic adaptogens that have a significant capacity to improve physical performance. The main representatives of this class are benzimidazole-derivative bemitil and adamantine-derivative bromantane. Nowadays, bemitil is manufactured in Ukraine and is widely used in the training of Ukrainian national sport teams. Bromantane is manufactured in Russia, and is employed mainly in the treatment of patients with asthenic and restless-asthenic frustration. Some other synthesized (new benzimidazole and adamantine derivatives, as well as thiazoindole, 3-hydroxypyridine derivatives, etc.) and even natural (including ginseng, chitosan) compounds are regarded as potential actoproptectors as well.
The reference actoprotector is bemitil. The mechanism of its pharmacological action is complex and has yet to be fully studied; however, is is established that this compound primarily (directly) stimulates protein synthesis. Other effects of bemitil (increasing physical and operator working capacity, antioxidation, antihypoxia, antimutagenic action, etc.) have been determined by proteins synthesized de novo in different organs.
All of the thoroughly studied actoprotectors have no serious side effects. Bemitil can cause dyspeptic disturbances (nausea, particularly on an empty stomach, though seldomly; vomiting; a general sense of discomfort in the region of the stomach and/or liver), psychoactivation effects (affective irritability, shortening of sleep quality and length), headache, and hyperemia of the face. Bromantane is characterized by the almost full absence of side effects including manifestations of withdrawal syndrome and hyperstimulation.
Actoprotectors initially were developed in the former Soviet Union for space, sports and military medicine applications, specifically for the purpose of increasing physical and operator work capacity under normal and extreme (hypoxia, hyperthermia) conditions; later, actoprotectors, owing to their wide-ranging pharmacological activity, high efficiency and safety, were found to offer a wider utility in other branches of practical medicine. Nowadays, bemitil is successfully employed in infectology, neurology, hepatology, cardiology, pulmonology, obstetrics and gynaecology, urology, dermatology, toxicology and other branches of clinical medicine. It is recommended for the rehabilitation of patients suffering a variety of diseases, not only as a symptomatic aid (for decrease of asthenic symptoms), but as a pathogenetic one as well.
Acknowledgments
This work was supported by a Korea Research Foundation grant (MRC, 2010-0029355) funded by the Korean Government (MEST).
References
- 1.Akilov M., Bobrovnyk V. I., Gunina L., Danko V., Dryukov V., Zemtsova I., Lysenko Y., Oliynyk S., Pavlenko Y., Platonov V., Shynkaruk O. Methods recommendations on the problems of preparation of Ukrainian athletes to the Games of XXIX Olympiad 2008 in Beijing. Science in Olympic Sports special issue. (2007):1–170. [Google Scholar]
- 2.Aleksandrovskii Iu. A., Bobkov Iu G., Neznamov G. G., Serebriakova T. V., Boiko S. S. Use of the new psychotropic preparation bemitil in treating asthenic disorders (clinico-pharmacological research). Zh. Nevropatol. Psikhiatr. Im. S. S. Korsakova. (1988);88:109–115. [PubMed] [Google Scholar]
- 3.Allen J. D., McLung J., Nelson A. G., Welsch M. Ginseng supplementation does not enhance healthy young adults' peak aerobic exercise performance. J. Am. Coll. Nutr. (1998);17:462–466. doi: 10.1080/07315724.1998.10718795. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Pellitero P., Sitja-Bobadilla A., Bermudez R., Quiroga M. I. Levamisole activates several innate immune factors in Scophthalmus maximus (L.) Teleostei. Int. J. Immunopathol. Pharmacol. (2006);19:727–738. doi: 10.1016/0165-1218(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 5.Arutyunyan R. M., Sarkisyan T. F., Oganesyan G. G., Durnev A. D. Comparative investigation of anticlastogenic effects in cell cultures of healthy donors and patients with nettle-rash. Mutat. Res. (1994);320:335–341. doi: 10.1016/0165-1218(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 6.Attele A. S., Wu J. A., Yuan C. S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem. Pharmacol. (1999);58:1685–1693. doi: 10.1016/S0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 7.Bloomer R. J., Goldfarb A. H. Anaerobic exercise and oxidative stress: a review. Can. J. Appl. Physiol. (2004);29:245–263. doi: 10.1139/h04-017. [DOI] [PubMed] [Google Scholar]
- 8.Bloomer R. J., Goldfarb A. H., Wideman L., McKenzie M. J., Consitt L. A. Effects of acute aerobic and anaerobic exercise on blood markers of oxidative stress. J. Strength. Cond. Res. (2005);19:276–285. doi: 10.1519/14823.1. [DOI] [PubMed] [Google Scholar]
- 9.Bloomer R. J., Smith W. A. Oxidative stress in response to aerobic and anaerobic power testing: influence of exercise training and carnitine supplementation. Res. Sports Med. (2009);17:1–16. doi: 10.1080/15438620802678289. [DOI] [PubMed] [Google Scholar]
- 10.Bobkov Iu. G., Epishkin A. K. Effect of actoprotectors on the work capacity of operators during modeling of various factors of space flight. Kosm. Biol. Aviakosm. Med. (1988);22:20–23. [PubMed] [Google Scholar]
- 11.Bogdan N. G., Kolotilinskaia N. V., Nadorov S. A., Iarkova M. A., Badyshtov B. A. Effect of afobazole on the psychophysiological state of healthy volunteers. Eksp. Klin. Farmakol. (2011);74:8–12. [PubMed] [Google Scholar]
- 12.Boǐko S. S., Bobkov Iu. G., Neznamov G. G., Serebriakova T. V. Pharmacokinetics and the clinical effect of bemitil after a single administration. Farmakol. Toksikol. (1986);49:17–20. [PubMed] [Google Scholar]
- 13.Boǐko S. S., Bobkov Iu. G., Dobrokhotova T. A., Kniazeva N. A., Neznamov G. G. Experimental and clinical data on the ability of bemetil to penetrate the hemato-encephalic barrier. Farmakol. Toksikol. (1987a);50:79–81. [PubMed] [Google Scholar]
- 14.Boǐko S. S., Bobkov Iu. G., Zherdev V. P., Dvorianinov A. A. Bemetil pharmacokinetics in an experiment on rats. Farmakol. Toksikol. (1987b);50:54–56. [PubMed] [Google Scholar]
- 15.Boǐko S. S., Zherdev V. P., Neznamov G. G. The role of bemitil pharmacokinetics in realizing its therapeutic efficacy. Farmakol. Toksikol. (1991);54:64–66. [PubMed] [Google Scholar]
- 16.Bors W., Michel C., Saran M., Lengfelder E. The involvement of oxygen radicals during the autoxidation of adrenalin. Biochim. Biophys. Acta. (1978);540:162–172. doi: 10.1016/0304-4165(78)90445-2. [DOI] [PubMed] [Google Scholar]
- 17.Burnat P., Payen A., Le Brumant-Payen C., Hugon M., Ceppa F. Bromontan, a new doping agent. Lancet . (1997);350:963–964. doi: 10.1016/s0140-6736(05)63310-7. [DOI] [PubMed] [Google Scholar]
- 18.Chen L. Y., Lin Y. L., Chiang B. L. Levamisole enhances immune response by affecting the activation and maturation of human monocyte-derived dendritic cells. Clin. Exp. Immunol. (2008);151:174–181. doi: 10.1111/j.1365-2249.2007.03541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi K. T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta. Pharmacol. Sin. (2008);29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 20.Christensen L. P. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv. Food Nutr. Res. (2009);55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 21.Daugel'-Dauge N. O., Durnev A. D., Kulakova A. V., Seredenin S. B., Velichkovskii B. T. Corpuscular mutagenesis and its prevention. Vestn. Ross. Akad. Med. Nauk. (1995);1:29–38. [PubMed] [Google Scholar]
- 22.De Feo P., Di Loreto C., Lucidi P., Murdolo G., Parlanti N., De Cicco A., Piccioni F., Santeusanio F. Metabolic response to exercise. J. Endocrinol. Invest. (2003);26:851–854. doi: 10.1007/BF03345235. [DOI] [PubMed] [Google Scholar]
- 23.Diliberto E. J. Jr., Allen P. L. Mechanism of dopamine-beta-hydroxylation. Semidehydroascorbate as the enzyme oxidation product of ascorbate. J. Biol. Chem. (1981);256:3385–3393. [PubMed] [Google Scholar]
- 24.Dillard C. J., Litov R. E., Savin W. M., Dumelin E. E., Tappel A. L. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J. Appl. Physiol. . (1978);45:927–932. doi: 10.1152/jappl.1978.45.6.927. [DOI] [PubMed] [Google Scholar]
- 25.Dubovik B. V., Bogomazov S. D. Multifactorial method for assessing the physical work capacity of mice. Farmakol. Toksikol. (1987);50:116–121. [PubMed] [Google Scholar]
- 26.Engels H. J., Wirth J. C. No ergogenic effects of ginseng (Panax ginseng C.A. Meyer) during graded maximal aerobic exercise. J. Am. Diet Assoc. (1997);97:1110–1115. doi: 10.1016/S0002-8223(97)00271-X. [DOI] [PubMed] [Google Scholar]
- 27.Engels H. J., Kolokouri I.,, Cieslak T. J. 2nd, Wirth J. C. Effects of ginseng supplementation on supramaximal exercise performance and short-term recovery. J. Strength Cond. Res. (2001);15:290–295. [PubMed] [Google Scholar]
- 28.Fabrizi F., Dixit V., Messa P., Martin P. Meta-analysis: levamisole improves the immune response to hepatitis B vaccine in dialysis patients. Aliment. Pharmacol. Ther. (2010);32:756–762. doi: 10.1111/j.1365-2036.2010.04410.x. [DOI] [PubMed] [Google Scholar]
- 29.Fisher-Wellman K., Bloomer R. J. Acute exercise and oxidative stress: a 30 year history. Dyn Med. (2009);8:1. doi: 10.1186/1476-5918-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fournier P. A., Brau L., Ferreira L. D., Fairchild T., Raja G., James A., Palmer T. N. Glycogen resynthesis in the absence of food ingestion during recovery from moderate or high intensity physical activity: novel insights from rat and human studies. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. (2002);133:755–763. doi: 10.1016/S1095-6433(02)00254-4. [DOI] [PubMed] [Google Scholar]
- 31.Gaivoronskaia V. V., Okovityi S. V., Shustov E. B., Smirnov A. V. Effects of bemethyl, ethomersol, and yakton on the liver regeneration after partial hepatectomy. Eksp. Klin. Farmakol. (2000);63:34–36. [PubMed] [Google Scholar]
- 32.Gavreev A. I., Marysheva V. V., Shabanov P. D. The actoprotective action of thiazoloindole antihypoxic agents. Eksp. Klin. Farmakol. (2010);73:25–30. [PubMed] [Google Scholar]
- 33.Grandhi A., Mujumdar A. M., Patwardhan B. A comparative pharmacological investigation of Ashwagandha and Ginseng. J. Ethnopharmacol. (1994);44:131–135. doi: 10.1016/0378-8741(94)01119-2. [DOI] [PubMed] [Google Scholar]
- 34.Iasnetsov V. V., Tsublova E. G., Karsanova S. K., Skachilova S. I. Actoprotective and antihypoxic action of new heteroaromatic antioxidants. Aviakosm. Ekolog. Med. (2011);45:51–54. [PubMed] [Google Scholar]
- 35.Iezhitsa I. N., Spasov A. A., Bugaeva L. I., Morozov I. S. Toxic effect of single treatment with bromantane on neurological status of experimental animals. Bull. Exp. Biol. Med. (2002);133:380–383. doi: 10.1023/A:1016206306875. [DOI] [PubMed] [Google Scholar]
- 36.Jung K., Kim I. H., Han D. Effect of medicinal plant extracts on forced swimming capacity in mice. J. Ethnopharmacol. (2004);93:75–81. doi: 10.1016/j.jep.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 37.Khasina E. I., Sgrebneva M. N., Yermak I. M., Gorbach V. I. Chitosan and nonspecific resistance of an organism. Vestnik DVO RAN (Herald of Far East Department of Russian Academy of Sciences) (2005);1:62–71. [Google Scholar]
- 38.Kibal'chich D. A., Belolipetskaia V. G., Blagodatskikh S. V., Martsevich S., Rudenko L. I., Iatsuk V. R. Pharmacokinetics of domestic actoprotector drug Metaprot in healthy volunteers. Eksp. Klin. Farmakol. (2011);74:30–32. [PubMed] [Google Scholar]
- 39.Kim S. H., Park K. S., Chang M. J., Sung J. H. Effects of Panax ginseng extract on exercise-induced oxidative stress. J. Sports Med. Phys. Fitness. (2005);45:178–182. [PubMed] [Google Scholar]
- 40.Kol's O. R., Limarenko I. M., Tarusov B. N. Formation of free radicals in nerve fiber under stimulation. Dokl. Akad. Nauk. SSSR. (1966);167:956–957. [PubMed] [Google Scholar]
- 41.Kosolapov V. A., Ostrovskii O. V., Spasov A. A. The protective action of ethomersol in acute hypobaric hypoxia and during the recovery after it. Eksp. Klin. Farmakol. (1996);59:51–53. [PubMed] [Google Scholar]
- 42.Kostka T. Aging, physical activity and free radicals. Pol. Merkur. Lekarski. (1999);7:202–204. [PubMed] [Google Scholar]
- 43.Krasikov S. I. Lipid peroxidation in skeletal muscles of rats during maximum physical exertion and its correction by ionol. Ukr. Biokhim. Zh. (1988);60:100–103. [PubMed] [Google Scholar]
- 44.Kulaputana O.,, Thanakomsirichot S., Anomasiri W. Ginseng supplementation does not change lactate threshold and physical performances in physically active Thai men. J. Med. Assoc. Thai. (2007);90:1172–1179. [PubMed] [Google Scholar]
- 45.Kurochka A. V., Agafonova O. V., Losev A. S., Mamaeva E. A., Bylikin S. Y., Negrebetsky V. V., Kramarova E. P., Shipov A. G., Baukov Y. I. Six- and seven-membered 1-oxa-4-aza-2-silacyclanes as possible correctors of adaptational mechanisms. Met. Based Drugs. (1998);5:25–33. doi: 10.1155/MBD.1998.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang M. T., Podolka T. D., Chuang W. J. Panax notoginseng supplementation enhances physical performance during endurance exercise. J. Strength Cond. Res. (2005);19:108–114. doi: 10.1519/00124278-200502000-00019. [DOI] [PubMed] [Google Scholar]
- 47.Litvintsev S. V., Davydov A. T., Uspenskii Iu. P., Zagrebel'nyi I. A., Balukova E. V. Using of aphobazol in the treatment of adaptation disorder in the contract service men, dismissed from the armed forces. Voen. Med. Zh. (2007);328:28–29. [PubMed] [Google Scholar]
- 48.Lobzin V. S., Saikova L. A., Chukhlovina M. L., Pustozerov V. G. The use of bemitil in patients with progressive muscular dystrophies. Lik Sprava. (1992);4:85–87. [PubMed] [Google Scholar]
- 49.Makarov V. I., Tiurenkov I. N., Klauchek S. V., Nalivaiko I., Antipova A. The enhancement of human thermal resistance by the single use of bemitil and fenibut. Eksp. Klin. Farmakol. (1997);60:68–71. [PubMed] [Google Scholar]
- 50.Marzatico F., Pansarasa O., Bertorelli L., Somenzini L., Della Valle G. Blood free radical antioxidant enzymes and lipid peroxides following long-distance and lactacidemic performances in highly trained aerobic and sprint athletes. J. Sports Med. Phys. Fitness. (1997);37:235–239. [PubMed] [Google Scholar]
- 51.Mironova O. P., Zarubina I. V., Shabanov P. D. Ethomerzol as an antioxidant. Biomed. Khim. (2003);49:434–442. [PubMed] [Google Scholar]
- 52.Morozov I. S., Ivanova I. A. Actoprotector and adaptogen properties of adamantane derivatives (a review). Pharmaceut. Chem. J. (2001);35:235–238. doi: 10.1023/A:1011905302667. [DOI] [Google Scholar]
- 53.Morozov I. S., Klimova N. V., Sergeeva S. A., Ivanova I. A., Barchukov V. G., Kovalev G. I., Piatin B. M., Avdiunina N. I. Adamantane derivatives enhancing body's resistance to emergencies. Vestn. Ross. Akad. Med. Nauk. (1999);3:28–32. [PubMed] [Google Scholar]
- 54.Morris A. C., Jacobs I., McLellan T. M., Klugerman A., Wang L. C., Zamecnik J. No ergogenic effect of ginseng ingestion. Int. J. Sport Nutr. (1996);6:263–271. doi: 10.1123/ijsn.6.3.263. [DOI] [PubMed] [Google Scholar]
- 55.Novikov V. S. Efficiency of preventive use of bemethyl during long-term voyages. Gig. Sanit. (1991);3:37–39. [PubMed] [Google Scholar]
- 56.Novikov V. S., Bortnovskii V. N. Effect of dibazol on indices of nonspecific resistance in human subjects in a hermetically sealed enclosure. Kosm. Biol. Aviakosm. Med. (1985);19:68–71. [PubMed] [Google Scholar]
- 57.Okovityi S. V., Gaivoronskaia V. V. Immune mechanisms of hepatoprotector effects of etomersol and thymogen. Eksp. Klin. Farmakol. (2002);65:44–46. [PubMed] [Google Scholar]
- 58.Oliynyk S. A study of the influence of the dietary supplement Antihot® on the parameters of working capacity in athletes specializing in Kyokushin karate. Int. J. Appl. Sports Sciences. (2009);21:48–63. [Google Scholar]
- 59.Oliynyk S., Koval I., Vdovenko N., Babenko L. Impact of the dietary supplement “Antihot” on the parameters of general endurance and antioxidant status of athlete’s body. Sporto mokslas (Vilnius) (2009);55:28–33. [Google Scholar]
- 60.Oliynyk S., Shevchenko V. Ergogenic aids as a means for increasing athlete working capacity in the high altitude environment. Int. J. Appl. Sports Sciences. (2009);21:61–73. [Google Scholar]
- 61.Pastushenkov V. A., Badyshtov B. A. Increase of human resistance to the effects of high ambient temperatures by using drugs. Med. Tr. Prom. Ekol. (1995);9:39–42. [PubMed] [Google Scholar]
- 62.Ping F. W., Keong C. C., Bandyopadhyay A. Effects of acute supplementation of Panax ginseng on endurance running in a hot & humid environment. Indian J. Med. Res. (2011);133:96–102. [PMC free article] [PubMed] [Google Scholar]
- 63.Plotnikov M. B., Kobzeva E. A., Plotnikova T. M. Antioxidant effects of antihypoxic drugs in cerebral ischemia. Biull. Eksp. Biol. Med. (1992);113:504–506. [PubMed] [Google Scholar]
- 64.Plotnikova T. M., Kulakova Z. V., Plotnikov M. B. Effects of ethomersol on cerebrovascular blood circulation and oxygen metabolism of the brain in acute transient ischemia and recirculation. Biull. Eksp. Biol. Med. (1991a);111:386–388. doi: 10.1007/BF00840906. [DOI] [PubMed] [Google Scholar]
- 65.Plotnikova T. M., Plotnikov M. B., Bazhenova T. G. The evaluation of the efficacy of antihypoxic agents lowering hemoglobin oxygen affinity in acute cerebral ischemia. Biull. Eksp. Biol. Med. (1991b);111:170–172. [PubMed] [Google Scholar]
- 66.Plotnikova T. M., Firsov N. N., Baizova O. E. The mechanism of the prevention by ethomersol of disorders in erythrocyte deformability in cerebral ischemia and recirculation. Eksp. Klin. Farmakol. (1992a);55:29–31. [PubMed] [Google Scholar]
- 67.Plotnikova T. M., Kulakova Z. V., Smol'iakova V. I., Plotnikov M. B. The mechanisms of the ethomersol correction of postischemic hypoperfusion. Eksp. Klin. Farmakol. (1992b);55:11–13. [PubMed] [Google Scholar]
- 68.Ratnikov V. I., Nesterenko V. G. Effect of a number of immunostimulating preparations on the intracellular bactericidal systems of peripheral blood neutrophils. Farmakol. Toksikol. (1984);47:81–84. [PubMed] [Google Scholar]
- 69.Rennie M. J., Tipton K. D. Protein and amino acid metabolism during and after exercise and the effects of nutrition. Annu. Rev. Nutr. (2000);20:457–483. doi: 10.1146/annurev.nutr.20.1.457. [DOI] [PubMed] [Google Scholar]
- 70.Rusin Vla. Influence of muscle training, adaptation to cold and dibazol administration on the resistance of certain tissues. Fiziol. Zh. SSSR Im. I. M. Sechenova. (1967);53:431–437. [PubMed] [Google Scholar]
- 71.Rusin Vla. On adaptation to cold and heat in muscular training and in dibazol administration. Patol. Fiziol. Eksp. Ter. (1962a);6:63–65. [PubMed] [Google Scholar]
- 72.Rusin Vla. Role of the adaptation to low temperatures and dibazol in increased resistance of mice to adverse factors. Fiziol. Zh. SSSR Im. I. M. Sechenova. (1962b);48:195–200. [PubMed] [Google Scholar]
- 73.Rusin Vla. The effect of dibazol and adaptation to muscular work and cold on animals with the Ehrlich tumor. Vopr Onkol. (1963a);18:60–66. [PubMed] [Google Scholar]
- 74.Rusin Vla. The effect of prolonged dibazol adminstration on the growth and resistance of white mice and their offspring. Fiziol. Zh. SSSR. Im. I. M. Sechenova. (1963b);49:632–638. [PubMed] [Google Scholar]
- 75.Rusin Vla. Resistance to cold and heat in animals receiving dibazol or subjected to muscular training and acclimatization. Fiziol. Zh. SSSR. Im. I. M. Sechenova. . (1963c);49:359–365. [PubMed] [Google Scholar]
- 76.Sedov A. V., Kustov V. V., Surovtsev N. A., Lukicheva T. A., Akin'shin A. V., Nazarov L., Stroganov V. P. Use of bemitil for increasing the resistance of the human body to combined effects of carbon monoxide and heating microclimate. Gig. Tr. Prof. Zabol. (1991);6:12–14. [PubMed] [Google Scholar]
- 77.Sedov A. V., Surovtsev T. A., Akin'shin N. A., Nazarov A. V., Miroshnik L. Experimental rationale for the use of drugs to increase the resistance of the human body to the combined action of carbon monoxide and hyperthermia. Med. Tr. Prom. Ekol. (1993);9-10:10–11. [PubMed] [Google Scholar]
- 78.Sedov A. V., Surovtsev N. A., Lukicheva T. A., Beliakova I. P. Tactics of protection of humans in accidents associated with combined exposure to chemical and physical factors. Med. Tr. Prom. Ekol. (1999);12:34–37. [PubMed] [Google Scholar]
- 79.Selamoglu S., Turgay F., Kayatekin B. M., Gonenc S., Yslegen C. Aerobic and anaerobic training effects on the antioxidant enzymes of the blood. Acta. Physiol. Hung. (2000);87:267–273. doi: 10.1556/APhysiol.87.2000.3.5. [DOI] [PubMed] [Google Scholar]
- 80.Seredenin S. B., Bobkov Iu G., Durnev A. D., Dubovskaia O. Mutagenic and antimutagenic properties of bemitil. Biull. Eksp. Biol. Med. . (1986);102:76–79. [PubMed] [Google Scholar]
- 81.Sergeeva S. A., Gulyaeva I. L. Distribution of bemitil in organs and tissues of rats after single or repeated administration. Bull. Exp. Biol. Med. (2006);141:596–598. doi: 10.1007/s10517-006-0230-0. [DOI] [PubMed] [Google Scholar]
- 82.Shabanov P. D. Chronicles of St. Petersburg pharmacology (on 210th anniversary of the Department of Pharmacology of the St. Petersburg State Military Medical Academy). Eksp. Klin. Farmakol. (2009a);72:46–51. [PubMed] [Google Scholar]
- 83.Shabanov P. D. Clinical pharmacology of metaprot, a new antiasthenic drug with psychoactivating properties. Obzori po klinichaskoi farmakologii i lekarstvennoi terapii (Reviews on clinical pharmacology and drug therapy) (St.Petersburg) (2009b);7:48–81. [Google Scholar]
- 84.Shahnazarov A. S., Makhnovskii V. P. Effect of bemitil on metabolic processes and work capacity at high altitude. Fiziol. Cheloveka. (1991);17:117–120. [PubMed] [Google Scholar]
- 85.Sidorova N. V., Kiikova O. I. Dibazol: a remedy for prophylaxis of acute respiratory infections among students of military colleges. Zh. Mikrobiol. Epidemiol. Immunobiol. (2000);6:122–124. [PubMed] [Google Scholar]
- 86.Spasov A. A, Kovalev G. V., Tsibanev A. V. Method of studying the effects of pharmacological substances on work capacity of animals in hypobaric hypoxia. Biull. Eksp. Biol. Med. (1990);110:164–166. [PubMed] [Google Scholar]
- 87.Syrov V. N., Shakhmurova G. A., Khushbaktova Z. A. Effects of phytoecdysteroids and bemithyl on functional, metabolic, and immunobiological parameters of working capacity in experimental animals. Eksp. Klin. Farmakol. (2008);71:40–43. [PubMed] [Google Scholar]
- 88.Udintsev S. N., Shakhov V. P., Borovskoi I. G., Ibragimova S. G. The effect of low concentrations of adaptogen solutions on the functional activity of murine bone marrow cells in vitro. Biofizika . (1991);36:105–108. [PubMed] [Google Scholar]
- 89.Uyanaev A. A., Fisenko V. P. Studies of long-term noopept and afobazol treatment in rats with learned helplessness neurosis. Bull. Exp. Biol. Med. (2006);142:202–204. doi: 10.1007/s10517-006-0327-5. [DOI] [PubMed] [Google Scholar]
- 90.Vaizova O. E., Plotnikova T. M., Plotnikov M. B. The effect of ethomersol on local cerebral blood flow and edema of the brain tissue in chronic ischemia. Eksp. Klin. Farmakol. (1994);57:25–27. [PubMed] [Google Scholar]
- 91.Viatleva O. A., Barchukov V. G., Morozov I. S., Salenko Iu. A., Zhirnov E. N. The neuro- and psychophysiological effects of bromantane. Voen. Med. Zh. (2000);321:61–65. [PubMed] [Google Scholar]
- 92.Voces J., Alvarez A. I., Vila L., Ferrando A., Cabral de Oliveira C., Prieto J. G. Effects of administration of the standardized Panax ginseng extract G115 on hepatic antioxidant function after exhaustive exercise. Comp. Biochem. Physiol. C. Pharmacol. Toxicol. Endocrinol. (1999);123:175–184. doi: 10.1016/S0742-8413(99)00025-0. [DOI] [PubMed] [Google Scholar]
- 93.Voskanian N. A., Dzhikidze E. K., Pochkhura M. A. Immunological status of monkeys during acclimatization and its correction with levamisole. Zh. Mikrobiol. Epidemiol. Immunobiol. (1986);3:62–65. [PubMed] [Google Scholar]
- 94.Wang L. C., Lee T. F. Effect of ginseng saponins on exercise performance in non-trained rats. Planta. Med. (1998);64:130–133. doi: 10.1055/s-2006-957389. [DOI] [PubMed] [Google Scholar]
- 95.Yeh T. S., Chan K. H., Hsu M. C., Liu J. F. Supplementation with soybean peptides, taurine, Pueraria isoflavone, and ginseng saponin complex improves endurance exercise capacity in humans. J. Med. Food. (2011);14:219–225. doi: 10.1089/jmf.2010.1096. [DOI] [PubMed] [Google Scholar]
- 96.Zarubina I. V., Mironova O. P. Effect of bemethyl on the glutathione system in the rat liver in acute hypoxia. Eksp. Klin. Farmakol. (2002);65:28–30. [PubMed] [Google Scholar]
- 97.Zarubina I. V., Shabanov P. D. The significance of individual resistance to hypoxia for correction of the consequences of craniocerebral trauma. Neurosci. Behav. Physiol. (2005);35:215–219. doi: 10.1007/s11055-005-0016-2. [DOI] [PubMed] [Google Scholar]
- 98.Zenina T. A., Gavrish I. V., Melkumyan D. S., Seredenina T. S., Seredenin S. B. Neuroprotective properties of afobazol in vitro. Bull. Exp. Biol. Med. (2005);140:194–196. doi: 10.1007/s10517-005-0443-7. [DOI] [PubMed] [Google Scholar]
- 99.Ziemba A. W., Chmura J., Kaciuba-Uscilko H., Nazar K., Wisnik P., Gawronski W. Ginseng treatment improves psychomotor performance at rest and during graded exercise in young athletes. Int. J. Sport Nutr. (1999);9:371–377. doi: 10.1123/ijsn.9.4.371. [DOI] [PubMed] [Google Scholar]


