Abstract
Atopic dermatitis is a chronic, inflammatory disease of the skin with increased transepidermal water loss. Both an abnormal inflammatory response and a defective skin barrier are known to be involved in the pathogenesis of atopic dermatitis. Protease activated receptor 2 (PAR2) belongs to a family of G-protein coupled receptors and is activated by both trypsin and a specific agonist peptide, SLIGKV-NH2. PAR2 is expressed in suprabasal layers of the epidermis and regulates inflammatory responses and barrier homeostasis. In this study, we show that nordihydroguaiaretic acid (NDGA) inhibits the PAR2-mediated signal pathway and plays a role in skin barrier recovery in atopic dermatitis. Specifically, NDGA reduces the mobilization of intracellular Ca2+ in HaCaT keratinocytes by down-regulating inflammatory mediators, such as interleukin-8, thymus and activation-regulated chemokine and intercellular cell adhesion molecule-1 in HaCaT keratinocytes. Also, NDGA decreases the protein expression of involucrin, a differentiation maker of keratinocyte, in both HaCaT keratinocytes and normal human epidermal keratinocytes. We examined NDGA-recovered skin barrier in atopic dermatitis by using an oxazolone-induced atopic dermatitis model in hairless mice. Topical application of NDGA produced an increase in transepidermal water loss recovery and a decrease in serum IgE level, without weight loss. Accordingly, we suggest that NDGA acts as a PAR2 antagonist and may be a possible therapeutic agent for atopic dermatitis.
Keywords: Protease activated receptor 2, Atopic dermatitis, Skin barrier, Nordihydroguaiaretic acid, PAR2 antagonist
INTRODUCTION
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by erythema, eruption, pruritus and hyperkeratosis accompanied with increased transepidermal water loss (TEWL) (Tsujii et al., 2009). AD has a complex etiology that involves abnormal immunological and inflammatory pathways that yield defective skin barrier, exposure to environmental agents, and neuropsychological factors (Jin et al., 2009). Epidermal barrier disruption increases the invasion of allergens and bacteria and worsens the inflammatory response. The epidermal barrier is developed when keratinocytes differentiate and secrete lamellar bodies.
Protease activated receptor 2 (PAR2) belongs to a subfamily of G protein-coupled receptors (Bohm et al., 1996) and is activated through proteolytic cleavage by specific serine proteases, such as mast cell tryptase and trypsin. These serine proteases cleave the extracellular N-terminal peptide in humans. Subsequently, the cleaved peptide sequence, as a tethered ligand, activates the receptor, itself (Vergnolle et al., 1999). In skin, PAR2 is expressed in suprabasal layers of epidermis (Nystedt et al., 1996). PAR2 expression is associated with the regulation of keratinocyte proliferation and differentiation, maintenance of the epidermal barrier and inflammation (Frateschi et al., 2011).
Skin barrier disruption increases the pH in the epidermis and subsequently increases activity of serine proteases (Hachem et al., 2006; Demerjian et al., 2008). PAR2 activation by a specific activating peptide was shown to delay barrier recovery, accelerate cornification and inhibit lamellar body (LB) secretion after tape-stripping in hairless mice (Hachem et al., 2006; Jeong et al., 2008). In another mouse model, acute barrier disruption caused terminal differentiation of keratinocytes in conjunction with caspase-14, and this differentiation was decreased by treatment with serine protease inhibitors (Demerjian et al., 2008). This event was shown to be regulated by PAR2-derived cytoskeletal and plasma membrane dynamics (Roelandt et al., 2011). In PAR2-/- mice, after tape-stripping, the skin barrier recovered with enhanced LB secretion (Hachem et al., 2006; Jeong et al., 2008; Roelandt et al., 2011). Also the transgenic mice, Grhl3PAR/+, displayed increased expression of PAR2 and exhibited phenotypes characterized by scaly skin, hyperplastic epidermis and scratching behavior (Frateschi et al., 2011).
PAR2 is also correlated with inflammatory responses. PAR2 activation increased the secretion of IL-8, NF-κB-dependent intercellular cell adhesion molecule-1 (ICAM-1) and prostaglandin E2 (PGE2) (Hou et al., 1998, Vergnolle et al., 1998, Buddenkotte et al., 2005).
We examined the effect of NDGA as a PAR2 antagonist on the recovery of skin barrier in atopic dermatitis. NDGA is a natural herbal antioxidant found in the creosote bush, Larrea tridentate (He et al., 2004). NDGA is well known as lipoxygenase inhibitor and anti-tumor agent (Eads et al., 2009; Mahajan et al., 2011; Rahman et al., 2011). Notably, NDGA inhibits the intracellular Ca2+ influx after treatment with trypsin or PAR2-activating peptide (SILGKV-NH2). In an oxazolone-induced atopic dermatitis model using hairless mice, we studied whether NDGA affects keratinocyte differentiation and inflammation by inhibiting PAR2 activation.
MATERIALS AND METHODS
Cell culture
Immortalized human keratinocyte cell line, HaCaT, cells were purchased from Amore Pacific (Korea). The cells were generally cultured in high glucose DMEM with FBS 10%, 100 units/ml of penicillin and 100 μg/ml of streptomycin (WelGENE Inc., Korea). Cells were incubated at 37℃ in a humidified atmosphere of 5% CO2 and 95% air.
Normal human epidermal keratinocytes (NHEKs) from neonatal origin were purchased from Invitrogen (USA). The NHEKs were used until passage number 5. The cells were cultured in EpiLife® medium with 60 μM calcium and human keratinocyte growth supplement (Invitrogen, USA) and incubated at 37℃ in a humidified atmosphere of 5% CO2 and 95% air.
Measurement of intracellular Ca2+
HaCaT cells were seeded on a 96-well plate (black wells and clear bottoms, Greiner bio-one, Germany) at 4×104 cells/well. After 24 hours, media were changed with 2 μM Fluo-4, AM (Invitrogen, USA), 0.02% Pluronic® F-127 (Invitrogen, USA) and 2.5 mM Probenecid (Sigma, USA) in Hank’s balanced salt solution (HBSS, Sigma, USA) containing 20 mM HEPES (Sigma, USA) for 30 minutes at 37℃ and protected from light. The cells were stabilized for 15 minutes at room temperature during shaking. Then, NDGA (Sigma, USA) were pretreated before activation of PAR2. After 30 minutes, 20 mM of PAR2-activating peptide (SLIGKV-NH2, Bachem, Switzerland) or 2 unit/ml of trypsin (Sigma, USA) was added with Flexstation 3 (Molecular Devices, USA), and intracellular Ca2+ was simultaneously measured using an excitation wavelength of 485 nm and an emission wavelength of 525 nm at room temperature. The data were analyzed with SoftMax® Pro (Molecular Devices, USA), and the mobilization of intracellular Ca2+ was calculated as the minimum value subtracted from the maximum value.
Measurement of secreted IL-8 chemokine
HaCaT cells were seeded on 96-well plate at 4×104 cells/well. After 24 hours, cells were starved with DMEM without FBS for 12 hours. NDGA were pretreated for 30 minutes. Then, 100 μM of SLIGKV-NH2 was added and cultured for 24 hours. Supernatants were harvested and analyzed with Human CXCL8/IL-8 DuoSet kit (R&D Systems, USA). Absorbance was measured at 450 nm and corrected at 540 nm using Infinite® M200 (TECAN, Switzerland).
Measurement of TARC level
HaCaT cells were seeded on a 96-well plate at 3×104 cells/well. After 24 hours, cells were starved using DMEM without FBS for 12 hours. Inflammatory responses were induced by treating with both INF-γ and TNF-α (ProSpec protein specialists, Korea) at 10 ng/ml for 24 hours. Then, NDGA was introduced for 72 hours. Dexamethasone (Sigma, USA) the positive control was also treated with 100 μg/ml. Supernatants were harvested and analyzed with Quantikine® Human CCL17/TARC immunoassay kit (R&D systems, USA). Absorbance was measured at 450 nm and corrected at 540 nm using Infinite® M200.
Western blot
The cells were treated with ice-cold lysis buffer, consisting of RIPA solution (Noble Bio, Korea), protease inhibitor cocktail (Sigma, USA) and 1 mM phenylmethanesulfonyl fluoride (PMSF, Sigma, USA) for 60 minutes. The lysates were sonicated and centrifuged at 13,000 × g and 4℃ for 20 minutes. Then, supernatants were harvested and protein concentration was normalized. The protein samples were stored at -20℃. The samples were mixed with LDS sample buffer and sample reducing buffer (Invitrogen, USA) and boiled at 95℃ for 5 minutes. The reduced samples were loaded on NuPAGE® 10% Bis-Tris gel and run with MOPS SDS running buffer (Invitrogen, USA). Then, the proteins were transferred to polyvinylidene fluoride transfer membrane (PVDF membrane, PALL corporation, USA). The membrane was blocked with 5% skim milk in TBS-T buffer (0.1% Tween® 20, 100 mM NaCl and 10 mM Tris-HCl, pH 7.5) and then probed using mouse monoclonal anti-involucrin antibody (1/1,000, Abcam, UK), sheep polyclonal anti-ICAM-1 antibody (1/1,000, R&D Systems, USA), mouse monoclonal anti-β-actin antibody (1/10,000, Sigma, USA), donkey anti-sheep IgG antibody (1/5,000, Bethyl Laboratories, Inc., USA) and goat anti-mouse IgG antibody (1/10,000, Bio-Rad, USA). Bands were detected with WEST-ZOL® plus Western Blot Detection system (iNtRON Biotechnology, Korea) and visualized with G : Box chemi (SYNGENE, USA). Densitometric analysis was performed using Image J software. The band density was normalized with β-actin intensity.
Animal study
Female hairless mice (SKH-1), aged 6-11 weeks old, were purchased from HOSHINO laboratory animals (Japan) and fed mouse diet and water ad libitum. All animal procedures were approved by the Institutional Animal Care and Use Committees of Gyeonggi Bio Center and performed in accordance with their guidelines. The following procedures made reference to those used by Man et al.(2008) All animals were sensitized with a single topical application of 10% oxazolone (100 μl/mouse) to the dorsal region except for the normal healthy mice group. To induce atopic dermatitis, 150 μl oxazolone 0.5% was topically applied with acetone : olive oil in a 4 : 1 (v/v) ratio twice daily for 9 days. After 1 day, TEWL was measured using VapoMeter (Delfin Technologies, Inc., USA). The mice were regrouped using TEWL levels. Then, 100 μl NDGA was applied with vehicle (propylene glycol : ethanol : water in a 5 : 3 : 2 (v/v/v) ratio) twice daily for an additional 5 weeks. Then one hour after NDGA application, oxazolone 0.5% was applied once daily. Dexamethasone 0.05% was used as positive control. TEWL was measured before and after (Day 0) oxazolone-induced atopic dermatitis and during NDGA treatment (Days 10, 22 and 26). Recovery of TEWL was analyzed with following formula : [1-(TEWL at indicated time- average TEWL of normal group at indicated time)/(TEWL after inducing atopic dermatitis- average TEWL of normal group at indicated time)×100] (Yun et al., 2011). TEWL data were measured four times in each mouse and the TEWL average of each mouse was used as a sample.
Measurement of serum IgE level
Retro orbital sinus blood samples were collected with MiniCollect® Tube (Greiner Bio-One, Austria) before and after oxazolone-induced atopic dermatitis and again on the final day (Day 27). Collected blood samples were left at room temperature for over 30 minutes to clot. The samples were centrifuged at 4℃ and 10,000 rpm for 10 minutes. Then, supernatants were stored at -80℃. Serum IgE concentration was diluted 1/40 with reagent diluent buffer and determined with Mouse IgE ELISA Quantitation kit (Bethyl Laboratories, Inc., USA) following instructions provided by the manufacturer. Absorbance was measured at 450 nm and corrected at 540 nm using Infinite® M200.
Statistics
All data are given as mean ± SEM. Statistical analyses for in vitro studies were performed by t-test using Excel (Microsoft, USA). The data of animal study were analyzed by one-way analysis of variance (ANOVA, SPSS, Inc., USA) followed by post hoc Duncan’s multiple range test to compare differences
among groups. Significance was defined as p-value < 0.05.
RESULTS
NDGA inhibited PAR2-induced intracellular Ca2+ influx in keratinocytes
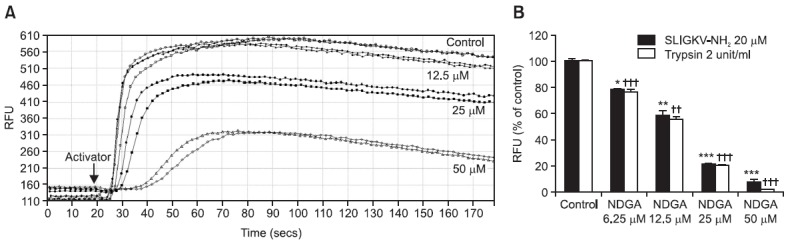
PAR2 activation induces intracellular Ca2+ mobilization. HaCaT keratinocytes were treated with SLIGKV-NH2 or trypsin to induce PAR2 activation, followed by several concentrations of NDGA treatment in order to induce changes in intracellular Ca2+ concentration. The chemical structure of NDGA is shown in Fig. 1. NDGA led to decreased SLIGKV-NH2- (20 μM) and trypsin- (2 unit/m) induced intracellular Ca2+ mobilization in a dose-dependent manner (Fig. 2). 6.25 μM, 12.5 μM, 25 μM and 50 μM of NDGA treatment inhibited intracellular Ca2+ mobilization in HaCaT keratinocytes by 22%, 42%, 79% and 92% respectively when stimulated by SLIGKV-NH2 and by 24%, 45%, 80% and 98% respectively when stimulated by trypsin relative to DMSO control (Fig. 2B). Calcium signals generated by HaCaT keratinocytes through release of Ca2+ were significantly reduced by NDGA.
Fig. 1. Chemical structure of NDGA.

Fig. 2. Mobilization of intracellular Ca2+ was decreased by pretreating with a PAR2 antagonist, NDGA. HaCaT keratinocytes were treated with various concentrations of NDGA, from 0.625 μM to 20 μM. After 30 minutes, PAR2-activating peptide, SLIGKV-NH2, or trypsin was added to activate PAR2, and the mobilization of intracellular Ca2+ was measured immediately by Flexstation 3. (A) Intracellular Ca2+ immediately increased with the addition of SLIGKV-NH2. The mobilization of intracellular Ca2+ was inhibited by pretreating with NDGA, in a dose-dependent manner. (B) NDGA decreased intracellular Ca2+ influx induced by both SLIGKV-NH2 and trypsin in HaCaT keratinocytes. Data are shown as Mean ± SEM, n=2. *p<0.05, ** (or ††) p<0.01, *** (or †††) p<0.001, compared with the control. RFU: relative fluorescence unit.
NDGA decreased PAR2-induced inflammatory responses in keratinocytes
PAR2 activation induces inflammatory responses. HaCaT keratinocytes were treated with SLIGKV-NH2 or TNF-α/IFN-γ to induce inflammatory responses. They were simultaneously exposed to various concentrations of NDGA. 100 μM of SLIGKV-NH2 raised the secretion of IL-8. NDGA was introduced at 1.25 μM, 2.5 μM, 5 μM, 10 μM and 20 μM. The secreted IL-8 level decreased significantly in a dose-dependent manner (Fig. 3A). 25 μM of SLIGKV-NH2 stimulated ICAM-1 expression, and 0.5 μM and 2 μM of NDGA inhibited ICAM-1 expression (Fig. 3C). Thymus and activation-regulated chemokine
Fig. 3. NDGA inhibited PAR2-mediated inflammatory response. (A) NDGA was introduced at different concentration, from 0.625 μM to 20 μM. Thirty minutes after pretreatment, SLIGKV-NH2 was added at 100 μM for 24 hours. The supernatants were harvested and immunoassay was performed. NDGA (1.25-20 μM) significantly decreased the secretion of IL-8 in a dose-dependent manner. (B) To stimulate inflammatory responses, INF-γ and TNF-α were introduced at 10 ng/ml for 24 hours. NDGA or dexamethasone was then applied for 72 hours. The supernatants were harvested and immunoassay was performed. NDGA treatment showed dose-dependent relationship with decreasing TARC levels in HaCaT keratinocytes. (C) SLIGKV-NH2 (25 μM) and NDGA (0.5 μM and 2 μM) was simultaneously introduced for 24 hrs. Western blot was performed to detect ICAM-1 expression. The intensity ratio (ICAM-1/β-actin) was described using the band of ICAM-1. SLIGKV-NH2 treatment stimulated the expression of ICAM-1 by 2.5-fold compared to the control (non-treated SLIGKV-NH2). Treatment of NDGA at 2 μM reduced the expression of ICAM-1. Data are shown as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, compared with the control. TARC: thymus and activation regulated chemokine, Dexa: dexamethasone, D: DMSO.
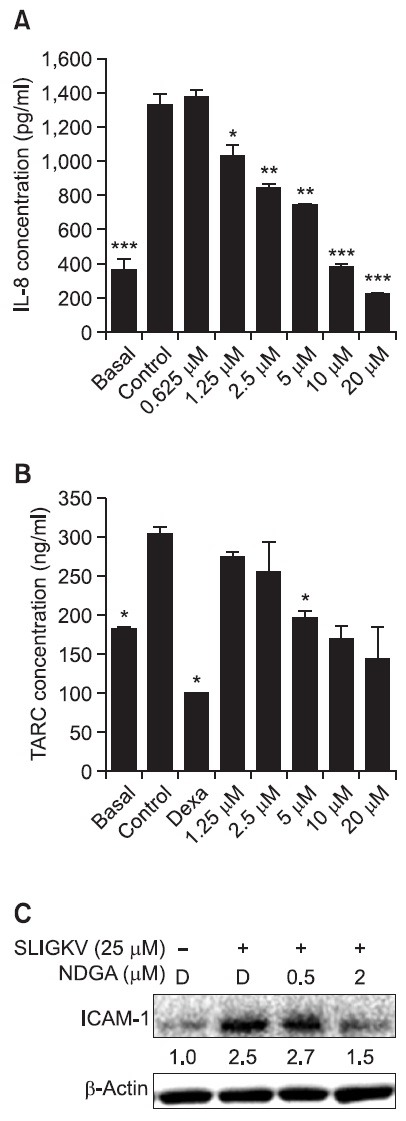
(TARC) was induced by exposure to TNF-α and IFN-γ at 10 ng/ml. 1.25 μM, 2.5 μM, 5 μM, 10 μM and 20 μM of NDGA decreased the secreted TARC level, though not statistically
significantly, in a dose-dependent manner (Fig. 3B). Dexamethasone (steroid) was used as positive control and decreased TARC level significantly at 250 μM.
NDGA inhibited PAR2-induced terminal differentiation in keratinocytes
PAR2 activation induced terminal differentiation of keratinocytes. In differentiating keratinocytes, many proteins such as involucrin, loricrin and filaggrin are upregulated (Steven and Steinert, 1994). We investigated whether NDGA decreased involucrin protein expression by inhibiting PAR2 activation in HaCaT keratinocytes and NHEKs. In HaCaT keratinocytes, treatment with 100 μM SLIGKV-NH2 for 72 hours showed a 3.4-fold increase in involucrin expression compared with control. NDGA exposure at 0.5 μM, 2 μM and 10 μM inhibited involucrin expression by 1.3-, 0.3- and 0.9-fold, respectfully, compared with treatment of SLIGKV-NH2 alone (Fig. 4A).
Fig. 4. NDGA inhibited PAR2-induced terminal differentiation in both HaCaT keratinocytes and NHEKs. To examine terminal differentiation of keratinocytes, we investigated the involucrin expression by using western blotting. The intensity ratio (involucrin/β-actin) was described under the band of involucrin. (A) In immortalized human HaCaT keratinocytes, SLIGKV-NH2 treatment stimulated the involucrin expression as a 3.1-fold increase. NDGA treatment for 72 hours inhibited the involucrin expression. (B) In NHEKs, normal human keratinocytes, SLIGKV-NH2 treatment increased the expression of involucrin, a 1.4-fold. NDGA treatment decreased the expression of involucrin, as dose-dependent manner. D: DMSO.
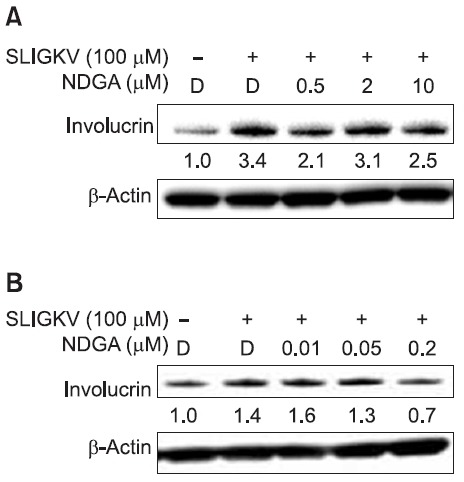
In NHEKs, 100 μM of SLIGKV-NH2 was introduced for 120 hours and upregulated involucrin protein level 1.4-fold. SLIGKV-NH2 was treated with 0.01 μM, 0.05 μM and 0.2 μM of NDGA and all doses except 0.01 μM dose-dependently downregulated the involucrin expression (Fig. 4B).
NDGA effects on oxazolone-induced atopic dermatitis in hairless mice
To investigate whether NDGA could recover skin barrier in atopic dermatitis in vivo, we induced atopic dermatitis in hairless mice using oxazolone 0.5% topically to dorsal skin for 9 days, followed by NDGA. Skin barrier recovery was checked by dorsal TEWL change. Dexamethasone 0.05% was used as a positive control. At 10 days after NDGA treatment (0.01%, 0.1% and 1%), skin barrier was recovered at 18%, 26% and 7% respectively, while vehicle treatment scarcely affect
the barrier recovery. The dexamethasone-treated group experienced 50% recovery at day 10. At day 16, treatment of NDGA (0.1% and 1%) recovered more than the vehicle, but the 0.01% concentration did not yield significant results. After 22 days, the dexamethasone-treated group had recovered almost 81% of the skin barrier, and other groups had recovered about 65% (Fig. 5A).
Fig. 5. NDGA recovered skin barrier and atopic dermatitis in hairless mice. To induce atopic dermatitis, mice were treated with oxazolone 0.05% for 9 days. Then they were treated with vehicle, dexamethasone (0.05%) and NDGA (0.01%, 0.1% and 1%) for additional 25 days. (A) Skin barrier recovery was checked by dorsal TEWL change. The dexamethasone-treated group recovered over 50% of the skin barrier at day 10. NDGA treated mice experienced the best results at day 10. NDGA treatment at 0.01%, 0.1% and 1% showed recovered skin permeability at 18%, 26% and 7%, respectively. At day 22, the NDGA and vehicle-treated groups showed similarly recovery. (B) To measure serum IgE level, blood samples were taken using retro orbital sinus blood collection. After the samples were centrifuged at 10,000 rpm for 10 minutes, supernatants were used in the following experiment. While the vehicle-treated group showed a 15% decrease in serum IgE level, dexamethasone and NDGA (0.01%, 0.1% and 1%)-treated groups showed decreases of 25%, 22%, 5% and 24%, respectively. (C) Dexamethasone causes weight loss and skin thinning when it is used chronically. During our experiment, the dexamethasone-treated group showed a significant decreased in weight. The data are shown as mean or mean ± SEM. *p<0.05, **p<0.01, compared with the control at indicated time.
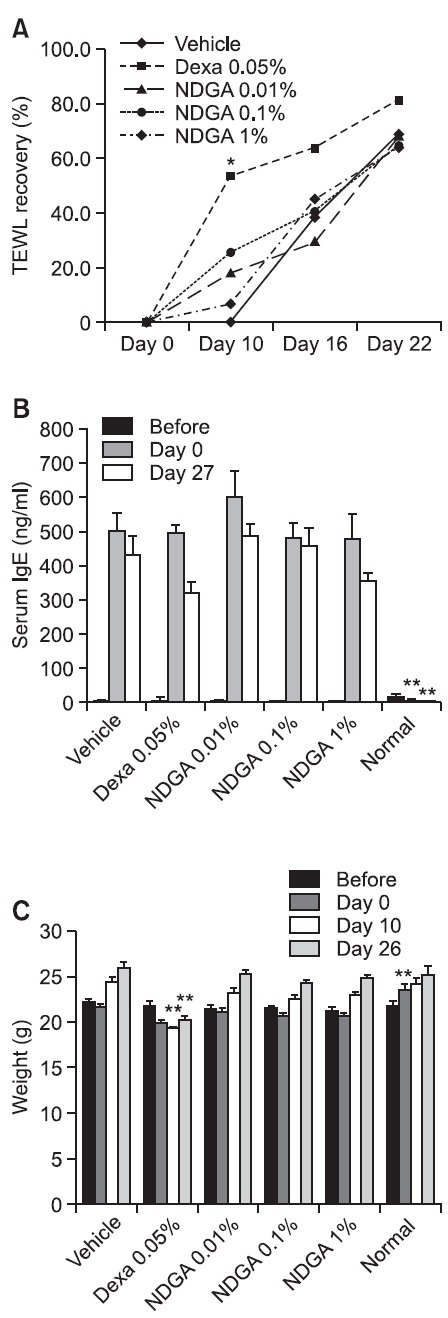
Serum IgE levels at basal condition were almost zero. Oxazolone treatment increased serum IgE level to approximately 500 ng/ml. Dexamethasone and NDGA-treated groups showed reduced serum IgE levels at day 27. IgE levels in the vehicle-treated group decreased 15%, levels in the dexamethasone (0.05 %) and NDGA (0.01%, 0.1% and 1%) groups decreased by 25%, 22%, 5% and 24%, respectively (Fig. 5B).
Dexamethasone side effects include weight loss and skin thinning. In this study, the dexamethasone-treated group displayed a significant decrease in weight at days 10 and 26 compared with the vehicle-treated group (Fig. 5C).
DISCUSSION
AD is a chronic inflammatory skin disease which occurs mainly in childhood and is a pervasive health problem in developed countries (Kang et al., 2008). Both abnormal inflammatory response and defective skin barrier are characteristic of AD (Jin et al., 2009). Barrier disruption increases the invasion of allergens, such as dust mite waste, pollen and pet dander and causes more severe inflammation (Elias and Steinhoff, 2008). This cycle comprises the “outside-inside-outside” model of AD pathogenesis (Elias and Steinhoff, 2008). PAR2 correlates with inflammatory responses and differentiation of keratinocytes. The proliferation and differentiation of keratinocytes in the epidermis control barrier permeability and homeostasis. The aim of this study is to find a new PAR2 antagonist to interfering with PAR2 activation, and consequently to halt symptoms of atopic dermatitis. Serine protease inhibitors, such as aprotinin and t-AMCHA, are known for inhibiting PAR2 activation by blocking cleavage of the N-terminus and subsequent inhibition of PAR2-activating peptide (He et al., 2004). We found that NDGA decreased intracellular Ca2+ mobilization induced by SLIGKV-NH2 and trypsin in HaCaT keratinocytes (Fig. 2). Our results indirectly indicated that PAR2 activation can be specifically inhibited by NDGA. Consequently, we have shown that NDGA inhibits PAR2-induced inflammation and keratinocyte differentiation.
PAR2 activation is known to increase the secretion of IL-8 and NF-κB-dependent ICAM-1 expression (Hou et al., 1998; Vergnolle et al., 1998). Additionally, PAR2 activation stimulates the mRNA expression of IL-1α, IL-8, TNF-α and human beta defensin (hBD)-2 in keratinocytes (Lee et al., 2010). Recent studies have also revealed the importance of PAR2 in itching and pain sensations. In rat and mouse DRG neurons, PAR2 was shown to be associated with long-lasting thermal and mechanical hyperalgesia by mediating the transient receptor potential vanilloid 4 ion channel (Grant et al., 2007; Jeong et al., 2008). We showed that NDGA inhibited PAR2-induced inflammation. SLIGKV-NH2 treatment increased IL-8 and ICAM-1 expression in HaCaT keratinocytes. NDGA treatment decreased IL-8 and ICAM-1 expression in a dose-dependent manner. Also, NDGA treatment decreased TARC secretion induced by TNF-α and IFN-γ in HaCaT keratinocytes (Fig. 3).
PAR2 activation induces the abnormal differentiation of keratinocytes. In previous studies, epidermal barrier disruption by repetitive tape-stripping caused a pH rise and an increase in serine protease activity. Serine protease was shown to cleave the PAR2 N-terminus, activating PAR2. PAR2 activation induced abnormal differentiation by stimulating cornification and inhibiting lamellar body secretion, thus delaying barrier recovery (Hachem et al., 2006, Demerjian et al., 2008; Jeong et al., 2008). To confirm the effects of NDGA on keratinocyte differentiation, we examined the expression of involucrin in HaCaT keratinocytes and NHEKs. NDGA decreased the expression of involucrin induced by SLIGKV-NH2 in both HaCaT keratinocytes and NHEKs (Fig. 4).
To determine the effects of NDGA on atopic dermatitis, we used an oxazolone-induced atopic dermatitis model. Oxazolone-induced atopic dermatitis was checked by measuring TEWL. Basal TEWL was about 20 g/m2h, and after AD induction TEWL was over 70 g/m2h. Topical treatment of NDGA decreased TEWL, and NDGA had the best effect on barrier recovery at day 10 (Fig. 5A). Also treatment with NDGA at concentrations of 0.01% and 1% reduced serum IgE level by 22%, 5% and 24%, respectively, compared with vehicle at day 27 (Fig. 5B). From these results, we concluded that NDGA could affect barrier recovery in atopic dermatitis without inducing weight loss.
In summary, NDGA blocked the PAR2-mediated signal pathway and promoted skin barrier recovery in atopic dermatitis. Our results suggest that NDGA is a PAR2 antagonist and may be a potential therapeutic agent for atopic dermatitis. For further study, we recommend examining the effects of NDGA on atopic dermatitis by using NC/Nga mice, a mice strain that spontaneously develops atopic dermatitis.
Acknowledgments
This study was supproted by a grant of the Korea Healthcare technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant No.: A103017).
References
- 1.Bohm S. K., Kong W., Bromme D., Smeekens S. P., Anderson D. C., Connolly A., Kahn M., Nelken N. A., Coughlin S. R., Payan D. G., Bunnett N. W. Molecular cloning, expression and potential functions of the human proteinase-activated receptor-2. Biochem. J. . (1996);314:1009–1016. doi: 10.1042/bj3141009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buddenkotte J., Stroh C., Engels I. H., Moormann C., Shpacovitch V. M., Seeliger S., Vergnolle N., Vestweber D., Luger T. A., Schulze-Osthoff K., Steinhoff M. Agonists of proteinase-activated receptor-2 stimulate upregulation of intercellular cell adhesion molecule-1 in primary human keratinocytes via activation of NF-kappa B. J. Invest. Dermatol. . (2005);124:38–45. doi: 10.1111/j.0022-202X.2004.23539.x. [DOI] [PubMed] [Google Scholar]
- 3.Demerjian M, Hachem J. P, Tschachler E, Denecker G, Declercq W, Vandenabeele P, Mauro T, Hupe M.,, Crumrine D, Roelandt T, Houben E, Elias P. M., Feingold K. R. Acute modulations in permeability barrier function regulate epidermal cornification: role of caspase-14 and the protease-activated receptor type 2. Am. J. Pathol. . (2008);172:86–97. doi: 10.2353/ajpath.2008.070161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eads D., Hansen R., Oyegunwa A., Cecil C., Culver C., Scholle F., Petty I., Laster S. Terameprocol, a methylated derivative of nordihydroguaiaretic acid, inhibits production of prostaglandins and several key inflammatory cytokines and chemokines. J. Inflamm (Lond). . (2009);6:2. doi: 10.1186/1476-9255-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elias P. M., Steinhoff M. "Outside-to-inside" (and now back to "outside") pathogenic mechanisms in atopic dermatitis. J. Invest. Dermatol. (2008);128:1067–1070. doi: 10.1038/jid.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frateschi S., Camerer E., Crisante G., Rieser S., Membrez M., Charles R. P., Beermann F., Stehle J. C., Breiden B., Sandhoff K., Rotman S., Haftek M., Wilson A., Ryser S., Steinhoff M., Coughlin S. R., Hummler E. AR2 absence completely rescues inflammation and ichthyosis caused by altered CAP1/Prss8 expression in mouse skin. Nat. Commun. . (2011);2:161. doi: 10.1038/ncomms1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant A. D., Cottrell G. S., Amadesi S., Trevisani M., Nicoletti P., Materazzi S., Altier C., Cenac N., Zamponi G. W., Bautista-Cruz F., Lopez C. B., Joseph E. K., Levine J. D., Liedtke W., Vanner S., Vergnolle N., Geppetti P., Bunnett N. W. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J. Physiol. (Lond). . (2007);578:715–733. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hachem J. P., Houben E., Crumrine D., Man M. Q., Schurer N., Roelandt T., Choi E. H., Uchida Y., Brown B. E., Feingold K. R., Elias P. M. Serine protease signaling of epidermal permeability barrier homeostasis. J. Invest. Dermatol. . (2006);126:2074–2086. doi: 10.1038/sj.jid.5700351. [DOI] [PubMed] [Google Scholar]
- 9.He S., Aslam A., Gaça M. D., He Y., Buckley M. G., Hollenberg M. D., Walls A. F. Inhibitors of tryptase as mast cell-stabilizing agents in the human airways: effects of tryptase and other agonists of proteinase-activated receptor 2 on histamine release. J. Pharmacol. Exp. Ther. . (2004);309:119–126. doi: 10.1124/jpet.103.061291. [DOI] [PubMed] [Google Scholar]
- 10.Hou L., Kapas S., Cruchley A. T., Macey M. G., Harriott P., Chinni C., Stone S. R., Howells G. L. Immunolocalization of protease-activated receptor-2 in skin: receptor activation stimulates interleukin-8 secretion by keratinocytes in vitro. Immunology. (1998);94:356–362. doi: 10.1046/j.1365-2567.1998.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeong S. K., Kim H. J., Youm J. K., Ahn S. K., Choi E. H., Sohn M. H., Kim K. E., Hong J. H., Shin D. M., Lee S. H. Mite and cockroach allergens activate protease-activated receptor 2 and delay epidermal permeability barrier recovery. J. Invest. Dermatol. (2008);128:1930–1939. doi: 10.1038/jid.2008.13. [DOI] [PubMed] [Google Scholar]
- 12.Jin H., He R., Oyoshi M., Geha R. S. Animal models of atopic dermatitis. J. Invest. Dermatol. . (2009);129:31–40. doi: 10.1038/jid.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang J. S., Yoon W. K., Han M. H., Lee H., Lee C. W., Lee K. H., Han S. B., Lee K., Yang K. H., Park S. K., Kim H. M. Inhibition of atopic dermatitis by topical application of silymarin in NC/Nga mice. Int. Immunopharmacol. (2008);8:1475–1480. doi: 10.1016/j.intimp.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Lee S. E., Kim J. M., Jeong S. K., Jeon J. E., Yoon H. J., Jeong M. K., Lee S. H. Protease-activated receptor-2 mediates the expression of inflammatory cytokines, antimicrobial peptides, and matrix metalloproteinases in keratinocytes in response to Propionibacterium acnes. Arch. Dermatol. Res. . (2010);302:745–756. doi: 10.1007/s00403-010-1074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahajan U. M., Gupta C., Wagh P. R., Karpe P. A., Tikoo K. Alteration in inflammatory/apoptotic pathway and histone modifications by nordihydroguaiaretic acid prevents acute pancreatitis in swiss albino mice. Apoptosis . (2011);16:1138–1149. doi: 10.1007/s10495-011-0643-8. [DOI] [PubMed] [Google Scholar]
- 16.Man M. Q., Hatano Y., Lee S. H., Man M., Chang S., Feingold K. R., Leung D. Y., Holleran W., Uchida Y., Elias P. M. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J. Invest. Dermatol. . (2008);128:79–86. doi: 10.1038/sj.jid.5701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nystedt S., Ramakrishnan V., Sundelin J. The proteinase-activated receptor 2 is induced by inflammatory mediators in human endothelial cells. Comparison with the thrombin receptor. J. Biol. Chem. . (1996);271:14910–14915. doi: 10.1074/jbc.271.25.14910. [DOI] [PubMed] [Google Scholar]
- 18.Rahman S., Ansari R. A., Rehman H., Parvez S., Raisuddin S. Nordihydroguaiaretic acid from creosote bush (Larrea tridentata) mitigates 12-O-tetradecanoylphorbol-13-acetate-induced inflammatory and oxidative stress responses of tumor promotion cascade in mouse skin. Evid. Based Complement Alternat. Med. . (2011);2011:734785. doi: 10.1093/ecam/nep076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roelandt T., Heughebaert C., Verween G., Giddelo C., Verbeken G., Pirnay J. P., Devos D., Crumrine D., Roseeuw D., Elias P. M., Hachem J. P. Actin dynamics regulate immediate PAR-2-dependent responses to acute epidermal permeability barrier abrogation. J. Dermatol. Sci. . (2011);61:101–109. doi: 10.1016/j.jdermsci.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Steven A. C., Steinert P. M. Protein composition of cornified cell envelopes of epidermal keratinocytes. J. Cell Sci. (1994);107:693–700. [PubMed] [Google Scholar]
- 21.Tsujii K., Andoh T., Ui, H., Lee J. B., Kuraishi Y. Involvement of tryptase and proteinase-activated receptor-2 in spontaneous itch-associated response in mice with atopy-like dermatitis. J. Pharmacol. Sci. (2009);109:388–395. doi: 10.1254/jphs.08332FP. [DOI] [PubMed] [Google Scholar]
- 22.Vergnolle N., Hollenberg M. D., Sharkey K. A., Wallace J. L. Characterization of the inflammatory response to proteinase-activated receptor-2 (PAR2)-activating peptides in the rat paw. Br. J. Pharmacol. (1999);127:1083–1090. doi: 10.1038/sj.bjp.0702634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vergnolle N., Macnaughton W. K., Al-Ani, B., Saifeddine M., Wallace J. L., Hollenberg M. D. Proteinase-activated receptor 2 (PAR2)-activating peptides: identification of a receptor distinct from PAR2 that regulates intestinal transport. Proc. Natl. Acad. Sci. USA. (1998);95:7766–7771. doi: 10.1073/pnas.95.13.7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yun J. W., Seo J. A., Jeong Y. S., Bae I. H., Jang W. H., Lee J., Kim S. Y., Shin S. S., Woo B. Y., Lee K. W., Lim K. M., Park Y. H. TRPV1 antagonist can suppress the atopic dermatitis-like symptoms by accelerating skin barrier recovery. J. Dermatol. Sci. (2009);62:8–15. doi: 10.1016/j.jdermsci.2010.10.014. [DOI] [PubMed] [Google Scholar]


