Abstract
suppresAvicularin, quercetin-3-α-L-arabinofuranoside, has been reported to possess diverse pharmacological properties such as anti-inflammatory and anti-infectious effects. However, the underlying mechanism by which avicularin exerts its anti-inflammatory activity has not been clearly demonstrated. This study aimed to elucidate the anti-inflammatory mechanism of avicularin in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophage cells. Avicularin significantly inhibited LPS-induced excessive production of pro-inflammatory mediators such as nitric oxide (NO) and PGE2 and the protein levels of iNOS and COX-2, which are responsible for the production of NO and PGE2, respectively. Avicularin also suppressed LPS-induced overproduction of pro-inflammatory cytokine IL-1β. Furthermore, avicularin significantly suppressed LPS-induced degradation of IκB, which retains NF-κB in the cytoplasm, consequently inhibiting the transcription of pro-inflammatory genes by NF-κB in the nucleus. To understand the underlying signaling mechanism of anti-inflammatory activity of avicularin, involvement of multiple kinases was examined. Avicularin significantly attenuated LPS-induced activation of ERK signaling pathway in a concentration-dependent manner. Taken together, the present study clearly demonstrates that avicularin exhibits anti-inflammatory activity through the suppression of ERK signaling pathway in LPS-stimulated RAW 264.7 macrophage cells.
Keywords: Avicularin, RAW 264.7 cells, Lipopolysaccharide, iNOS, COX-2, NF-κB
INTRODUCTION
Macrophages are the primary immune cells that respond to harmful stimuli and play a crucial role in host defense (Rehman et al., 2012). However, aberrant activation of macrophages has been reported to play pathogenic roles in inflammatory disorders such as rheumatoid arthritis, atherosclerosis, and sepsis. (Kim et al., 2012). In pathologic conditions, abnormally activated macrophages produce excessive amount of various pro-inflammatory mediators such as NO and cytokines via NF-κB activation, which could lead to the aggravation of the conditions (Itharat and Hiransai, 2012). Lipopolysacharide (LPS), a component of the outer membrane of Gram-negative bacteria, is one of the most potent activators of macrophages (Rietschel and Brade, 1992). LPS activation of macrophages results in a wide range of responses, including secretion of pro-inflammatory mediators, expression of adhesion molecules and coagulation factors, phagocytosis, and cytoskeletal rearrangement (Sweet and Hume, 1996). Therefore, suppression of aberrant activation of macrophages may have valuable therapeutic potential for the treatment of inflammation-related disorders.
Avicularin, quercetin-3-α-L-arabinofuranoside, is a glycoside of quercetin, which has been reported to possess a variety of biological properties such as anti-inflammatory, anti-allergic, anti-oxidant, hepatoprotective, and anti-tumor activities (Hertog et al., 1993; Williams et al., 2004). Although biological activities of quercetin, aglycone of avicularin, have been extensively examined, biological properties of avicularin itself was not fully examined. Avicularin has been reported to protect cardiomyocytes and hepatocytes against oxidative stress-induced apoptosis (Kim et al., 2011a; Kim et al., 2011b). In addition, avicularin has been reported to play a protective role by inhibiting ureases, which are virulene factors implicated in the pathogenesis of many clinical conditions such as pyelonephritis and hepatic coma (Shabana et al., 2010). However, anti-inflammatory property of avicularin has not been reported. Furthermore, the underlying mechanism by which avicularin may exert the anti-inflammatory action has not been demonstrated. Avicularin has been reported to be present in many medicinal herbs including Lespedeza cuneata (Kim et al., 2011b), Lindera erythrocarpa (Kim et al., 2011a), and Psidium guajava (Shabana et al., 2010). Avicularin, isolated from Rhododendron schlipenbachii, was used in the present study (Kim et al., 2006).
The goal of this study was to provide a novel pharmacological agent that could suppress abnormally activated immune responses of macrophages by examining the anti-inflammatory activity of avicularin and its underlying mechanism in LPS-stimulated RAW264.7 macrophage cells.
MATERIALS AND METHODS
Reagents and cell culture
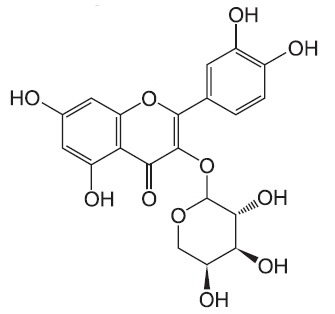
Bacterial lipopolysaccharide (LPS) from Escherichia coli serotype 055:B5 was purchased from Sigma-Aldrich (St. Louis, MO, USA). Avicularin was isolated and identified from the leaves of Rhododendron schlipenbachii (Kim et al., 2006) (Fig. 1). The compound was dissolved in dimethyl sulfoxide (DMSO) and added to the cell culture at the desired concentrations. The macrophage RAW 264.7 cell line was maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco BRL, Grand Island, NY, USA) containing 5% heat-inactivated fetal bovine serum and penicillin-streptomycin (Gibco BRL) at 37℃, 5% CO2. In all experiments, cells were incubated in the presence of the indicated concentration of avicularin before the addition of LPS (200 ng/ml).
Fig. 1. Chemical structure of avicularin.
Cell viability
Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The RAW 264.7 cells were seeded at 5×105 cells per well and incubated with avicularin at various concentrations for 24 hr at 37℃. After incubation, MTT (0.5 mg/ml in PBS) was added to each well, and the cells were incubated for 3 hr at 37℃ and 5% CO2. The resulting formazan crystals were dissolved in dimethyl sulfoxide (DMSO). Absorbance was determined at 540 nm. The results were expressed as a percentage of surviving cells over control cells.
Nitrite quantification assay
The production of NO was estimated by measuring the amount of nitrite, a stable metabolite of NO, using the Griess reagent as described (Lee et al., 2012). After avicularin-pretreated RAW 264.7 macrophage cells were stimulated with
LPS in 12-well plates for 24 hr, 100 μl of the cell supernatant was mixed with an equal volume of Griess reagent. Light absorbance was read at 540 nm. The results were expressed as a concentration of released NO from RAW 264.7 cells. To
prepare a standard curve, sodium nitrite was used to prepare a standard curve.
Western blot analysis
The RAW 264.7 macrophage cells were pretreated with avicularin for 1 hr and then stimulated with LPS. Cells were washed with PBS and lysed in PRO-PREP lysis buffer (iNtRON Biotechnology, Seongnam, Korea). Equal amounts of protein were separated on 10% SDS-polyacrylamide gel. Proteins were transferred to Hypond PVDF membrane (Amersham Biosciences, Piscataway, NJ, USA) and blocked in 5% skim milk in TBST for 1 hr at room temperature. Specific antibodies against iNOS, COX-2, extracellular signal-regulated kinase (ERK), phosphorylated (p)-ERK, p38, p-p38, c-Jun N-terminal kinase (1:1,000; Cell signaling Technology), IκB-α (1:1,000; Santa Cruz Biotechnology Inc), and β-actin (1:2,500; Sigma) were diluted in 5% skim milk. After thoroughly washing with TBST, horseradish peroxidase-conjugated secondary antibodies were applied. The blots were developed by the enhanced chemiluminescence detection (Amersham Biosciences).
ELISA assay for cytokines
The RAW 264.7 macrophage cells were treated with avicularin in the absence or presence of LPS. After 24 hr incubation, TNF-α and IL-1β levels in culture media were quantified using monoclonal anti-TNF-α or IL-1β antibodies according to the manufacturer’s instruction (R&D Systems).
Statistical analysis
All values shown in the figures are expressed as the mean ± SD obtained from at least three independent experiments. Statistical significance was analyzed by two-tailed Student’s t-test. Data with values of p<0.05 were considered as statistically significant. Single (* and #) and double (** and ##) marks represent statistical significance in p<0.05 and p<0.01, respectively.
RESULTS
Avicularin suppresses NO and PGE2 production in LPS-stimulated RAW 264.7 macrophage cells
Excessive production of NO and PGE2 has been widely considered as a characteristic feature of pro-inflammatory condition of immune cells (Ock et al., 2009; Lee et al., 2012). Therefore, suppressive effects of avicularin on NO and PGE2 production in LPS-stimulated RAW 264.7 macrophage cells were examined. Cells were incubated with indicated concentrations of avicularin for 1 hr prior to LPS treatment and, for NO
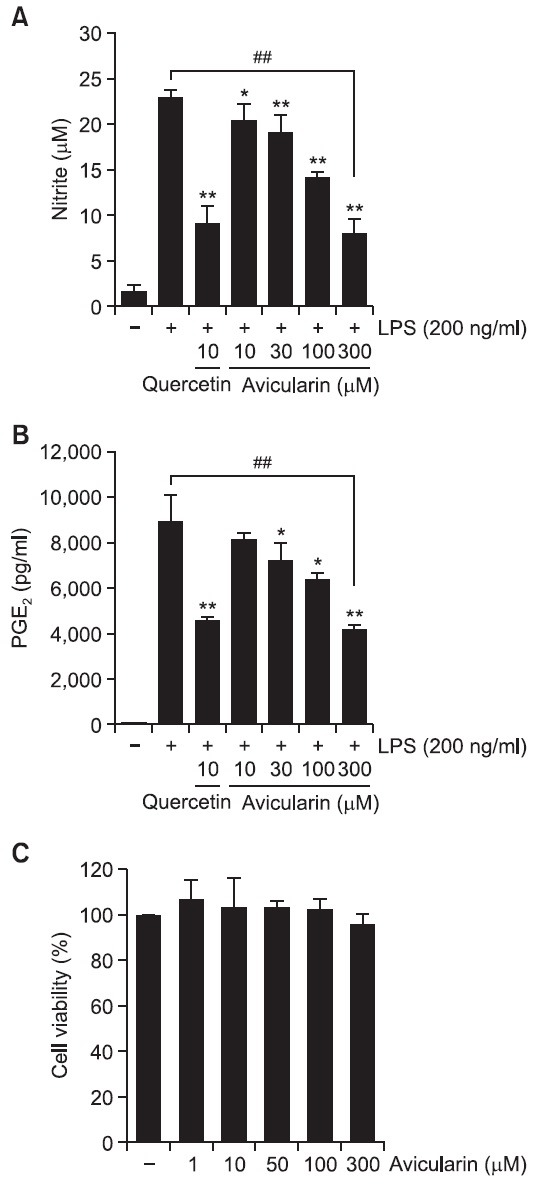
measurement, Griess reaction was used as an index for NO synthesis. As shown in Fig. 2A, LPS markedly increased NO production in RAW 264.7 macrophage cells. Avicularin significantly inhibited NO production in LPS-stimulated RAW 264.7 cells in a concentration dependent manner (Fig. 2A). Aavicularin significantly attenuated LPS-induced PGE2 production, which was measured with ELISA assay, in a concentration-dependent manner (Fig. 2B). In addition, avicularin showed no noticeable cytotoxicity in concentration ranges (Fig. 2C).
Fig. 2. Avicularin significantly attenuated LPS-induced overproduction of NO (A) and PGE2 (B) in RAW 264.7 macrophage cells. RAW 264.7 cells were pretreated with various concentrations of avicularin for 1 hr before incubation with LPS (200 ng/ml) for 24 hr. (A) The amount of nitrite in the supernatants was measured using Griess reagent. Avicularin exhibited a significant suppression of LPS-induced NO production in a concentration-dependent manner. (B) To measure the amount of PGE2 secretion, culture media were subjected to PGE2 ELISA. PGE2 secretion was significantly suppressed with avicularin in a concentration-dependent manner. (C) Effect of avicularin on the viability of RAW 264.7 cells. No noticeable cell death was observed with avicularin concentrations used in the present study. The data are expressed as mean ± S.D. (n=3), and are representative of three or more independent experiments. *p<0.05 and **p<0.01 indicate statistically significant differences from treatment with LPS alone. ##p<0.01 indicates statistically significant difference between indicated groups.
Avicularin inhibits LPS-induced protein expression of iNOS and COX-2
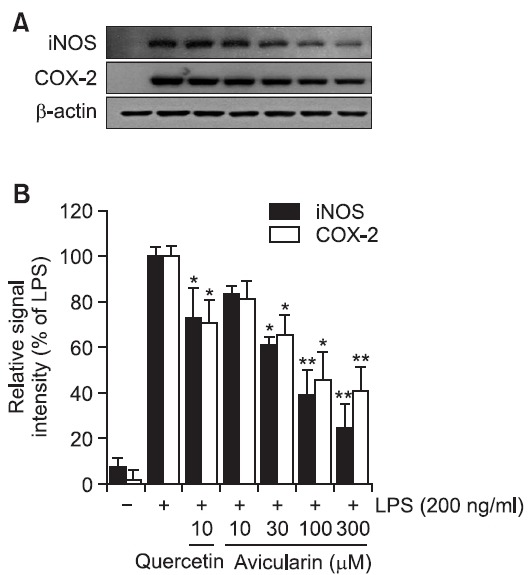
As avicularin was found to inhibit LPS-induced NO and PGE2 production in RAW 264.7 cells (Fig. 2), protein expression levels of iNOS and COX-2 with avicularin treatment were examined using Western blot analysis. LPS resulted in markedly increased expression of iNOS and COX-2 proteins and avicularin significantly suppressed their protein levels in a concentration-dependent manner (Fig. 3), demonstrating that suppressed production of NO and PGE2 is due to decreased expression of their responsible proteins, iNOS and COX-2, respectively, with avicularin treatment.
Fig. 3. Avicularin significantly suppressed LPS-induced iNOS and COX-2 expressions in RAW 264.7 cells. (A) The cell lysates were subjected to SDS-PAGE, and then protein levels of iNOS and COX-2 were determined by Western blot analysis. (B) Quantitative analysis of iNOS and COX-2 expression was performed by densitometric analysis. The β-actin was used as an internal control. Avicularin significantly attenuated LPS-induced overexpression of iNOS and COX-2. Images are representative of three independent experiments that shows consistent results. *p<0.05 and **p<0.01 indicate statistically significant differences from treatment with LPS alone.
Avicularin attenuates LPS-induced release of pro-inflammatory cytokine IL-1β
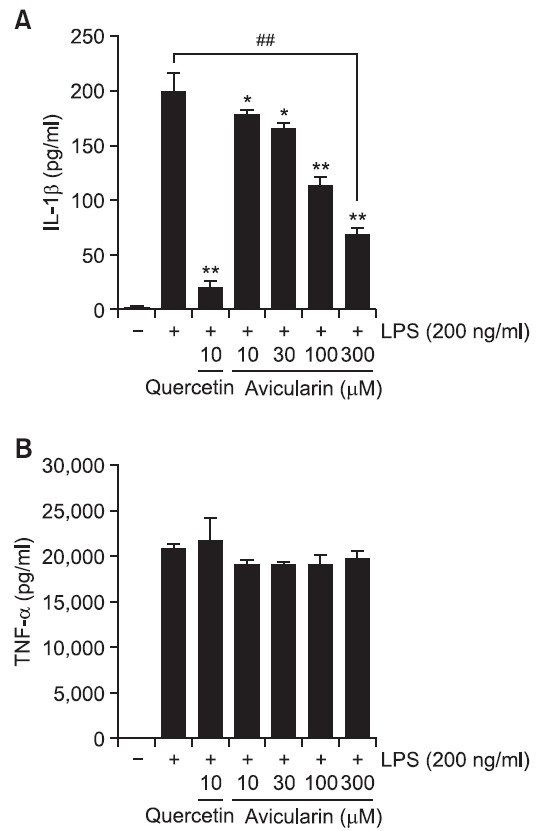
To examine the effects of avicularin on the pro-inflammatory cytokines such as TNF-α and IL-1β, production of these cytokines was examined using ELISA assay in LPS-stimulated RAW 264.7 cells. LPS treatment resulted in excessive extracellular release of TNF-α and IL-1β in RWA 264.7 cells. Avicularin significantly attenuated LPS-induced extracellular release of IL-1β (Fig. 4A). However, avicularin has no effect on LPS-induced extracellular release of TNF-α (Fig. 4B). Further studies are necessary to elucidate the differential role of
Fig. 4. Avicularin inhibited the release IL-1β (A), but not TNF-α (B) in LPS-stimulated RAW 264.7 macrophage cells. Cells were pretreated with the indicated concentrations of avicularin for 1 hr before LPS treatment (200 ng/ml), cell culture media were collected and subjected to TNF-α and IL-1β ELISA. Avicularin significantly attenuated LPS-induced IL-1β secretion, but did not affect on TNF-α. Data represent three independent experiments in triplicate and are expressed as mean ± SD. *p<0.05 and **p<0.01 indicate statistically significant differences from treatment with LPS alone. ##p<0.01 indicates statistically significant difference between indicated groups.
avicularin on these pro-inflammatory cytokines.
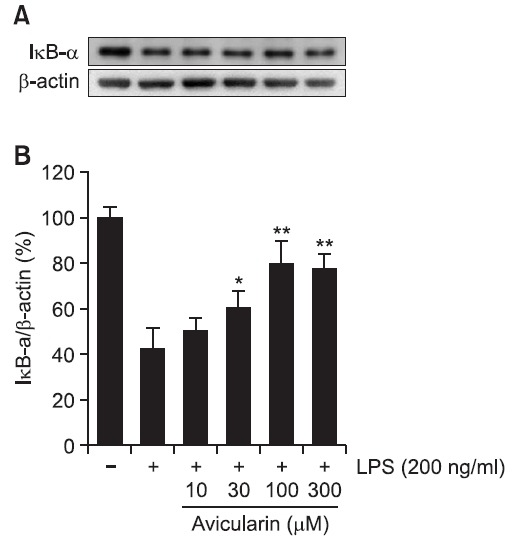
Avicularin attenuates LPS-induced degradation of IKB
The transcription factor NF-κB has been demonstrated to be a predominant regulator of numerous pro-inflammatory genes and cytosolic NF-κB has to translocate to the nucleus for transcription of pro-inflammatory genes. IκB inhibits the nuclear translocation of NF-κB by sequestering NF-κB in the cytoplasm (Zheng et al., 2008b; Ock et al., 2009). In the present
study, whether avicularin inhibits LPS-induced degradation of cytosolic IκB was examined. LPS treatment resulted in noticeable degradation of IκB and avicularin significantly attenuated LPS-induced degradation of IκB in a concentration-dependent manner (Fig. 5), suggesting that avicularin may suppress LPS-induced NF-κB activity in RAW 264.7 macrophage cells.
Fig. 5. Avicularin suppressed LPS-induced IκB-α degration in RAW 264.7 macrophage cells. (A) Total cell lysates obtained 15 min after the LPS stimulation were subjected to Western blotting to measure the levels of IκB-α degradation. (B) Quantification of IκB-α degradation was performed by densitometric analysis. The β-actin was used as an internal control. Data from triplicate determination are shown (mean ± S.D.). *p<0.05 and **p<0.01 indicate statistically significant differences from treatment with LPS alone.
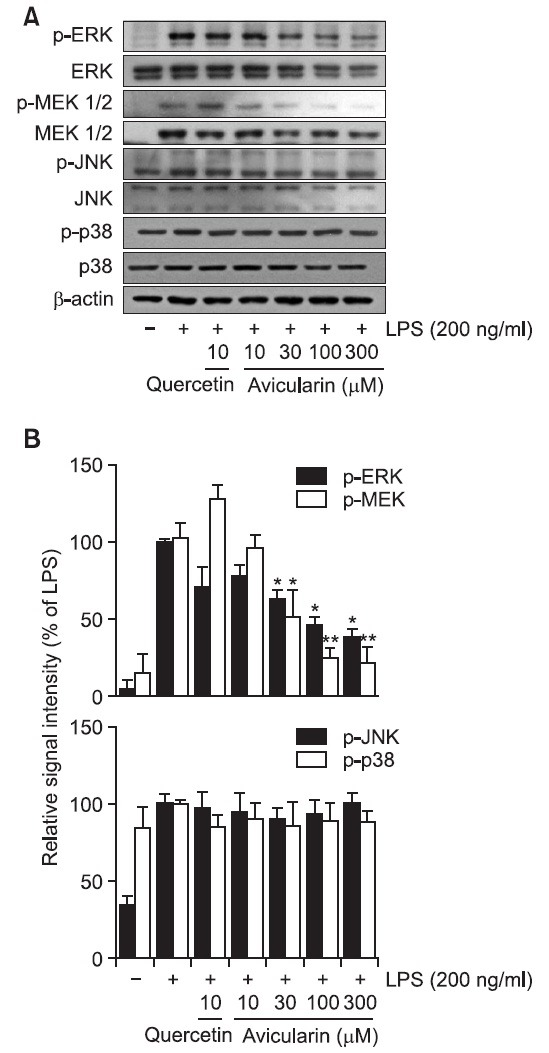
Avicularin attenuates LPS-induced phosphorylation of ERK
To investigate the underlying mechanism by which avicularin exerts its anti-inflammatory property, possible involvement of MAP kinase signaling pathway was examined. The effect of avicularin on LPS-stimulated phosphorylation of JNK, ERK, and p38 kinase in RAW 264.7 macrophage cells was measured. Cells were pretreated with avicularin at various concentrations and then were treated with LPS (200 ng/ml) for 30 min. As shown in Fig. 6, avicularin significantly suppressed the phosphorylation of ERK in a concentration-dependent manner in the LPS-stimulated RAW 264.7 cells. Furthermore, phosphorylation of MEK, upstream kinase of ERK, also significantly suppressed withn. However, no noticeable inhibitory effect was observed on the phosphorylation of JNK and p38. Although aviculairn significantly attenuated LPS-induced ERK phosphorylation, quercetin exhibited negligible effects on MAP kinases, suggesting that quercetin might modulate other signaling pathways to exert its anti-inflammatory properties. However, further studies are necessary to elucidate the exact mechanism by which quercetin exerts its anti-inflammatory effects in RAW 264.7 cells.
Fig. 6. Avicularin inhibited LPS-stimulated ERK signaling pathway in RAW 264.7 macrophage cells. RAW 264.7 cells were stimulated with 200 ng/ml LPS in the absence or presence of avicularin. (A) Western blot analysis was then performed to evaluate the activation of MAP kinases signaling pathways. (B) Ratio of phosphorylated forms to native forms was carried out by densitometric analysis. LPS-induced phosphorylation of ERK was significantly attenuated with aviculairn treatment, but phosphorylation of JNK and p38 was not decreased with avicularin treatment. Phosphorylation of MEK, upstream kinase of ERK, was accordingly attenuated with avicularin, suggesting that MEK/ERK might play an important role in the LPS-induced overactivation of RAW 264.7 cells. β-Actin was used as an internal control. Images are representative of three independent experiments that shows reproducible results. *p<0.05 and **p<0.01 indicate statistically significant differences from treatment with LPS alone.
DISCUSSION
The present study clearly demonstrated that avicularin possesses anti-inflammatory properties through the suppression of ERK signaling pathway in LPS-stimulated RAW 264.7 macrophage cells. Avicularin significantly suppressed LPS-induced overproduction of NO and PGE2 and expression of their responsible proteins, iNOS and COX-2, respectively. Avicularin also suppressed the secretion of pro-inflammatory cytokine IL-1β and significantly attenuated LPS-induced IκB degradation. Furthermore, avicularin significantly attenuated LPS-induced activation of ERK signaling pathway.
Avicularin, quercetin-3-α-L-arabinofuranoside, belongs to a group of flavonoid glycosides. Flavonoids have been extensively reported to possess a variety of biological activities including anti-inflammatory, anti-oxidant, anti-tumor, and neuroprotective actions (Havsteen, 1983; Dixon and Steele, 1999). Although significant amount of flavonoids has been identified to be present as glycosides in medicinal plants, determination of biological activities of flavonoids were mainly carried out with aglycones of flavonoids (Kim et al., 1999; Aligiannis et al., 2001; Ha et al., 2003). It has been previously demonstrated that aglycones of flavonoid glycosides exhibit more potent free radical scavenging activity than their glycosides (Hou et al., 2004). The attenuated biological activities of glycosides might be attributed to the decreased intracellular transport of glycosides due to hydrophilicity of sugar residues. In accordance, it has been reported that even though some glycosides exhibited comparable antioxidant potentials in in vitro DPPH radical scavenging assay, they showed significantly attenuated biological activity such as NO suppression in a cell model of BV2 microglia (Kwon et al., 2004), suggesting that hydrophilic sugar residues might cause decreased uptake of glycosides into cells. Accordingly, the present study showed that avicularin exhibited significantly attenuated biological activities compared to quercetin, aglycone of avicularin. It has been reported that glycosides are hydrolyzed to the aglycones by gycosidases in the intestines and the liver (Akao et al., 1994). However, it has been also reported that types of sugar moiety determine the bioavailability of glycosides (Arts et al., 2004). Further studies are necessary to clearly elucidate the effects of glycosidation on the biological activities of glycosides in terms of the position, degree, and types of sugar substitution.
Macrophages play essential roles in inflammation and mobilization of the host defense against bacterial infection (Rehman et al., 2012). However, aberrantly activated macrophages also play a key role in sepsis and other inflammation-related disorders by producing a wide variety of pro-inflammatory mediators (Rietschel and Brade, 1992). It has been reported that macrophages mediate LPS-induced gene transcription through the binding of LPS to its membrane receptor, TLR4, and that LPS bound to TLR4 induces signal transduction pathways leading to the phosphorylation of kinases such as IκB kinases and MAPKs, which in turn activates various transcription factors including NF-κB and AP-1 families (O'Connell et al., 1998; Guha and Mackman, 2001). In accordance, the present study showed that LPS exhibited noticeable degradation of IκB, which indicates nuclear translocation of NF-κB. In addition, LPS resulted in ERK signaling pathway in RAW 264.7 cells. However, avicularin significantly abolished LPS-induced IκB degradation and ERK activation. Although avicularin inhibited LPS-induced extracellular secretion of IL-1β, it showed negligible effect on another key pro-inflammatory cytokine TNF-α. Interestingly, quercetin either did not affect LPS-induced TNF-α release. However, effects of avicularin on other pro-inflammatory cytokines and transcription factors other than NF-κB have not been examined. Therefore, further studies are necessary to clearly elucidate the effect of avicularin on wide range of cytokines and transcription factors, which are known to be implicated in inflammation.
NF-κB is an important transcription factor for pro-inflammatory mediators such as iNOS, IL-1β, and TNF-α (Siebenlist et al., 1994; Kuprash et al., 1995) and inappropriate regulation of NF-κB and its downstream genes have been associated with various pathological conditions including cancer and autoimmune diseases (Karin et al., 2001; Li and Verma, 2002). It has been reported that LPS causes the nuclear translocation of p65 subunit of NF-κB through IκB degradation (Moon et al., 2007; Zheng et al., 2008a). In accordance with these reports, the present study showed that avicularin significantly attenuated LPS-induced IκB degradation, which presumably prevents subsequent nuclear translocation of p65 in LPS-induced RAW 264.7 macrophages.
In conclusion, the results clearly demonstrate that avicularin exhibits anti-inflammatory activity such as suppression of NO and PGE2 production and cytokine release by presumably inhibiting nuclear transclocation of NF-κB in LPS-stimulated RAW 264.7 macrophage cells. The present study strongly suggests that avicularin might be a valuable therapeutic agent in the treatment of inflammation-related pathologies such as rheumatoid arthritis, atherosclerosis, and sepsis. However, further studies are necessary to clearly demonstrate the exact mechanism by which avicularin inhibits LPS-induced activation of ERK signaling pathway.
Acknowledgments
This study was supported by Kangwon National University (YS Kwon).
References
- 1.Akao T., Kobashi K., Aburada M. Enzymic studies on the animal and intestinal bacterial metabolism of geniposide. Biol. Pharm. Bull. (1994);17:1573–1576. doi: 10.1248/bpb.17.1573. [DOI] [PubMed] [Google Scholar]
- 2.Aligiannis N., Mitaku S., Mitrocotsa D., Leclerc S. Flavonoids as cycline-dependent kinase inhibitors: inhibition of cdc 25 phosphatase activity by flavonoids belonging to the quercetin and kaempferol series. Planta Med. . (2001);67:468–470. doi: 10.1055/s-2001-15807. [DOI] [PubMed] [Google Scholar]
- 3.Arts I. C., Sesink A. L., Faassen-Peters M., Hollman P. C. The type of sugar moiety is a major determinant of the small intestinal uptake and subsequent biliary excretion of dietary quercetin glycosides. Br. J. Nutr. (2004);91:841–847. doi: 10.1079/BJN20041123. [DOI] [PubMed] [Google Scholar]
- 4.Dixon R. A., Steele C. L. Flavonoids and isoflavonoids - a gold mine for metabolic engineering. Trends Plant Sci. . (1999);4:394–400. doi: 10.1016/s1360-1385(99)01471-5. [DOI] [PubMed] [Google Scholar]
- 5.Guha M., Mackman N. LPS induction of gene expression in human monocytes. Cell. Signal. . (2001);13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 6.Ha H. J., Kwon Y. S., Park S. M., Shin T., Park J. H., Kim H. C., Kwon M. S., Wie M. B. Quercetin attenuates oxygen-glucose deprivation- and excitotoxin-induced neurotoxicity in primary cortical cell cultures. Biol. Pharm. Bull. . (2003);26:544–546. doi: 10.1248/bpb.26.544. [DOI] [PubMed] [Google Scholar]
- 7.Havsteen B. Flavonoids, a class of natural products of high pharmacological potency. Biochem. Pharmacol. . (1983);32:1141–1148. doi: 10.1016/0006-2952(83)90262-9. [DOI] [PubMed] [Google Scholar]
- 8.Hertog M. G., Feskens E. J., Hollman P. C., Katan M. B., Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. (1993);342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 9.Hou L., Zhou B., Yang L., Liu Z. L. Inhibition of free radical initiated peroxidation of human erythrocyte ghosts by flavonols and their glycosides. Org. Biomol. Chem. . (2004);2:1419–1423. doi: 10.1039/b401550a. [DOI] [PubMed] [Google Scholar]
- 10.Itharat A., Hiransai P. Dioscoreanone suppresses LPS-induced nitric oxide production and inflammatory cytokine expression in RAW 264.7 macrophages by NF-kappaB and ERK1/2 signaling transduction. J. Cell. Biochem. (2012);113:3427–3435. doi: 10.1002/jcb.24219. [DOI] [PubMed] [Google Scholar]
- 11.Karin M., Takahashi T., Kapahi P., Delhase M., Chen Y., Makris C., Rothwarf D., Baud V., Natoli G., Guido F., Li N. Oxidative stress and gene expression: the AP-1 and NF-kappaB connections. Biofactors . (2001);15:87–89. doi: 10.1002/biof.5520150207. [DOI] [PubMed] [Google Scholar]
- 12.Kim G. B., Shin K. S., Kim C. M., Kwon Y. S. Flavonoids from the leaves of Rhododendron schlipenbachii. Kor. J. Pharmacogn. (2006);37:177–183. [Google Scholar]
- 13.Kim H. K., Cheon B. S., Kim Y. H., Kim S. Y., Kim H. P. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure-activity relationships. Biochem. Pharmacol. (1999);58:759–765. doi: 10.1016/s0006-2952(99)00160-4. [DOI] [PubMed] [Google Scholar]
- 14.Kim J. A., Jung Y. S., Kim M. Y., Yang S. Y., Lee S., Kim Y. H. Protective effect of components isolated from Lindera erythrocarpa against oxidative stress-induced apoptosis of H9c2 cardiomyocytes. Phytother. Res. . (2011a);25:1612–1617. doi: 10.1002/ptr.3465. [DOI] [PubMed] [Google Scholar]
- 15.Kim S. M., Kang K., Jho E. H., Jung Y. J., Nho C. W., Um B. H., Pan C. H. Hepatoprotective effect of flavonoid glycosides from Lespedeza cuneata against oxidative stress induced by tert-butyl hyperoxide. Phytother. Res. (2011b);25:1011–1017. doi: 10.1002/ptr.3387. [DOI] [PubMed] [Google Scholar]
- 16.Kim Y. J., Shin Y., Lee K. H., Kim T. J. Anethum graveloens flower extracts inhibited a lipopolysaccharide-induced inflammatory response by blocking iNOS expression and NF-kappaB activity in macrophages. Biosci. Biotechnol. Biochem. (2012);76:1122–1127. doi: 10.1271/bbb.110950. [DOI] [PubMed] [Google Scholar]
- 17.Kuprash D. V., Udalova I. A., Turetskaya R. L., Rice N. R., Nedospasov S. A. Conserved kappa B element located downstream of the tumor necrosis factor alpha gene: distinct NF-kappa B binding pattern and enhancer activity in LPS activated murine macrophages. Oncogene. (1995);11:97–106. [PubMed] [Google Scholar]
- 18.Kwon Y. S., Kim S. S., Sohn S. J., Kong P. J., Cheong I. Y., Kim C. M., Chun W. Modulation of suppressive activity of lipopolysaccharide-induced nitric oxide production by glycosidation of flavonoids. Arch. Pharm. Res. . (2004);27:751–756. doi: 10.1007/BF02980144. [DOI] [PubMed] [Google Scholar]
- 19.Lee J. W., Bae C. J., Choi Y. J., Kim S. I., Kim N. H., Lee H. J., Kim S. S., Kwon Y. S., Chun W. 3,4,5-Trihydroxycinnamic Acid Inhibits LPS-Induced iNOS Expression by Suppressing NF-kappaB Activation in BV2 Microglial Cells. Korean J. Physiol. Pharmacol. (2012);16:107–112. doi: 10.4196/kjpp.2012.16.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Q., Verma I. M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. (2002);2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 21.Moon D. O., Park S. Y., Lee K. J., Heo M. S., Kim K. C., Kim M. O., Lee J. D., Choi Y. H., Kim G. Y. Bee venom and melittin reduce proinflammatory mediators in lipopolysaccharide-stimulated BV2 microglia. Int. Immunopharmacol. (2007);7:1092–1101. doi: 10.1016/j.intimp.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 22.O'Connell M. A., Bennett B. L., Mercurio F., Manning A. M., Mackman N. Role of IKK1 and IKK2 in lipopolysaccharide signaling in human monocytic cells. J. Biol. Chem. (1998);273:30410–30414. doi: 10.1074/jbc.273.46.30410. [DOI] [PubMed] [Google Scholar]
- 23.Ock J., Kim S., Suk K. Anti-inflammatory effects of a fluorovinyloxyacetamide compound KT-15087 in microglia cells. Pharmacol. Res. . (2009);59:414–422. doi: 10.1016/j.phrs.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Rehman M. U., Yoshihisa Y., Miyamoto Y., Shimizu T. The anti-inflammatory effects of platinum nanoparticles on the lipopolysaccharide-induced inflammatory response in RAW 264.7 macrophages. Inflamm. Res. (2012);61:1177–1185. doi: 10.1007/s00011-012-0512-0. [DOI] [PubMed] [Google Scholar]
- 25.Rietschel E. T., Brade H. Bacterial endotoxins. Sci. Am. (1992);267:54–61. doi: 10.1038/scientificamerican0892-54. [DOI] [PubMed] [Google Scholar]
- 26.Shabana S., Kawai A., Kai K., Akiyama K., Hayashi H. Inhibitory activity against urease of quercetin glycosides isolated from Allium cepa and Psidium guajava. Biosci. Biotechnol. Biochem. . (2010);74:878–880. doi: 10.1271/bbb.90895. [DOI] [PubMed] [Google Scholar]
- 27.Siebenlist U., Franzoso G., Brown K. Structure, regulation and function of NF-kappa B. Annu. Rev. Cell Biol. . (1994);10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 28.Sweet M. J., Hume D. A. Endotoxin signal transduction in macrophages. J. Leukoc. Biol. (1996);60:8–26. doi: 10.1002/jlb.60.1.8. [DOI] [PubMed] [Google Scholar]
- 29.Williams R. J., Spencer J. P., Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic. Biol. Med. (2004);36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Zheng L. T., Ock J., Kwon B. M., Suk K. Suppressive effects of flavonoid fisetin on lipopolysaccharide-induced microglial activation and neurotoxicity. Int. Immunopharmacol. (2008a);8:484–494. doi: 10.1016/j.intimp.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Zheng L. T., Ryu G. M., Kwon B. M., Lee W. H., Suk K. Anti-inflammatory effects of catechols in lipopolysaccharide-stimulated microglia cells: inhibition of microglial neurotoxicity. Eur. J. Pharmacol. (2008b);588:106–113. doi: 10.1016/j.ejphar.2008.04.035. [DOI] [PubMed] [Google Scholar]


