Abstract
Although the immense efforts have been made for cancer prevention, early diagnosis, and treatment, cancer morbidity and mortality has not been decreased during last forty years. Especially, lung cancer is top-ranked in cancer-associated human death. Therefore, effective strategy is strongly required for the management of lung cancer. In the present study, we found that novel daphnane diterpenoids, yuanhualine (YL), yuanhuahine (YH) and yuanhuagine (YG) isolated from the flower of Daphne genkwa (Thymelaeaceae), exhibited potent anti-proliferative activities against human lung A549 cells with the IC50 values of 7.0, 15.2 and 24.7 nM, respectively. Flow cytometric analysis revealed that the daphnane diterpenoids induced cell-cycle arrest in the G0/G1 as well as G2/M phase in A549 cells. The cell-cycle arrests were well correlated with the expression of checkpoint proteins including the up-regulation of cyclin-dependent kinase inhibitor p21 and p53 and down-regulation of cyclin A, cyclin B1, cyclin E, cyclin dependent kinase 4, cdc2, phosphorylation of Rb and cMyc expression. In the analysis of signal transduction molecules, the daphnane diterpenoids suppressed the activation of Akt, STAT3 and Src in human lung cancer cells. The daphnane diterpenoids also exerted the potent anti-proliferative activity against anticancer-drug resistant cancer cells including gemcitabine-resistant A549, gefitinib-, erlotinib-resistant H292 cells. Synergistic effects in the growth inhibition were also observed when yuanhualine was combined with gemcitabine, gefitinib or erlotinib in A549 cells. Taken together, these findings suggest that the novel daphnane diterpenoids might provide lead candidates for the development of therapeutic agents for human lung cancers.
Keywords: Daphnane diterpenoids, Anti-proliferation, Cell-cycle arrest, Akt, Drug-resistant, Human lung cancer cells
INTRODUCTION
Cancer is leading causes of human death in the world. In particular, the incidence and mortality of lung cancer have been continuously increased during last 40 years (Siegel et al., 2012). For most cancers, including non-small cell lung cancer (NSCLC), traditional cytotoxic chemotherapy has a limited efficacy, and patients develop resistance to targeted inhibitors. Therefore, it is important to develop anticancer agents with novel structures and/or mechanisms of action for the treatment of lung cancer.
In our continuous efforts to search for anticancer agents from natural products, we recently found that the isolates from the flower buds of Daphne genkwa exhibited potent anti-proliferative activity against human lung cancer cells (Hong et al., 2010). Daphne genkwa Sieb. et Zucc. (Thymelaeaceae) is mainly distributed in mainland China and Korea. It has been traditionally used for the treatment for asthma, edema and cancer (Zhan et al., 2005; Zhang et al., 2006; Park et al., 2007). In our previous study, several daphnane diterpenoids were isolated from D. genkwa including two novel compounds yuanhualine and yuanhuahine (Hong et al., 2010). Previous reports showed that daphnane diterpenoids yuanhuacine and yuanhuadine have the moderate nuerotrophic activity, inhibitory activities on DNA topoisomerase I and on the growth of human cancer cell lines (He et al., 2002; Zhan et al., 2005; Zhang et al., 2006). In our previous study, we also reported that the growth-inhibitory activity of yuanhuadine was associated with the modulation of mTOR, EGFR or cMet signal transduction pathways in A549 cells (Hong et al., 2011). Consequently, in the present study, we further investigated the mechanism of action of novel daphnane diterpenoids in the anti-proliferative activity of human lung cancer cells. In addition, the anti-proliferative activities of the daphnane diterpenoids were also examined in anticancer drug-resistant human lung cancer cells as well as the synergistic effects in combination with anticancer agents.
MATERIALS AND METHODS
Chemicals
Fetal bovine serum (FBS), antibiotics-antimycotic solution, and trypsin_EDTA were purchased from Invitrogen (Grand Island, NY, USA). Bovine serum albumin, sulforhodamine B (SRB), trichloroacetic acid, propidium iodide, mouse monoclonal anti-b-actin antibody, gemcitabine and ellipticine were obtained from Sigma-Aldrich (St. Louis, MO, USA). Gefitinib and Erlotinib were provided by AstraZeneca (Wilmington, DE, USA) and Roche (Indianapolis, IN, USA), respectively. Mouse monoclonal anti-p53, rabbit polyclonal anti-cyclin A, anti-cyclin B1, anti-cdk4, anti-cdc2, anti-p21, anti-p27 and anti-STAT3 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse monoclonal anti-cyclin D1 and anti-cyclin E antibodies were purchased from BD Biosciences (San Diego, CA, USA). Rabbit polyclonal anti-phospho-Akt (Ser473), anti-Akt and anti-phospho-STAT3 (Ser 727) were purchased from Cell Signaling Technology (Beverly, MA, USA). Yuanhualine, yuanhuahine and yuanhuagine (purity >98.5%) were isolated and characterized from a CHCl3-soluble extract of the flowers of Daphne genkwa, as described previously (Hong et al., 2010).
Cell culture
The human non-small-cell lung cancer cell lines, A549, SK-MES-1 and H358, were provided from the Korean Cell Line Bank (Seoul, Korea). H292 and H1993 and MRC-5 cells were obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in RPMI 1640 supplemented with 10% heat-inactivated FBS and antibiotic_antimycotic solution (100 units/ml penicillin G sodium, 100 μg/ml streptomycin, and 250 ng/ml amphothericin B). Cells were maintained at 37℃ in a humidified atmosphere with 5% CO2.
Establishment of acquired resistant cells to anticancer drugs
During a 6-month period, cancer cells in culture were continuously exposed to increasing concentration of drugs. A549-Gem cells were treated with gemcitabine from concentration of 1 nM to 1 μM. H292-Gef and H292-Erl cells were treated with gefitinib and erlotinib, respectively, in stepwise increase of concentration from 1 nM to 1 μM. The concentrations were increased until the cells of each group resumed growth kinetics similar to the untreated parental cells.
Cell proliferation assay
Cells (5 ×104 cells/ml) were treated with various concentrations of daphnane diterpenes for 3 days. After treatment, cells were fixed with 10% trichloroacetic acid (TCA) solution, and cell viability was determined with a sulforhodamine B (SRB) assay (Skehan et al., 1990; Lee et al., 1998). The results are expressed as percentages, relative to solvent-treated control incubations, and IC50 values were calculated using non-linear regression analysis (percent survival versus concentration).
Analysis of cell cycle distribution
The analysis of cell-cycle dynamics was performed by flow cytometry as described previously (Lee et al., 1998) . Briefly, A549 cells were plated at a density of 1×105 cells per 100 mm culture dish and incubated for 24 h. Fresh medium containing test samples was added to the culture dishes. After 24 or 48 h, adherent and floating cells were harvested, washed twice with PBS, fixed with 100% methanol, and incubated with a staining solution containing 0.2% NP-40, propidium iodide (50 μg/ml), and RNase A (50 μg/ml) in phosphate-citrate buffer (pH 7.2) for 30 min at room temperature. Cellular DNA content was analyzed by FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). At least 20,000 cells were used for each analysis, and the results are displayed as histograms. The distribution in each phase of the cell cycle was determined using the ModFit LT 2.0 program.
Western blot analysis
Cells were incubated with various concentrations of daphnane diterpenes. The proteins from cell lysates were resolved by 6-15% SDS-PAGE and transferred onto PVDF membranes (Millipore, Bedford, MA, USA). Membranes were blocked with blocking buffer (5% nonfat dry milk in PBS containing 0.1% Tween-20 (PBST) for 1 h at room temperature. After washing three times with PBST, membranes were incubated with primary antibodies diluted in 3% nonfat dry milk in PBST (1:200-1:2,000) overnight at 4℃. Membranes were washed three times with PBST and incubated with corresponding secondary antibodies diluted in 3% nonfat dry milk in PBST (1:1,000-1:5,000) for 2 h at room temperature. Membranes were washed three times with PBST and detected using ECL reagent (Lab Frontier, Suwon, Korea). Blots were imaged by LAS 3000 (Fuji Film Corp., Tokyo, Japan).
Analysis of combination effect
Cells (1×104 cells/well) were plated in 96-well plates with various concentrations of yuanhualine and drugs (gemcitabine, gefitinib and elrotinib). After 72 h incubation, the growth inhibition was measured by the SRB assay. The combination effect of yuanhualine plus drug was analyzed by the calculation of the combination index (CI), which was calculated using the following equation: CI=D1/(Dx)1+D2/(Dx)2. Thus, D1 and D2 are the concentrations of yuanhualine and drug in the mixture that achieve the expected effect, and (Dx)1 and (Dx)2 are the concentrations that achieve the same effect when the compounds are used alone. In this study, the effective level was chosen at 50% inhibition. The calculated CI was then compared to the reference values reported by Chou (Chou, 2006).
Statistical analysis
Data are presented as means ± SE for the indicated number of independently performed experiments. Statistical significance (p<0.05) was assessed by one-way analysis of variance (ANOVA) coupled with the Dunnett’s t-test.
RESULTS
Inhibitory effect of daphnane diterpenoids on the proliferation of human NSCLC cells
Anti-proliferative potential of the daphnane diterpenoids (yuanhualine, yuanhuahine and yuanhuagine) against human lung cancer cells was evaluated by a colorimetric SRB protein dye staining method (Skehan et al., 1990; Lee et al., 1998). As summarized in Table 1 and Fig. 1, the daphnane diterpenoids exhibited a potent anti-proliferative effect with IC50 values ranging from 3.7 to 24.7 nM. Especially, the A549, H292, H1993 and SK-MES-1 cells were sensitive compared to other NSCLC cells such as H1299 and H358 cells. When compared to human normal lung epithelial cells (MRC-5), the daphanane diterpenoids inhibited preferentially the proliferation of the lung cancer cell lines (Table 1). In A549 cells,ane diterpenoids inhibited the cell proliferation in a concentration-dependent manner for 72 h, and the IC50 values were 7.0 nM for yuanhualin (YL), 9.7 nM for yuanhuahine (YH), and 24.7 nM for yuanhuagine (YG), respectively (Table 1 and Fig. 2). Morphological changes were also examined using a phase-contrast microscope. As treated with the daphnane diterpenoids, the numbers of cells were decreased by comparison with control cells, and cells were mostly remained attached on dishes with shrank morphology (Fig. 3).
Table 1.
Growth inhibitory effects of daphnane diterpenoids from Daphne genkwa on the proliferation of several human lung cancer cells
| Cell line | IC50 | ||
|---|---|---|---|
| YL | YH | YG | |
| A549 (nM) | 7.0 | 9.7 | 24.7 |
| SK-MES-1 (nM) | 13.2 | 17.1 | 22.8 |
| H292 (nM) | 3.7 | 6.2 | 5.1 |
| H1993 (nM) | 65.3 | 35.1 | 74.4 |
| H358 (μM) | 4.7 | 0.3 | 9.0 |
| MRC-5 (μM) | 15.2 | >20 | >20 |
YL: yunahualine, YH: yuanhuahine, YG: yuanhuagine
Fig. 1. Structures of daphnane diterpenoids isolated from Daphne genkwa.
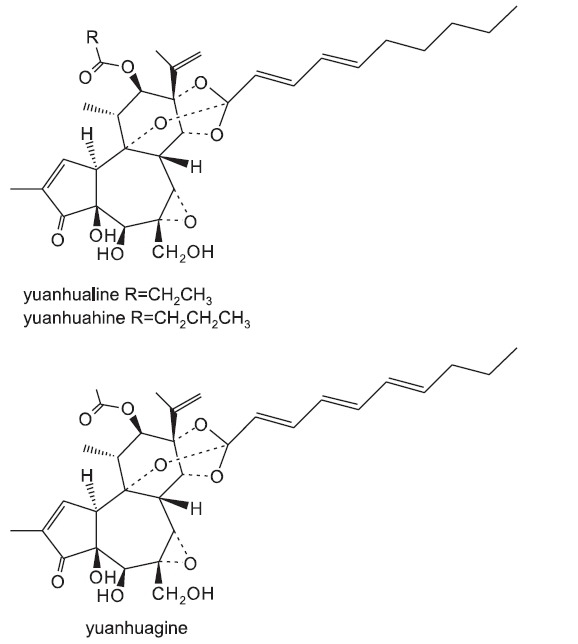
Fig. 2. Dose-dependent inhibitory effects of daphnane diterpenoids from Daphne genkwa on the proliferation of A549 human lung cancer cells (YL: yuanhualine, YH: yuanhuahine, YG: yuanhuagine). Cells were treated with or without various concentrations of daphnane diterpenoids for 72 h. Anti-proliferative effect was determined using SRB assay.
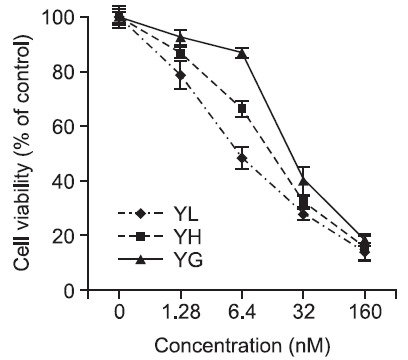
Fig. 3. Morphological changes mediated by daphnane diterpenoids in A549 cells (24 h). Cells were treated with or without various concentrations of daphnane diterpenoids for 24 h. Cells were photographed by inverted microscopy (magnification 100×).
Inhibitory effect on the proliferation of anticancer drug-resistant cells
Chemoresistance is commonly found in lung cancer and the resistance of drug has been an issue in lung cancer treatment (Shepherd 2003; Pfister et al. 2004; Sève and Dumontet 2005; Chang, 2011). With repeated exposure of stepwise increasing concentrations of anticancer drug for over 6 months, gemcitabine-resistant A549 cells (A549-Gem), gefitinib- and erlotinib-resistant H292 cells (H292-Gef and H292-Erl, respectively) were generated. A549-Gem cells was shown to be approximately 320-fold more resistant to gemcitabine than the parental A549 cells. H292-Gef and H292-Erl cells were also shown to be 40- and 37-fold more resistant to geftinib and erlotinib than the parental H292 cells, respectively (Table 2). The daphnane diterpenoids, however, were found to be equally effective in inhibiting growth in both parental and drug-resistant A549-Gem, H292-Gef and H292-Erl cells, indicating that the daphnane diterpenoids might be effective and overcome resistance to gemcitabine, geftinib or erlotinib-resistant human
Table 2.
Growth inhibitory effects mediated by daphnane diterpenoids and gemcitabine in drug-resistant cell lines
| Cell line | IC50 (nM) | |||||
|---|---|---|---|---|---|---|
| YL | YH | YG | Gem | Gef | Erl | |
| A549 | 7.0 | 9.7 | 24.7 | 41.7 | nd* | nd* |
| A549-Gem | 9.4 | 15.2 | 24.3 | 1,300 | nd* | nd* |
| H292 | 3.7 | 6.2 | 5.1 | nd* | 16.2 | 26.0 |
| H292-Gef | <0.2 | <0.2 | <0.2 | nd* | 646.1 | 189.0 |
| H292-Erl | 0.4 | <0.2 | <0.2 | nd* | 464.2 | 954.8 |
*nd: not detected, Gem: gemcitabine, Gef: geftinib, Erl: Erlotinib.
lung cancer cells.
Effects of the daphnane diterpenoids on the regulation of cell cycle progression
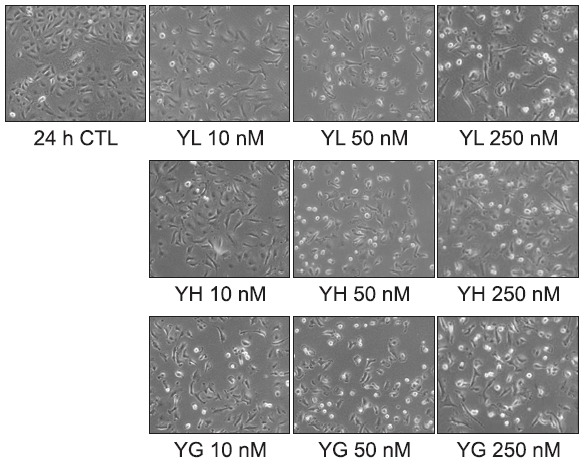
Further study was designed to elucidate the mechanism of action for the anti-proliferative activity of the daphnane diterpenoids against A549 human lung cancer cells. A549 cells were treated with the daphnane diterpenoids for 24 h, and the cell cycle distributions were analyzed by flow cytometry. G0/G1 and G2/M phase were effectively increased by the compounds in a concentration-dependent manner, but the S phase was decreased significantly (Fig. 4). However, the sub-G1 peaks were not much accumulated even with a high concentration (250 nM) treatment, suggesting that the daphnane diterpenoids yuanhualine, yuanhuahine and yuanhuagine caused cell cycle arrest without the induction of apoptosis. To further examine whether cell-cycle arrest is associated with the expression of cell-cycle regulatory proteins, the cells were treated with various concentrations of the daphnane diterpenoids for 24 h, and then Western blot analysis was performed. As shown in Fig. 5, the daphnane diterpenoids suppressed the expression of G1 cell cycle checkpoint proteins such as CDK4 and also downregulated the expression of biomarkers such as cyclin A and phospho-Rb (Ser780) in the transition of
Fig. 4. Effect of daphnane diterpenoids on the regulation of cell cycle distribution in A549 cells (24 h). Cells were treated with or without various concentrations of daphnane diterpenoids for the indicted times. Cell cycle distribution was analyzed by flow cytometer.
Fig. 5. Effect of daphnane diterpenoids on the expression of cell cycle checkpoint proteins in A549 cells. Cells were treated with the indicated concentrations for 24 h. The level of protein was analyzed by Western blot.
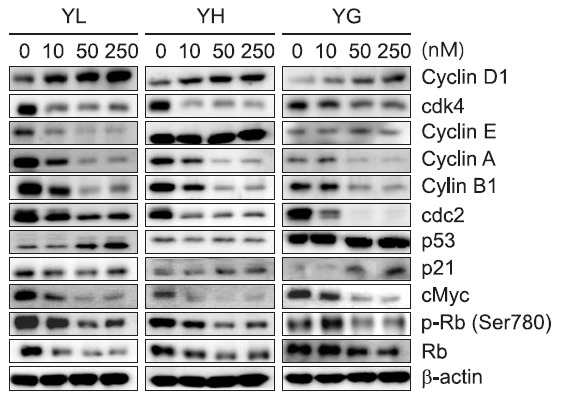
G1 to S phase, but the expression of cyclin D1 was exceptionally increased. Cyclin dependent kinase inhibitor p21 was increased by yuanhualine and p53 was increased by yuanhuagine. c-Myc, a protein that regulates cell cycle progression and cell proliferation, was also down-regulated by the daphnane diterpenoids. In addition, the changes of protein expression involved in regulating progression from G2 to M phase were examined. Cyclin B1/cell division cycle 2 (CDC2) complex plays a central role on the regulation of cell-cycle progression from G2 to M phase (Smits and Medema, 2001). When treated with the daphnane diterpenoids, the expressions of cyclin B1 and CDC2 were downregulated. These findings indicate that the induction of cell-cycle arrest by the daphnane diterpenoids was associated with the modulation of the expression of cell-cycle regulators in A549 cells.
Effects on the activation of signal transduction pathway
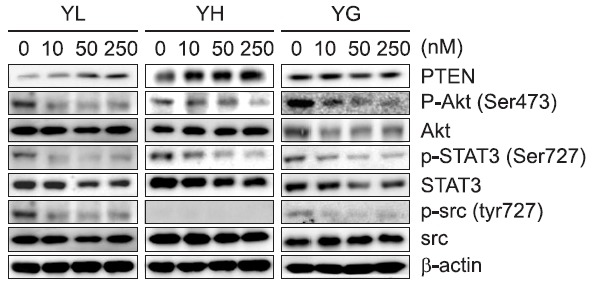
To further determine the mechanisms of action underlying the daphnane diterpenoids-mediated anti-proliferative activities on the A549 lung cancer cells, relevant overexpressed or activated signaling proteins in the cancer cells were examined. The expression of PTEN, which negatively regulates the PI3K/Akt/mTOR signaling pathway by counteracting with PI3K, was upregulated by yuanhualine and yuanhuahine (Fig. 6). The daphnane diterpenoids also suppressed the activation of signal transducer and activator of transcription 3 (STAT3), which is constitutively activated and malignant progression in human cancers. Furthermore, yuanhualine and yuanhuagine also suppressed the activation of Src with the downregulation of phospho-Src (Tyr727).
Fig. 6. Effect of daphnane diterpenoids on the expression of signal transduction-associated proteins in A549 cells. Cells were treated with the indicated concentrations for 24 h. The level of protein was analyzed by Western blot.
Anti-proliferation effects with combination of cancer chemotherapeutic agents
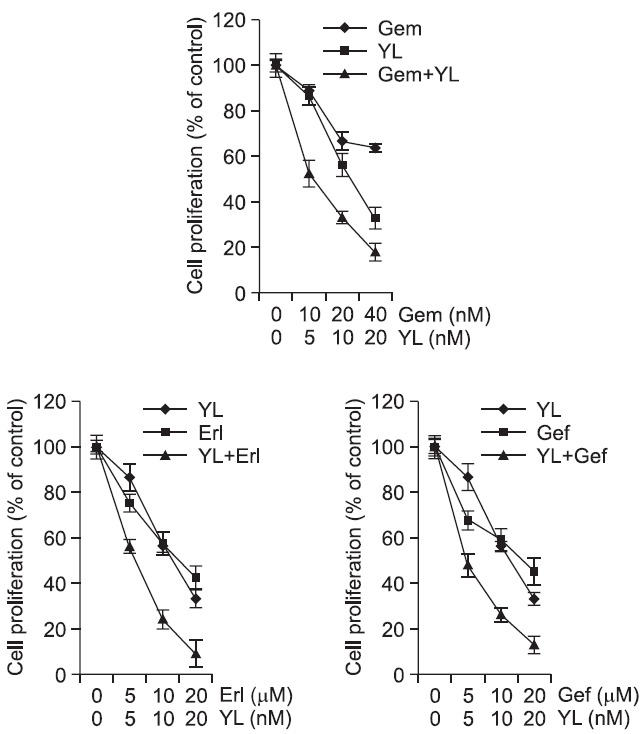
Conventional therapeutic plateau has been reached with many of the chemotherapeutic agents and there always had concerns with resistance of the agents. Thus, to avoid these matters combination therapy using agents simultaneously that have different mechanism has been generally carried out. Therefore, we evaluated the anti-proliferative effects of yuanhualine in A549 cells in combination with cytotoxic agents generally clinically used against NSCLC. Combinations of yuanhualine with gemcitabine or gefitinib displayed the synergistic effects. In addition, when yuanhualine was combined with erlotinib, the moderately synergistic effect was observed (Table 3 and Fig. 7). Taken together, these results suggest that yuanhualine exhibits the enhancement of gemcitabine, gefitinib or erlotinib-induced anti-proliferative activity in the A549 cells.
Table 3.
The mean of combination index (CI) value at IC50 for each chemotherapeutic agent combined with YL in the treatment of A549 cells
| Sample | Combination Indix (CI) | Interact with YL |
|---|---|---|
| Gemcitabine + YL | 0.65 | Synergism |
| Gefitinib + YL | 0.69 | Synergism |
| Erlotinib + YL | 0.78 | Moderate Synergism |
YL: yuanhualine.
Fig. 7. Growth inhibitory effects of yuanhualine in combination with anticancer agents in A549 cells (Gem: gemcitabine, Gef: gefitinib, Erl: Erlotinib). Cells were treated with yuanhualine, anticancer agent, or in combination for 72 h. Anti-proliferative activity was measured using SRB assay.
DISCUSSION
Natural products have been played an important role in the development of cancer chemotherapeutic drugs (Mann, 2002). In our program to identify anti-tumor agents from natural products, we recently reported several daphnane-type diterpenoids exhibit potential growth inhibitory effects against human lung cancer cells (Hong et al., 2010). In the present study, we report herein the additional information for the anti-proliferative mechanisms of actions of these daphnane diterpenoids through modulating cell cycle and cell signaling pathways in A549 human lung cancer cells.
In accordance with previously reported results on the growth inhibition of A549 lung cancer cells (Hong et al., 2010), yuanhualine (YL), yuanhuahine (YH) and yuanhuagine (YG) exerted potent anti-proliferative activity with IC50 value of 7.0, 9.7 and 24.7 nM, respectively, in the A549 cells. These data also suggest that yuanhualine and yuanhuahine are more potent than yuanhuagine in the growth-inhibitory activity of the A549 cells. In addition, the anti-proliferative effects of the daphnane diterpenoids in drug-resistant cancer cells including gemcitabine-resistant (A549-Gem), gefitinib-resistant (H292-Gef) and erlotinib-resistant (H292-Erl) lung cancer cells were similar to that in parent cancer cells (Table 2). These results assumed that the daphnane diterpenoids might have a potential to overcome multidrug resistance in human lung cancer cells, but further detailed mechanistic studies remain to be elucidated.
Based on the potent anti-proliferative activity, further investigation on the mechanism of action of the daphnane diterpenoids in the regulation of cell proliferation was performed in A549 cells. In general, cell cycle arrest is one of most common
causes of the inhibition of cell proliferation. The daphnane diterpenoids induced the accumulation of cells in the G0/G1 and G2/M phase, but dose-dependent modulation of cell cycle progression was not noticeable, suggesting modulation of cell cycle progression might partially contribute to its anti-proliferative effects of daphnane diterpenoids (Fig. 4). In addition, although we treated with a high concentration of daphnane diterpenoids (250 nM) for 24 h, sub-G1 peaks were not detected, indicating that the daphnane diterpenoids did not evoke obvious apoptotic cell death compared to the conventional cytotoxic anticancer agents. Cell cycle control is a highly regulated process that involves a complex cascade of events. Cyclins and CDKs are considered as cell cycle regulators and play an important role through the formation of cyclin/CDK complex and CDK inhibitors (Johnson and Walker, 1999; Shapiro, 2006). Especially, the transition from G1 to S phase was finely regulated by cyclin E-cdk2 and cyclin D1-cdk4 complexes, inactivation of pRb tumor suppressor, and cdk inhibitors (Fig. 5). Cyclin D1 expression was increased by the daphnane diterpenoids, however, the downregulation of cdk4 might result in the suppression of cyclin D1-cdk4 complex, thus eventually activating the G0/G1 phase arrest. The inductions of G2/M phase cell cycle arrest by the daphnane diterpenoids were also associated with the suppression of cyclin B1 and cdc2 expressions in A549 cells.
Further study was designed to identify the anti-proliferative effect of the daphnane diterpenoids with the regulation of cellular signal transduction pathway. Akt is serine/threonine kinase that promotes cell survival, and triggers a network that positively regulates G0/G1 cell cycle progression through regulation of substrates of Akt such as p21, p53 and c-myc (Liang and Slingerland, 2003). Therefore, the inhibition of Akt/c-myc and Akt/p21 signaling by the daphnane diterpenoids resulted in the suppression of cyclin E/cdk2 expression, and
leading to the dephosphorylation of the Rb protein, and then these events might evoke to arrest the G0/G1 phase cell cycle progression. Moreover, the daphnane diterpenoids also upregulated the expression of PTEN, an upstream of Akt, and these effects might enhance the negatively regulation of the overall Akt signaling pathway by the daphnane diterpenoids (Fig. 6). mTOR is another downstream effector of the PI3K/Akt signaling pathway and a central modulator of cell proliferation in lung cancer (Wullschleger et al., 2006). In our previous study, yuanhuadine downregulated the activation of mTOR, however, in the present study, the daphnane diterpenoids (YL, YH and YG) did not inhibit the mTOR signaling pathway (data not shown). Thus, despite of the similarity of the chemical structures, it is considerable that the difference of these mechanisms of action might affect to their potency in the anti-proliferative activities.
In the examination of combination effect, yuanhualine exerted the enhancement of the anticancer drugs-mediated anti-proliferative activities in the A549 cells (Table 3 and Fig. 7). Recent reports have shown that the sensitivity of NSCLC cell lines to gefitinib is dependent on the inhibition of the PI3K/Akt pathway (Janmaat et al., 2006). Therefore, it seems that the suppression of Akt signaling by yuanhualine might enhance the anti-proliferative activities of gefitinib in the A549 cells.
In summary, the present findings demonstrate that the daphnane diterpenoids yuanhualine, yuanhuahine and yuanhuagine isolated from the flower of Daphne genkwa exhibited the potent growth inhibitory activity against human lung cancer cells as well as anticancer drug-resistant human lung cancer cells. The growth inhibition of the daphnane diterpenoids is highly related to the cell cycle arrest in the G0/G1 and G2/M phase. The growth inhibition is also associated with the modulation of signal transduction pathway in the Akt, STAT3 and Src signaling. Synergistic anti-proliferative effects of the daphnane diterpenoids were also found in combination with the anticancer agents. These findings demonstrate that the daphnane diterpenoids might be a promising new chemotherapeutic candidate in the management of human lung cancer cells.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (20100024361).
References
- 1.Chang A. Chemotherapy, chemoresistance and changing treatment landscape for NSCLC. Lung Cancer . (2011);71:3–10. doi: 10.1016/j.lungcan.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Chou T. C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. (2006);58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 3.He W., Cik M., Appendino G., Puyvelde L. V., Leysen J. E., De Kimpe N. Daphnane-type diterpene orthoesters and their biological activities. Mini Rev. Med. Chem. (2002);2:185–200. doi: 10.2174/1389557024605492. [DOI] [PubMed] [Google Scholar]
- 4.Hong J. Y., Chung H. J., Lee H. J., Park H. J., Lee S. K. Growth inhibition of human lung cancer cells via down-regulation of epidermal growth factor receptor signaling by yuanhuadine, a daphnane diterpene from Daphne genkwa. J. Nat. Prod. (2011);74:2102–2108. doi: 10.1021/np2003512. [DOI] [PubMed] [Google Scholar]
- 5.Hong J. Y., Nam J. W., Seo E. K., Lee S. K. Daphnane diterpene esters with anti-proliferative activities against human lung cancer cells from Daphne genkwa. Chem. Pharm. Chem. Pharm. Bull. (2010);58:234–237. doi: 10.1248/cpb.58.234. [DOI] [PubMed] [Google Scholar]
- 6.Janmaat M. L., Rodriguez J. A., Gallegos-Ruiz M., Kruyt F. A., Giaccone G. Enhanced cytotoxicity induced by gefitinib and specific inhibitors of the Ras or phosphatidyl inositol-3 kinase pathways in non-small cell lung cancer cells. Int. J. Cancer . (2006);118:209–214. doi: 10.1002/ijc.21290. [DOI] [PubMed] [Google Scholar]
- 7.Johnson D. G., Walker C. L. Cyclins and cell cycle checkpoints. Annu. Rev. Pharmacol. Toxicol. (1999);39:295–312. doi: 10.1146/annurev.pharmtox.39.1.295. [DOI] [PubMed] [Google Scholar]
- 8.Lee S. K., Cui B., Mehta R. R., Kinghorn A. D., Pezzuto J. M. Cytostatic mechanism and antitumor potential of novel 1H-cyclopental[b]benzofuran lignans isolated from Aglaia elliptica. Chem. Biol. Interact. . (1998);115:215–225. doi: 10.1016/s0009-2797(98)00073-8. [DOI] [PubMed] [Google Scholar]
- 9.Liang J., Slingerland J. M. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle . (2003);2:339–345. [PubMed] [Google Scholar]
- 10.Mann J. Natural products in cancer chemotherapy: past, present and future. Nat. Rev. Cancer . (2002);2:143–148. doi: 10.1038/nrc723. [DOI] [PubMed] [Google Scholar]
- 11.Park B. Y., Min B. S., Ahn K. S., Kwon O. K., Joung H., Bae K. H., Lee H. K., Oh S. R. Daphnane diterpene esters isolated from flower buds of Daphne genkwa induce apoptosis in human myelocytic HL-60 cells and suppress tumor growth in Lewis lung carcinoma (LLC)-inoculated mouse model. J. Ethnopharmacol. (2007);111:496–503. doi: 10.1016/j.jep.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Pfister D. G., Johnson D. H., Azzoli C. G., Sause W., Smith T. J., Baker S. Jr., Olak J., Stover D., Strawn J. R., Turrisi A. T. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: Update. J. Clin. Oncol. (2004);22:330–353. doi: 10.1200/JCO.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 13.Sève P., Dumontet C. Chemoresistance in non-small cell lung cancer. Curr. Med. Chem. Anticancer Agents. (2005);5:73–88. doi: 10.2174/1568011053352604. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro G. I. Cyclin-dependent kinase pathways as targets for cancer treatment. J. Clin. Oncol. . (2006);24:1770–1783. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- 15.Shepherd F. A. Second-line chemotherapy from non-small lung cancer. Expert Rev. Anticancer Ther. (2003);3:435–442. doi: 10.1586/14737140.3.4.435. [DOI] [PubMed] [Google Scholar]
- 16.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2012. CA Cancer J. Clin. . (2012);62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 17.Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J. T., Bokesch H., Kenney S., Boyd M. R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. (1990);82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 18.Smits V. A., Meedema R. H. Checking out the G(2)/M transition. Biochim. Biophys. Acta. . (2001);28:1–12. doi: 10.1016/s0167-4781(01)00204-4. [DOI] [PubMed] [Google Scholar]
- 19.Wullschleger S., Loewith R., Hall M. N. TOR signaling in growth and metabolism. Cell . (2006);124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Zhan Z. J., Fan C. Q., Ding J., Yue J. M. Novel diterpenoids with potent inhibitory activity against endothelium cell HMEC and cytotoxic activities from a well-known TCM plant Daphne genkwa. Bioorg. Med. Chem. (2005);13:645–655. doi: 10.1016/j.bmc.2004.10.054. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S., Li X., Zhang F., Yang P., Gao X., Song Q. Preparation of yuanhuacine and relative daphne diterpene esters from Daphne genkwa and structure-activity relationship of potent inhibitory activity against DNA topoisomerase I. Bioorg. Med. Chem. (2006);14:3888–3895. doi: 10.1016/j.bmc.2006.01.055. [DOI] [PubMed] [Google Scholar]


