Abstract
Although pruritus is the critical symptom of atopic dermatitis that profoundly affect the patients’ quality of life, controlling and management of prurirtus still remains as unmet needs mainly due to the distinctive multifactorial pathogenesis of pruritus in atopic dermatitis. Based on the distinct feature of atopic dermatitis that psychological state of patients substantially influence on the intensity of pruritus, various psychotropic drugs have been used in clinic to relieve pruritus of atopic dermatitis patients. Only several psychotropic drugs were reported to show real antipruritic effects in atopic dermatitis patients including naltrexone, doxepin, trimipramine, bupropion, tandospirone, paroxetine and fluvoxamine. However, the precise mechanisms of antipruritic effect of these psychotropic drugs are still unclear. In human skin, serotonin receptors and serotonin transporter protein are expressed on skin cells such as keratinocytes, melanocytes, dermal fibroblasts, mast cells, T cells, natural killer cells, langerhans cells, and sensory nerve endings. It is noteworthy that serotonergic drugs, as well as serotonin itself, showed immune-modulating effect. Fenfluramine, fluoxetine and 2, 5-dimethoxy-4-iodoamphetamine significantly decreased lymphocyte proliferation. It is still questionable whether these serotonergic drugs exert the immunosuppressive effects via serotonin receptor or serotonin transporter. All these clinical and experimental reports suggest the possibility that antipruritic effects of selective serotonin reuptake inhibitors in atopic dermatitis patients might be at least partly due to their suppressive effect on T cells. Further studies should be conducted to elucidate the precise mechanism of neuroimmunological interaction in pruritus of atopic dermatitis.
Keywords: Pruritus, Atopic dermatitis, Neuromediator, Serotonin, T cells
Pruritus is defined as an unpleasant sensation eliciting the urge to scratch. It is one of the signature symptoms in inflammatory skin diseases. Atopic dermatitis (AD) is one of the most pruritic skin diseases and pruritus is an essential feature in diagnosis and treatment of AD (Koblenzer, 1999). Long-lasting pruritus has a profound impact on the patients’ quality of life (Metz et al., 2011). In many cases, patients scratch the skin lesions to cause erosions, ulcerations, bleeding, and lichenification which can aggravate AD symptoms in turn. The sleep disturbances following prolonged nocturnal scratching and psychological difficulties such as cicatrization and social isolation also result in dramatic impairment in the quality of life in AD patients (Buske-Kirschbaum et al., 2001). Thus, proper treatment of pruritus is the critical part of therapeutic approach to AD.
Based on early studies, pruritus was regarded as a low-intensity pain for a long time. Nowadays, it is clear that the sensation of pruritus is distinct from the sensation of pain and there exists a sensory system for pruritoception that is different from the sensory system for nociception (Andrew and Craig, 2001; Paus et al., 2006; Handwerker and Schmelz, 2009). Pruritus can be triggered by various endogenous and exogenous stimuli via free endings of specific nonmyelinated, slow-conducting C-type nerve fiber in the epidermis and dermis (Ständer et al., 2002). The stimuli run along the dorsal root ganglion via the spinal cord and reach different areas of the cortex, where the scratching reflex is initiated.
A number of reports indicated that skin immune system is closely related to the provocation and intensity of pruritus in AD patients. In this review, the neuroimmunological mechanism of pruritus in AD would be discussed based on the reports about the antipruritic effects of several psychotropic drugs, especially focused on the role of serotonin in skin.
SPECIFIC FEATURES OF PRURITUS IN ATOPIC DERMATITIS
Although the pathophysiology of pruritus in AD has not been fully defined yet, a number of reports demonstrated that pruritogenic mechanisms involved in AD are different from those of normal pruritus. The skin lesions of AD often have increased density of cutaneous nerve fibers (Tobin et al., 1992; Urashima and Mihara, 1998). AD lesional and nonlesional skin showed increased substance P-positive and calcitonin gene related peptide (CGRP)-positive fibers associated with increased mast cell-nerve fiber contacts (Järvikallio et al., 2003; Elenkov, 2004). In addition, the skin of AD patients have reduced pruritus threshold and prolonged pruritus duration to pruritic stimuli as compared with healthy skin, resulting in a higher tendency to pruritus upon stimulation (Morren et al., 1994).
So far, many laboratory and clinical data indicated that a number of genetic, environmental and psychological factors are related to pruritus in AD (Pastar et al., 2005; Weidinger et al., 2008). A group of pruritogens including inflammatory lipids, cytokines, neuropeptides, neurotransmitters such as histamine and serotonin (5-hydroxytryptamine, 5-HT), proteases, proteinase-activating receptors, and opioid peptides appears to be related to pruritus in AD (Darsow et al., 1997; Steinhoff et al., 2006; Ständer et al., 2008; Lee, 2010). Among these factors, histamine has been the most well-known pruritogen. Histamine was found at high concentrations in AD lesions and most of the cells involved in the inflammatory responses express the histamine 1 receptor (H1R) and histamine 2 receptor (H2R)(Mommert et al., 2011). There is a specific chemosensitive subpopulation of C-fibers that mediate histamine-induced pruritus (Schmelz, 2001; Schmelz et al., 2003). In this context, H1R antagonists have been tried and demonstrated to reduce pruritus in numerous clinical trials (Sugimoto et al., 2004; Paus et al., 2006). However, in AD patients, antihistamines showed only limited clinical efficacy to pruritus (Klein and Clark, 1999) suggesting that histamine is unlikely to be a major peripheral mediator of pruritus in AD (Rukwied et al., 2000).
Recently, the fourth histamine receptor (H4R) brought histamine back into focus in the pathophysiology of pruritus in AD. Several studies showed that the H4R, which is differentially expressed on immune and nonimmune cells, plays an important role in pruritus of AD patients (Dijkstra et al., 2007; Gutzmer et al., 2009). Pruritus was selectively inhibited by the treatment with a H4R antagonist in a Th2 cell-mediated mouse skin inflammation model that mimics several features of AD (Cowden et al., 2010) or in H4R knockout mice (Dunford et al., 2007).
Despite of the newly discovered role of H4R in pruritus of AD lesion, it is still accepted that histamine is not the sole mediator of pruritus in AD and mediators other than histamine such as cytokines and neuropeptides may be involved in induction of intractable, recurrent pruritus in AD. This assumption is supported by the evidence of histamine-independent pruritus-specific fibers in the skin (Nakano et al., 2008).
ANTIPRURITIC EFFECTS OF IMMUNOMODULATING THERAPY IN ATOPIC DERMATITIS
Controlling and treatment of chronic pruritus is one of the major unmet needs in the therapeutic approach to AD. Because of the multifactorial pathogenesis of AD, no specific, universally effective and well-tolerated antipruritic agent for AD has been developed yet (Lee, 2010). Until now, management of pruritus in AD is mainly confined to imunomodulators such orgas glucocorticoids, cyclosporin A and topical calcineurin inhibitors (tacrolimus and pimecrolimus) (Wahlgren et al., 1990; Hoare et al., 2000; Hanifin et al., 2001; Luger et al., 2001). These immunomodulating therapies applied in AD often succeed in reducing pruritus via suppressing the inflammatory mechanisms underlying the induction of pruritus (Yosipovitch et al., 1996). The reports that cyclosporin A treatment reduced pruritus in AD patients clearly showed that interleukin-2 and T cells are at the center of AD pruritus (Wahlgren et al., 1990). Even a single intracutaneous injection of IL-2 resulted in intermittent local pruritus perception in AD patients (Wahlgren et al., 1995; Darsow et al., 1997).
ROLE OF SKIN T CELLS IN THE PATHOPHYSIOLOGY OF PRURITUS IN ATOPIC DERMATITIS
The skin of AD patients show infiltrating CD11c+ dendritic cells and CD3+ T cells (Novak and Leung, 2011) mainly composed of CD4+ and cutaneous lymphocyte associated antigen (CLA+ T cells (Leung and Bieber, 2003; Leung et al., 2004). The infiltrates predominantly consist of interleukin 4 -producing Th2 type cells in the initial phase of AD, whereas chronic lesions frequently show infiltrates with Th1 type (Leung and Bieber, 2003). It has been reported that Th2-type cytokines are involved in the interplay between nerves and T lymphocytes indicating the roles of cytokines in pruritus (Sonkoly et al., 2006; Cevikbas et al., 2011). However, a recent report from biopsies taken from atopy patch test revealed that in addition to CD4+ T cells, CD8+ T cells might play a pivotal role for the development of eczema (Hennino et al., 2011).
Recently, a novel T cell-driven cytokine interleukin 31(IL-31) has been found to be significantly upregulated in pruritic AD patients compared with healthy, nonatopic subjects (Sonkoly et al., 2006). Actually IL-31 induced severe pruritus and dermatitis in transgenic mice (Sonkoly et al., 2006). The sources of IL-31 include the activated skin infiltrating CLA-positive T cells (Bilsborough et al., 2006). All these results back up the earlier reports suggesting that skin-infiltrating T cells might play a major role in pruritus in AD (Wahlgren et al., 1990; Greaves and Khalifa., 2004).
There is a specific type of T cells in the skin epidermis and intestinal epithelia, the γδ T cells. In contrast to the classical αβ T cells, γδ T cells have limited specificities of T cell receptor (TCR), unusual dendritic shape, and functions distinct from αβ T cells in many ways. These cells have been found to play crucial roles in tissue homeostasis and damage repair by recognition of damaged self (Jameson et al., 2002; Strid et al., 2009), whereas αβ T cells function in foreign antigen recognition. Epithelial γδ T cells express special costimulatory molecules such as junctional adhesion molecule-like protein (JAML) (Witherden et al., 2010). Once activated, epithelial γδ T cells express cytokines that promote inflammation and stimulate repair of the skin tissue (Sharp et al., 2005). However, it is unknown what molecules are recognized by γδ T cells and by what mechanisms γδ T cells are activated.
Interestingly, activation of H4R on Th2 cells increased the production of IL-31, which is one of important inducers of pruritus in AD (Gutzmer et al., 2009), suggesting the possibility that receptors of neuromediators might be involved in the activation process of skin T cells.
INTERACTIONS BETWEEN NERVOUS SYSTEM AND IMMUNE SYSTEM IN ATOPIC DERMATITIS
In the skin, the nervous and immune systems bidirectionally interplay via the action of neuromediators (neuropeptides and neurotransmitters) that are released by skin nerves and the immune cells (Luger, 2002; Straub, 2004). The cells of both systems are able to release neuromediators and respond to them via specific receptors expressed on their surface. For instance, skin immune cells such as mast cells and dendritic cells release neuropeptides which induce activation of nerve ending, vasodilatation, and plasma extravasation (Roosterman et al., 2006). In turn, neuropeptides such as vasoactive intestinal peptide (VIP) and CGRP modulate the functions of macrophages and T cells (González-Rey et al., 2007) and langerhans cells (Cevikbas et al., 2007) respectively, while substance P affects lymphocyte proliferation and mast cell degranulation (Turner et al., 2007). Thus it seems quite reasonable that neuromediators are involved in the pathogenesis of pruritus in AD (Roosterman et al., 2006). Actually, stressed patients with AD had increased numbers of 5-HT-receptive mast cells (Lonne-Rahm et al., 2008).
ANTIPRURITIC EFFECTS OF PSYCHOTROPIC DRUGS IN ATOPIC DERMATITIS AND THE ROLE OF 5-HT IN NEUROIMMUNE INTERACTION
Another distinct feature of AD is the substantial influence of psychological state on the intensity of pruritus. The majority of patients with AD reported that psychological stress aggravated their pruritus, suggesting that pruritus might be closely related with emotional distress in AD (Wahlgren, 1992). In this context, various psychotropic drugs have been tried in clinic to relieve pruritus. However, only several psychotropic drugs showed real antipruritic effects in AD patients. Table 1 summarizes the Ki (binding affinity) values of the antipruritic psychotropic drugs to receptors and transporters for various neurotransmitters. The opioid receptor antagonist naltrexone showed antipruritic effect in patients with AD (Heyer and Hornstein, 1999). A tricyclic antidepressant doxepin showed antipruritic effect topically and systemically for its additive antihistaminic and anticholinergic effects (Drake and Millikan, 1995). Tricyclic antidepressant trimipramine, that antagonizes H1 receptor and H2 receptor, reduced nocturnal scratching in patients with AD (Savin et al., 1979). Bupropion is an antidepressant
Table 1.
Binding affinities of antipruritic psychotropic drugs to receptors or transporters for various neuromediators
| Name | Mechanism | Ki (nM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Histamine receptor 1 | Opioid receptor | Cholinergic, muscarinic receptor | Adrenergic receptor | Norepine-phrine transporter | Dopamine Trans-porter | Dopamin receotor 2 | 5-HT transporter | 5-HT receptor | ||
| Doxepin | Tricyclic anti- depressant | 0.24 | 23 | α1: 23.5 (brain) | 29.5 | >10,000 | 360 (brain) | 68 | 1A: 276 (brain) | |
| α2: 1,270 (brain) | 2: 27 (brain) | |||||||||
| Trimi- pramine | Tricyclic anti- depressant | 1.4 (cortex) | 2,450 | 3,780 | 149 | |||||
| Bupropion | Atypical anti- depressant | >10,000 (cloned) | >10,000 | α1: 4,200 (brain) | >10,000 | 541 | >10,000 (brain) | >10,000 | 1A : >10,000 (brain) | |
| α2: >10,000 (brain) | 2 : >10,000 (brain) | |||||||||
| Naltrexone | Opioid receptor antagonist | δ: 26.6 | ||||||||
| κ: 1.75 | ||||||||||
| μ: 0.39 | ||||||||||
| Paroxetine | Selective serotonin reuptake inhibitor | >10,000 (brain) | 108 (brain) | α1: 1,868 (cortex) | 242 | 963 | >10,000 (brain) | 0.58 | 1A : >10,000 (brain) | |
| α2: 3,915 (cortex) | 2 : >10,000 (brain) | |||||||||
| Fluvox- amine | Selective serotonin reuptake inhibitor | >10,000 | α1: 1,288 (cortex) | 2,931 | >10,000 | 12.5 | ||||
Ki values mean the binding affinities of drugs to receptors and transporters for various neurotransmitters. Ki values used in this plot were adopted from PDSP Ki data base (http://pdsp.med.unc.edu/pdsp.php).
( ) means the source of receptor or transporter. Only the Ki values obtained using human source were identified.
acting via inhibition of both noradrenaline and dopamine reuptake. Case reports have shown that patients with AD can have symptomatic improvement with oral bupropion treatment (Gonzalez et al., 2006). Fig. 1 summarizes the Ki values of antipruritic psychotropic drugs to various receptors or transporters for neuromediators.
Fig. 1. Chemical structures of psychotropic drugs with antipruritic effects in patients of atopic dermatitis.

In addition, psychotropic drugs that act on 5-HT receptor or serotonin transporter (SERT) showed antipruritic effects in AD. An anxiolytic 5-HT agonist for 5-HT1A receptor, tandospirone citrate showed a significant improvement in SCORAD (SCORing Atopic Dermatitis) indices (Kawana et al., 2010). The selective serotonin reuptake inhibitor (SSRI) paroxetine and fluvoxamine considerably reduced pruritus in patients with AD in an open label two-armed proof of concept study (Diehn and Tefferi, 2001; Ständer et al., 2009). Yaris et al. and Zylicz et al. suggested that antipruritic effect of paroxetine might be predominantly due to its central action rather than peripheral effects (Yaris et al., 2003; Zylicz et al., 2003). However, considering the reports that intradermal administration of 5-HT could induce pruritus (Yamaguchi et al., 1999) and that selective 5-HT1A receptor agonist, tandospirone citrate inhibited stress-enhanced degranulation of skin mast cells (Shimoda et al., 2010), further studies should be conducted to elucidate whether the antipruritic effects of these serotonergic drugs are due to the central sedative effect based on their additive antihistaminic or anticholinergic action or peripheral effect. 5-HT is one of the key neurotransmitter that acts in both central and peripheral nervous system (Slominski et al., 2005). Dysregulation of 5-HT leads to sleep disorder, anxiety, depression, and aggressiveness. In vitro results show that 5-HT exerts variable effects on skin cells (Slominski et al., 2003). It stimulates growth of dermal fibroblasts in a dose-dependent manner (Seuwen and Pouyssegur, 1990). Immortalized epidermal melanocytes exhibit serotonin-stimulated growth when the cells had been cultured without melanocyte growth supplements (Slominski et al., 2003). In addition, recent reports showed that 5-HT induces melanogenesis via 5-HT receptor 2A(5-HT2A)(Lee et al., 2011).
In skin, 5-HT is involved in vasodilaion, inflammation, immunomodulation and pruritogenic effects via interaction with membrane-bound receptors, which are categorized into 7 families (5-HT1-7) with at least 21 subtypes (Mössner and Lesch, 1998; Kroeze et al., 2002; Slominski et al., 2003).
SERT determines the magnitude and duration of the serotonergic response via recycling released 5-HT in the synaptic cleft. Because SERT can terminate the action of 5-HT on nerve, the SSRIs targeting SERT have been used as antidepressants and anxiolytics.
However, 5-HT receptors and SERT are not confined to nerves. 5-HT receptors were found to be expressed on lymphocytes, dendritic cells and macrophages (Meredith et al., 2005). Expression of SERT on human blood lymphocytes (Faraj et al., 1994), murine peritoneal macrophages and dendritic cells (Rudd et al., 2005) has been reported.
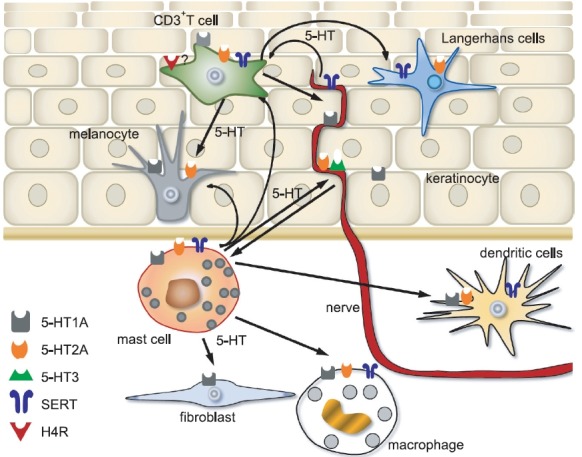
In human skin, Slominski et al. reported an expression of the serotonergic receptors on human keratinocytes, melanocytes and dermal fibroblasts (Slominski et al., 2003). 5-HT1A receptors were found on mast cells and melanocyte-like cells, 5-HT2A receptors and SERT on lymphocytes, NK cells and langerhans cells (LCs) in the eczematous skin of patients suffering allergic contact dermatitis (El-Nour et al., 2007). Pharmacological studies indicate that 5-HT3 receptors are also expressed on sensory nerve endings (Weisshaar et al., 1997). CD3+ cells in skin co-expressed 5-HT2A and SERT (El-Nour et al., 2007). Moreover, skin mast cells showed increased expression of serotonin receptor 5-HT1A, 5-HT2A, SERT in lesional skin of patients with stress-associated AD, compared with non-lesional skin (Lonne-Rahm et al., 2008). Fig. 2 summarizes the reports about the role of serotonin in neuroimmunologicalinteraction in skin of atopic dermatitis patients.
Fig. 2. Graphic summary about the role of serotonin in neuroimmunological interaction in skin of atopic dermatitis patients.
A recent paper suggested a possible association between polymorphisms in the SERT gene and aggravation of AD. Among the three known polymorphisms affecting transcription of SERT gene, a tendency towards high prevalence of the short (10-copy) variant of STin2 was found in AD patients. All AD patients with high-anxiety traits carried the short variant of STin2. In the corresponding healthy control group, the prevalences of the 10-and 12-copy variants were 62% and 38%, respectively (p<0.01) (de Mel et al., 2012). Interestingly, 5-HT is also reported to modulate T-cell activation and differentiation
strongly suggesting 5-HT as one of key mediators in signalling between nervous system and immune system (Aune et al., 1993; Aune et al., 1994; Gordon and Barnes, 2003). Thus it is not surprising that serotonergic drugs showed modulating effect on cells of the immune system (Frank et al., 1999; Pellegrino and Bayer, 2000). Release of 5-HT by fenfluramine treatment has been shown to decrease whole blood lymphocyte proliferation in rats (Connor et al., 2000). In addition, a SSRI fluoxetine and 5-HT2 receptor agonist 2, 5-dimethoxy-4-iodoamphetamine (DOI) administration resulted in a significant decrease in concanavalin A-induced lymphocyte proliferation (Pellegrino and Bayer, 2002). Pellegrino et al. suggested the effects of fluoxetine on lymphocyte proliferation were the result of elevated central serotonin neurotransmission and activation of central 5-HT2 receptors, because pretreatment with the 5-HT2 antagonist ritanserin or ketanserin almost completely antagonized the decrease in lymphocyte proliferation by fluoxetine (Pellegrino and Bayer, 2002). On the other hand, a couple of reports showed that fluoxetine promoted the Ca2+-mediated proteolysis of protein kinase C and increased cyclic AMP levels, impairing proliferation of human peripheral blood T cells, when optimal mitogenic stimuli was used (Edgar et al., 1999). CD3+ cells in skin co-expressed 5-HT2A and SERT (El-Nour et al., 2007). Thus the question whether the cyclic AMP-mediated suppression of T cell proliferations by fluoxetine might work in skin T cells and by other SSRI needs further study.
Considering all these results and the critical roles of skin T cells in the pathophysiology of AD, the antipruritic effects of SSRIs in AD patients might be due to their suppressive effect on T cells at least partly. However, it is still unclear whether the antipruritic effects of neuromodulators are mediated directly via suppression of lymphocyte function or indirectly by the modulation of neuro-immune interaction.
SUMMARY
Controlling and management of prurirtus still remains as unmet needs mainly due to the distinctive multifactorial pathogenesis of pruritus in AD. Based on the distinct feature of AD that psychological state of patients substantially influence the intensity of pruritus, various psychotropic drugs have been used in clinic to relieve pruritus of AD patients. Only several psychotropic drugs including SSRI were reported to show real antipruritic effects in AD patients and the precise mechanisms of antipruritic effect of these drugs are still unclear. Interestingly, drugs that increased 5-HT concentration showed significant decrease in lymphocyte proliferation, suggesting that antipruritic effects of SSRIs in AD patients might at least partly due to their suppressive effect on T cells considering the critical roles of skin T cells in the pathophysiology of atopic dermatitis. However, further studies should be conducted to elucidate the precise mechanism of neuro-immune interaction in pruritus of AD.
Acknowledgments
The present research was conducted by the research fund of Dankook University in 2011.
References
- 1.Andrew D., Craig A. D. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat. Neurosci. (2001);4:72–77. doi: 10.1038/82924. [DOI] [PubMed] [Google Scholar]
- 2.Aune T. M., McGrath K. M., Sarr T., Bombara M. P., Kelley K. A. Expression of 5HT1a receptors on activated human T cells. Regulation of cyclic AMP levels and T cell proliferation by 5-hydroxytryptamine. J. Immunol. (1993);151:1175–1183. [PubMed] [Google Scholar]
- 3.Aune T. M., Golden H. W., McGrath K. M. Inhibitors of serotonin synthesis and antagonists of serotonin 1A receptors inhibit T lymphocyte function in vitro and cell-mediated immunity in vivo. J. Immunol. . (1994);153:489–498. [PubMed] [Google Scholar]
- 4.Bilsborough J., Leung D. Y., Maurer M., Howell M., Boguniewicz M., Yao L., Storey H., LeCiel C., Harder B., Gross J. A. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J. Allergy Clin. Immunol. (2006);117:418–425. doi: 10.1016/j.jaci.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 5.Buske-Kirschbaum A., Geiben A., Hellhammer D. Psychobiological aspects of atopic dermatitis: an overview. Psychother. Psychosom. (2001);70:6–16. doi: 10.1159/000056219. [DOI] [PubMed] [Google Scholar]
- 6.Cevikbas F., Steinhoff A., Homey B., Steinhoff M. Neuroimmune interactions in allergic skin diseases. Curr. Opin. Allergy Clin. Immunol. . (2007);7:365–373. doi: 10.1097/ACI.0b013e3282a644d2. [DOI] [PubMed] [Google Scholar]
- 7.Cevikbas F., Steinhoff M., Ikoma A. Role of spinal neurotransmitter receptors in itch: new insights into therapies and drug development. CNS Neurosci. Ther. (2011);17:742–749. doi: 10.1111/j.1755-5949.2010.00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connor T. J., Kelly J. P., Leonard B. E. An assessment of the acute effects of serotonin releasers methylenedioxymethamphetamine, methylenedioxyamphetamine and fenfluramine on immunity in rats. Immunopharmacology . (2000);46:223–235. doi: 10.1016/s0162-3109(99)00180-0. [DOI] [PubMed] [Google Scholar]
- 9.Cowden J. M., Zhang M., Dunford P. J., Thurmond R. L. The histamine H4 receptor mediates inflammation and pruritus in Th2-dependent dermal inflammation. J. Invest. Dermatol. (2010);130:1023–1033. doi: 10.1038/jid.2009.358. [DOI] [PubMed] [Google Scholar]
- 10.Darsow U., Scharein E., Bromm B., Ring J. Skin testing of the pruritogenic activity of histamine and cytokines (interleukin-2 and tumour necrosis factor-alpha) at the dermal-epidermal junction. Br. J. Dermatol. . (1997);137:415–417. [PubMed] [Google Scholar]
- 11.de Mel S., Nordlind K., Holst M., Frohm-Nilsson M., Lonne-Rahm S. B. Polymorphisms in the serotonin transporter gene of patients with atopic dermatitis-association with personality traits related to high level of anxiety. Immunopharmacol. Immunotoxicol. (2012);34:534–538. doi: 10.3109/08923973.2011.632636. [DOI] [PubMed] [Google Scholar]
- 12.Diehn F., Tefferi A. Pruritus in polycythaemia vera: prevalence, laboratory correlates and management. Br. J. Haematol. (2001);115:619–621. doi: 10.1046/j.1365-2141.2001.03161.x. [DOI] [PubMed] [Google Scholar]
- 13.Dijkstra D., Leurs R., Chazot P., Shenton F. C., Stark H., Werfel T., Gutzmer R. Histamine downregulates monocyte CCL2 production through the histamine H4 receptor. J. Allergy Clin. Immunol. (2007);120:300–307. doi: 10.1016/j.jaci.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Drake L. A., Millikan L. E. The antipruritic effect of 5% doxepin cream in patients with eczematous dermatitis. Doxepin Study Goup. Arch. Dermatol. . (1995);131:1403–1408. [PubMed] [Google Scholar]
- 15.Dunford P. J., Williams K. N., Desai P. J., Karlsson L., McQueen D., Thurmond R. L. Histamine H4 receptor antagonists are superior to traditional antihistamines in the attenuation of experimental pruritus. J. Allergy Clin. Immunol. 2007);119:176–183. doi: 10.1016/j.jaci.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 16.Edgar V. A., Sterin-Borda L., Cremaschi G. A., Genaro A. M. Role of protein kinase C and cAMP in fluoxetine effects on human T-cell proliferation. Eur. J. Pharmacol. (1999);372:65–73. doi: 10.1016/s0014-2999(99)00142-9. [DOI] [PubMed] [Google Scholar]
- 17.El-Nour H., Lundeberg L., Abdel-Magid N., Lonne-Rahm S. B., Azmitia E. C., Nordlind K. Serotonergic mechanisms in human allergic contact dermatitis. Acta Derm. Venereol. (2007);87:390–396. doi: 10.2340/00015555-0288. [DOI] [PubMed] [Google Scholar]
- 18.Elenkov I. J. Glucocorticoids and the Th1/Th2 balance. Ann. N. Y. Acad. Sci. . (2004);1024:138–146. doi: 10.1196/annals.1321.010. [DOI] [PubMed] [Google Scholar]
- 19.Faraj B. A., Olkowski Z. L., Jackson R. T. Expression of a high-affinity serotonin transporter in human lymphocytes. Int. J. Immunopharmacol. . (1994);16:561–567. doi: 10.1016/0192-0561(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 20.Frank M. G., Hendricks S. E., Johnson D. R., Wieseler J. L., Burke W. J. Antidepressants augment natural killer cell activity: in vivo and in vitro. Neuropsychobiology. (1999);39:18–24. doi: 10.1159/000026555. [DOI] [PubMed] [Google Scholar]
- 21.Gonzaléz-Rey E., Chorny A., Delgado M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat. Rev. Immunol. (2007);7:52–63. doi: 10.1038/nri1984. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez E., Sanguino R. M., Franco M. A. Bupropion in atopic dermatitis. Pharmacopsychiatry. (2006);39:229. doi: 10.1055/s-2006-951384. [DOI] [PubMed] [Google Scholar]
- 23.Gordon J., Barnes N. M. Lymphocytes transport serotonin and dopamine: agony or ecstasy? Trends Immunol. . (2003);24:438–443. doi: 10.1016/s1471-4906(03)00176-5. [DOI] [PubMed] [Google Scholar]
- 24.Greaves M. W., Khalifa N. Itch: more than skin deep. Int. Arch. Allergy Immunol. (2004);135:166–172. doi: 10.1159/000080898. [DOI] [PubMed] [Google Scholar]
- 25.Gutzmer R., Mommert S., Gschwandtner M., Zwingmann K., Stark H., Werfel T. The histamine H4 receptor is functionally expressed on Th2 cells. J. Allergy Clin. Immunol. . (2009);123:619–625. doi: 10.1016/j.jaci.2008.12.1110. [DOI] [PubMed] [Google Scholar]
- 26.Handwerker H. O., Schmelz M. Pain: itch without pain-a labeled line for itch sensation? Nat. Rev. Neurol. (2009);5:640–641. doi: 10.1038/nrneurol.2009.191. [DOI] [PubMed] [Google Scholar]
- 27.Hanifin J. M., Ling M. R., Langley R., Breneman D., Rafal E. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part I, efficacy. J. Am. Acad. Dermatol. (2001);44:S28–S38. doi: 10.1067/mjd.2001.109810. [DOI] [PubMed] [Google Scholar]
- 28.Hennino A., Jean-Decoster C., Giordano-Labadie F., Debeer S., Vanbervliet B., Rozières A., Schmitt A. M., Nicolas J. F. CD8+ T cells are recruited early to allergen exposure sites in atopy patch test reactions in human atopic dermatitis. J. Allergy Clin. Immunol. (2011);127:1064–1067. doi: 10.1016/j.jaci.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 29.Heyer G. R., Hornstein O. P. Recent studies of cutaneous nociception in atopic and non-atopic subjects. J. Dermatol. (1999);26:77–86. doi: 10.1111/j.1346-8138.1999.tb03516.x. [DOI] [PubMed] [Google Scholar]
- 30.Hoare C., Li Wan Po A., Williams H. Systematic review of treatments for atopic eczema. Health Technol. Assess. (2000);4:1–191. [PMC free article] [PubMed] [Google Scholar]
- 31.Jameson J., Ugarte K., Chen N., Yachi P., Fuchs E., Boismenu R., Havran W. L. A role for skin gammadelta T cells in wound repair. Science . (2002);296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 32.Järvikallio A., Harvima I. T., Naukkarinen A. Mast cells, nerves and neuropeptides in atopic dermatitis and nummular eczema. Arch. Dermatol. Res. (2003);295:2–7. doi: 10.1007/s00403-002-0378-z. [DOI] [PubMed] [Google Scholar]
- 33.Kawana S., Kato Y., Omi T. Efficacy of a 5-HT1a receptor agonist in atopic dermatitis. Clin. Exp. Dermatol. (2010);35:835–840. doi: 10.1111/j.1365-2230.2009.03771.x. [DOI] [PubMed] [Google Scholar]
- 34.Klein P. A., Clark R. A. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch. Dermatol. (1999);135:1522–1525. doi: 10.1001/archderm.135.12.1522. [DOI] [PubMed] [Google Scholar]
- 35.Koblenzer C. S. Itching and the atopic skin. J. Allergy Clin. Immunol. (1999);104:S109–S113. doi: 10.1016/s0091-6749(99)70052-7. [DOI] [PubMed] [Google Scholar]
- 36.Kroeze W. K., Kristiansen K., Roth B. L. Molecular biology of serotonin receptors structure and function at the molecular level. Curr. Top. Med. Chem. . (2002);2:507–528. doi: 10.2174/1568026023393796. [DOI] [PubMed] [Google Scholar]
- 37.Lee C. H. Progress of pruritus research in atopic dermatitis. Biomol. Ther. (2010);18:246–256. [Google Scholar]
- 38.Lee H. J., Park M. K., Kim S. Y., Park Choo H. Y., Lee A. Y., Lee C. H. Serotonin induces melanogenesis via serotonin receptor 2A. Br. J. Dermatol. (2011);165:1344–1348. doi: 10.1111/j.1365-2133.2011.10490.x. [DOI] [PubMed] [Google Scholar]
- 39.Leung D. Y., Bieber T. Atopic dermatitis. Lancet . (2003);361:151–160. doi: 10.1016/S0140-6736(03)12193-9. [DOI] [PubMed] [Google Scholar]
- 40.Leung D. M., Boguniewicz M., Howell M. D., Nomura I., Hamid Q. A. New insights into atopic dermatitis. J. Clin. Invest. (2004);113:651–657. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lonne-Rahm S. B., Rickberg H., El-Nour H., Mårin P., Azmitia E. C., Nordlind K. Neuroimmune mechanisms in patients with atopic dermatitis during chronic stress. J. Eur. Acad. Dermatol. Venereol. (2008);22:11–18. doi: 10.1111/j.1468-3083.2007.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luger T. A. Neuromediators ? a crucial component of the skin immune system. J. Dermatol. Sci. (2002);30:87–93. doi: 10.1016/s0923-1811(02)00103-2. [DOI] [PubMed] [Google Scholar]
- 43.Luger T., Van Leent E. J., Graeber M., Hedgecock S., Thurston M., Kandra A., Berth-Jones J., Bjerke J., Christophers E., Knop J., Knulst A. C., Morren M., Morris A., Reitamo S., Roed-Petersen J., Schoepf E., Thestrup-Pedersen K., Van Der Valk P. G., Bos J. D. SDZ ASM 981: an emerging safe and effective treatment for atopic dermatitis. Br. J. Dermatol. . (2001);144:788–794. doi: 10.1046/j.1365-2133.2001.04134.x. [DOI] [PubMed] [Google Scholar]
- 44.Meredith E. J., Chamba A., Holder M. J., Barnes N. M., Gordon J. Close encounters of the monoamine kind: immune cells betray their nervous disposition. Immunology . (2005);115:289–295. doi: 10.1111/j.1365-2567.2005.02166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Metz M., Grundmann S., Ständer S. Pruritus: an overview of current concepts. Veterinary. Dermatology . (2011);22:121–131. doi: 10.1111/j.1365-3164.2010.00945.x. [DOI] [PubMed] [Google Scholar]
- 46.Mommert S., Gschwandtne M., Gutzmer R., Werfel T. The role of the histamine H4 receptor in atopic dermatitis. Curr. Allergy Asthma Rep. (2011);11:21–28. doi: 10.1007/s11882-010-0162-7. [DOI] [PubMed] [Google Scholar]
- 47.Morren M. A., Przybilla B., Bamelis M., Reynaers A., Degreef H. Atopic dermatitis: triggering factors. J. Am. Acad. Dermatol. (1994);31:467–473. doi: 10.1016/s0190-9622(94)70213-6. [DOI] [PubMed] [Google Scholar]
- 48.Mössner R., Lesch K. P. Role of serotonin in the immune system and in neuroimmune interactions. Brain. Behav. Immun. (1998);12:249–271. doi: 10.1006/brbi.1998.0532. [DOI] [PubMed] [Google Scholar]
- 49.Nakano T., Andoh T., Lee J. B., Kuraishi Y. Different dorsal horn neurons responding to histamine and allergic itch stimuli. Neuroreport . (2008);19:723–726. doi: 10.1097/WNR.0b013e3282fdf6c5. [DOI] [PubMed] [Google Scholar]
- 50.Novak N., Leung D. Y. Advances in atopic dermatitis. Curr. Opin. Immunol. (2011);23:778–783. doi: 10.1016/j.coi.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pastar Z., Lipozencic J., Ljubojevic S. Etiopathogenesis of atopic dermatitis - an overview. Acta Dermatovenerol. Croat. (2005);13:54–62. [PubMed] [Google Scholar]
- 52.Paus R., Schmelz M., Bíró T., Steinhoff M. Frontiers in pruritus research: scratching the brain for more effective itch therapy. J. Clin. Invest. (2006);116:1174–1186. doi: 10.1172/JCI28553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pellegrino T. C., Bayer B. M. Specific serotonin reuptake inhibitor-induced decreases in lymphocyte activity require endogenous serotonin release. Neuroimmunomodulation . (2000);8:179–187. doi: 10.1159/000054278. [DOI] [PubMed] [Google Scholar]
- 54.Pellegrino T. C., Bayer B. M. Role of central 5-HT (2) receptors in fluoxetine-induced decreases in T lymphocyte activity. Brain Behav. Immun. (2002);16:87–103. doi: 10.1006/brbi.2001.0625. [DOI] [PubMed] [Google Scholar]
- 55.Roosterman D., Goerge T., Schneider S.W., Bunnett N. W., Steinhoff M. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol. Rev. (2006);86:1309–1379. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- 56.Rudd M. L., Nicolas A. N., Brown B. L., Fischer-Stenger K., Stewart J. K. Peritoneal macrophages express the serotonin transporter. J. Neuroimmunol. (2005);159:113–118. doi: 10.1016/j.jneuroim.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 57.Rukwied R., Lischetzki G., McGlone F., Heyer G., Schmelz M. Mast cell mediators other than histamine induce pruritus in atopic dermatitis patients: a dermal microdialysis study. Br. J. Dermatol. (2000);142:1114–1120. doi: 10.1046/j.1365-2133.2000.03535.x. [DOI] [PubMed] [Google Scholar]
- 58.Savin J. A., Paterson W. D., Adam K., Oswald I. Effects of trimeprazine and trimipramine on nocturnal scratching in patients with atopic eczema. Arch. Dermatol. (1979);115:313–315. [PubMed] [Google Scholar]
- 59.Schmelz M. A neural pathway for itch. Nat. Neurosci. . (2001);4:9–10. doi: 10.1038/82956. [DOI] [PubMed] [Google Scholar]
- 60.Schmelz M., Hilliges M., Schmidt R., Ørstavik K., Vahlquist C., Weidner C., Handwerker H. O., Torebjörk H. E. Active “ itch fibers” in chronic pruritus. Neurology. (2003);61:564–566. doi: 10.1212/01.wnl.0000078193.64949.08. [DOI] [PubMed] [Google Scholar]
- 61.Seuwen K., Pouysségur J. Serotonin as a growth factor. Biochem. Pharmacol. (1990);39:985–990. doi: 10.1016/0006-2952(90)90276-q. [DOI] [PubMed] [Google Scholar]
- 62.Sharp L. L., Jameson J. M., Witherden D. A., Komori H. K., Havran W. L. Dendritic epidermal T-cell activation. Crit. Rev. Immunol. (2005);25:1–18. doi: 10.1615/critrevimmunol.v25.i1.10. [DOI] [PubMed] [Google Scholar]
- 63.Shimoda T., Liang Z., Suzuki H., Kawana S. Inhibitory effects of antipsychotic and anxiolytic agents on stress induced degranulation of mouse dermal mast cells. Clin. Exp. Dermatol. . (2010);35:531–536. doi: 10.1111/j.1365-2230.2009.03650.x. [DOI] [PubMed] [Google Scholar]
- 64.Slominski A., Pisarchik A., Zbytek B., Tobin D. J., Kauser S., Wortsman J. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J. Cell. Physiol. (2003);196:144–153. doi: 10.1002/jcp.10287. [DOI] [PubMed] [Google Scholar]
- 65.Slominski A., Wortsman J., Tobin D. J. The cutaneous serotonergic/melatonergic system: securing a place under the sun. FASEB J. . (2005);19:176–194. doi: 10.1096/fj.04-2079rev. [DOI] [PubMed] [Google Scholar]
- 66.Sonkoly E., Mulle A., Lauerma A., Pivarcsi A., Soto H., Kemeny L., Alenius H., Dieu-Nosjean M. C., Meller S., Rieker J., Steinhoff M., Hoffmann T. K., Ruzicka T., Zlotnik A., Homey B. IL-31: A new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol. (2006);117:411–417. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 67.Ständer S., Gunzer M., Metze D., Luger T., Steinhoff M. Localization of mu-opioid receptor 1A on sensory nerve fibers in human skin. Regul. Pept. (2002);110:75–83. doi: 10.1016/s0167-0115(02)00159-3. [DOI] [PubMed] [Google Scholar]
- 68.Ständer S., Weisshaar E., Luger T. A. Neurophysiological and neurochemical basis of modern pruritus treatment. Exp. Dermatol. (2008);17:161–169. doi: 10.1111/j.1600-0625.2007.00664.x. [DOI] [PubMed] [Google Scholar]
- 69.Ständer S., Böckenholt B., Schürmeyer-Horst F., Weishaupt C., Heuft G., Luger T. A., Schneider G. Treatment of chronic pruritus with the selective serotonin re-uptake inhibitors paroxetine and fluvoxamine: results of an open-labelled, two-arm proof-of concept study. Acta Derm. Venereol. (2009);89:45–51. doi: 10.2340/00015555-0553. [DOI] [PubMed] [Google Scholar]
- 70.Steinhoff M., Bienenstock J., Schmelz M., Maurer M., Wei E., Bíró T. Neurophysiological, neuroimmunological, and neuroendocrine basis of pruritus. J. Invest. Dermatol. (2006);126:1705–1718. doi: 10.1038/sj.jid.5700231. [DOI] [PubMed] [Google Scholar]
- 71.Straub R. H. Complexity of the bi-directional neuroimmune junction in the spleen. Trends Pharmacol. Sci. . (2004);25:640–646. doi: 10.1016/j.tips.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 72.Strid J., Tigelaar R. E., Hayday A. C. Skin immune surveillance by T cells--a new order? Semin. Immunol. (2009);21:110–120. doi: 10.1016/j.smim.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 73.Sugimoto Y., Iba Y., Nakamura Y., Kayasuga R., Kamei C. Pruritus-associated response mediated by cutaneous histamine H3 receptors. Clin. Exp. Allergy . (2004);34:456–459. doi: 10.1111/j.1365-2222.2004.01876.x. [DOI] [PubMed] [Google Scholar]
- 74.Tobin D., Nabarro G., Baart de la Faille H., van Vloten W. A., van der Putte S. C., Schuurman H. J. Increased number of immunoreactive nerve fibers in atopic dermatitis. J. Allergy Clin. Immunol. (1992);90:613–622. doi: 10.1016/0091-6749(92)90134-n. [DOI] [PubMed] [Google Scholar]
- 75.Turner D. J., Martin P. C., Rao J. N., Greenspon J., Zou T., Bass B. L., Wang J. Y., Strauch E. D. Substance P regulates migration in rat intestinal epithelial cells. Ann. Surg. (2007);245:408–414. doi: 10.1097/01.sla.0000245549.57076.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Urashima R., Mihara M. Cutaneous nerves in atopic dermatitis. A histological, immunohistochemical and electron microscopic study. Virchows. Arch. (1998);432:363–370. doi: 10.1007/s004280050179. [DOI] [PubMed] [Google Scholar]
- 77.Wahlgren C. F., Scheynius A., Hägemark O. Antipruritic effect of oral cyclosporin A in atopic dermatitis. Acta. Derm. Venereol. (1990);70:323–329. [PubMed] [Google Scholar]
- 78.Wahlgren C. F. Pathophysiology of itching in urticaria and atopic dermatitis. Allergy. (1992);47:65–75. doi: 10.1111/j.1398-9995.1992.tb05091.x. [DOI] [PubMed] [Google Scholar]
- 79.Wahlgren C. F., Tengvall, Linder M., Hägemark O., Scheynius A. Itch and inflammation induced by intradermally injected interleukin-2 in atopic dermatitis patients and healthy subjects. Arch. Dermatol. Res. (1995);287:572–580. doi: 10.1007/BF00374079. [DOI] [PubMed] [Google Scholar]
- 80.Weidinger S., Gieger C., Rodriguez E., Baurecht H., Mempel M., Klopp N., Gohlke H., Waqenpfeil S., Ollert M., Ring J., Behrendt H., Heinrich J., Novak N., Bieber T., Krämer U., Berdel D., von Berg A., Bauer C. P., Herbarth O., Koletzko S., Prokisch H., Mehta D., Meitinger T., Depner M., von Mutius E., Liang L., Moffatt M., Cookson W., Kabesch M., Wichmann, H. E., Illig T. Genome-wide scan on total serum IgE levels identifies FCER1A as novel susceptibility locus. PLoS. Genet. (2008);4:e1000166. doi: 10.1371/journal.pgen.1000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weisshaar E., Ziethen B., Gollnick H. Can a serotonin type 3 (5-HT3) receptor antagonist reduce experimentally-induced itch? Inflamm. Res. (1997);46:412–416. doi: 10.1007/s000110050213. [DOI] [PubMed] [Google Scholar]
- 82.Witherden D. A., Verdino P., Rieder S. E., Garijo O., Mills R. E., Teyton L., Fischer W. H., Wilson I. A., Havran W. L. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial gammadelta T cell activation. Science. (2010);329:1205–1210. doi: 10.1126/science.1192698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamaguchi T., Nagasawa T., Satoh M., Kuraishi Y. Itch-associated response induced by intradermal serotonin through 5-HT2 receptors in mice. Neurosci. Res. (1999);35:77–83. doi: 10.1016/s0168-0102(99)00070-x. [DOI] [PubMed] [Google Scholar]
- 84.Yaris E., Kesim M., Kadioglu M., Kalyoncu N. I., Ulku C., Ozyavuz R. The effects of paroxetine on rat isolated vas deferens. Pharmacol. Res. (2003);48:335–345. doi: 10.1016/s1043-6618(03)00157-9. [DOI] [PubMed] [Google Scholar]
- 85.Yosipovitch G., Szolar C., Hui X. Y., Maibach H. High-potency topical corticosteroid rapidly decreases histamine-induced itch but not thermal sensation and pain in human beings. J. Am. Acad. Dermatol. (1996);35:118–120. doi: 10.1016/S0190-9622(96)90524-1. [DOI] [PubMed] [Google Scholar]
- 86.Zylicz Z., Krajnik M., Sorge A. A., Costantini M. Paroxetine in the treatment of severe non-dermatological pruritus: a randomized, controlled trial. J. Pain. Symptom Manage. (2003);26:1105–1112. doi: 10.1016/j.jpainsymman.2003.05.004. [DOI] [PubMed] [Google Scholar]


