Abstract
The tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a member of the tumor necrosis factor family of cytokines. TRAIL selectively induces apoptotic cell death in various tumors and cancer cells, but it has little or no toxicity in normal cells. Agonism of TRAIL receptors has been considered to be a valuable cancer-therapeutic strategy. However, more than 85% of primary tumors are resistant to TRAIL, emphasizing the importance of investigating how to overcome TRAIL resistance. In this report, we have found that nemadipine-A, a cell-permeable L-type calcium channel inhibitor, sensitizes TRAIL-resistant cancer cells to this ligand. Combination treatments using TRAIL with nemadipine-A synergistically induced both the caspase cascade and apoptotic cell death, which were blocked by a pan caspase inhibitor (zVAD) but not by autophagy or a necrosis inhibitor. We further found that nemadipine-A, either alone or in combination with TRAIL, notably reduced the expression of survivin, an inhibitor of the apoptosis protein (IAP) family of proteins. Depletion of survivin by small RNA interference (siRNA) resulted in increased cell death and caspase activation by TRAIL treatment. These results suggest that nemadipine-A potentiates TRAIL-induced apoptosis by down-regulation of survivin expression in TRAIL resistant cells. Thus, combination of TRAIL with nemadipine-A may serve a new therapeutic scheme for the treatment of TRAIL resistant cancer cells, suggesting that a detailed study of this combination would be useful.
Keywords: TRAIL, Nemadipine-A, Sensitization, Cell death, H1299 cells
INTRODUCTION
The tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a member of the tumor necrosis factor family, which includes Fas ligand and TNF-α. TRAIL triggers typical apoptotic signaling by binding to its receptors, death receptor 4 (DR4) and death receptor 5 (DR5), thereby recruiting a death-inducing signaling complex (DISC), which activates the caspase cascade (Johnstone et al., 2008; Abdulghani and El-Deiry, 2010). Unlike Fas ligand and TNF-α, because TRAIL functions preferentially in tumor cells, TRAIL has attracted enormous interest for cancer therapy (Bellail et al., 2009). However, accumulating evidence has now shown that primary tumors are resistant to TRAIL-induced cell death through multiple mechanisms. For example, mutations in apoptosis regulatory proteins are occasionally found in various types of tumor cells (Abdulghani and El-Deiry, 2010). In addition, dysfunction of death receptors and abnormal expression of anti-apoptotic proteins results in TRAIL resistance in tumor cells (van Geelen et al., 2011). This phenomenon is understandable because TRAIL is now characterized as a part of the human immune system response against primary tumors, which implies that primary tumors have already evaded TRAIL. Thus, TRAIL alone is not effective in killing primary tumors. To overcome the resistance to TRAIL that develops in cancer cells, and to further understand the main mechanism underlying this resistance, numerous studies have been performed and suggest that combination therapy with specific agents significantly enhances the cytotoxicity of TRAIL in TRAIL-resistant cancer cells. For example, anti-cancer therapeutic agents such as histone deacetylase inhibitors and DNA-damaging agents, including VP-16 and CDDP, have the potential to treat various cancer types in combination with TRAIL (Ammann et al., 2009; Sung et al., 2010; Sung et al., 2012; Park et al., 2012).
Accordingly, we have previously identified several novel TRAIL sensitizers in TRAIL-resistant cancer cells (Oh et al., 2012). In our present study, we investigated the effects of nemadipine-A as a TRAIL sensitizer. Nemadipine-A is similar to the class of anti-hypertension drugs that antagonize a particular type of calcium channel. Indeed, nemadipine-A was first identified as an antagonist of the α-subunit of L-type calcium channels in C. elegans (Kwok et al., 2006; Hui et al., 2009). Nemadipine-A induces morphological defects in egg laying in worms. However, the role of this agent in cell death and cancer therapy has not been elucidated. We found in our current experiments that nemadipine-A strongly potentiates TRAIL-induced apoptosis in TRAIL-resistant lung cancer cells via the down-regulation of the anti-apoptotic protein survivin.
MATERIALS AND METHODS
Cells and reagents
H1299 and A549 lung cancer cells were obtained from the American Type Culture Collection (ATCC). The cells were maintained in RPMI medium containing 10% FBS and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) Recombinant human TRAIL and zVAD-FMK were obtained from R&D Systems (Minneapolis, MN, USA). Nemadipine-A was obtained from Enzo Life Science (San Diego, CA, USA). Bafilomycin, and Necrostatin-1 were purchased from Sigma-aldrich (St. Louis, MO, USA). The previously validated siRNA that targets survivin (5’-GAAGCAGUUUGAAGAAUUA-3’) (Okamoto et al., 2010) and negative scrambled siRNA (5’-CCUACGCCACCAAUUUCGU-3’) were synthesized by Bioneer (Daejeon, Korea).
Cell viability and cell death assay
Cell viability was determined using a cell counting kit-8 (CCK-8) in accordance with the manufacturer’s protocol (Dojindo Corporation, Japan). Briefly, cells incubated with a test compound in a 96-well plate received 10 μl of CCK-8 solution, and were then incubated for one hour in a CO2 incubator. The subsequent colorimetric change was measured using a Victor microtiter plate reader (PerkinElmer) set to monitor changes in absorbance at 450 nm. To determine cell death, the cells treated with indicated agents were stained with Annexin V and PI (BD Bioscience, San Jose, CA) and measured dead cells with NucleoCounter NC3000 (Chemometec, Denmark).
Western blotting analysis
All cells lysates were prepared with 2× Laemmli sample buffer (62.5 mM Tris-HCl, pH 6.8, 25% glycerol, 2% SDS, 5% β-mercaptoethanol, 0.01% bromophenol blue) (BioRad, Hercules, CA, USA). Proteins (approximately 50 μg) were measured by using the Bradford solution (BioRad) according to the manufacture’s instruction. Then the samples were separated by SDS-polyacrylamide gel electrophoresis, and transferred to PVDF membrane (BioRad). After blocking with 4% skim milk in TBST (25 mM Tris, 3 mM 140 mM NaCl, 0.05% Tween20), the membranes were incubated over-night with specific primary antibodies. For the protein detection, the membranes were incubated with HRP-conjugated secondary antibodies (Pierce, Rockford, IL, USA). And the protein level was analyzed by densitometer (BioRad).
Antibody
Anti-actin (MAB1501) antibody was obtained from Millipore (Temecula, CA, USA). Anti- Bcl-xl (#2764), anti-Bax (#2772), anti-Bik (#4592), anti-Bak (#6947), anti-p-AKT (#9271), anti-ERK1/2 (#9102), anti-p-ERK1/2 (#9101), anti-Survivin (#2803), anti-cleaved caspase-3 (#9661), and anti-Mcl-1(#5453) antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA); anti-DR4 (ab13890), anti-DR5, (ab47179), and anti-Noxa (ab13687) antibodies were purchased from Abcam (Cambridge, MA, USA). Anti-Bim (Sc-11425), anti-EIF2 (Sc11386), and anti-p-EIF2 (Sc-101170) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-GRP94 (ADI-SPA-827) antibody was obtained from Enzo Life Science (San Diego, CA, USA). Anti-caspase-10 (IMG-4150) antibody was obtained from Imgenex (San Diego, CA, USA). Anti-p-p38 (AF869) antibody was purchased from R&D system (Minneapolis, MN, USA). Anti-XIAP (61062) antibody was obtained from BD Bioscience (San Jose, CA, USA). And the antibody against caspase-8 used in this experiment was generated in our laboratory (Kim et al., 2000).
Statistical analysis
Data were obtained from least three independent experiments, and presented as means ± SEM Statistical evaluation of the results was performed with one-way ANOVA. Data represent ± standard error of the mean (SEM) from more than three independent experiments (n>3).
RESULTS
Nemadipine-A sensitizes H1299 lung cancer cells towards TRAIL-induced cytotoxicity
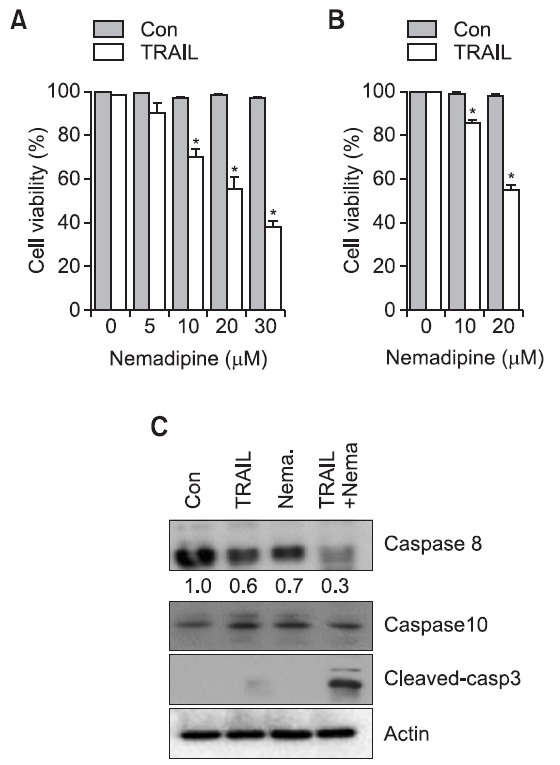
Since TRAIL preferentially kills tumor cells, it has been developed as a promising anti-cancer agent. However, several types of cancer cells develop resistance to TRAIL-induced cell death. H1299 lung adenocarcinoma cells, are also resistant to the apoptotic effects of TRAIL, but the co-administration of nemadipine-A and TRAIL (Nema/TRAIL) in these cells resulted in notably increased cytotoxicity in a dose-dependent manner (Fig. 1A). Moreover, activation of caspase-8 and caspase-3 were also synergistically enhanced in Nema/TRAIL-exposed H1299 cells. In contrast neither TRAIL alone nor nemadipine-A alone strongly activated caspase-8 or caspase-3 (Fig. 1B). These results suggest that nemadipine-A sensitizes H1299 cells to TRAIL-induced cytotoxicity.
Fig. 1. Nemadipine-A sensitizes TRAIL-induced cell death in lung cancer cells. (A, B) H1299 and A549 cells were treated with TRAIL (20 ng/ml) with increasing concentration of nemadipine-A (1 to 30 μM) for 8 hours. Cell viability and caspase activation were then determined using the CCK-8 assay. (C) H1299 cells were either untreated (Con.) or treated with TRAIL (20 ng/ml), nemadipine-A (Nema. (20 μM), or a combination of nemadipine-A and TRAIL for 8 hours. The cells were then harvested, and analyzed by western blotting with the indicated antibodies. Actin was used as an internal loading control. The protein levels were measured by densitometry analysis. The data were obtained from at least three different experiments, and are presented ± standard error of the mean (SEM), (n=3, *<0.05).
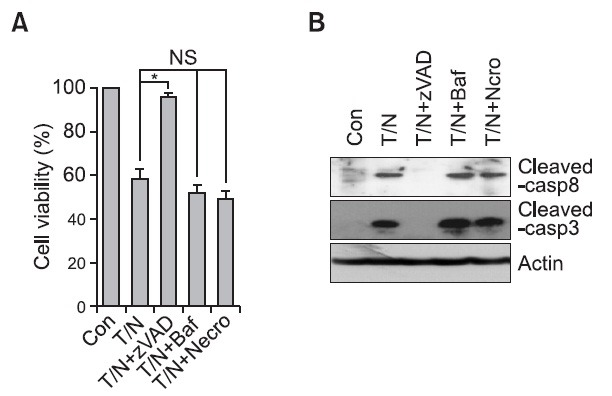
Although TRAIL is a well-known apoptosis inducer, previous evidences suggested that necrosis or autophagy is involved in TRAIL-mediated cell death (Cho et al., 2009; Hou et al, 2008; Jouan-Lanhouet et al., 2012). Therefore, we questioned whether Nema/TRAIL-mediated cell death is apoptotic or not. We examined inhibition of cell death with specific chemical inhibitors such as zVAD (a pan caspase inhibitor), Bafilomycin (an autophagy inhibitor, which interferes with the maturation of autophagosomes to autolysomes), and necrostatin-1 (an inhibitor of necrotic cell death). H1299 cells were treated with Nema/TRAIL in the presence or absence of the inhibitor. Inhibition of caspases by zVAD treatment significantly reduced the Nema/TRAIL-induced cell death while bafilomycin or necrostatin-1 did not (Fig. 2A). According to the cell death results, both caspase-8 and caspase-3 activation were only inhibited in zVAD-treated cells (Fig. 2B), suggesting that combination of nemadipine-A with TRAIL induces caspase-mediated apoptotic cell death.
Fig. 2. Caspase inhibition suppressed nemadipine-A/TRAIL-induced cell death in H1299 cells. (A, B) H1299 cells were co-treated with TRAIL and nemadipine-A (T/N; 20 ng/ml and 20 μM respectively) with or without different cell death inhibitors (40 μM zVAD, 100 nM bafilomycin (Baf), 10 μM necrostatin-1 (Necro)). Cell viability and caspase activation were then analyzed by CCK-8 assay and western blotting. Values are the mean ± SEM (n=3, *<0.01, NS: not signicant).
Survivin expression is down-regulated by nemadipine-A in lung cancer cells
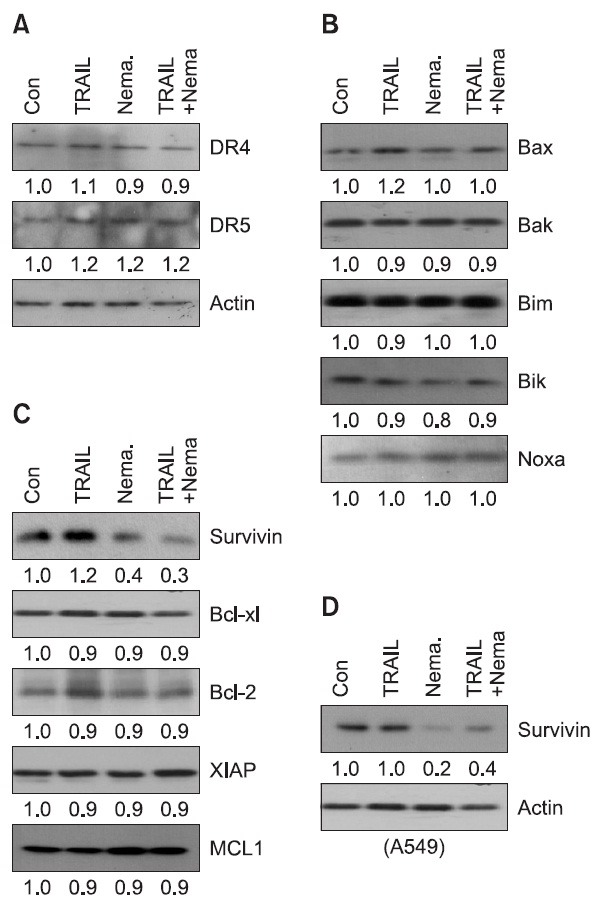
TRAIL resistance can be occur at several steps in the cell death signaling cascade and can include several distinct mechanisms (Mahalingam et al., 2009). To investigate the responsible event for nemadipine-A-mediated sensitization, we further investigated the expression levels of proteins that regulate the TRAIL signaling pathway such as TRAIL receptors, the anti-apoptotic or pro-apoptotic Bcl-2 family proteins, and IAP protein (Fig. 3A-C). Expression of TRAIL receptors DR4 and DR5 were not changed by nemadipine-A. In addition, several pro-apoptotic Bcl-2 proteins, including Bax, Bak, Bim, Bik, and Noxa, were not altered by nemadipine-A treatment. However, an anti-apoptotic protein, survivin, was remarkably decreased following the treatment of nemadipine-A alone or Nema/TRAIL, while Bcl-2, Bcl-xl, Mcl-1, and XIAP proteins were not altered. Down-regulation of survivin by nemadipine-A was further examined in A549 cells, another TRAIL-resistant lung cancer cell line (Fig. 3D). These results suggested that survivin is a key mediator for the TRAIL-sensitizing effect of nemadipine-A in lung cancer cells.
Fig. 3. Nemadipine-A down-regulates survivin expression. (A-C) H1299 cells were treated with TRAIL (20 ng/ml), nemadipine-A (Nema., 20 μM), or with a combination of both agents for 8 hours. The cells were then harvested for western blotting. The expression of death receptors, anti-apoptotic proteins and pro-apototic Bcl-2 proteins were analyzed using the indicated antibodies. (D) A549 cells were treated with TRAIL (20 ng/ml), nemadipine-A (Nema., 20 μM), or a combination of both for 8 hours. Survivin expression was then determined by western blotting. The protein levels were measured by densitometry analysis.
Depletion of survivin expression sensitizes TRAIL-induced cell death and the caspase cascade
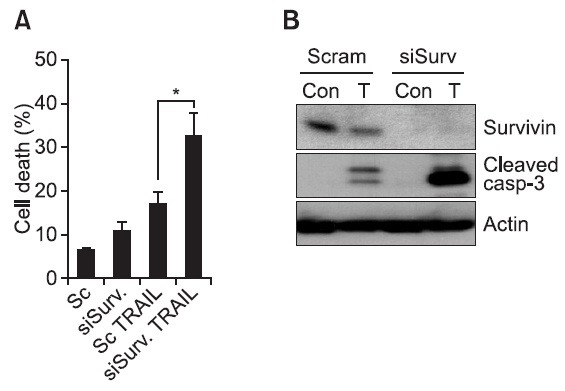
Survivin is a member of the inhibitor of apoptosis protein (IAP) family. Based on this notion, it was reported that suppression of survivin increased cell death in various cancer cells. Since we addressed that nemadipin-A decreases survivin expression in lung cancer cells, we further investigated the role of survivin in TRAIL-induced caspase activation as well as cell death. As shown in Fig. 4, depletion of survivin by siRNA not only significantly increased TRAIL-induced apoptotic cell death but also remarkably increased caspase-3 activation compared with the control siRNA. Thus, down-regulation of survivin is responsible for enhancement or restoration of TRAIL sensitivity after nemadipine-A treatment in lung cancer cells.
Fig. 4. Depletion of survivin expression potentiates TRAIL-mediated apoptosis. H1299 cells were transiently transfected with scrambled negative siRNA (Scram) or survivin specific siRNA (siSurvi). After three days, the cells were treated with TRAIL (20 ng/ml) for 10 hours. Then cell death was determined with Annexin V/PI staining (A). The cells were then harvested, and subjected to Western blot analysis with the indicated antibodies (B), (n=3, *<0.05).
ER stress and MAP kinases do not mediate the effect of nemadipine-A on TRAIL sensitization
Nemadipine-A was identified as a calcium channel antagonist in C. elegance and as an inducer of calcium homeostasis dysfunction that stresses the endoplasmic reticulum (ER) (Kwok et al., 2006). In addition, ER stress could potentiate TRAIL-induced apoptosis in cancer cells (Jiang et al., 2007; Zhang et al., 2011). To investigate ER stress is involved in nemadipine-A-mediated TRAIL sensitization, we examined the known ER stress response proteins such as p-EIF2 and GRP94 following nemadipine-A treatment. As shown in Fig. 5A, the expression of ER stress proteins and activity of ER stress marker proteins were not notably altered.
Fig. 5. ER stress and MAP kinases are not associated with nemadipin-A-mediated TRAIL sensitization. (A, B) H1299 cells were treated with TRAIL (20 ng/ml), nemadipine-A (Nema., 20 μM), or a combination of both agents for 8 hours. The cells were then harvested for western blotting of ER stress response proteins (A) and activated MAP kinase and AKT proteins (B). The protein levels were measured by densitometry analysis.
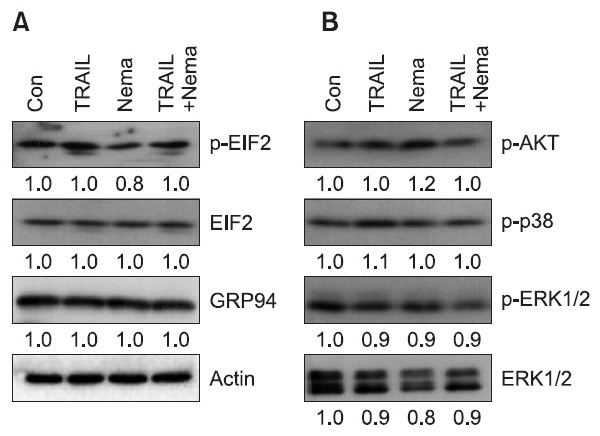
It has been shown that AKT and MAPK signaling pathways are dysregulated in cancer cells and that these pathways are associated with their apoptotic responses to anti-cancer agents (Jin et al., 2007). Recent studies also suggest that targeting these pathways can modulate TRAIL resistance in cancer cells (Lamy et al., 2011; Li et al., 2011). Thus, we further investigated the effects of nemadipine-A or Nema/TRAIL on these molecules. Unlike previous results, these proteins were found not to be activated by nemapidine-A or Nema/TRAIL treatment (Fig. 5B). These results suggest that neither ER stress nor dysregulated AKT or MAP kinase pathways are associated with TRAIL sensitization by nemadpine-A in H1299 cells.
DISCUSSION
Since TRAIL is preferentially cytotoxic to tumor cells but not normal cells, it is considered to have strong potential as an anticancer agent. However, about 50% of tumor cell lines and the majority of primary tumors derived from cancer patients have shown resistance to TRAIL. Thus, overcoming this resistance will be an important step in any therapeutic strategies involving TRAIL (Mahalingam et al., 2009). Accumulating evidence indicates that a combination of chemotherapeutic drugs with TRAIL effectively enhances the cytotoxicity of TRAIL. In this study, we examined the effects of nemadipine-A, an L-type calcium channel blocker, on TRAIL-induced cell death in TRAIL-resistant cancer cells.
Nemadipine-A was originally identified as a calcium channel antagonist, which can block L-type voltage gated calcium channel (VGCC)-mediated calcium influx in C. elegans (Stout and Parpura, 2011). VGCC channels have been associated with a large and sustained calcium entry and response (Frøkjaer-Jensen et al., 2006). Interestingly, Kaddour-Djebbar et al. showed that a combination of calcium channel blockers with TRAIL potentiates TRAIL-induced apoptosis. Inhibition of Na+/Ca2+ exchange by benzodiazepine CGP-37157 also synergistically increases TRAIL-induced apoptosis in prostate cancer cells (Kaddour-Djebbar et al., 2006). Accordingly, we also found that nemadipine-A-sensitizes TRAIL induced cell death in lung cancer cells. An imbalance of Ca2+ homeostasis is related to ER stress and it has been reported that ER stress inducers such as tumicamycine and thapsigargine could sensitize cancer cells to TRAIL-induced apoptosis (Jiang et al., 2007; Zhang et al., 2011). Thus, we examined the effect of nemadipin-A on ER stress response. However, nemadipine-A did not increase ER stress-response proteins, which suggests that it is not strongly associated with ER stress-mediated TRAIL sensitization. Nonetheless, further analyses regarding ER stress may help to elucidate the mechanism of nemadipine-A on TRAIL sensitization.
TRAIL resistance can occur in several steps such as receptor binding, formation of DISC complex, and caspase cascase in the apoptosis signaling pathway (Kim and Seol, 2003; Mahalingam et al., 2009). In our present study, in order to find the mechanism by which nemadipine-A overcomes TRAIL resistance, we examined several apoptosis-related proteins that function in the TRAIL response. Neither the TRAIL receptors DR4 and DR5, nor various proteins from the pro-apoptotic Bcl-2 family of proteins, including Bax, Bak, Bim, Bik, and Noxa, showed altered expression upon treatment with nemadipine-A alone or with a combination of nemadipine-A and TRAIL. In addition, the expression of several anti-apoptotic members of the Bcl-2 family of proteins such as Bcl-2, Bcl-xL, and Mcl-1 were also unchanged (Fig. 3). However, the expression level of survivin was remarkably reduced in nemadipine-A treated cells. It has been well documented that the down-regulation of survivin by chemotherapeutic agents sensitizes cancer cells to TRAIL-induced apoptosis (Kim et al., 2004; Lu et al., 2008; Raviv et al., 2011). Consistently, we have also found that nemadipine-A potentiates TRAIL-induced apoptosis by reducing survivin expression. Although, the precise mechanisms are not clear, it was recently reported that survivin expression could be up-regulated by cellular calcium level (Ortiz and Chou, 2012). Survivin is a member of the IAP family proteins, which are frequently highly expressed in cancer cells (Ryan et al., 2009; Altieri, 2010). Survivin also regulates apoptosis and cell proliferation by interaction with the second mitochondria-derived activator of caspase (SMAC/Diablo), caspases, heat shock protein 90, and the chromosomal passenger complex (Tamm et al., 1998; Vader et al., 2006; Ryan et al., 2009; Kanwar et al., 2011). Survivin is considered to be an attractive target for anti-cancer therapy because it shows low expression in most normal cells but high expression in cancer cells. In addition, survivin regulates cell proliferation and angiogenesis (Kawasaki et al., 2001) as well as apoptosis, which are essential features for cancer treatment (Hanahan and Weinberg 2000). Moreover, the down-regulation of survivin enhances the response to multiple types of conventional cancer therapies (Pennati et al., 2002). For these reasons, several small molecules that target survivin are currently undergoing clinical trials (Ryan et al., 2009). We also demonstrated here that nemadipine-A sensitizes TRAIL-induced apoptosis by reducing survivin expression in TRAIL-resistant cells. Therefore, the targeting of survivin by nemadipine-A is a potential future therapeutic strategy for lung cancer.
Acknowledgments
This research was supported by a grant from Kyung Hee University in 2010 (KHU-20101839).
References
- 1.Abdulghani J., El-Deiry W. S. TRAIL receptor signaling and therapeutics. Expert Opin. Ther. Targets. (2010);14:1091–1108. doi: 10.1517/14728222.2010.519701. [DOI] [PubMed] [Google Scholar]
- 2.Altieri D. C. Survivin and IAP proteins in cell-death mechanisms. Biochem. J. (2010);430:199–205. doi: 10.1042/BJ20100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ammann J. U., Haag C., Kasperczyk H., Debatin K. M., Fulda S. Sensitization of neuroblastoma cells for TRAIL-induced apoptosis by NF-kappaB inhibition. Int. J. Cancer. (2009);124:1301–1311. doi: 10.1002/ijc.24068. [DOI] [PubMed] [Google Scholar]
- 4.Bellail A. C., Qi L., Mulligan P., Chhabra V., Hao C. TRAIL agonists on clinical trials for cancer therapy; the promises and the challenges. Rev. Recent Clin. Trials. (2009);4:34–41. doi: 10.2174/157488709787047530. [DOI] [PubMed] [Google Scholar]
- 5.Cho D. H., Jo Y. K., Hwang J. J., Lee Y. M., Roh S. A., Kim J. C. Caspase-mediated cleavage of ATG6/Beclin-1 links apoptosis to autophagy in HeLa cells. Cancer Lett. (2009);274:95–100. doi: 10.1016/j.canlet.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Frøkjaer-Jensen C., Kindt K. S., Kerr R. A., Suzuki H., Melnik-Martinez K., Gerstbreih B., Driscol M., Schafer W. R. Effects of voltage-gated calcium channel subunit genes on calcium influx in cultured C. elegans mechanosensory neurons. J. Neurobiol. (2006);66:1125–1139. doi: 10.1002/neu.20261. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D., Weinberg R. A. The hallmarks of cancer. Cell. (2000);100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 8.Hou W., Han J., Lu C., Goldstein L. A., Rabinowich H. Enhancement of tumor-TRAIL susceptibility by modulation of autophagy. Autophagy. (2008);4:940–943. doi: 10.4161/auto.6769. [DOI] [PubMed] [Google Scholar]
- 9.Hui K., Kwok T. C., Kostelecki W., Leen J., Roy P. J., Feng Z. P. Differential sensitivities of CaV1.2 IIS5-S6 mutants to 1,4-dihydropyridine analogs. Eur. J. Pharmacol. (2009);602:255–261. doi: 10.1016/j.ejphar.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 10.Jiang C. C., Chen L. H., Gillespie S., Kiejda K. A., Mhaidat N., Wang Y. F., Thorne R., Zhang X. D., Hersey P. Tunicamycin sensitizes human melanoma cells to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by up-regulation of TRAIL-R2 via the unfolded protein response. Cancer Res. (2007);67:5880–5888. doi: 10.1158/0008-5472.CAN-07-0213. [DOI] [PubMed] [Google Scholar]
- 11.Jin C. Y., Moon D. O., Lee J. D., Heo M. S., Choi Y. H., Lee C. M., Park Y. M., Kim G. Y. Sulforaphane sensitizes tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis through downregulation of ERK and Akt in lung adenocarcinoma A549 cells. Carcinogenesis. (2007);28:1058–1066. doi: 10.1093/carcin/bgl251. [DOI] [PubMed] [Google Scholar]
- 12.Johnstone R. W., Frew A. J., Smyth M. J. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat. Rev. Cancer. (2008);8:782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 13.Jouan-Lanhouet S., Arshad M. I., Piquet-Pellorce C., Martin-Chouly C., Le Moigne-Muller G., Van Herreweghe F., Takahashi N., Sergent O., Lagadic-Gossmann D., Vandenabeele P., Samson M., Dimanche-Boitrel M. T. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. (2012);19:2003–2014. doi: 10.1038/cdd.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaddour-Djebbar I., Lakshmikanthan V., Shirley R. B., Ma Y., Lewis R. W., Kumar M.V. Therapeutic advantage of combining calcium channel blockers and TRAIL in prostate cancer. Mol. Cancer Ther. (2006);5:1958–1966. doi: 10.1158/1535-7163.MCT-06-0011. [DOI] [PubMed] [Google Scholar]
- 15.Kanwar J. R., Kamalapuram S. K., Kanwar R. K. Targeting survivin in cancer: the cell-signalling perspective. Drug Discov. Today. (2011);16:485–494. doi: 10.1016/j.drudis.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Kawasaki H., Toyoda M., Shinohara H., Okuda J., Watanabe I., Yamamoto T., Tanaka K., Tenjo T., Tanigawa N. Expression of survivin correlates with apoptosis, proliferation, and angiogenesis during human colorectal tumorigenesis. Cancer. (2001);91:2026–2032. doi: 10.1002/1097-0142(20010601)91:11<2026::aid-cncr1228>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.Kim I. K., Chung C. W., Woo H. N., Hong G. S., Nagata S., Jung Y. K. Reconstitution of caspase-8 sensitizes JB6 cells to TRAIL. Biochem. Biophys. Res. Commun. (2000);277:311–316. doi: 10.1006/bbrc.2000.3673. [DOI] [PubMed] [Google Scholar]
- 18.Kim S., Kang J., Qiao J., Thomas R. P., Evers B. M., Chung D. H. Phosphatidylinositol 3-kinase inhibition down-regulates survivin and facilitates TRAIL-mediated apoptosis in neuroblastomas. J. Pediatr. Surg. (2004);39:516–521. doi: 10.1016/j.jpedsurg.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y., Seol D. W. TRAIL, a mighty apoptosis inducer. Mol. Cells. (2003);15:283–293. [PubMed] [Google Scholar]
- 20.Kwok T. C., Ricker N., Fraser R., Chan A. W., Burns A., Stanley E. F., McCourt P., Cutler S. R., Roy P. J. A small-molecule screen in C. elegans yields a new calcium channel antagonist. Nature. (2006);441:91–95. doi: 10.1038/nature04657. [DOI] [PubMed] [Google Scholar]
- 21.Lamy V., Bousserouel S., Gossé F., Minker C., Lobstein A., Raul F. Lupulone triggers p38 MAPK-controlled activation of p53 and of the TRAIL receptor apoptotic pathway in human colon cancer-derived metastatic cells. Oncol. Rep. (2011);26:109–114. doi: 10.3892/or.2011.1273. [DOI] [PubMed] [Google Scholar]
- 22.Li S.S., Yang S., Wang S., Yang X. M., Tang Q. L., Wang S. H. Latent membrane protein 1 mediates the resistance of nasopharyngeal carcinoma cells to TRAIL-induced apoptosis by activation of the PI3K/Akt signaling pathway. Oncol. Rep. (2011);26:1573–1579. doi: 10.3892/or.2011.1423. [DOI] [PubMed] [Google Scholar]
- 23.Lu M., Strohecker A., Chen F., Kwan T., Bosman J., Jordan V. C., Cryns V. L. Aspirin sensitizes cancer cells to TRAIL-induced apoptosis by reducing survivin levels. Clin. Cancer Res. (2008);14:3168–3176. doi: 10.1158/1078-0432.CCR-07-4362. [DOI] [PubMed] [Google Scholar]
- 24.Mahalingam D., Szegezdi E., Keane M., de Jong S., Samali A. TRAIL receptor signalling and modulation: Are we on the right TRAIL? Cancer Treat Rev. (2009);35:280–288. doi: 10.1016/j.ctrv.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Oh B., Park S., Pak J. H., Kim I. Downregulation of Mcl-1 by daunorubicin pretreatment reverses resistance of breast cancer cells to TNF-related apoptosis-inducing ligand. Biochem. Biophys. Res. Commun. (2012);422:42–47. doi: 10.1016/j.bbrc.2012.04.093. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto K., Okamoto I., Okamoto W., Tanaka K., Takezawa K., Kuwata K., Yamaguchi H., Nishio K., Nakagawa K. Role of survivin in EGFR inhibitor-induced apoptosis in non-small cell lung cancers positive for EGFR mutations. Cancer Res. (2010);70:10402–10410. doi: 10.1158/0008-5472.CAN-10-2438. [DOI] [PubMed] [Google Scholar]
- 27.Ortiz J., Chou L. L. Calcium up-regulated survivin expression and associated osteogenesis of normal human osteoblasts. J. Biomed. Mater. Res. (2012);100:1770–1776. doi: 10.1002/jbm.a.34103. [DOI] [PubMed] [Google Scholar]
- 28.Park S. J., Park S. H., Kim J., Kim J. H., Park S. J., Hwang J. J., Jin D.H., Jeong S., Lee S.J., Kim J.C., Kim I., Cho D.H. Carnitine sensitizes TRAIL-resistant cancer cells to TRAIL-induced apoptotic cell death through the up-regulation of Bax. Biochem. Biophys. Res. Commun. (2012);428:185–190. doi: 10.1016/j.bbrc.2012.10.038. [DOI] [PubMed] [Google Scholar]
- 29.Pennati M., Colella G., Folini M., Citti L., Daidone M. G., Zaffaroni N. Ribozyme-mediated attenuation of survivin expression sensitizes human melanoma cells to cisplatin-induced apoptosis. J. Clin. Invest. (2002);109:285–286. doi: 10.1172/JCI14891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raviv Z., Zilberberg A., Cohen S., Reischer-Pelech D., Horrix C., Berger M. R., Rosin-Arbesfeld R., Flescher E. Methyl jasmonate down-regulates survivin expression and sensitizes colon carcinoma cells towards TRAIL-induced cytotoxicity. Br. J. Pharmacol. (2011);164:1433–1444. doi: 10.1111/j.1476-5381.2011.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan B. M., O'Donovan N., Duffy M. J. Survivin: a new target for anti-cancer therapy. Cancer Treat. Rev. (2009);35:553–562. doi: 10.1016/j.ctrv.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 32.Stout R. F. Jr., Parpura V. Voltage-gated calcium channel types in cultured C. elegans CEPsh glial cells. Cell Calcium. (2011);50:98–108. doi: 10.1016/j.ceca.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 33.Sung E. S., Kim A., Park J. S., Chung J., Kwon M. H., Kim Y. S. Histone deacetylase inhibitors synergistically potentiate death receptor 4-mediated apoptotic cell death of human T-cell acute lymphoblastic leukemia cells. Apoptosis. (2010);10:1256–1269. doi: 10.1007/s10495-010-0521-9. [DOI] [PubMed] [Google Scholar]
- 34.Sung E. S., Park K. J., Choi H. J., Kim C. H., Kim Y. S. The proteasome inhibitor MG132 potentiates TRAIL receptor agonist-induced apoptosis by stabilizing tBid and Bik in human head and neck squamous cell carcinoma cells. Exp. Cell Res. (2012);318:1564–1576. doi: 10.1016/j.yexcr.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Tamm I., Wang Y., Sausville E., Scudiero D. A., Vigna N., Oltersdorf T., Reed J. C. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. (1998);58:5315–5320. [PubMed] [Google Scholar]
- 36.Vader G., Kauw J. J., Medema R. H., Lens S. M. Survivin mediates targeting of the chromosomal passenger complex to the centromere and midbody. EMBO Rep. (2006);7:85–92. doi: 10.1038/sj.embor.7400562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Geelen C. M., Pennarun B., Le P.T., de Vries E. G., de Jong S. Modulation of TRAIL resistance in colon carcinoma cells: different contributions of DR4 and DR5. BMC Cancer. (2011);11:39. doi: 10.1186/1471-2407-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X., Inukai T., Akahane K., Hirose K., Kuroda I., Honna H., Goi K., Kagami K., Tauchi T., Yagita H., Sugita K. Endoplasmic reticulum stress inducers, but not imatinib, sensitize Philadelphia chromosome-positive leukemia cells to TRAIL-mediated apoptosis. Leuk. Res. (2011);35:940–949. doi: 10.1016/j.leukres.2011.03.016. [DOI] [PubMed] [Google Scholar]


