Abstract
Polyamines, putrescine, spermidine and spermine, are ubiquitous in living cells and are essential for eukaryotic cell growth. These polycations interact with negatively charged molecules such as DNA, RNA, acidic proteins and phospholipids and modulate various cellular functions including macromolecular synthesis. Dysregulation of the polyamine pathway leads to pathological conditions including cancer, inflammation, stroke, renal failure and diabetes. Increase in polyamines and polyamine synthesis enzymes is often associated with tumor growth, and urinary and plasma contents of polyamines and their metabolites have been investigated as diagnostic markers for cancers. Of these, diacetylated derivatives of spermidine and spermine are elevated in the urine of cancer patients and present potential markers for early detection. Enhanced catabolism of cellular polyamines by polyamine oxidases (PAO), spermine oxidase (SMO) or acetylpolyamine oxidase (AcPAO), increases cellular oxidative stress and generates hydrogen peroxide and a reactive toxic metabolite, acrolein, which covalently incorporates into lysine residues of cellular proteins. Levels of protein-conjuagated acrolein (PC-Acro) and polyamine oxidizing enzymes were increased in the locus of brain infarction and in plasma in a mouse model of stroke and also in the plasma of stroke patients. When the combined measurements of PC-Acro, interleukin 6 (IL-6), and C-reactive protein (CRP) were evaluated, even silent brain infarction (SBI) was detected with high sensitivity and specificity. Considering that there are no reliable biochemical markers for early stage of stroke, PC-Acro and PAOs present promising markers. Thus the polyamine metabolites in plasma or urine provide useful tools in early diagnosis of cancer and stroke.
Keywords: Polyamine metabolites, Acrolein, Diacetylspemine, Diagnostic marker, Cancer, Stroke
INTRODUCTION
The polyamines, putrescine [NH2(CH2)4NH2], spermidine [NH2(CH2)4NH(CH2)3NH2] and spermine [NH2(CH2)3NH(CH2)4 NH(CH2)3NH2], are organic polycations present in all eukaryotes and are essential for cell proliferation (Tabor and Tabor, 1984; Igarashi and Kashiwagi, 2010; Pegg and Casero, 2011). Since their primary and secondary amino groups are protonated at physiological pH, these polyamines interact electrostatically with negatively charged molecules such as DNA, RNA, proteins and phospholipids (Bachrach, 2005) and they have been proposed to regulate cellular activities at transcriptional, translational and post-translational levels. The polyamines differ from inorganic cations like Mg2+ or Ca2+ in that their positive charges are spaced at defined distances by flexible methylene chains that can participate in hydrophobic interactions. Thus polyamines engage in stronger and more specific interactions with nucleic acids and acidic macromolecules than inorganic cations do (Igarashi and Kashiwagi, 2000; Thomas and Thomas, 2001; Bachrach, 2005; Igarashi and Kashiwagi, 2010). Although net cellular concentrations of polyamines are generally at millimolar levels in eukaryotic cells (Igarashi and Kashiwagi, 2000), most intracellular polyamines are compartmentalized and/or bound to nucleic acids and other negatively charged molecules. Hence, the concentrations of free polyamines are much lower than the total cellular concentration.
Normally, polyamine homeostasis is elaborately maintained by intricate multiple feedback mechanisms at the levels of biosynthesis, catabolism, uptake and efflux (Pegg, 2009; Igarashi and Kashiwagi, 2010; Pegg and Casero, 2011). Over-accumulation of polyamines has been associated with cell transformation or apoptosis, whereas their reduction/depletion leads to inhibition of cell growth, migration, and embryonic development. Enhanced levels of polyamines and polyamine biosynthetic enzymes, ornithine decarboxylase (ODC) and S-adenosylmethionine decarboxylase (SAMDC) are often associated with hyper-proliferation and cancer. NIH3T3 cells over-expressing ODC are tumorigenic in nude mice (Auvinen et al., 1992) and increased expression of ODC enhances tumor development in initiated premalignant epidermal cells (Clifford et al., 1995). Activation of the polyamine catabolic pathway causes an increased oxidative stress and also may contribute to aging and pathological conditions resulting from cellular damages (Cerrada-Gimenez et al., 2011).
POLYAMINE METABOLISM IN MAMMALS
Cellular polyamines can be interconverted by cycling of biosynthesis and catabolism and their content is regulated by a complex mechanism in mammalian cells (Fig. 1) (Igarashi and Kashiwagi, 2010; Pegg and Casero, 2011). The main sources for polyamines in mammals are cellular synthesis, food intake and microbial synthesis in the gut. The diamine putrescine is synthesized from ornithine which is formed from arginine by arginase. The rate limiting enzyme in polyamine biosynthesis is ODC. The polyamine spermidine is synthesized from putrescine by spermidine synthase, by transfer of an aminopropyl moiety from decarboxylated S-adenosylmethionine (DCSAM), to an amino group of putrescine. Spermine is formed by addition of an aminopropyl moiety at the aminobutyl moiety of spermidine by spermine synthase. Spermine and spermidine are converted back to putrescine by polyamine oxidases, spermine oxidase (SMO) or acetylpolyamine oxidase (AcPAO), a peroxisomal enzyme. Spermine oxidase oxidatively degrades spermine to spermidine. Alternatively, spermine and spermidine can be converted to lower polyamines by way of two consecutive enzymatic reactions. First, spermine or spermidine is acetylated at the aminopropyl end by spermidine/spermine N1-acetyltransferase 1 (SSAT1) (Pegg, 2008), a key regulatory enzyme, and the acetylated spermine and spermidine subsequently undergo oxidative cleavage between C3 and N4 to generate a lower polyamine. Alternatively, the monoacetyl-spermine can be acetylated at the other aminopropyl end to form diacetyl-spermine (Fig. 2). The mono- and diacetyl- polyamines can be excreted from cells and are detected in cell culture medium, in animal body fluids, urine and plasma.
Fig. 1. Polyamine metabolism and regulation in mammalian cells. The biosynthetic enzymes are indicated by yellow ovals, catabolic enzymes in aquablue ovals, and hypusine modification enzymes in green ovals. Inhibition is indicated by broken red lines and stimulation by solid blue lines. ODC: ornithine decarboxylase; SAMDC: S-adenosylmethionine decarboxylase; SPDS: spermidine synthase; SPMS: spermine synthase, SSAT1: spermidine/spermine N1-acetyltransferase; AcPAO: acetylpolyamine oxidase; SMO: spermine oxidase; eIF5A: eukaryotic initiation factor 5A; eIF5A (Lys): eIF5A lysine form; eIF5A (Dhp): eIF5A deoxyhypusine form; eIF5A (Hpu): eIF5A hypusine form; DHS: deoxyhypusine synthase; DOHH: deoxyhypusine hydroxylase; Az: antizyme; AzI: antizyme inhibitor.
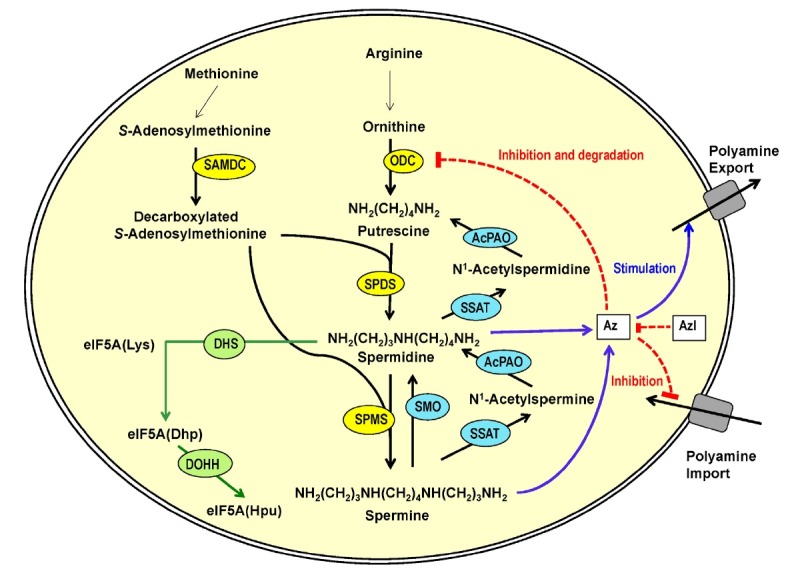
Fig. 2. Generation of acrolein and protein-conjugated acrolein by polyamine oxidase reactions. Acrolein generated by SMO, AcPAO or BSAO reacts with ε-amino group of lysine residues of proteins to form FDP-lysine containing adducts. BSAO: bovine serum amine oxidase; FDP-Lys: N-(3-formyl-3,4-dehydropiperidino)lysine.
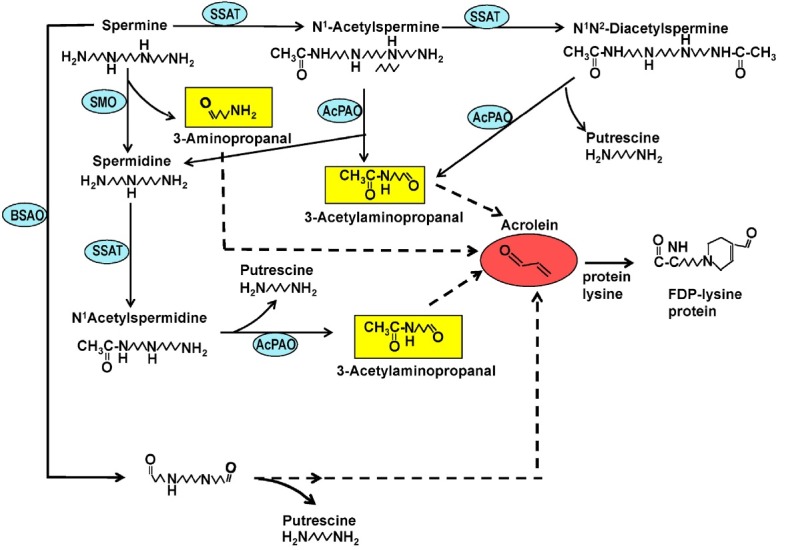
Polyamine oxidase (SMO or AcPAO) mediated reactions cause oxidative stress to cells and tissues. In addition to a lower polyamine, SMO generates H2O2 and 3-aminopropanal and AcPAO generates H2O2 and 3-acetylaminopropanal (Fig. 2). These aldehydes are unstable and spontaneously decompose to acrolein after deamination. Acrolein can be also generated extracellularly from spermine or spermidine in cell culture medium containing bovine serum amine oxidase (BSAO) -like activity that oxidizes at the terminal amino group of polyamines (Sharmin et al., 2001). Acrolein, an unsaturated reactive aldehyde, is highly toxic and readily reacts with lysine residues of proteins to form Nε-(3-formyl-3,4-dehydropiperidino)lysine (FDP-lysine) (Uchida et al., 1998).
In addition to the polyamine interconversion pathway, a small amount of cellular spermidine is covalently incorporated into a specific cellular protein eIF5A to form an unusual amino acid hypusine [Nε-(4-amino-2-hydroxybutyl)lysine] (Fig.1) (Park, 2006; Park et al., 2010). The first step enzyme, deoxyhypusine synthase (DHS) (Joe et al., 1995) catalyzes the NAD-dependent cleavage of spermidine between N4 and C5 and the transfer of aminobutyl moiety of spermidine to the ε-amino-group of a specific lysine residue of the eIF5A precursor, eIF5A(Lys), to form a deoxyhypusine-containing intermediate, eIF5A(Dhp). This intermediate is subsequently hydroxylated by deoxyhypusine hydroxylase (DOHH) (Park et al., 2006) to form hypusine and the biologically active, mature eIF5A, eIF5A(Hpu).
The polyamine biosynthetic enzymes and catabolic enzymes ODC, SAMDC and SSAT1 are highly regulated by polyamines and by various stimuli such as growth factors, hormones, 12-O-tetradecanoylphorbol-1 3-acetate (TPA), etc (Pegg, 2009). In the presence of high levels of spermidine and spermine in cells or medium, ODC and SAMDC are downregulated while SSAT1 is upregulated. Reciprocally, when cellular polyamine levels are reduced, ODC and SAMDC are upregulated and SSAT1 is downregulated. The regulation occurs at the transcriptional, translational, and posttranslational levels and specific mechanisms of regulation of these enzymes by polyamines have been extensively investigated (Pegg, 2009; Igarashi and Kashiwagi, 2010). When the cellular polyamine level increases, ODC is downregulated by induction of antizyme (Az) translation by frame shifting (Ivanov et al., 1998; Coffino, 2001). The antizyme forms a complex with the ODC monomer to inactivate the enzyme and directs it to degradation by the 26S proteasome. In addition, antizyme inhibits polyamine uptake and stimulates its secretion. Further complexity of ODC regulation is introduced by an antizyme inhibitor (AzI) (Fujita et al., 1982), which shares high sequence similarity to ODC, but is devoid of the enzyme activity.
PROPOSED FUNCTIONS OF POLYAMINES
From gene disruption studies in yeast and mice (Jänne et al., 2004), it is well established that polyamines are essential for eukaryotic cell growth and mammalian development (Pegg, 2009). As mentioned above (Fig. 1), one well defined function of polyamine is the requirement for spermidine for hypusine modification in eIF5A (Park, 2006; Park et al., 2010). Since eIF5A and its deoxyhypusine/hypusine modification are essential for eukaryotic cell growth and mouse development (Nishimura et al., 2012), the eIF5A modification defines one specific function of polyamines. Due to a narrow specificity of DHS for spermidine, only natural polyamine or an analog with closely related structure to spermidine can support long-term growth of mammalian cells (Byers et al., 1994; Hyvönen et al., 2007). In addition to eIF5A modification, polycationic polyamines are also essential for proliferation of mammalian cells (Nishimura et al., 2005). These organic polycations fulfill unique cellular functions that cannot be substituted by inorganic cations, such as Mg2+ in macromolecular synthesis or in cell growth (Oredsson et al., 1984). Numerous studies have been reported on the effects of polyamines on DNA structure (Thomas and Thomas, 2001), chromatin condensation (Matthews, 1993), RNA structure (Igarashi and Kashiwagi, 2010) and protein synthesis in vitro (Ogasawara et al., 1989). However, the molecular mechanism of polycationic polyamine actions in vivo has remained obscure for decades.
Insights on the cellular function of polyamines in protein synthesis have been offered by their cellular distribution, estimated from the individual dissociation constant for each of polyamine-partner binding under the physiological concentrations of K+ and Mg2+ of E. coli and mammalian cells (Igarashi and Kashiwagi, 2010). In mammalian cells and tissues, a majority of cellular spermidine and spermine (57-85%) appear to be bound to RNA, whereas free polyamines and the fractions bound to other components (DNA, ATP or phospholipids) are relatively small. Furthermore, the crystal structure of yeast tRNAPhe revealed two molecules of spermine (Quigley et al., 1978), suggesting specific interactions between polyamines and RNA. In in vitro translation assays, polyamines not only lowered the Mg2+ requirement, but also stimulated protein synthesis beyond the maximum level achieved by high Mg2+ alone (Ogasawara et al., 1989). In addition, polyamines were reported to exert several other effects on translation, including stimulation of the assembly of the 30S ribosome, rat liver Ile-tRNA formation, an increase in the fidelity of protein synthesis and frame shifting (Igarashi and Kashiwagi, 2010).
More recently, further evidence for a primary function of polyamines in translation was obtained in mammalian cells over-expressing SSAT1. Rapid arrest in protein synthesis and cell growth occurred in these cells depleted of spermidine and spermine by SSAT1 over-expression, whereas there was little or no inhibition of synthesis of DNA and RNA (Mandal et al., 2013). These findings are consistent with specific interactions of polyamines with RNA (Igarashi and Kashiwagi, 2010), and their stimulatory effects on protein synthesis in vitro (Ogasawara et al., 1989), and corroborate an important function of the polyamines spermidine and spermine in translation. Thus, polyamines appear to contribute to mammalian cell growth mainly through their participation in eIF5A hypusine modification (Park, 2006) and by stimulating translation initiation by enhancing ribosome assembly and stability (Ogasawara et al., 1989). Specific genes influenced by cellular polyamines at translation include Az, SAMDC, and SSAT1 and their regulation involves different mechanisms, including ribosome frame shifting (for Az) (Ivanov et al., 1998), a small upstream open reading frame (for SAMDC) (Mize et al., 1998), and polyamine response element in the promoter region (for SSAT1) (Wang et al., 1998).
In addition, polyamines have been proposed to play several other important roles in cellular regulation and animal physiology (Pegg, 2009; Minois et al., 2011). Due to their interaction with DNA structure (Thomas and Thomas, 2001), their effects on chromatin condensation (Matthews, 1993) and enzymes such as HAT and HDAC (Hobbs et al., 2002) and protein kinases/phosphatases (Lawson et al., 2005), polyamines may also regulate transcription and gene expression, directly or indirectly. The two oncogenes, c-Myc and c-Jun were reported to be transcriptionally controlled by polyamines in cells treated with DFMO (Patel and Wang, 1997). The polyamine effects on global transcription have been investigated by microarray analyses in mammalian cells partially depleted of polyamine by treatment with DFMO (Landau et al., 2012).
Polyamines also bind and regulate glutamate receptor ion channels (Dingledine et al., 1999), inwardly rectifying potassium channels (Kir) (Stanfield and Sutcliffe, 2003), and other channels that affect intracellular calcium signaling or Na+ transport (Fleidervish et al., 2008). Although spermine is not required for mammalian cell growth, spermine synthase defective mice (Gy mice) are totally deaf (Wang et al., 2009), suggesting the importance of homeostasis of spermidine and spermine in animal development and physiology.
POLYAMINES, AGING AND HUMAN DISEASES
Since polyamines are essential for cell growth and viability (Pendeville et al., 2001; Nishimura et al., 2002), polyamine levels change with aging, and under several disease conditions (Minois et al., 2011). Decrease in polyamines with aging was evaluated with mouse (Nishimura et al., 2006), and the decrease in polyamine content was observed in almost all tissues, and it was especially notable in skin, heart and muscle. The decrease was not significant in brain and pancreas. It also became clear that polyamine-rich food decreases mortality in aged mice (Soda et al., 2009).
Deregulation of the polyamine pathway enzymes has been implicated in various pathological conditions, including cancer (Gerner and Meyskens, 2004; Casero and Marton, 2007), inflammation (Babbar et al., 2007), stroke (Tomitori et al., 2005; Yoshida et al., 2010; Igarashi and Kashiwagi, 2011b; Saiki et al., 2011), renal failure (Igarashi et al., 2006) and diabetes (Kramer et al., 2008).
As for cancer, Russell and Snyder first reported high levels of ODC activity in regenerating rat liver and in several human cancers (Russell and Snyder, 1968). Since then, effects of inhibitors of polyamine biosynthesis; i.e., α-difluoromethylornithine (DFMO) (Mamont et al., 1978), an inhibitor of ODC, and methylglyoxal bis(guanylhaydrazone) (MGBG) (Williams-Ashman and Schenone, 1972), an inhibitor of SAMDC, on cancer growth were extensively studied with mice bearing various cancers. The combination of DFMO-MGBG has been shown to be more effective therapeutically than either drug alone in a number of in vivo model systems, which include Ehrlich ascites carcinoma (Seppänen et al., 1983), murine L1210 and P388 leukemia (Kramer et al., 1985; Nakaike et al., 1988), and murine renal adenocarcinoma (Herr et al., 1984). Furthermore, the stronger antitumor effects were exerted when mice were fed a polyamine-deficient diet (Nakaike et al., 1988), suggesting that dietary control enhances the effect of cancer chemotherapy. Yet, an anticancer strategy in human clinical trials, use of inhibitors of polyamine biosynthesis has been generally ineffective thus far (Gerner and Meyskens, 2004). However, the use of DFMO together with non-steroidal anti-inflammatory drugs (NSAIDS) such as sulindac, an inhibitor of cyclooxygenase 2 (COX2), appears to be effective in the prevention of recurrence of colon cancer (Gerner and Meyskens, 2004). In clinical trial of the combination of DFMO and sulindac, the 3-year treatment resulted in a 70% reduction of recurrence of advanced and/or multiple adenomas, that are closely associated with the development of colon cancers in humans (Gerner and Meyskens, 2009).
The shortcoming of DFMO treatment in cancer chemotherapy is probably due to the fact that DFMO causes depletion of putrescine and spermidine only, but not of cellular spermine and exhibits cytostatic/noncytotoxic effects. A number of polyamine analogs/derivatives that mimic natural polyamines in causing downregulation of polyamine biosynthesis, and induction of polyamine catabolism were developed (Casero and Marton, 2007). While these analogs cause a rapid depletion of cellular polyamines, they fail to perform critical functions of natural polyamines, thereby leading to growth arrest and cell death (Oredsson et al., 2007). Alternatively, another strategy to deplete both spermidine and spermine using SSAT1 adenoviral gene therapy, may be explored in anticancer therapy in the future.
An inherited human disease that is associated with altered polyamine metabolism is Snyder-Robinson syndrome (SRS), an X-linked mental-retardation and developmental disease. This is caused by an alteration in the SpmS gene that encodes spermine synthase (Becerra-Solano et al., 2009; Pegg, 2009). Reduction of spermine in the SRS patient brain may causes a neurological disorder by influencing the activity of neurotransmitter receptors and ion channels including NMDA receptors (Dingledine et al., 1999; Jin et al., 2008), AMPA receptors (Shin et al., 2005), K+ channels (Stanfield and Sutcliffe, 2003) and Ca2+ channels (Gomez and Hellstrand, 1995). Alteration in the regulation of another enzyme, SSAT over-expression may be linked to a human disease, Keratosis follicularis spinulosa decalvans (KFSD)- a rare X-linked disease. A patient with this disease has a gene duplication that includes the region that encodes SSAT (Gimelli et al., 2002). Low SSAT expression has been observed in psychiatric patients prone to suicide (Sequeira et al., 2006). A reduced activity and spatial learning impairment observed in SSAT transgenic mice (Kaasinen et al., 2004) further suggest a role for polyamines in behavioral changes.
Altered polyamine metabolism may contribute to an increase in oxidative stress and tissue damage in chronic renal failure and stroke (Igarashi and Kashiwagi, 2011a; 2011b). During metabolism of spermine and spermidine released from ribosomes (Watanabe et al., 1991), two toxic compounds, i.e. acrolein (CH2=CH-CHO) and hydrogen peroxide (H2O2) are produced. Of the two compounds, it was determined that acrolein was more toxic than H2O2 (Sharmin et al., 2001). Actually, the levels of protein-conjugated acrolein (PC-Acro) in plasma were well correlated with the seriousness of chronic renal failure (Igarashi et al., 2006) and brain stroke (Tomitori et al., 2005). A close correlation between brain infarction and PC-Acro was confirmed using a photochemically induced thrombosis model in mice (Saiki et al., 2009).
URINARY DIACETYL POLYAMINE DERIVATIVES AS MARKERS FOR HUMAN CANCERS
Since polyamines are well correlated with growth of cancer cells, initially urinary polyamine levels were measured to see if polyamines, putrescine, spermidine and spermine would be useful markers in diagnosis of various human cancers (Russell et al., 1971). Although the amount of polyamines excreted in urine was generally elevated in urine of cancer patients and appeared to correlate with progression of the disease in the initial report, follow-up studies did not support urinary polyamines as consistent indicators of malignant diseases.
When spermine and spermidine are accumulated in excess amounts in cells, they are acetylated, and then excreted into urine. Therefore, it was tested whether diacetylspermine (DiAcSpm) and diacetylspermidine (DiAcSpd) in urine are reliable biochemical markers for cancer using an enzyme-linked immunosorbent assay (ELISA) systems. A marked increase in urinary DiAcSpm was associated with all types of human cancers examined, including colorectal, prostate, testicular, renal, pancreatic, hepatocellular carcinoma, breast, lung and brain cancers (Kawakita and Hiramatsu, 2006). Sensitivity of the detection of DiAcSpm in urine was compared with that of commonly used biomarkers, serum carcinoembryonic antigen (CEA), cancer antigen19-9 (CA19-9) and cancer antigen 15-3 (CA15-3) (Hiramatsu et al., 2005; Kawakita and Hiramatsu, 2006). The sensitivity of urinary DiAcSpm for colon cancer patients was 75.8%, which was markedly higher than the sensitivity of serum CEA (39.5%) and CA19-9 (14.1%). DiAcSpm was elevated in 60% of tumor-node-metastasis cancer stage 0+1 patients, whereas only 10% and 5% of these patients were CEA and CA19-9 positive, respectively. The sensitivity of DiAcSpm for breast cancer was 60.2%, while that of CEA and CA15-3 was 37.3% and 37.3%, respectively. DiAcSpm was elevated in 28% of tumor-node-metastasis stage 1+2 patients, whereas only 3% and 0% of these patients were CEA and CA15-3 positive, respectively. These observations indicate that urinary DiAcSpm is a more sensitive marker than CEA, CA19-9 and CA15-3 in various human cancers and that it can efficiently detect colorectal and breast cancers at early stages. DiAcSpm may also be useful as a follow-up marker that is efficient in detection of recurrence and sensitive to changes in the clinical condition of patients.
The molecular basis for the increased DiAcSpm in urine of cancer patients is not clear. The rise in the acetylated polyamines in urine or plasma may result from increased cellular polyamines, increased SSAT activity, a decrease in oxidative degradation by AcPAO, and increased excretion of actylated polyamines from cells or lysis of necrotic tumor cells. In breast tumors, it was found that AcPAO was decreased while SSAT increased (Wallace et al., 2000). Thus the levels of both enzymes may contribute to an increase in acetylated polyamines excreted in urine.
PC-ACRO AS MARKERS FOR STROKE AND RENAL FAILURE
Oxidative stress can lead to various disorders and is thought to be caused by two types of compounds – first, reactive oxygen species (ROS) such as the superoxide anion radical (O2-∙), H2O2 and the hydroxyl radical (∙OH) (Giorgio et al., 2007), and second, an unsaturated aldehyde such as acrolein (Stevens and Maier, 2008). Addition of SPD and SPM to culture medium containing ruminant serum causes inhibition of cell proliferation (Higgins et al., 1969; Agostinelli et al., 2010). This effect is caused by oxidation products of the polyamines by ruminant amine oxidase, H2O2 and acrolein (Fig. 2). In a cell culture system, complete inhibition of cell growth was accomplished with 10 μM acrolein, 100 μM H2O2 and 20 μM hydroxylradical produced by 20 μM H2O2 and 1 mM Vitamin C (Yoshida et al., 2009), suggesting acrolein as the major toxic agent. These findings led us to test whether acrolein or protein-conjugated acrolein (PC-Acro) is a good biochemical marker for stroke and chronic renal failure.
At present, there is no reliable biochemical marker for diagnosis of the early stage of stroke. Thus, the activities of spermine oxidase (SMO) and acetylpolyamine oxidase (AcPAO) were measured along with the level of PC-Acro in plasma of patients with stroke (Tomitori et al., 2005). PC-Acro was metowardasured by ELISA using anti-FDP-lysine antibody (Uchida et al., 1998). The median levels of PC-Acro and total polyamine oxidases (SMO and AcPAO) were significantly higher in the plasma of patients with stroke. The median levels of PC-Acro and total polyamine oxidase activity (PAO) in plasma increased from 14.4 to 21.3 nmol/ml plasma and from 4.5 to 8.0 nmol SPD produced from SPM by PAO/ml plasma, respectively. It was then examined whether the increases in PC-Acro and PAO are correlated with the severity of stroke (size of the infarct). Because the maximal increase in PAO precedes that in PC-Acro, the multiplied value of PAO by PC-Acro was compared with the size of the infarct. Statistical significance between stroke patients and no-stroke subjects became greater in the multiplied value (p=9.3×10−7) than PAO (p=7.0×10−5) or PC-Acro (p=6.6×10−6). The size of the infarct was nearly parallel with the value of PAO activity multiplied by PC-Acro amount (Tomitori et al., 2005).
There are reports that silent brain infarction (SBI) increases the risk of subsequent stroke (Vermeer et al., 2007), dementia (Vermeer et al., 2007), and mild cognitive impairment (Lopez et al., 2003). Furthermore, it has been reported that carotid atherosclerosis (CA) is a risk factor for stroke and SBI (Inoue et al., 2007), and that SBI and white matter hyperintensity (WMH) increase the risk for stroke and mortality (Bokura et al., 2006). Thus, it was tested how PC-Acro is correlated with SBI, WMH and CA by collecting blood from 790 elderly healthy volunteers. Since the levels of CRP and IL-6 are reported to increase in the serum of apparently healthy individuals with SBI (Hoshi et al., 2005), the levels of CRP and IL-6 were measured along with PC-Acro (Yoshida et al., 2010). SBI (affected areas ≥3 mm diameter) and WMH were estimated by MRI, CA by carotid ultrasound examination, and CRP and IL-6 in plasma by ELISA. PC-Acro, IL-6 and CRP were significantly higher in SBI and CA compared with the control. PC-Acro was most strongly correlated with CA, and IL-6 and CRP with SBI (Yoshida et al., 2010). Thus, CA is likely the primary risk factor related to stroke in this analysis.
It was next determined whether SBI, CA and WMH could be detected by altered levels of PC-Acro, IL-6 and CRP using a receiver operating characteristic (ROC) curve – a commonly used technique for assessing diagnostic and predictive accuracy in disease management (Linden, 2006). Since age was an important factor among various markers evaluated for the detection of SBI, CA and WMH, these three values were analyzed together with age as a factor. The median RRV (relative risk value) for SBI with CA (93 subjects), SBI (214 subjects), CA (263 subjects), WMH with CA (90 subjects), WMH (245 subjects) and control (260 subjects) was 0.90, 0.80, 0.76, 0.65, 0.46 and 0.14, respectively. Although PC-Acro is well correlated with brain infarction, measurement of IL-6 and CRP along with PC-Acro was necessary to increase sensitivity and specificity to detect SBI.
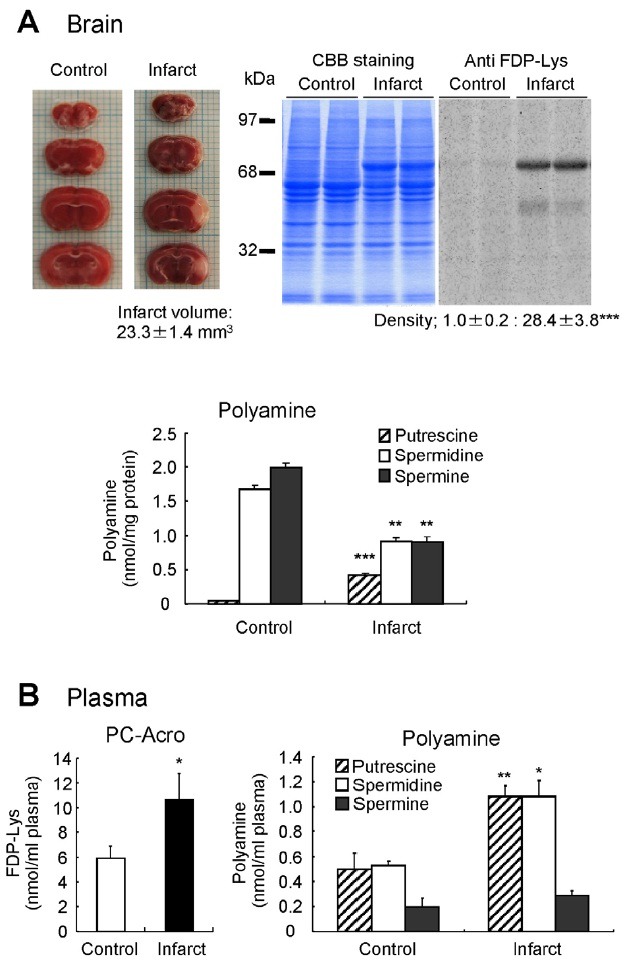
The role of PC-Acro and polyamine oxidases was also evaluated in an animal model of stroke by using photochemically induced thrombosis (PIT) model mice (Fig. 3) (Saiki et al., 2009). In this model, it was determined whether acrolein is produced from polyamines at the locus of infarction. The volume of infarcted tissue was determined by staining 2-mm- thick coronal slices with triphenyltetrazolium, which stains the viable brain tissue red whereas infarcted tissue remains unstained. The average volume of infarction at 24 h of PIT model mice was 23 mm3 (Fig. 3A). The PC-Acro determined by westem blotting using anti FDP-Lys antibody was 28 fold higher in infarcted tissue than that in the normal tissue and the major protein (68 kDa) containing acrolein was identified as albumin using an albumin antibody. The levels of spermidine and spermine were reduced whereas putrescine was increased in infarcted tissues compared to normal tissues, suggesting activated polyamine catabolism. Consistent with this finding, both SMO and AcPAO activities were higher at the locus of infarction. The levels of PC-Acro and all three polyamines were significantly higher in the plasma of PIT mice compared to control mice (Fig. 3B). These results provide strong evidence that polyamine oxidation and production of PC-Acro play important role in PIT model mice of stroke. Furthermore, brain infarction correlated more closely with PC-Acro than with H2O2 (Saiki et al., 2011).
Fig. 3. Correlation between brain infarction and PC-Acro in PIT model mice. (A) Infarction volume at 24 h after the induction of infarction, the level of PC-Acro estimated by Western blotting using anti-FDP-Lys antibody, and polyamine levels at the locus of brain infarction and at the corresponding locus in normal mice are shown. (B) Increase in PC-Acro and polyamines in plasma of PIT model mice with brain infarction is shown. *p<0.05, **p<0.01, ***p<0.001. Data were adapted from Saiki et al. (2009).
In the case of chronic renal failure, a very strong correlation with PC-Acro was observed (Igarashi and Kashiwagi, 2011a). Severity of chronic renal failure is classified by the level of creatinine, i.e. in normal, <1.2 mg/100 ml plasma; moderate, <8 mg/100 ml plasma and severe, ≥8 mg/100 ml plasma. PC-Acro level of normal (n=19), moderate (n=13) and severe (n=19) cases was 31.2, 138 and 170 nmol/ml plasma, respectively. The results indicate that the level of PC-Acro in plasma is well correlated with the severity of chronic renal failure. Furthermore, the level of PC-Acro returned to normal after patients (n=7) had undergone hemodialysis. The results indicate that together with creatinine, PC-Acro is a good biochemical marker for chronic renal failure.
CONCLUDING REMARKS
Cellular polyamine homeostasis is regulated by intricate mechanisms to maintain normal physiology of mammalian cells and organisms. Dysregulation of polyamine metabolic pathway is associated with various pathological conditions, including cancer, inflammation, atherosclerosis, stroke, renal failure and diabetes. Thus the polyamine pathway has been extensively explored as targets of cancer chemotherapy and chemoprevention and holds potential for intervention in other human diseases. In this review, we focused on the potential application and utilities of polyamine metabolites as diagnostic markers for various human cancers, silent brain infarction, stroke and renal failure, where early clinical diagnostic markers are not known. Recent data support the utility of urinary diacetyl spermine (DiAcSpm), which is markedly increased in urine of patients of all types of human cancers examined. DiAcSpm which can be accurately determined by a simple ELISA assay, is an excellent diagnostic and prognostic indicator of various human cancers, with superior scores than known diagnostic markers, CEA, CA19-9 and CA15-3. Activated polyamine catabolism may increase oxidative stress and tissue damage, contributing to renal failure and stroke. Acrolein derived from polyamine oxidation reacts with proteins to form acrolein-conjugated proteins (PC-Acro), which can be readily measured by a simple ELISA assay. The increase in the plasma levels of PC-Acro, and polyamine oxidases, and their multiplied value exhibit excellent correlation with the severity of stroke and detects even SBI (silent brain infarction) with high sensitivity. Thus, these polyamine metabolites provide highly sensitive, cost-effective means in the early diagnosis of human diseases, cancer, stroke and renal failure.
Acknowledgments
The research was supported in part by the Intramural Research Program of National Institute of Dental and Craniofacial Research (NIDCR), NIH. We thank Edith C. Wolff (NIDCR, NIH) for helpful suggestions on the manuscript.
References
- 1.Agostinelli E., Tempera G., Viceconte N., Saccoccio S., Battaglia V., Grancara S., Toninello A., Stevanato R. Potential anticancer application of polyamine oxidation products formed by amine oxidase: a new therapeutic approach. Amino Acids. (2010);38:353–368. doi: 10.1007/s00726-009-0431-8. [DOI] [PubMed] [Google Scholar]
- 2.Auvinen M., Paasinen A., Andersson L. C., Holtta E. Ornithine decarboxylase activity is critical for cell transformation. Nature. (1992);360:355–358. doi: 10.1038/360355a0. [DOI] [PubMed] [Google Scholar]
- 3.Babbar N., Murray-Stewart T., Casero R. A., Jr. Inflammation and polyamine catabolism: the good, the bad and the ugly. Biochem. Soc. Trans. (2007);35:300–304. doi: 10.1042/BST0350300. [DOI] [PubMed] [Google Scholar]
- 4.Bachrach U. Naturally occurring polyamines: interaction with macromolecules. Curr. Protein Pept. Sci. (2005);6:559–566. doi: 10.2174/138920305774933240. [DOI] [PubMed] [Google Scholar]
- 5.Becerra-Solano L. E., Butler J., Castaneda-Cisneros G., McCloskey D. E., Wang X., Pegg A. E., Schwartz C. E., Sanchez-Corona J., Garcia-Ortiz J. E. A missense mutation, p.V132G, in the X-linked spermine synthase gene (SMS) causes Snyder-Robinson syndrome. Am. J. Med. Genet. A. (2009);149A:328–335. doi: 10.1002/ajmg.a.32641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bokura H., Kobayashi S., Yamaguchi S., Iijima K., Nagai A., Toyoda G., Oguro H., Takahashi K. Silent brain infarction and subcortical white matter lesions increase the risk of stroke and mortality: a prospective cohort study. J. Stroke Cerebrovasc. Dis. (2006);15:57–63. doi: 10.1016/j.jstrokecerebrovasdis.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Byers T. L., Lakanen J. R., Coward J. K., Pegg A. E. The role of hypusine depletion in cytostasis induced by S-adenosyl-L-methionine decarboxylase inhibition: new evidence provided by 1-methylspermidine and 1,12-dimethylspermine. Biochem. J. (1994);303:363–368. doi: 10.1042/bj3030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casero R. A., Jr., Marton L. J. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug Discov. (2007);6:373–390. doi: 10.1038/nrd2243. [DOI] [PubMed] [Google Scholar]
- 9.Cerrada-Gimenez M., Pietila M., Loimas S., Pirinen E., Hyvonen M. T., Keinanen T. A., Janne J., Alhonen L. Continuous oxidative stress due to activation of polyamine catabolism accelerates aging and protects against hepatotoxic insults. Transgenic Res. (2011);20:387–396. doi: 10.1007/s11248-010-9422-5. [DOI] [PubMed] [Google Scholar]
- 10.Clifford A., Morgan D., Yuspa S. H., Soler A. P., Gilmour S. Role of ornithine decarboxylase in epidermal tumorigenesis. Cancer Res. (1995);55:1680–1686. [PubMed] [Google Scholar]
- 11.Coffino P. Regulation of cellular polyamines by antizyme. Nat. Rev. Mol. Cell Biol. (2001);2:188–194. doi: 10.1038/35056508. [DOI] [PubMed] [Google Scholar]
- 12.Dingledine R., Borges K., Bowie D., Traynelis S. F. The glutamate receptor ion channels. Pharmacol. Rev. (1999);51:7–61. [PubMed] [Google Scholar]
- 13.Fleidervish I. A., Libman L., Katz E., Gutnick M. J. Endogenous polyamines regulate cortical neuronal excitability by blocking voltage-gated Na+ channels. Proc. Natl. Acad. Sci. USA. (2008);105:18994–18999. doi: 10.1073/pnas.0803464105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujita K., Murakami Y., Hayashi S. A macromolecular inhibitor of the antizyme to ornithine decarboxylase. Biochem. J. (1982);204:647–652. doi: 10.1042/bj2040647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerner E. W., Meyskens F. L. Jr. Polyamines and cancer: old molecules, new understanding. Nat. Rev. Cancer. (2004);4:781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 16.Gerner E. W., Meyskens F. L. Jr. Combination chemoprevention for colon cancer targeting polyamine synthesis and inflammation. Clin. Cancer Res. (2009);15:758–761. doi: 10.1158/1078-0432.CCR-08-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gimelli G., Giglio S., Zuffardi O., Alhonen L., Suppola S., Cusano R., Lo Nigro C., Gatti R., Ravazzolo R., Seri M. Gene dosage of the spermidine/spermine N(1)-acetyltransferase ( SSAT) gene with putrescine accumulation in a patient with a Xp21.1p22.12 duplication and keratosis follicularis spinulosa decalvans (KFSD). Hum. Genet. (2002);111:235–241. doi: 10.1007/s00439-002-0791-6. [DOI] [PubMed] [Google Scholar]
- 18.Giorgio M., Trinei M., Migliaccio E., Pelicci P. G. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. (2007);8:722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 19.Gomez M., Hellstrand P. Effects of polyamines on voltage-activated calcium channels in guinea-pig intestinal smooth muscle. Pflugers Arch. (1995);430:501–507. doi: 10.1007/BF00373886. [DOI] [PubMed] [Google Scholar]
- 20.Herr H. W., Kleinert E. L., Conti P. S., Burchenal J. H., Whitmore W.F., Jr. Effects of alpha-difluoromethylornithine and methylglyoxal bis(guanylhydrazone) on the growth of experimental renal adenocarcinoma in mice. Cancer Res. (1984);44:4382–4385. [PubMed] [Google Scholar]
- 21.Higgins M. L., Tillman M. C., Rupp J. P., Leach F. R. The effect of polyamines on cell culture cells. J. Cell. Physiol. (1969);74:149–154. doi: 10.1002/jcp.1040740206. [DOI] [PubMed] [Google Scholar]
- 22.Hiramatsu K., Takahashi K., Yamaguchi T., Matsumoto H., Miyamoto H., Tanaka S., Tanaka C., Tamamori Y., Imajo M., Kawaguchi M., et al. N(1),N(12)-Diacetylspermine as a sensitive and specific novel marker for early- and late-stage colorectal and breast cancers. Clin. Cancer Res. (2005);11:2986–2990. doi: 10.1158/1078-0432.CCR-04-2275. [DOI] [PubMed] [Google Scholar]
- 23.Hobbs C. A., Paul B. A., Gilmour S. K. Deregulation of polyamine biosynthesis alters intrinsic histone acetyltransferase and deacetylase activities in murine skin and tumors. Cancer Res. (2002);62:67–74. [PubMed] [Google Scholar]
- 24.Hoshi T., Kitagawa K., Yamagami H., Furukado S., Hougaku H., Hori M. Relations of serum high-sensitivity C-reactive protein and interleukin-6 levels with silent brain infarction. Stroke. (2005);36:768–772. doi: 10.1161/01.STR.0000158915.28329.51. [DOI] [PubMed] [Google Scholar]
- 25.Hyvönen M. T., Keinanen T. A., Cerrada-Gimenez M., Sinervirta R., Grigorenko N., Khomutov A. R., Vepsalainen J., Alhonen L., Janne J. Role of hypusinated eukaryotic translation initiation factor 5A in polyamine depletion-induced cytostasis. J. Biol. Chem. (2007);282:34700–34706. doi: 10.1074/jbc.M704282200. [DOI] [PubMed] [Google Scholar]
- 26.Igarashi K., Kashiwagi K. Polyamines: mysterious modulators of cellular functions. Biochem. Biophys. Res. Commun. (2000);271:559–564. doi: 10.1006/bbrc.2000.2601. [DOI] [PubMed] [Google Scholar]
- 27.Igarashi K., Kashiwagi K. Modulation of cellular function by polyamines. Int. J. Biochem. Cell Biol. (2010);42:39–51. doi: 10.1016/j.biocel.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Igarashi K., Kashiwagi K. Use of polyamine metabolites as markers for stroke and renal failure. Methods Mol. Biol. (2011a);720:395–408. doi: 10.1007/978-1-61779-034-8_25. [DOI] [PubMed] [Google Scholar]
- 29.Igarashi K., Kashiwagi K. Protein-conjugated acrolein as a biochemical marker of brain infarction. Mol. Nutr. Food Res. (2011b);55:1332–1341. doi: 10.1002/mnfr.201100068. [DOI] [PubMed] [Google Scholar]
- 30.Igarashi K., Ueda S., Yoshida K., Kashiwagi K. Polyamines in renal failure. Amino Acids. (2006);31:477–483. doi: 10.1007/s00726-006-0264-7. [DOI] [PubMed] [Google Scholar]
- 31.Inoue K., Matsumoto M., Shono T., Toyokawa S., Moriki A. Increased intima media thickness and atherosclerotic plaques in the carotid artery as risk factors for silent brain infarcts. J. Stroke Cerebrovasc. Dis. (2007);16:14–20. doi: 10.1016/j.jstrokecerebrovasdis.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Ivanov I. P., Gesteland R. F., Matsufuji S., Atkins J. F. Programmed frameshifting in the synthesis of mammalian antizyme is +1 in mammals, predominantly +1 in fission yeast, but -2 in budding yeast. RNA. (1998);4:1230–1238. doi: 10.1017/s1355838298980864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jänne J., Alhonen L., Pietila M., Keinanen T. A. Genetic approaches to the cellular functions of polyamines in mammals. Eur. J. Biochem. (2004);271:877–894. doi: 10.1111/j.1432-1033.2004.04009.x. [DOI] [PubMed] [Google Scholar]
- 34.Jin L., Miyazaki M., Mizuno S., Takigawa M., Hirose T., Nishimura K., Toida T., Williams K., Kashiwagi K., Igarashi K. The pore region of N-methyl-D-aspartate receptors differentially influences stimulation and block by spermine. J. Pharmacol. Exp. Ther. (2008);327:68–77. doi: 10.1124/jpet.108.140459. [DOI] [PubMed] [Google Scholar]
- 35.Joe Y. A., Wolff E. C., Park M. H. Cloning and expression of human deoxyhypusine synthase cDNA. Structure-function studies with the recombinant enzyme and mutant proteins. J. Biol. Chem. (1995);270:22386–22392. doi: 10.1074/jbc.270.38.22386. [DOI] [PubMed] [Google Scholar]
- 36.Kaasinen S. K., Oksman M., Alhonen L., Tanila H., Janne J. Spermidine/spermine N1-acetyltransferase overexpression in mice induces hypoactivity and spatial learning impairment. Pharmacol. Biochem. Behav. (2004);78:35–45. doi: 10.1016/j.pbb.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Kawakita M., Hiramatsu K. Diacetylated derivatives of spermine and spermidine as novel promising tumor markers. J. Biochem. (2006);139:315–322. doi: 10.1093/jb/mvj068. [DOI] [PubMed] [Google Scholar]
- 38.Kramer D. L., Diegelman P., Jell J., Vujcic S., Merali S., Porter C. W. Polyamine acetylation modulates polyamine metabolic flux, a prelude to broader metabolic consequences. J. Biol. Chem. (2008);283:4241–4251. doi: 10.1074/jbc.M706806200. [DOI] [PubMed] [Google Scholar]
- 39.Kramer D. L., Paul B., Porter C. W. Effect of pretreatment with alpha-difluoromethylornithine on the selectivity of methylglyoxal bis(guanylhydrazone) for tumor tissue in L1210 leukemic mice. Cancer Res. (1985);45:2512–2515. [PubMed] [Google Scholar]
- 40.Landau G., Ran A., Bercovich Z., Feldmesser E., Horn-Saban S., Korkotian E., Jacob-Hirsh J., Rechavi G., Ron D., Kahana C. Expression profiling and biochemical analysis suggest stress response as a potential mechanism inhibiting proliferation of polyamine-depleted cells. J. Biol. Chem. (2012);287:35825–35837. doi: 10.1074/jbc.M112.381335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawson K., Larentowicz L., Laury-Kleintop L., Gilmour S. K. B23 is a downstream target of polyamine-modulated CK2. Mol. Cell. Biochem. (2005);274:103–114. doi: 10.1007/s11010-005-3066-4. [DOI] [PubMed] [Google Scholar]
- 42.Linden A. Measuring diagnostic and predictive accuracy in disease management: an introduction to receiver operating characteristic (ROC) analysis. J. Eval. Clin. Pract. (2006);12:132–139. doi: 10.1111/j.1365-2753.2005.00598.x. [DOI] [PubMed] [Google Scholar]
- 43.Lopez O. L., Jagust W. J., Dulberg C., Becker J. T., DeKosky S. T., Fitzpatrick A., Breitner J., Lyketsos C., Jones B., Kawas C., et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 2. Arch. Neurol. (2003);60:1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 44.Mamont P. S., Duchesne M. C., Grove J., Bey P. Anti-proliferative properties of DL-alpha-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem. Biophys. Res. Commun. (1978);81:58–66. doi: 10.1016/0006-291x(78)91630-3. [DOI] [PubMed] [Google Scholar]
- 45.Mandal S., Mandal A., Johansson H. E., Orjalo A. V., Park M. H. Depletion of cellular polyamines, spermidine and spermine, causes a total arrest in translation and growth in mammalian cells. Proc. Natl. Acad. Sci. USA in press published ahead of print January 23, 2013. (2013) doi: 10.1073/pnas.1219002110. [DOI] [PMC free article] [PubMed]
- 46.Matthews H. R. Polyamines, chromatin structure and transcription. Bioessays. (1993);15:561–566. doi: 10.1002/bies.950150811. [DOI] [PubMed] [Google Scholar]
- 47.Minois N., Carmona-Gutierrez D., Madeo F. Polyamines in aging and disease. Aging (Albany NY) (2011);3:716–732. doi: 10.18632/aging.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mize G. J., Ruan H., Low J. J., Morris D. R. The inhibitory upstream open reading frame from mammalian S-adenosylmethionine decarboxylase mRNA has a strict sequence specificity in critical positions. J. Biol. Chem. (1998);273:32500–32505. doi: 10.1074/jbc.273.49.32500. [DOI] [PubMed] [Google Scholar]
- 49.Nakaike S., Kashiwagi K., Terao K., Iio K., Igarashi K. Combined use of alpha-difluoromethylornithine and an inhibitor of S-adenosylmethionine decarboxylase in mice bearing P388 leukemia or Lewis lung carcinoma. Jpn. J. Cancer Res. (1988);79:501–508. doi: 10.1111/j.1349-7006.1988.tb01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishimura K., Lee S. B., Park J. H., Park M. H. Essential role of eIF5A-1 and deoxyhypusine synthase in mouse embryonic development. Amino Acids. (2012);42:703–710. doi: 10.1007/s00726-011-0986-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishimura K., Murozumi K., Shirahata A., Park M. H., Kashiwagi K., Igarashi K. Independent roles of eIF5A and polyamines in cell proliferation. Biochem. J. (2005);385:779–785. doi: 10.1042/BJ20041477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nishimura K., Nakatsu F., Kashiwagi K., Ohno H., Saito T., Igarashi K. Essential role of S-adenosylmethionine decarboxylase in mouse embryonic development. Genes Cells. (2002);7:41–47. doi: 10.1046/j.1356-9597.2001.00494.x. [DOI] [PubMed] [Google Scholar]
- 53.Nishimura K., Shiina R., Kashiwagi K., Igarashi K. Decrease in polyamines with aging and their ingestion from food and drink. J. Biochem. (2006);139:81–90. doi: 10.1093/jb/mvj003. [DOI] [PubMed] [Google Scholar]
- 54.Ogasawara T., Ito K., Igarashi K. Effect of polyamines on globin synthesis in a rabbit reticulocyte polyamine-free protein synthetic system. J. Biochem. (1989);105:164–167. doi: 10.1093/oxfordjournals.jbchem.a122633. [DOI] [PubMed] [Google Scholar]
- 55.Oredsson S. M., Alm K., Dahlberg E., Holst C. M., Johansson V. M., Myhre L., Soderstjerna E. Inhibition of cell proliferation and induction of apoptosis by N1,N11-diethylnorspermine-induced polyamine pool reduction. Biochem. Soc. Trans. (2007);35:405–409. doi: 10.1042/BST0350405. [DOI] [PubMed] [Google Scholar]
- 56.Oredsson S. M., Anehus S., Heby O. Reversal of the growth inhibitory effect of alpha-difluoromethylornithine by putrescine but not by other divalent cations. Mol. Cell. Biochem. (1984);64:163–172. doi: 10.1007/BF00224773. [DOI] [PubMed] [Google Scholar]
- 57.Park J. H., Aravind L., Wolff E. C., Kaevel J., Kim Y. S., Park M. H. Molecular cloning, expression, and structural prediction of deoxyhypusine hydroxylase: a HEAT-repeat-containing metalloenzyme. Proc. Natl. Acad. Sci. USA. (2006);103:51–56. doi: 10.1073/pnas.0509348102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park M. H. The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A). J. Biochem. (2006);139:161–169. doi: 10.1093/jb/mvj034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park M. H., Nishimura K., Zanelli C. F., Valentini S. R. Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids. (2010);38:491–500. doi: 10.1007/s00726-009-0408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patel A. R., Wang J. Y. Polyamines modulate transcription but not posttranscription of c-myc and c-jun in IEC-6 cells. Am. J. Physiol. (1997);273:C1020–1029. doi: 10.1152/ajpcell.1997.273.3.C1020. [DOI] [PubMed] [Google Scholar]
- 61.Pegg A. E. Spermidine/spermine-N1-acetyltransferase: a key metabolic regulator. Am. J. Physiol. Endocrinol. Metab. (2008);294:E995–1010. doi: 10.1152/ajpendo.90217.2008. [DOI] [PubMed] [Google Scholar]
- 62.Pegg A. E. Mammalian polyamine metabolism and function. IUBMB Life. (2009);61:880–894. doi: 10.1002/iub.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pegg A. E., Casero R. A. Jr. Current status of the polyamine research field. Methods Mol. Biol. (2011);720:3–35. doi: 10.1007/978-1-61779-034-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pendeville H., Carpino N., Marine J. C., Takahashi Y., Muller M., Martial J. A., Cleveland J. L. The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol. Cell. Biol. (2001);21:6549–6558. doi: 10.1128/MCB.21.19.6549-6558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quigley G. J., Teeter M. M., Rich A. Structural analysis of spermine and magnesium ion binding to yeast phenylalanine transfer RNA. Natl. Acad. Sci. USA. (1978);75:64–68. doi: 10.1073/pnas.75.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc. Natl. Acad. Sci. USA. (1968);60:1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Russell D. H., Levy C. C., Schimpff S. C., Hawk I. A. Urinary polyamines in cancer patients. Cancer Res. (1971);31:1555–1558. [PubMed] [Google Scholar]
- 68.Saiki R., Nishimura K., Ishii I., Omura T., Okuyama S., Kashiwagi K., Igarashi K. Intense correlation between brain infarction and protein-conjugated acrolein. Stroke . (2009);40:3356–3361. doi: 10.1161/STROKEAHA.109.553248. [DOI] [PubMed] [Google Scholar]
- 69.Saiki R., Park H., Ishii I., Yoshida M., Nishimura K., Toida T., Tatsukawa H., Kojima S., Ikeguchi Y., Pegg A. E., et al. Brain infarction correlates more closely with acrolein than with reactive oxygen species. Biochem. Biophys. Res. Commun. (2011);404:1044–1049. doi: 10.1016/j.bbrc.2010.12.107. [DOI] [PubMed] [Google Scholar]
- 70.Seppänen P., Alhonen-Hongisto L., Janne J. Combined use of 2-difluoro-methylornithine and methylglyoxal bis (guanylhydrazone) in normal and leukemia-bearing mice. Cancer Lett. . (1983);18:1–10. doi: 10.1016/0304-3835(83)90111-8. [DOI] [PubMed] [Google Scholar]
- 71.Sequeira A., Gwadry F. G., Ffrench-Mullen J. M., Canetti L., Gingras Y., Casero R. A. Jr., Rouleau G., Benkelfat C., Turecki G. Implication of SSAT by gene expression and genetic variation in suicide and major depression. Arch. Gen. Psychiatry. (2006);63:35–48. doi: 10.1001/archpsyc.63.1.35. [DOI] [PubMed] [Google Scholar]
- 72.Sharmin S., Sakata K., Kashiwagi K., Ueda S., Iwasaki S., Shirahata A., Igarashi K. Polyamine cytotoxicity in the presence of bovine serum amine oxidase. Biochem. Biophys. Res. Commun. (2001);282:228–235. doi: 10.1006/bbrc.2001.4569. [DOI] [PubMed] [Google Scholar]
- 73.Shin J., Shen F., Huguenard J. R. Polyamines modulate AMPA receptor-dependent synaptic responses in immature layer v pyramidal neurons. J. Neurophysiol. (2005);93:2634–2643. doi: 10.1152/jn.01054.2004. [DOI] [PubMed] [Google Scholar]
- 74.Soda K., Dobashi Y., Kano Y., Tsujinaka S., Konishi F. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp. Gerontol. (2009);44:727–732. doi: 10.1016/j.exger.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 75.Stanfield P. R., Sutcliffe M. J. Spermine is fit to block inward rectifier (Kir) channels. J. Gen. Physiol. (2003);122:481–484. doi: 10.1085/jgp.200308957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stevens J. F., Maier C. S. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol. Nutr. Food Res. (2008);52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tabor C. W., Tabor H. Polyamines. Ann. Rev. Biochem. . (1984);53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- 78.Thomas T., Thomas T. J. Polyamines in cell growth and cell death: molecular mechanisms and therapeutic applications. Cell. Mol. Life Sci. (2001);58:244–258. doi: 10.1007/PL00000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tomitori H., Usui T., Saeki N., Ueda S., Kase H., Nishimura K., Kashiwagi K., Igarashi K. Polyamine oxidase and acrolein as novel biochemical markers for diagnosis of cerebral stroke. Stroke. (2005);36:2609–2613. doi: 10.1161/01.STR.0000190004.36793.2d. [DOI] [PubMed] [Google Scholar]
- 80.Uchida K., Kanematsu M., Morimitsu Y., Osawa T., Noguchi N., Niki E. Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J. Biol. Chem. (1998);273:16058–16066. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- 81.Vermeer S. E., Longstreth W. T. Jr., Koudstaal P. J. Silent brain infarcts: a systematic review. Lancet Neurol. (2007);6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 82.Wallace H. M., Duthie J., Evans D. M., Lamond S., Nicoll K. M., Heys S. D. Alterations in polyamine catabolic enzymes in human breast cancer tissue. Clin. Cancer Res. (2000);6:3657–3661. [PubMed] [Google Scholar]
- 83.Wang X., Levic S., Gratton M. A., Doyle K. J., Yamoah E. N., Pegg A. E. Spermine synthase deficiency leads to deafness and a profound sensitivity to alpha-difluoromethylornithine. J. Biol. Chem. (2009);284:930–937. doi: 10.1074/jbc.M807758200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y., Xiao L., Thiagalingam A., Nelkin B. D., Casero R. A. Jr. The identification of a cis-element and a trans-acting factor involved in the response to polyamines and polyamine analogues in the regulation of the human spermidine/spermine N1-acetyltransferase gene transcription. J. Biol. Chem. (1998);273:34623–34630. doi: 10.1074/jbc.273.51.34623. [DOI] [PubMed] [Google Scholar]
- 85.Watanabe S., Kusama-Eguchi K., Kobayashi H., Igarashi K. Estimation of polyamine binding to macromolecules and ATP in bovine lymphocytes and rat liver. J. Biol. Chem. (1991);266:20803–20809. [PubMed] [Google Scholar]
- 86.Williams-Ashman H. G., Schenone A. Methyl glyoxal bis(guanylhydrazone) as a potent inhibitor of mammalian and yeast S-adenosylmethionine decarboxylases. Biochem. Biophys. Res. Commun. (1972);46:288–295. doi: 10.1016/0006-291x(72)90661-4. [DOI] [PubMed] [Google Scholar]
- 87.Yoshida M., Higashi K., Kobayashi E., Saeki N., Wakui K., Kusaka T., Takizawa H., Kashiwado K., Suzuki N., Fukuda K., et al. Correlation between images of silent brain infarction, carotid atherosclerosis and white matter hyperintensity, and plasma levels of acrolein, IL-6 and CRP. Atherosclerosis. (2010);211:475–479. doi: 10.1016/j.atherosclerosis.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 88.Yoshida M., Tomitori H., Machi Y., Hagihara M., Higashi K., Goda H., Ohya T., Niitsu M., Kashiwagi K., Igarashi K. Acrolein toxicity: Comparison with reactive oxygen species. Biochem. Biophys. Res. Commun. (2009);378:313–318. doi: 10.1016/j.bbrc.2008.11.054. [DOI] [PubMed] [Google Scholar]


