Abstract
Prostate cancer is one of the most prevalent non-skin related cancers. It is the second leading cause of cancer deaths among males in most Western countries. If prostate cancer is diagnosed in its early stages, there is a higher probability that it will be completely cured. Prostatic acid phosphatase (PAP) is a non-specific phosphomonoesterase synthesized in prostate epithelial cells and its level proportionally increases with prostate cancer progression. PAP was the biochemical diagnostic mainstay for prostate cancer until the introduction of prostate-specific antigen (PSA) which improved the detection of early-stage prostate cancer and largely displaced PAP. Recently, however, there is a renewed interest in PAP because of its usefulness in prognosticating intermediate to high-risk prostate cancers and its success in the immunotherapy of prostate cancer. Although PAP is believed to be a key regulator of prostate cell growth, its exact role in normal prostate as well as detailed molecular mechanism of PAP regulation is still unclear. Here, many different aspects of PAP in prostate cancer are revisited and its emerging roles in other environment are discussed.
Keywords: Prostatic acid phosphatase (PAP), Prostate cancer, Biomarker, Prognosis, Diagnosis, Immunotherapy
INTRODUCTION
Prostate cancer is one of the most prevalent non-skin cancers in men. In America, prostate cancer related death is ranked second out of all cancer related deaths in men, but its etiology has not been clearly identified yet (Hsing and Chokkalingam, 2006). However, in contrast to many other cancers that are difficult to treat, prostate cancer can be completely cured if it is detected in its early stage. Many prostate cancer markers including prostate-specific antigen (PSA), prostate specific membrane antigen (PSMA), prostate acid phosphatase (PAP), and prostate stem cell antigen (PSCA) have been identified so far (Truong et al., 1993; Hobisch et al., 1998; Bussemakers et al., 1999, Gupta et al., 2009; Madu and Lu, 2010; Batta et al., 2012), which all together can help to increase the chance of earlier detection of prostate cancer (Table 1).
Table 1.
Biomarkers of prostate cancer. Different classes of prostate cancer biomarkers are shown. The list is not exhaustive. The markers are mostly proteins in blood. DNA, RNA, and metabolite are also shown
| Biomarker | Composition | Change | Purpose | Reference |
|---|---|---|---|---|
| Prostatic acid phosphatase (PAP) | Protein | Increase | Diagnosis/Prognosis | Taskén et al., 2005; Veeramani et al., 2005; Makarov et al., 2009 |
| Prostate-specific antigen (PSA) | Protein | Increase | Diagnosis | Li and Beling, 1973; Ercole et al., 1987; Stamey et al., 1987 |
| Biomarker candidate | ||||
| α-Methylacyl coenzyme A racemase (AMACR) | Protein | Increase | Diagnosis | Rogers et al., 2004 |
| B7-H3 | Protein | Increase | Diagnosis/Prognosis | Roth et al., 2007 |
| Caveolin-1 (Cav-1) | Protein | Decrease | Prognosis | Thompson et al., 2010 |
| Chromogranin A (CGA, GRN-A) | Protein | Increase | Prognosis | Deftos, 1998 |
| DAB2 interacting protein (DAB2IP) | Protein | Decrease | Diagnosis | Chen et al., 2002 |
| Endoglin (CD 105) | Protein | Increase | Prognosis | Wikström et al., 2002 |
| Early prostate cancer antigen (EPCA) | Protein | Increase | Diagnosis | Getzenberg et al., 1991 |
| Goligiphosphoprotein 2 (GOLPH2) | Protein | Increase | Diagnosis | Kristiansen et al., 2008 |
| Glutathione S-transferase P1 gene (GSTP1) | DNA | Hypermethylation (decrease) | Diagnosis | Lee et al., 1994 |
| Human kallikrein 2 (hK2) | Protein | Increase | Diagnosis | Becker et al., 2000 |
| Interleukin-6 (IL-6) | Protein | Increase | Prognosis | Hobisch et al., 1998 |
| Ki-67 | Protein | Increase | Diagnosis | Gerdes et al., 1984; |
| P504S/p63 | Protein | Increase | Diagnosis | Harvey et al., 2010 |
| Prolactin-inducible protein (PIP/GCTFP15) | Protein | Increase | Diagnosis | Tian et al., 2004 |
| Prostate cancer antigen-1 (PCA-1) | Protein | Increase | Diagnosis | Liu et al., 2007 |
| Prostate cancer antigen 3 (PCA3 or DD3) | RNA | Increase | Diagnosis | Bussemakers et al., 1999 |
| PDLIM4 gene (PDLIM4) | DNA | Hypermethylation (decrease) | Diagnosis | Vanaja et al., 2006 |
| Prostate stem cell antigen (PSCA) | Protein | Increase | Prognosis | Reiter et al., 1998 |
| Prostate-specific membrane antigen (PSMA) | Protein | Increase | Diagnosis | Brawer et al., 1992 |
| Sarcosine | Metabolite (Chemical) | Increase (in urine) | Diagnosis | Sreekumar et al., 2009 |
| STAMP1 | Protein | Increase | Diagnosis | Korkmaz et al., 2002 |
| STAMP2 | Protein | Increase | Diagnosis | Korkmaz et al., 2005 |
| STEAP | Protein | Increase | Diagnosis | Hubert et al., 1999 |
| Transforming growth factor-β1 (TGF-β1) | Protein | Increase | Prognosis | Truong et al., 1993 |
| Urokinase plasminogen activation (uPA) | Protein | Increase | Diagnosis/Prognosis | Gupta et al., 2009 |
In 1938, which is 85 years after first identification of prostate cancer (Fig. 1), it was discovered that the activity of prostatic acid phosphatase (PAP) was increased in the circulation of the patients with prostate cancer (Gutman and Gutman, 1938). This elevated PAP activity was especially higher in those patients with bone metastasis (Small et al., 2006; Sheridan et al., 2007). Later on, it was established that blood PAP activity correlates with prostate cancer progression in prostate cancer patients and that PAP could serve as a biochemical indicator for cancer treatment (Veeramani et al., 2005). Subsequently, serum PAP was widely studied as a surrogate marker for prostate cancer until the establishment of prostate-specific antigen (PSA) as the new standard (Veeramani et al., 2005). The introduction of total PSA testing in blood has revolutionized the detection and management of patients with prostate cancer. Indeed, PSA has been regarded as a strong prognostic marker for long-term risk of prostate cancer. The patients who will eventually develop prostate cancer have increased total PSA levels years or decades before the cancer is diagnosed. However, there is a growing need for novel biomarkers that could aid in clinical decision making about biopsy and initial treatment. This is due to the inherent biological variability of total PSA levels which inevitably affects the interpretation of clinical data. For example, total PSA velocity improves the predictiveness of total PSA only marginally, limiting its value for prostate cancer screening and prognostication (Shariat et al., 2011). In this regard, it is encouraging that Swedish group recently developed a novel miRNA index quote (miQ = [miR-96-5p×miR-183-5p]/[miR-145-5p×miR221-5p]) as an early marker for prostate cancer with aggressive progression characteristics (Larne et al., 2012). They measured the expression of microRNAs (miRNA) using qRT-PCR in FFPE prostatic tissue samples from a Swedish cohort of 49 patients with prostate cancer and 25 without cancer and found eight of 14 preselected miRNAs to discriminate between the two groups. Subsequently four discriminatory miRNAs were combined to a quota. The miQ was found to successfully predict diagnosis (p<0.0001) with high accuracy (AUC=0.931), significantly outperforming PSA. On the other hand, novel blood-based biomarkers including human glandular kallikrein 2 (hK2, Becker et al., 2000), urokinase plasminogen activator (uPA) and its receptor (uPAR, Gupta et al., 2009), transforming growth factor-beta 1 (TGF-β1, Truong et al., 1993), interleukin-6 (IL-6) and its receptor (IL-6R, Hobisch et al., 1998) were identified and they also are expected to supplement or replace PSA for better diagnosis, staging, prognostication, and monitoring (Kraus et al., 2010). Recently, PCA3 (Bussemakers et al., 1999), and T2:ERG (Young et al., 2012) was suggested to be a novel biomarker for prostate cancer. PCA3 is a noncoding RNA that is found at high levels in prostate cancer compared to noncancerous prostate cells. T2:ERG is a fusion of the TMPRSS2 gene that is regulated by androgens and ERG oncogene. Found in 50% of prostate cancers, this fusion gene is hypothesized to have a role in the development of prostate cancer (Young et al., 2012).
Fig. 1. History of PAP development. Illustrated timelines for prostate cancer and its biomarkers. The major breakthroughs and advances in prostate cancer research are shown. The rise, fall, and revival of PAP along with its emerging diverse roles are also depicted.
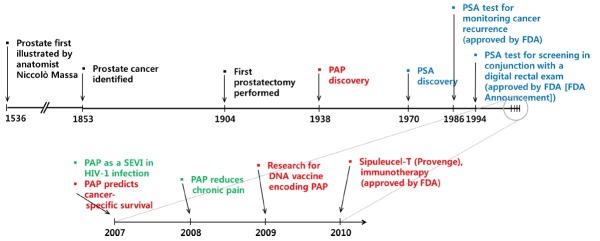
In this review, however, we will focus on PAP molecule because an increasing number of studies have identified PAP as a significant prognostic factor for patients with intermediate- to high-risk prostate cancer (Taira et al., 2007) and it is recently used as a therapeutic target for immunotherapy (Drake, 2012; Sims, 2012). In addition, novel non-canonical functions of PAP such as pain suppression and involvement in viral infection will be discussed.
HUMAN PAP
Human PAP, also known as Acpp or prostatic specific acid phosphatase (PSAP), is a secreted glycoprotein (100 kDa) enzyme (E.C. 3.1.3.2) that is synthesized in the prostate gland’s epithelial cells (Vihko et al., 1978). Although used as a prostate cancer marker and studied widely in the past few decades, its physiological role is not clearly understood yet. As the name suggests, PAP shows phosphatase activity in acidic condition (pH 4-6) (Zelivianski et al., 1998). PAP enzymatic activity occurs when PAP forms a homodimer that consists of two catalytically inactive subunits (50 kDa) bound by non-covalent bonds (Kuciel et al., 1990; Lee et al., 1991). Each subunit comprises two domains. The larger domain is an α/β type composed of a central seven-stranded mixed β-sheet with α-helices on both sides, while the smaller α-domain contains six α-helices and is formed mostly by long-chain excursions from the first domain (Ortlund et al., 2003; Hassan et al., 2010). The inter-subunit hydrogen bonds observed are the side chain of Gln 33 to main chain His 67 interactions, side chain of Gln 40 to main chain of Val 97, and side chain of His 112 to side chain of Asp 76 (Jakob et al., 2000).
After cleavage of the 32 amino acids that make up PAP’s signal peptide, PAP becomes approximately 41 kDa which is its mature form (Roiko et al., 1990; Zelivianski et al., 1998). The PAP monomer has 6 conserved cysteine residues that form 3 disulfide bonds (Cys129-Cys340, Cys183-Cys281 and Cys315-Cys319) together with three putative N-linked glycosylation sites (Van Etten et al., 1991). A high mannose-type carbohydrate binds to Asn61 and Asn301, while Asn188 residue partially sialylates (Jakob et al., 2000). Interestingly, these glycosylation sites and active sites are conserved in all mammalian PAPs (Ostanin et al., 1994). The structure and active site of PAP has been extensively characterized from various species (Hassan et al., 2010). Multiple sequence analyses of human PAP with that of other mammalian PAPs revealed close resemblance among one another. Interestingly, the human PAP showed approximately 99% sequence homology with the panther, 94% with the monkey, 81% with the cow, 83% with the mouse and 80% with the rat.
The major action of PAP is to dephosphorylate macromolecules with the help of catalytic residues (His12 and Asp258) that are located in the cleft between two domains (Hassan et al., 2010). Site-directed mutagenesis of amino acid residues of PAP revealed that His12 and Asp 258 are critical residues for enzymatic activity of PAP because H12D and/or D258A mutant could not decrease phosphorylation level of ErbB-2 (Zhang et al., 2001). Histidine (H257) and arginine (R11, R15, R54, R79) residues are also important for PAP activity (Ostanin et al., 1994). The dephosphroylation mechanism of PAP is similar to that of fructose-2,6-bisphosphatase (Okar et al., 2000). The His12 acts as a nucleophile and conjugates with the substrate to form a phosphohistidine intermediate. Then, for recycling of the enzyme and dephosphorylation, Asp258 hydrolyzes phosphohistidine (Ostanin et al., 1994; Sharma and Juffer, 2009). Although the dephosphorylation mechanisms and catalytic active site of PAP are well-known, there are very few substrates that have been identified so far. The few that have been identified include AMP, phosphotyrosine, phosphocholine, phosphocreatine and ErbB-2 (Dave and Rindani, 1988). Because PAP has the potential to act as the protein tyrosine phosphatase, there could be many other substrates that have yet to be identified. Identification of such substrates would help to delineate the signal transduction pathways of PAP, which can contribute to better diagnosis, treatment and prevention of prostate cancer.
TISSUE EXPRESSION OF PAP
In human, PAP is one of the major proteins secreted by prostate columnar epithelium secretory cells following puberty (Graddis et al., 2011). PAP protein has been determined to be about 0.5 mg/g wet weight of prostate tissue (Goldfarb et al., 1986) and approximately 1 mg/ml in seminal fluid (Ronnberg et al., 1981). PAP expression is associated with the sex hormone testosterone which determines secondary sexual characteristics (Goldfarb et al., 1986). PAP may be found in increased amounts in men who have prostate cancer. Indeed, robust expression of PAP was detected in high Gleason score prostate cancer (Gunia et al., 2009). But PAP expression is also enriched in normal prostate cells as well as in prostate cancer tissue as determined by real-time qPCR (Graddis et al., 2011). When compared with other tissue, PAP mRNA level is 50-5,000 fold higher in normal prostate tissue, and 110-6,000 fold higher in prostate cancer tissue. PAP can also be detected in various tissues other than prostate such as brain, kidney, liver, lung, placenta, salivary gland, spleen, thyroid and thymus cells (Solin et al., 1990). PAP is absent in breast carcinoma tissue in contrast to normal breast tissue where PAP can be detected (Wang et al., 2005). More recently, however, PAP was discovered in large quantities in breast cyst fluid (BCF), especially in metaplastic epithelium (intracystic Na/Klessthan, 3 type I), suggesting the role of PAP in protecting several carcinomas by activating TGF-β as a similar molecule to PSA (Erbas et al., 2007). Further study is needed to elucidate the role of PAP on TGF-β activation. In colon carcinoma, PAP was detected in only 40% of samples at a lower level than normal prostate or prostate carcinoma (Wang et al., 2005). The acid phosphatase that is expressed in placenta and liver is mostly located in lysosome, and are therefore termed lysosomal acid phosphatase (LAP) (Shan et al., 2003).
REGULATION OF PAP GENE EXPRESSION
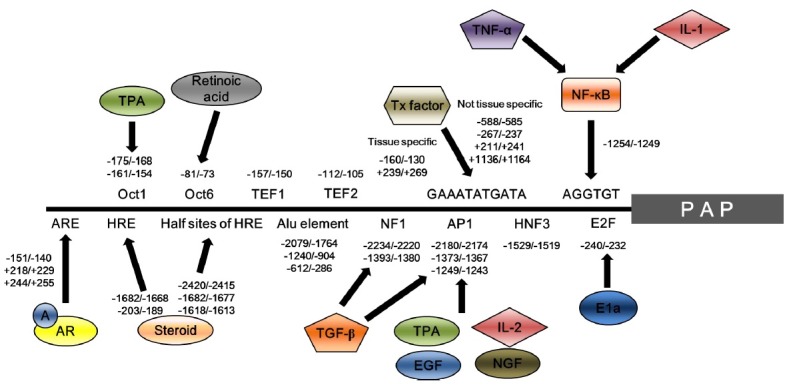
The PAP gene is located in chromosome 3q21-23 in humans (Winqvist et al., 1989). Alternative splicing generates two types of PAP transcripts; transmembrane PAP which consists of 11 exons (exon 1-9, 10a and 11), and cellular and secretory PAPs having 10 exons (exon 1-9 and 10b) (Zelivianski et al., 1998; Veeramani et al., 2005; Kong et al., 2011). The length of 3’-UTR of transmembrane PAP is shorter than those of cellular and secretory PAPs: 405 bp versus 874 bp (Kong et al., 2011). The molecular mechanisms underlying PAP gene regulation are not fully understood. However, many trans-acting factors including androgen/androgen receptor, NF-κB, TNF-α and IL-1 are involved in the regulation of PAP gene expression (Fig. 2). In human prostatic carcinoma, two major transcription initiation sites exist, one at 56bp and the other 91bp upstream of ATG codon (Zelivianski et al., 1998). The androgen and androgen receptor (AR) complex seems to be essential for prostate development and function. In PAP regulatory regions, an androgen response element (ARE) exists in 3 sites (−151/−140, +218/+229, +244/+255) which are androgen-AR complex binding motifs. When androgen levels are normal, the −151/−140 site is involved in enhancing PAP transcription level. However, when androgen level is low, the +218/+229 and +244/+255 sites act as negative regulators of PAP transcription (Porvari et al., 1995; Kong et al., 2011). These findings imply that the androgen is an essential factor for human PAP expression. Upstream deletion analysis confirmed that 577bp in -1356/-799 region is cis-acting enhancer region. Furthermore, prostate cancer specific PAP expression is increased due to NF-κB binding to AGGTGT motif in −1254/−1249 region that is located in the PAP promoter region (Zelivianski et al., 2004). Chloramphenicol acetyl transferase (CAT) reporter gene assay of the human PAP promoter revealed that the −1258 /−779 elements are essential for cell type-specific PAP expression (Zelivianksi et al., 2004). In addition, GAAATATGATA-like element necessary for transcription factor binding were found to exist in 6 sites in PAP regulatory regions (Shan et al., 2005). Based on electrophoretic mobility shift assay data, it was confirmed that −160/−130 and +239/+269 sites are related to tissue-specific PAP expression (Shan et al., 2003; Shan et al., 2005). Additionally, other sites should exist that regulate tissue-specific regulation of PAP in promoter regions.
Fig. 2. Binding sites and their corresponding factors which regulate human PAP expression. Trans-acting factors involved in the regulation of PAP are schematically represented together with their binding sites. NF-κB binds to AGGTGT motif at the -1254/-1249 region, which acts as a cis-acting element. This leads to tissue-specific upregulation of PAP expression through TNF-α or IL-1. Transcription factors enhance PAP transcription through the GAAATATGATA motif. −588/−585, −267/−237, +211/+241 and +1136/+1164 regions are associated with not tissue-specific upregulation of PAP, whereas −160/−130 and +239/+269 regions are associated with prostate tissue-specific upregulation. Androgen (A) - androgen receptor (AR) complex binds to androgen response element (ARE) for positive and/or negative regulation of PAP transcription. ARE located in −151/140 region is involved in enhancing PAP transcription, whereas, +218/+229 and +244/+255 regions are associated with transcriptional inhibition of PAP. A: androgen; AR: androgen receptor; NGF: nerve growth factor; EGF: epidermal growth factor; TPA: tissue plasminogen activator; IL: interleukin; TGF-β: Transforming growth factor-β; NF-κB: nuclear factor kappa B; TNF-α: tumor necrosis factor-α.
DIFFERENT FORMS OF PAP
Although the level of PAP is increased in the circulation of patients with prostate cancer, its intracellular level and activity are greatly diminished in prostate cancer cells. This apparent discrepancy can be explained by the fact that there are two forms of PAP in prostate epithelial cells; the cellular form (cPAP) and secretory form (sPAP). The two forms of PAP differ in their biochemical properties such as hydrophobicity, isoelectric points and glycosylation patterns (Van Etten, 1982; Veeramani et al., 2005). sPAP is expressed only in the prostate (Solin et al., 1990) and is mostly released into seminal fluid (Ronnberg et al., 1981). The expression of cPAP becomes very high in normal prostate epithelial cells. But its level decreases in prostate cancer cells compared to neighboring normal cells (Reif et al., 1973; Lin et al., 2001). This decreased expression of cPAP results in hyperphosphorylation of HER-2 at tyrosine residues and activation of downstream extracellular signal-regulated kinase (ERK)/mitogen activated protein kinase (MAPK) signaling, which can lead to androgen-independent cell growth and prostate cancer development. Consistent with this, prolonged passage of LNCaP cells led to a decrease in the cPAP level, which corresponds to the loss of their androgen sensitivity and an increase in the growth rate and tumorigenicity. Conversely, ectopic expression of cPAP in AR-positive, PAP-null cells could restore their androgen sensitivity and a decrease in their growth rate and tumorigenicity (Lin et al., 1998; Meng et al., 2000; Lin et al., 2001). Moreover, the level of cPAP inversely correlated with prostate cancer progression despite an elevated level of blood sPAP (Abrahamsson et al., 1988). In another aspect, PAP proteins isolated from prostate cancer patients had lower pI values and longer half-lives than normal tissues (Veeramani et al., 2005). Lin et al. could show that the decreased clearance rate of this cancerous PAP in animal model can be explained by altered post-translational modification such as increased sialyation (lower pI). Based on these findings, it was suggested that the elevated blood PAP in prostate cancer patients is due to combined effects of increased tumor mass and increased half-life of sPAP (Veeramani et al., 2005). On the other hand, recent studies revealed that the splice variant (TM-PAP) was expressed in nonprostatic tissues, including brain, kidney, liver, lung, skeletal muscle, placenta, salivary gland, spleen, thyroid, and thymus (Azumi et al., 1991; Hsing and Chokkalingam, 2006). TM-PAP was also expressed in fibroblast, Schwann, and LNCaP cells, but not in PC-3 cells. This type I transmembrane (TM) protein had the extracellular NH2-terminal phosphatase activity and the COOH-terminal lysosomal targeting signal (YxxΦ). TM-PAP was localized in the plasma membrane-endosomal-lysosomal pathway and found to colocalize with the lipid raft marker flotillin-1 (Quintero et al., 2007). These findings emphasize the fact that the expression of PAP may not be exclusive to prostatic tissue, and that this issue together with non-canonical functions of PAP has to be taken into account for the success of PAP-based immunotherapy without unwanted side effects (Antonarakis and Drake, 2010; Garcia, 2011; Gerritsen, 2012).
CELL SIGNALING REGULATION BY PAP
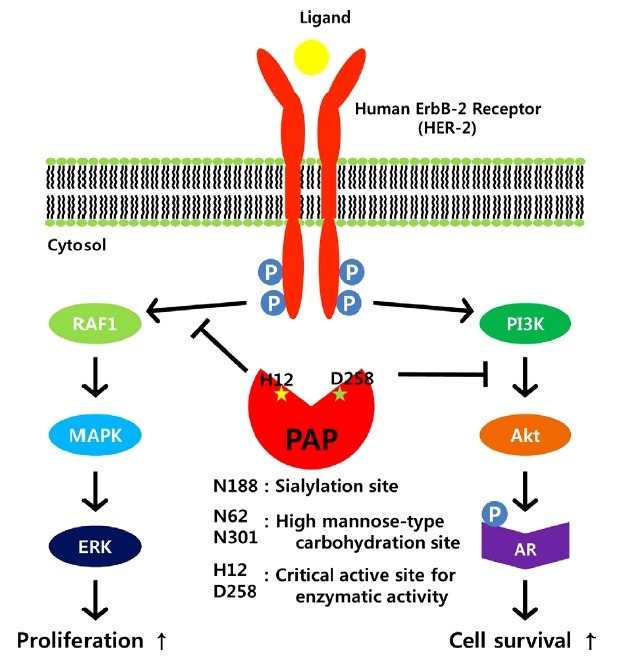
PAP can regulate prostate cell growth in two signaling pathways (Fig. 3). Human ErbB-2 (HER-2) can be homodimerized when it is phosphorylated at a tyrosine residue in early developmental stages of prostate cancer (Lin et al., 1994). Dimerized HER-2 then activates downstream ERK1/2 and MAPK, which in turn increases cell proliferation (Meng et al., 2000). In another pathway, activated HER-2 stimulates PI3K signaling. Upon activation, PI3K accumulates and activates Akt. Activated Akt then leads to phosphorylation and activation of AR and phosphorylated AR stimulates cell proliferation (Vihko et al., 2005). PAP can act as a negative regulator of both pathways of HER-2 by dephosphorylation. By blocking Akt, PAP can inhibit androgen-independent prostate cell growth. This is consistent with the observation that PAP expression has a negative correlation with prostate cancer development (Saito et al., 2007). Indeed, late stage prostate cancer had a low level of PAP, suggesting a high risk for malignant tumor formation (Merrick et al., 2005). In this regard, PAP can be regarded as a tumor suppressor mediating inhibition of cell growth (Veeramani et al., 2005).
Fig. 3. Molecular inhibitory mechanism of cPAP to block proliferation and survival of prostate cells. When human ErbB-2 receptor (HER-2) is activated by hyperphosphorylation, it transduces signaling for two pathways: RAF1/MAPK/ERK pathway and PI3K/Akt/AR pathway. Blocking of these two pathways by prostatic acid phosphatase (PAP) can lead to inhibition of cell proliferation and survival. Functionally important residues for PAP activity together with critical active sites (H12 & D258) are indicated. AR: androgen receptor.
Measuring levels of the active form of the protein EGFR in the tumor and its vicinity can provide a more reliable prognosis for individuals with prostate cancer. EGFR belongs to the same family as the prognosis marker HER-2 (Rubenstein et al., 2012), which is used today for breast cancer to determine the aggressiveness of a tumor that is to be treated with inhibitors of HER2 (Herceptin). In a similar way, it may be possible in the future to screen for the active form of EGFR to select patients with a poor prognosis and are suitable for treatment with inhibitors of EGFR. In order to use EGFR as a prognosis marker clinically in the future, further studies will need to target its expressions in other and larger material in prostate tumors.
PAP AS A USEFUL MARKER FOR PROSTATE CANCER
Despite the great progress in our understanding of the disease process and standardization of diagnostic criteria for prostate cancer, the majority of prostate tumors are detected at early stages with uncertain prognosis (Larne et al., 2012). Previous studies have shown that PAP can serve as a prostate cancer marker by proportionally increasing secretory PAP expression as prostate cancer progresses (Azumi et al., 1991; Wang et al., 2005; Gunia et al., 2009). High levels of PAP expression were detected in high Gleason score prostate cancers as determined by immunohistochemistry (Gunia et al., 2009). However, the introduction and widespread adoption of PSA has largely displaced PAP in the diagnosis and treatment of prostate cancer. This was because PSA was more sensitive than PAP in the detection of prostate cancer in the serum. However, the use of PSA has also led to over-diagnosis and overtreatment of prostate cancer resulting in controversy about its use for screening (Vihko et al., 2005; Shariat et al., 2011). Indeed, there are still some significant controversies over PSA screening because no study has successfully shown any significant correlation between such screening and a decline in mortality rate. (Madu and Lu, 2010). PSA also has limited predictive accuracy for predicting outcomes after treatment and for making clinical decisions about adjuvant and salvage therapies (Huang et al., 1993; Madu and Lu, 2010). Hence, there has been an urgent need for novel biomarkers to supplement PSA for detection and management of prostate cancer. Under these circumstances, there is now a renewed interest in PAP again because it has significantly higher correlation with prostate cancer progression (Zimmermann, 2009). The cancer-specific survival (CSS) study, which tested 193 patients’ serum, showed that, when PAP concentration is <1.5 U/L, 1.5-2.4 U/L and >2.5 U/L, the progression of prostate cancer is 93%, 87% and 75% (p=0.013), respectively. However, when PSA concentration is <10 ng/ml, 10-20 ng/ml and > 20 ng/ml, the progression of prostate cancer is 92%, 76% and 83% (p=0.393), respectively (Fang et al., 2008). These results strongly suggest that PAP may be a more suitable marker for prostate cancer and CSS than PSA. PAP appears to be particularly valuable in predicting distant failure in higher-risk patients for whom high levels of local control are achieved with aggressive initial local treatment. As prostate cancer care becomes increasingly focused on identifying the minority of patients who would benefit from aggressive systemic therapy, a reevaluation of the potential contribution of the PAP test seems timely (Taira et al., 2007). Investigation of any potential interplay between PAP and PSA and between PAP and other markers are also warranted. Recently, diagnostic utility of P504S/p63 cocktail in verifying prostatic carcinoma involvement in seminal vesicles were evaluated (Harvey et al., 2010). The use of the single-color P504S/p63 immunohistochemical stain cocktail was recommended for identifying prostatic carcinoma involving the seminal vesicle and for distinguishing benign prostatic glands from prostatic carcinoma when there is a question of seminal vesicle invasion. It was argued that P504S/p63 cocktail is superior to PSA or PAP when sections contain both seminal vesicle and benign glands because PSA and PAP cannot distinguish benign from malignant glands (Harvey et al., 2010).
PAP AS A USEFUL ANTIGEN FOR PROSTATE CANCER THERAPY
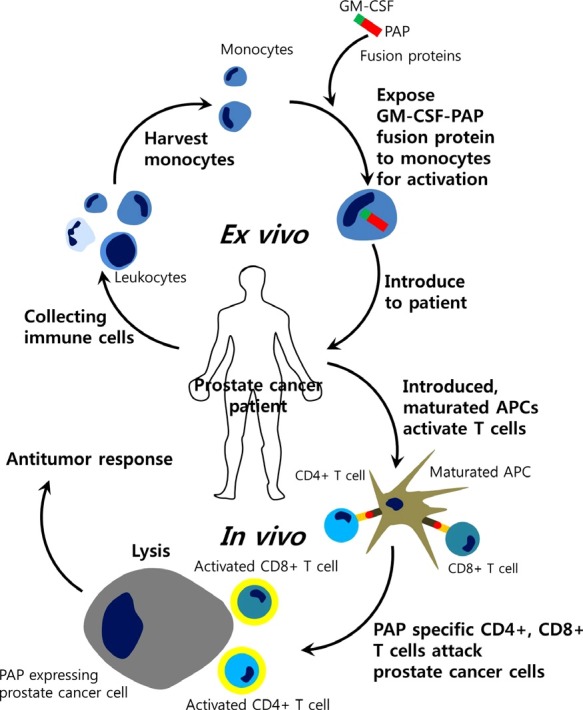
Based on the good prognostic value of PAP and the potential usefulness of PAP as an antigen, an immunotherapy employing autologous PAP-loaded dendritic cells was initiated (Drake, 2010). This FDA-approved therapy termed PROVENGE (Sipuleucel-T) works on the basic idea that over 95% of prostate cancer cells express PAP (Drake, 2012). Treatment with sipuleucel-T comprises a number of stages (Fig. 4). First, autologous peripheral blood mononuclear cells (PBMCs) including antigen-presenting cells (APCs) are pulsed ex vivo and activated in vitro with a recombinant fusion protein (PA2024) that couples the vaccine target (PAP) to granulocyte-macrophage colony-stimulating factor (GM-CSF) (Garcia, 2011; Gerritsen, 2012). This PAP and GM-CSF fusion protein is presented to antigen presenting cells (APCs) that are collected from the patient. These activated APCs are then introduced to the patient for induction of T cells in vivo. Activated T cells now attack prostate cancer cells in the patient, thus treating the cancer (Cheever and Higano., 2011; Sims, 2012). In the phase III IMPACT (Immunotherapy Prostate AdenoCarcinoma Treatment) trial of 512 patients with asymptomatic or minimally symptomatic metastatic CRPC, which served as the basis for the licensing approval of sipuleucel-T, vaccine treatment resulted in a 4.1 month improvement in median overall survival compared with placebo (25.8 months versus 21.7 months, respectively) with a 22% relative reduction in the risk of death [hazard ratio (HR): 0.78, p=0.03] (Gerritsen, 2012). The delayed onset of response reflected in the late separation of survival curves has been reported in many clinical trials of immunotherapeutic agents and is known to impact on clinical dynamics, highlighting the need for suitable end points to assess efficacy (Gerritsen, 2012). On the other hand, the PAP encoded DNA vaccine is currently undergoing clinical trials that aim to prevent and treat prostate cancer. Ten of twenty-two patients showed antigen-specific T cell proliferation and upregulation of CD8+INFγ (McNeel et al., 2009, Lubaroff, 2012). DNA vaccines encoding PAP are expected to be an effective way to prevent and treat prostate cancer. To date, a lot of prostate cancer-associated antigens such as PSA, PAP, or PSMA have been cloned and are being tested as a component of investigational therapeutic cancer vaccines (Vieweg and Dannull, 2005). But because prostate cancer is known to be a heterogeneous disease with a number of different genetic make-ups, personalized therapeutic strategies guided by the use of novel molecular imaging will be necessary to successfully test the utility of such targeted agents in patients whose tumors will depend upon that antigen target for tumor growth and/or survival.
Fig. 4. Schematic diagram of Provenge trial. The stages of Sipuleucel-T treatment for patients with prostate cancer are shown. Sipuleucel-T treatment is similar to a dendritic cell (DC) vaccine. It is a United States Food and Drug Administration (FDA)-approved autologous cell-based immunotherapy that targets prostatic acid phosphatase (PAP) as a treatment for advanced prostate cancer. Modified from Garcia (2011) and Gerritsen (2012). GM-CSF: granulocyte-macrophage colony-stimulating factor; APC: antigen-presenting cells.
INVERSE CORRELATION BETWEEN PAP AND OLIGOSPERMIA
PAP has an essential role not only in prostate cancer but also in many other physiological functions (Fig. 5). PAP is also expressed in normal prostate tissue, which is an indication that PAP has a prostate-specific physiological role. PAP is abundant in seminal fluid and is therefore thought to be an important factor in fertilization, helping to increase the mobility of sperm (Afzal et al., 2003). On the other hand, a previous study of 365 semen samples has shown that PAP concentration is inversely associated with sperm concentration (Dave and Rindani, 1988; Singh et al., 1996). Moreover, other group showed that the highest phosphatase activity was detected in azoospermic men and, when phosphatase activity was decreased, the concentration of sperm tended to recover to normal concentration (Dave and Rindani, 1988; Collins and Bennett, 2011). Although molecular mechanisms are still unclear as to how PAP induces oligospermia, PAP can be used as an effective marker for oligospermia (Coussens and Werb, 2002).
Fig. 5. Diverse roles of PAP including non-canonical functions. Traditionally, PAP was a molecule mainly involved in prostate cancer diagnosis and treatment. Recently, however, inherent phosphatase activity of PAP broadens its role in other areas such as oligospermia, SEVI, and pain suppression.
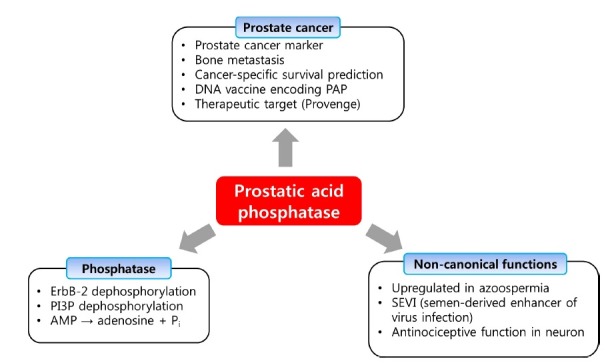
ANTINOCICEPTIVE EFFECT OF PAP
While PAP was classically considered to be a non-specific phosphomonoesterase (E.C. 3.1.3.2) (Ostrowski and Kuciel, 1994), sPAP and transmembrane PAP could function as ectonucleotidases that hydrolyzes extracellular adenosine 5′-monophosphate (AMP) to adenosine and Pi. This extracellular adenosine leads to a decrease in chronic pain by activating A1R in nociceptive neurons (Zylka et al., 2008). sPAP is glycosylated at three asparagine residues (N62, N188, N301) and has potent antinociceptive effects when administered to mice (Hurt et al., 2012a). Secretion and post-translational carbohydrate modifications were found to be required for PAP protein stability and catalytic activity. Also, it was found that deletion of PAP reduces extracellular AMP hydrolysis in nociceptive neurons and in the dorsal spinal cord (Street et al., 2011). Intrathecal injection of sPAP had three day long antinociceptive effects in mouse models of inflammatory pain and neuropathic pain (Sowa et al., 2009). In addition, sPAP had enduring (>7 days) A1R-dependent antinociceptive effects if injected intrathecally before nerve injury or inflammation (Sowa et al., 2010). These findings altogether suggest that a recombinant version of human sPAP could be used as a treatment for chronic pain or for preemptive analgesia (Hurt et al., 2012b).
SEMEN-DERIVED ENHANCER OF VIRUS INFECTION (SEVI)
PAP may play an important role in the transmission of HIV. By screening a complex peptide/protein library derived from human semen, German group could show that naturally occurring fragments of the abundant semen marker prostatic acidic phosphatase (PAP) form amyloid fibrils (Münch et al., 2007). These fibrils, termed Semen-derived Enhancer of Virus Infection (SEVI), capture HIV virions and promote their attachment to target cells, thereby enhancing the infectious virus titer by several orders of magnitude. Physiological concentrations of SEVI amplified HIV infection of T cells, macrophages, ex vivo human tonsillar tissues, and transgenic rats in vivo, as well as trans-HIV infection of T cells by dendritic or epithelial cells. Since amyloidogenic PAP fragments are abundant in seminal fluid and boost semen-mediated enhancement of HIV infection, PAP may be a future target to combat the spread of HIV infection. In another instance, SEVI greatly increased xenotropic murine leukemia virus-related virus (XMRV) infections of primary prostatic epithelial and stromal cells (Hong et al., 2009). Recently, it was shown that Cu(II) and Zn(II) inhibit fibrillization of SEVI, suggesting that the metals may modulate SEVI fibrillization under physiological conditions (Sheftic et al., 2012).
SUMMARY AND CONCLUSIONS
Prostate cancer research in the past decade has made huge stride in the understanding of the disease process and standardization of diagnostic criteria. Although great progress has been made, there still remain many areas of uncertainty and debate. The revolution towards a synthesis of diagnosis and therapy together with sound prognostic models is only just beginning. One of the major hurdles in prostate cancer therapy is that more than 70% of patients fall into a group where very little can be said about their prognosis with today's markers. This in turn means that certain patients are over-treated with therapies that can lead to serious side effects and that other patients who really need intensive treatment do not get it or get it too late. Therefore, a panel of sound biomarkers will be needed to achieve sufficient degree of certainty in guiding clinical decisions. PAP has a significantly higher correlation with the morphological characteristics of prostate cancer and can provide a more efficient prognosis than any other markers currently available. Since PAP is a proportional measure of prostate cancer progression, it can also be used in immunotherapy of prostate cancer. However, utility of PSA and other potential markers must also be considered to ensure best diagnosis and prognosis of prostate cancer. More molecular studies on PAP increase in prostate cancer and different forms of PAP including transmembrane PAP are needed to unveil the detailed mechanism of PAP in prostate cancer. Although PAP has been used as a marker of prostate cancer for decades, normal physiological functions of PAP must still be identified. Recent characterization of PAP’s involvement in pain suppression, oligospermia, and viral infection is shedding newer lights on the role played by PAP. To better understand the diverse roles of PAP in vivo, a systematic and integrated approach will be needed.
Acknowledgments
The present research was conducted by the research fund of Dankook University in 2010.
References
- 1.Abrahamsson P. A., Lilja H., Falkmer S., Wadström L. B. Immunohistochemical distribution of the three predominant secretory proteins in the parenchyma of hyperplastic and neoplastic prostate glands. Prostate. (1988);12:39–46. doi: 10.1002/pros.2990120106. [DOI] [PubMed] [Google Scholar]
- 2.Afzal S., Ahmad M., Mushtaq S., Mubarik A., Qureshi A. H., Khan S. A. Morphological features correlation with serum tumor markers in prostatic carcinoma. J. Coll. Physicians. Surg. Pak. (2003);13:511–514. [PubMed] [Google Scholar]
- 3.Antonarakis E. S., Drake C. G. Current status of immunological therapies for prostate cancer. Curr. Opin. Urol. (2010);20:241–246. doi: 10.1097/MOU.0b013e3283381793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azumi N., Traweek S. T., Battifora H. Prostatic acid phosphatase in carcinoid tumors: Immunohistochemical and immunoblot studies. Am. J. Surg. Pathol. (1991);15:758–790. doi: 10.1097/00000478-199108000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Batta A., Panag KMDS., Singh J. Diagnosis of prostate cancer --- Role of biomarkers. Int. J. Cur. Biomed. Phar. Res. (2012);2:339–345. [Google Scholar]
- 6.Becker C., Piironen T., Pettersson K., Björk T., Wojno K. J., Oesterling J. E., Lilja H. Discrimination of men with prostate cancer from those with benign disease by measurements of human glandular kallikrein 2 (HK2) in serum. J. Urol. (2000);163:311–316. [PubMed] [Google Scholar]
- 7.Brawer M. K., Chetner M. P., Beatie J., Buchner D. M., Vessella R. L., Lange P. H. Screening for prostatic carcinoma with prostate specific antigen. J. Urol. (1992);147:841–845. doi: 10.1016/s0022-5347(17)37401-3. [DOI] [PubMed] [Google Scholar]
- 8.Bussemakers M. J., van Bokhoven A., Verhaegh G. W., Smit F. P., Karthaus H. F., Schalken J. A., Debruyne F. M., Ru N., Isaacs W. B. DD3: a new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res. (1999);59:5975–5979. [PubMed] [Google Scholar]
- 9.Cheever M. A., Higano C. S. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. (2011);17:3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 10.Chen H., Pong R. C., Wang Z., Hsieh J. T. Differential regulation of the human gene DAB2IP in normal and malignant prostatic epithelia: cloning and characterization. Genomics. (2002);79:573–581. doi: 10.1006/geno.2002.6739. [DOI] [PubMed] [Google Scholar]
- 11.Collins K. A., Bennett A. T. Persistence of spermatozoa and prostatic acid phosphatase in specimens from decreased individuals during varied postmortem intervals. Am. J. Forensic Med. Pathol. (2011);22:228–232. doi: 10.1097/00000433-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Coussens L. M., Werb Z. Inflammation and cancer. Nature. (2002);420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dave B. N., Rindani T. H. Acid phosphatase activity in human semen. Int. J. Fertil. (1988);33:45–47. [PubMed] [Google Scholar]
- 14.Deftos L. J. Granin-A, parathyroid hormone-related protein, and calcitonin gene products in neuroendocrine prostate cancer. Prostate Suppl. (1998);8:23–31. doi: 10.1002/(sici)1097-0045(1998)8+<23::aid-pros5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 15.Drake C. G. Prostate cancer as a model for tumour immunotherapy. Nat. Rev. Immunol. (2010);10:580–593. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drake C. G. Interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J. Natl. Cancer Inst. (2012);104:1422. doi: 10.1093/jnci/djs340. [DOI] [PubMed] [Google Scholar]
- 17.Erbas H., Erten O., Irfanoglu M. E. Prostatic acid phosphatase in breast cyst fluid. Malays. J. Pathol. (2007);29:95–99. [PubMed] [Google Scholar]
- 18.Ercole C. J., Lange P. H., Mathisen M., Chiou R. K., Reddy P. K., Vessella R. L. Prostatic specific antigen and prostatic acid phosphatase in the monitoring and staging of patients with prostatic cancer. J. Urol. (1987);138:1181–1184. doi: 10.1016/s0022-5347(17)43543-9. [DOI] [PubMed] [Google Scholar]
- 19.Fang L.C., Dattoli M., Taira A., True L., Sorace R., Wallner K. Prostatic acid phosphatase adversely affects cause-specific survival in patients with intermediate to high-risk prostate cancer treated with brachytherapy. Urology. (2008);71:146–150. doi: 10.1016/j.urology.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 20.Garcia J. A. Sipuleucel-T in patients with metastatic castration-resistant prostate cancer: an insight for oncologists. Ther. Adv. Med. Oncol. (2011);3:101–108. doi: 10.1177/1758834010397692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. (1984);133:1710–1715. [PubMed] [Google Scholar]
- 22.Gerritsen W. R. The evolving role of immunotherapy in prostate cancer. Ann. Oncol. (2012);23:viii22–27. doi: 10.1093/annonc/mds259. [DOI] [PubMed] [Google Scholar]
- 23.Getzenberg R. H., Pienta K. J., Huang E. Y., Coffey D. S. Identification of nuclear matrix proteins in the cancer and normal rat prostate. Cancer Res. (1991);51:6514–6520. [PubMed] [Google Scholar]
- 24.Goldfarb D. A., Stein B. S., Shamszadeh M., Petersen R. O. Age-related changes in tissue level of prostatic acid phosphatase and prostate specific antigen. J. Urol. (1986);136:1266–1269. doi: 10.1016/s0022-5347(17)45310-9. [DOI] [PubMed] [Google Scholar]
- 25.Graddis T. J., McMahan C. J., Tamman J., Page K. J., Trager J. B. Prostatic acid phosphatase expression in human tissues. Int. J. Clin. Exp. Pathol. (2011);4:295–306. [PMC free article] [PubMed] [Google Scholar]
- 26.Gunia S., Koch S., May M., Dietel M., Erbersdobler A. Expression of prostatic acid phosphatase (PSAP) in transurethral resection specimens of the prostate is predictive of histopathologic tumor stage in subsequent radical prostatectomies. Virchows. Arch. (2009);454:573–579. doi: 10.1007/s00428-009-0759-1. [DOI] [PubMed] [Google Scholar]
- 27.Gupta A., Lotan Y., Ashfaq R., Roehrborn C. G., Raj G. V., Aragaki C. C., Montorsi F., Shariat S. F. Predictive value of the differential expression of the urokinase plasminogen activation axis in radical prostatectomy patients. Eur. Urol. (2009);55:1124–1133. doi: 10.1016/j.eururo.2008.06.054. [DOI] [PubMed] [Google Scholar]
- 28.Gutman A. B., Gutman E. B. An “acid” phosphatase occurring in the serum of patients with metastasizing carcinoma of the prostate gland. J. Clin. Invest. (1938);17:473–478. doi: 10.1172/JCI100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harvey A. M., Grice B., Hamilton C., Truong L. D., Ro J. Y., Ayala A. G., Zhai Q. J. Diagnostic utility of P504S/p63 cocktail, prostate-specific antigen, and prostatic acid phosphatase in verifying prostatic carcinoma involvement in seminal vesicles: a study of 57 cases of radical prostatectomy specimens of pathologic stage pT3b. Arch. Pathol. Lab. Med. (2010);134:983–988. doi: 10.5858/2009-0277-OA.1. [DOI] [PubMed] [Google Scholar]
- 30.Hassan M. I., Aijaz A., Ahmad F. Structural and functional analysis of human prostatic acid phosphatase. Expert. Rev. Anticancer Ther. (2010);10:1055–1068. doi: 10.1586/era.10.46. [DOI] [PubMed] [Google Scholar]
- 31.Hobisch A., Eder I. E., Putz T., Horninger W., Bartsch G., Klocker H., Culig Z. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res. (1998);58:4640–4645. [PubMed] [Google Scholar]
- 32.Hong S., Klein E. A., Das Gupta J., Hanke K., Weight C. J., Nguyen C., Gaughan C., Kim K. A., Bannert N., Kirchhoff F., Munch J., Silverman R. H. Fibrils of prostatic acid phosphatase fragments boost infections with XMRV (xenotropic murine leukemia virus-related virus), a human retrovirus associated with prostate. J. Virol. (2009);83:6995–7003. doi: 10.1128/JVI.00268-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsing A. W., Chokkalingam A. P. Prostate cancer epidemiology. Front. Biosci. (2006);11:1388–1413. doi: 10.2741/1891. [DOI] [PubMed] [Google Scholar]
- 34.Huang C. L., Brassil D., Rozzell M., Schellhammer P. F., Wright G. L. Jr. Comparison of prostate secretory protein with prostate specific antigen and prostatic acid phosphatase as a serum biomarker for diagnosis and monitoring patients with prostate carcinoma. Prostate. (1993);23:201–212. doi: 10.1002/pros.2990230303. [DOI] [PubMed] [Google Scholar]
- 35.Hubert R. S., Vivanco I., Chen E., Rastegar S., Leong K., Mitchell S. C., Madraswala R., Zhou Y., Kuo J., Raitano A. B., Jakobovits A., Saffran D. C., Afar D. E. STEAP: A prostate-specific cell-surface antigen highly expressed in human prostate tumors. Proc. Natl. Acad. Sci. USA. (1999);96:14523–14528. doi: 10.1073/pnas.96.25.14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurt J. K., Fitzpatrick B. J., Norris-Drouin J., Zylka M. J. Secretion and N-linked glycosylation are required for prostatic acid phosphatase catalytic and antinociceptive activity. PLoS One. (2012a);7:e32741. doi: 10.1371/journal.pone.0032741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurt J. K., Zylka M. J. PAPupuncture has localized and long-lasting antinociceptive effects in mouse models of acute and chronic pain. Mol. Pain. (2012b);8:28. doi: 10.1186/1744-8069-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jakob C. G., Lewinski K., Kuciel R., Ostrowski W., Lebioda L. Crystal structure of human prostatic acid phosphatase. Prostate. (2000);42:211–218. doi: 10.1002/(sici)1097-0045(20000215)42:3<211::aid-pros7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 39.Kong H. Y., Lee H. J., Byun J. Roles of prostatic acid phosphatase in prostate cancer. J. Life Sci. (2011);21:893–900. [Google Scholar]
- 40.Korkmaz C. G., Korkmaz K. S., Kurys P., Elbi C., Wang L., Klokk T. I., Hammarstrom C., Troen G., Svindland A., Hager G. L., Saatcioglu F. Molecular cloning and characterization of STAMP2, an androgen-regulated six transmembrane protein that is overexpressed in prostate cancer. Oncogene. (2005);24:4934–4945. doi: 10.1038/sj.onc.1208677. [DOI] [PubMed] [Google Scholar]
- 41.Korkmaz K. S., Elbi C., Korkmaz C. G., Loda M., Hager G. L., Saatcioglu F. Molecular cloning and characterization of STAMP1, a highly prostate-specific six transmembrane protein that is overexpressed in prostate cancer. J. Biol. Chem. (2002);277:36689–36696. doi: 10.1074/jbc.M202414200. [DOI] [PubMed] [Google Scholar]
- 42.Kraus T. S., Cohen C., Siddiqui M. T. Prostate-specific antigen and hormone receptor expression in male and female breast carcinoma. Diagn. Pathol. (2010);5:63. doi: 10.1186/1746-1596-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kristiansen G., Fritzsche F. R., Wassermann K., Jäger C., Tölls A., Lein M., Stephan C., Jung K., Pilarsky C., Dietel M., Moch H. GOLPH2 protein expression as a novel tissue biomarker for prostate cancer: implications for tissue-based diagnostics. Br. J. Cancer. (2008);99:939–948. doi: 10.1038/sj.bjc.6604614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuciel R., Bakalova A., Mazurkiewicz A., Bilska A., Ostrowski W. Is the subunit of prostatic phosphatase active?Reversibledenaturation of prostatic acid phosphatase. Biochem. Int. (1990);22:329–334. [PubMed] [Google Scholar]
- 45.Larne O., Martens-Uzunova E., Hagman Z., Edsjö A., Lippolis G., den Berg M. S., Bjartell A., Jenster G., Ceder Y. miQ - a novel microRNA based diagnostic and prognostic tool for prostate cancer. Int. J. Cancer. (2012) doi: 10.1002/ijc.27973.. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Lee H., Chu T. M., Li S. S., Lee C. L. Homodimer and heterodimer subunits of human prostatic acid phosphatase. Biochem. J. (1991);277:759–765. doi: 10.1042/bj2770759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee W. H., Morton R. A., Epstein J. I., Brooks J. D., Campbell P. A., Bova G. S., Hsieh W. S., Isaacs W. B., Nelson W. G. Cytidine methylation of regulatory sequences near the pi-class glutathione S-transferase gene accompanies human prostatic carcinogenesis. Proc. Natl. Acad. Sci. USA. (1994);91:11733–11737. doi: 10.1073/pnas.91.24.11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li T. S., Beling C. G. Isolation and characterization of two specific antigens of human seminal plasma. Fertil. Steril. (1973);24:134–144. doi: 10.1016/s0015-0282(16)39496-1. [DOI] [PubMed] [Google Scholar]
- 49.Lin M. F., Garcia-Arenas R., Xia X. Z., Biela B., Lin F. F. The cellular level of prostatic acid phosphatase and the growth of human prostate carcinoma cells. Differentiation. (1994);57:143–149. doi: 10.1046/j.1432-0436.1994.5720143.x. [DOI] [PubMed] [Google Scholar]
- 50.Lin M. F., Lee M. S., Zhou X. W., Andressen J. C., Meng T. C., Johansson S. L., West W. W., Taylor R. J., Anderson J. R., Lin F. F. Decreased expression of cellular prostatic acid phosphatase increases tumorigenicity of human prostate cancer cells. J. Urol. (2001);166:1943–1950. [PubMed] [Google Scholar]
- 51.Lin M. F., Meng T. C., Rao P. S., Chang C., Schonthal A. H., Lin F. F. Expression of human prostatic acid phosphatase correlates with androgen-stimulated cell proliferation in prostate cancer cell lines. J. Biol. Chem. (1998);273:5939–5947. doi: 10.1074/jbc.273.10.5939. [DOI] [PubMed] [Google Scholar]
- 52.Liu B. Q., Wu Y. D., Li P. H., Wei J. X., Zhang T., Liu R. L. Prostate cancer antigen-1 as a potential novel marker for prostate cancer. Asian J. Androl. (2007);9:821–826. doi: 10.1111/j.1745-7262.2007.00279.x. [DOI] [PubMed] [Google Scholar]
- 53.Lubaroff D. M. Prostate cancer vaccines in clinical trials. Expert. Rev. Vaccines. (2012);11:857–868. doi: 10.1586/erv.12.54. [DOI] [PubMed] [Google Scholar]
- 54.Madu C. O., Lu Y. Novel diagnostic biomarkers for prostate cancer. J. Cancer . (2010);1:150–177. doi: 10.7150/jca.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Makarov D. V., Loeb S., Getzenberg R. H., Partin A. W. Biomarkers for prostate cancer. Ann. Rev. Medicine. (2009);60:139–151. doi: 10.1146/annurev.med.60.042307.110714. [DOI] [PubMed] [Google Scholar]
- 56.McNeel D. G., Dunphy E.J., Davies J. G., Frye T. P., Johnson L. E., Staab M. J., Horvath D. L., Straus J., Alberti D., Marnocha R., Liu G., Eickhoff J. C., Wilding G. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with D0 prostate cancer. J. Clin. Oncol. (2009);27:4047–4054. doi: 10.1200/JCO.2008.19.9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meng T. C., Lee M. S., Lin M. F. Interaction between protein tyrosine phosphatase and protein tyrosine kinase is involved in androgen-promoted growth of human prostate cancer cells. Oncogene. (2000);19:2664–2677. doi: 10.1038/sj.onc.1203576. [DOI] [PubMed] [Google Scholar]
- 58.Merrick G. S., Butler W. M., Wallner K. E., Allen Z., DeFilippo J. L., Adamovich E. Enzymatic prostatic acid phosphatase in the clinical staging of patients diagnosed with prostate cancer. W. V. Med. J. (2005);101:116–119. [PubMed] [Google Scholar]
- 59.Münch J., Rücker E, Ständker L., Adermann K., Goffinet C., Schindler M., Wildum S., Chinnadurai R., Rajan D., Specht A., Giménez-Gallego G., Sánchez P. C., Fowler D. M., Koulov A., Kelly J. W., Mothes W., Grivel J. C., Margolis L., Keppler O. T., Forssmann W. G., Kirchhoff F. Semen-derived amyloid-fibrils drastically enhance HIV infection. Cell. (2007);131:1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 60.Okar D. A., Live D. H., Devany M. H., Lange A. J. Mechanism of the bisphosphatase reaction of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase probed by (1)H-(15)N NMR spectroscopy. Biochemistry. (2000);39:9754–9762. doi: 10.1021/bi000815k. [DOI] [PubMed] [Google Scholar]
- 61.Ortlund E., LaCount M. W., Lebioda L. Crystal structure of human prostatic acid phosphatase in complex with a phosphate ion and a-benzylaminobenzylphosphonic acid update the mechanistic picture and offer new insight into inhibitor design. Biochemistry. (2003);42:383–389. doi: 10.1021/bi0265067. [DOI] [PubMed] [Google Scholar]
- 62.Ostanin K., Saeed A., Van Etten R. L. Heterologous expression of human prostatic acid phosphatase and site-directed mutagenesis of the enzyme active site. J. Biol. Chem. (1994);269:8971–8978. [PubMed] [Google Scholar]
- 63.Ostrowski W. S., Kuciel R. Human prostatic acid phosphatase: selected properties and practical applications. Clin. Chim. Acta. (1994);226:121–129. doi: 10.1016/0009-8981(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 64.Porvari K., Kurkela R., Kivinen A., Vihko P. Differential androgen regulation of rat prostatic acid phosphatase transcripts. Biochem. Biophys. Res. Commun. (1995);213:861–868. doi: 10.1006/bbrc.1995.2208. [DOI] [PubMed] [Google Scholar]
- 65.Quintero I. B., Araujo C. L., Pulkka A. E., Wirkkala R. S., Herrala A. M., Eskelinen E. L., Jokitalo E., Hellström P. A., Tuominen H. J., Hirvikoski P. P., Vihko P. T. Prostatic acid phosphatase is not a prostate specific target. Cancer Res. (2007);67:6549–6554. doi: 10.1158/0008-5472.CAN-07-1651. [DOI] [PubMed] [Google Scholar]
- 66.Reif A. E., Schlesinger R. M., Fish C. A., Robinson C. M. Acid phosphatase isoenzymes in cancer of the prostate. Cancer. (1973);31:689–699. doi: 10.1002/1097-0142(197303)31:3<689::aid-cncr2820310331>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 67.Reiter R. E., Gu Z., Watabe T., Thomas G., Szigeti K., Davis E., Wahl M., Nisitani S., Yamashiro J., Le Beau M. M., Loda M., Witte O. N. Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc. Natl. Acad. Sci. USA. (1998);95:1735–1740. doi: 10.1073/pnas.95.4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rogers C. G., Yan G., Zha S., Gonzalgo M. L., Isaacs W. B., Luo J., De Marzo A. M., Nelson W. G., Pavlovich C. P. Prostate cancer detection on urinalysis for alpha methylacyl coenzyme A racemase protein. J. Urol. (2004);172:1501–1503. doi: 10.1097/01.ju.0000137659.53129.14. [DOI] [PubMed] [Google Scholar]
- 69.Roiko K., Jänne O. A., Vihko P. Primary structure of rat secretory acid phosphatase and comparison to other acid phosphatase. Gene. (1990);89:223–229. doi: 10.1016/0378-1119(90)90009-g. [DOI] [PubMed] [Google Scholar]
- 70.Ronnberg L., Vihko P., Sajanti E., Vihko R. Clomiphene citrate administration to normogonadotrophic subfertile men: blood hormone changes and activation of acid phosphatase in seminal fluid. Int. J. Androl. (1981);4:372–378. doi: 10.1111/j.1365-2605.1981.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 71.Roth T. J., Sheinin Y., Lohse C. M., Kuntz S. M., Frigola X., Inman B. A., Krambeck A. E., McKenney M. E., Karnes R. J., Blute M. L., Cheville J. C., Sebo T. J., Kwon E. D. B7-H3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. (2007);67:7893–7900. doi: 10.1158/0008-5472.CAN-07-1068. [DOI] [PubMed] [Google Scholar]
- 72.Rubenstein M., Hollowell C. M., Guinan P. Differentiated prostatic antigen expression in LNCaP cells following treatment with bispecific antisense oligonucleotides directed against BCL-2 and EGFR. Med. Oncol. (2012);29:835–841. doi: 10.1007/s12032-011-9977-x. [DOI] [PubMed] [Google Scholar]
- 73.Saito T., Hara N., Kitamura Y., Komatsubara S. Prostate-specific antigen/ prostatic acid phosphatase ratio is significant prognostic factor in patients with stage IV prostate cancer. Urology. (2007);70:702–705. doi: 10.1016/j.urology.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 74.Shan J., Porvari K., Kivinen A., Patrikainen L., Halmekytö M., Jönne J., Vihko P. Tissue-specific expression of the prostatic acid phosphatase promoter constructs. Biochem. Biophys. Res. Commun. (2003);311:864–869. doi: 10.1016/j.bbrc.2003.10.071. [DOI] [PubMed] [Google Scholar]
- 75.Shan J., Porvari K., Vihko P. GAAAATATGATA-like elements in androgen-associated regulation of the prostatic acid phosphatasegene. J. Steroid. Biochem. Mol. Biol. (2005);96:245–249. doi: 10.1016/j.jsbmb.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 76.Shariat S. F., Scherr D. S., Gupta A., Bianco F. J. Jr, Karakiewicz P. I., Zeltser I. S., Samadi D. B., Akhavan A. Emerging biomarkers for prostate cancer diagnosis, staging, and prognosis. Arch. Esp. Urol. (2011);64:681–694. [PubMed] [Google Scholar]
- 77.Sharma S., Juffer A. H. Hydrolysis of phosphohistidine in water and in prostatic acid phosphatase. Chem. Commun (Camb). (2009);14:6385–6387. doi: 10.1039/b910451h. [DOI] [PubMed] [Google Scholar]
- 78.Sheftic S., R. Snell, J. M., Jha S., Alexandrescu A. T. Inhibition of semen-derived enhancer of virus infection (SEVI) fibrillogenesis by zinc and copper. Eur. Biophys J. (2012);41:695–704. doi: 10.1007/s00249-012-0846-0. [DOI] [PubMed] [Google Scholar]
- 79.Sheridan T., Herawi M., Epstein J. I., Illei P. B. The role of P501S and PSA in diagnosis of metastatic adenocarcinoma of the prostate. Am. J. Surg. Pathol. (2007);31:1351–1355. doi: 10.1097/PAS.0b013e3180536678. [DOI] [PubMed] [Google Scholar]
- 80.Sims R. B. Development of sipuleucel-T: autologous cellular immunotherapy for the treatment of metastatic castrate resistant prostate cancer. Vaccine. (2012);30:4394–4397. doi: 10.1016/j.vaccine.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 81.Singh G., Adaikan P. G., Ng Y. K. Is seminal prostatic acid phosphatase a reliable marker for male infertility? Singapore Med. J. (1996);37:598–599. [PubMed] [Google Scholar]
- 82.Small E. J., Schellhammer P. F., Higano C. S., Redfern C. H., Nemunaitis J. J., Valone F. H., Verjee S. S., Jones L. A., Hershberg R. M. Placebo-controlled Phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J. Clin. Oncol. (2006);24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 83.Solin T., Kontturi M., Pohlmann R., Vihko P. Gene expression and prostate specificity of human prostatic acid phosphatase (PAP) : evaluation by RNA blot analyses. Biochim. Biophys. Acta. (1990);1048:72–77. doi: 10.1016/0167-4781(90)90024-v. [DOI] [PubMed] [Google Scholar]
- 84.Sowa N. A., Street S. E., Vihko P., Zylka M. J. Prostatic acid phosphatase reduces thermal sensitivity and chronic pain sensitization by depleting phosphatidylinositol 4,5-bisphosphate. J. Neurosci. (2010);30:10282–10293. doi: 10.1523/JNEUROSCI.2162-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sowa N. A., Vadakkan K. I., Zylka M. J. Recombinant mouse PAP has pH-dependent ectonucleotidase activity and acts through A(1)-adenosine receptors to mediate antinociception. PLoS One. (2009);4:e4248. doi: 10.1371/journal.pone.0004248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sreekumar A., Poisson L. M., Rajendiran T. M., Khan A. P., Cao Q., Yu J., Laxman B., Mehra R., Lonigro R. J., Li Y., Nyati M. K., Ahsan A., Kalyana-Sundaram S., Han B., Cao X., Byun J., Omenn G. S., Ghosh D., Pennathur S., Alexander D. C., Berger A., Shuster J. R., Wei J. T., Varambally S., Beecher C., Chinnaiyan A. M. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. (2009);457:910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 87.Stamey T. A., Yang N., Hay A. R., McNeal J. E., Freiha F. S., Redwine E. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N. Engl. J. Med. (1987);317:909–916. doi: 10.1056/NEJM198710083171501. [DOI] [PubMed] [Google Scholar]
- 88.Street S. E., Walsh P. L., Sowa N. A., Taylor-Blake B., Guillot T. S., Vihko P., Wightman R. M., Zylka M. J. PAP and NT5E inhibit nociceptive neurotransmission by rapidly hydrolyzing nucleotides to adenosine. Mol. Pain. (2011);7:80. doi: 10.1186/1744-8069-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Taira A., Merrick G., Wallner K., Dattoli M. Reviving the acid phosphatase test for prostate cancer. Oncology. (2007);21:1003–1010. [PubMed] [Google Scholar]
- 90.Taskén K. A., Angelsen A., Svindland A., Eide T., Berge V., Wahlquist R., Karlsen S. Markers for diagnosis, prediction and prognosis of prostate cancer. Tidsskr. Nor. Laegeforen. (2005);125:3279–3282. [PubMed] [Google Scholar]
- 91.Thompson T. C., Tahir S. A., Li L., Watanabe M., Naruishi K., Yang G., Kadmon D., Logothetis C. J., Troncoso P., Ren C., Goltsov A., Park S. The role of caveolin-1 in prostate cancer: clinical implications. Prostate Cancer Prostatic. Dis. (2010);13:6–11. doi: 10.1038/pcan.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tian W., Osawa M., Horiuchi H., Tomita Y. Expression of the prolactin-inducible protein (PIP/GCDFP15) gene in benign epithelium and adenocarcinoma of the prostate. Cancer Sci. (2004);95:491–495. doi: 10.1111/j.1349-7006.2004.tb03238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Truong L. D., Kadmon D., McCune B. K., Flanders K. C., Scardino P. T., Thompson T. C. Association of transforming growth factor-beta 1 with prostate cancer: An immunohistochemical study. Hum. Pathol. (1993);24:4–9. doi: 10.1016/0046-8177(93)90055-l. [DOI] [PubMed] [Google Scholar]
- 94.Van Etten R. L. Human prostatic acid phosphatase: a histidine phosphatase. Ann. N. Y. Acad. Sci. (1982);390:27–51. doi: 10.1111/j.1749-6632.1982.tb40302.x. [DOI] [PubMed] [Google Scholar]
- 95.Van Etten R. L., Davidson R., Stevis P. E., MacArthur H., Moore D. L. Covalent structure, disulfide binding, and identification of reactive surface and active site residues of human prostatic acid phosphatase. J. Biol. Chem. (1991);266:2313–2319. [PubMed] [Google Scholar]
- 96.Vanaja D. K., Ballman K. V., Morlan B. W., Cheville J. C., Neumann R. M., Lieber M. M., Tindall D. J., Young C. Y. PDLIM4 repression by hypermethylation as a potential biomarker for prostate cancer. Clin. Cancer Res. (2006);12:1128–1136. doi: 10.1158/1078-0432.CCR-05-2072. [DOI] [PubMed] [Google Scholar]
- 97.Veeramani S., Yuan T. C., Chen S. J., Lin F. F., Petersen J. E., Shaheduzzaman S., Srivastava S., MacDonald R. G., Lin M. F. Cellular prostatic acid phosphatase : a protein tyrosine phosphatase involved in androgen-independent proliferation of phosphatase. Endocr. Relat. Cancer. (2005);12:805–822. doi: 10.1677/erc.1.00950. [DOI] [PubMed] [Google Scholar]
- 98.Vieweg J., Dannull J. Technology insight: vaccine therapy for prostate cancer. Nat. Clin. Pract. Urol. (2005);2:44–51. doi: 10.1038/ncpuro0079. [DOI] [PubMed] [Google Scholar]
- 99.Vihko P., Herrala A., Härkönen P., Isomaa V., Kaija H., Kurkela R., Li Y., Patrikainen L., Pulkka A., Soronen P., Törn S. Enzymes as modulators in malignant transformation. J. Steroid Biochem. Mol. Biol. (2005);93:277–283. doi: 10.1016/j.jsbmb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 100.Vihko P., Kontturi M., Korhonen L. K. Purification of human prostatic acid phosphatase by affinity chromatography and isoelectric focusing. Part I. Clin. Chem. (1978);24:466–470. [PubMed] [Google Scholar]
- 101.Wang Y., Harada M., Yano H., Ogasawara S., Takedatsu H., Arima Y., Matsueda S., Yamada A., Itoh K. Prostatic acid phosphatase as a target molecule in specific immunotherapy for patients with nonprostate adenocarcinoma. J. Immunother. (2005);28:535–541. doi: 10.1097/01.cji.0000175490.26937.22. [DOI] [PubMed] [Google Scholar]
- 102.Wikström P., Lissbrant I. F., Stattin P., Egevad L., Bergh A. Endoglin (CD105) is expressed on immature blood vessels and is a marker for survival in prostate cancer. Prostate. (2002);51:268–275. doi: 10.1002/pros.10083. [DOI] [PubMed] [Google Scholar]
- 103.Winqvist R., Virkkunen P., Grzeschik K. H., Vihko P. Chromosomal localization to 3q21----qter and two TaqI RFLPs of the human prostatic-specific acid phosphatase gene (ACPP). Cytogenet. Cell Genet. (1989);52:68–71. doi: 10.1159/000132842. [DOI] [PubMed] [Google Scholar]
- 104.Young A., Palanisamy N., Siddiqui J., Wood D. P., Wei J. T., Chinnaiyan. A. M., Kunju L. P., Tomlins S. A. Correlation of Urine TMPRSS2:ERG and PCA3 to ERG and Total Prostate Cancer Burden. Am. J. Clin. Pathol. (2012);138:685–696. doi: 10.1309/AJCPU7PPWUPYG8OH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zelivianski S., Comeau D., Lin M. F. Cloning and analysis of the promoter activity of the human prostatic acid phosphatase gene. Biochem. Biophys. Res. Commun. (1998);245:108–112. doi: 10.1006/bbrc.1998.8386. [DOI] [PubMed] [Google Scholar]
- 106.Zelivianski S., Glowacki R., Lin M. F. Transcriptional activation of the human prostatic acid phosphatase gene by NF-κB via a novel hexanucleotide-binding site. Nucleic. Acid. Res. (2004);32:3566–3580. doi: 10.1093/nar/gkh677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang X. Q., Lee M. S., Zelivianski S., Lin M. F. Characterization of a prostate-specific tyrosine phosphatase by mutagenesis and expression in human prostate cancer cells. J. Biol. Chem. (2001);276:2544–2550. doi: 10.1074/jbc.M006661200. [DOI] [PubMed] [Google Scholar]
- 108.Zimmermann H. Prostatic acid phosphatase, a neglected ectonucleotidase. Purinergic Signal. (2009);5:273–275. doi: 10.1007/s11302-009-9157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zylka M. J., Sowa N. A., Taylor-Blake B., Twomey M. A., Herrala A., Voikar V., Vihko P. Prostatic acid phosphatase is an ectonucleotidase and suppresses pain by generating adenosine. Neuron. (2008);60:111–122. doi: 10.1016/j.neuron.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]


