Abstract
The purpose of the present study was to examine the effect of Lycii Radicis Cortex (LRC) and betaine (BT) on immobility and neurochemical change in the forced swimming test (FST) in the rat. LRC, BT or fluoxentine was administered intraperitoneally to Sprague-Dawley rats three times (1, 5 and 23.5 h) before the FST. To investigate antidepressant-like effect, serotonin (5-HT) and norepinephrine (NE) were examined in the hippocampus and hypothalamus of rats. LRC (100 mg/kg) and BT (30, 100 mg/kg) significantly decreased the immobility time in the FST. LRC (100 mg/kg) significantly increased both 5-HT and NE levels in the hypothalamus of rats exposed to FST. BT (100 mg/kg) significantly increased 5-HT levels in the hypothalamus and hippocampus of rats. Taken together, these results demonstrated that improvement in the behavioral changes after LRC and BT administration may be mediated by elevation of 5-HT level in the hypothalamus and hippocampus, indicating a possible antidepressant-like activity. The present results suggest that the efficacy of LRC and BT in an animal model of depression may provide anti-depressant effects in human, which remains to be determined.
Keywords: Lycii Radicis Cortex, Betaine, Depression, Norepinephrine, Serotonin
INTRODUCTION
Depression is a present major mental disorder, and it is a disturbance in mood characterized by a typical collection of symptoms, including a lowered mood and a loss of interest or pleasure in the events of life that interferes with the ability to work and logically communicate, and these disturbances could eventually lead to suicide (Johnson et al., 1992; Bland, 1997). The neurochemical imbalance of the central monoamines, in particular serotonin (5-HT), norepinephrine (NE) and dopamine (DA), has been related with the depressive state (Blier and de Montigny, 1994; Takahashi, 2011). Most of the currently used antidepressants are monoamine based, and they affect the inhibition of re-uptake or the metabolism of 5-HT, NE and DA (Hollister, 1990; Wong and Liu, 2012). However, these treatments are effective in only 60-70% of the depressed patients and they inevitably produce a variety of undesirable adverse effects such as cardiovascular disease and the delayed onset of symptom relief or pharmacological action (Möller and Volz, 1996; Taylor et al., 2011). Thus, there is a great need to develop more effective antidepressants. Clearly, screening compounds that come from traditional medicinal Abstractplants will be an important way to obtain new antidepressants.
Lycium chinense Mill. (family Solanaceae), namely Lycii Radicis Cortex (LRC) is a medicinal plant that is extensively consumed in Korea for the purpose of health improvement, and it is used in traditional oriental medicine in its unpurified form as a tonic agent. It is known to be remarkably effective in eliminating physical and mental fatigue. Previous papers have described the lipotropic action, the hypoglycemic and the hypocholesterolemic effects of the plant (Tang and Eisenbrand, 1992). The extract of the roots of LRC has been clinically prescribed as an oriental medicine for centuries. It has hypotensive, hypoglycaemic, antipyretic and anti-stress ulcer activities in experimental animal models (Ponasik et al., 1995; Han et al., 2002). LRC contained approximately 2-5 mg/g of betaine (BT) which is a derivative of choline and this is known to have a pharmacological action (Nishiyama, 1963; Schwab et al., 2006). BT (known as trimethylglycine) is the major bioactive compound obtained from LRC, and it has been described as having ability to alleviate fatty liver, inhibit the accumulation of triglyceride and cholesterol in the plasma and liver, enhance the regeneration of hepatocytes and prevent hypotension (Sprince et al., 1969; Barak et al., 1996; Kim et al., 1997; Eikelboom et al., 1999; Schwab et al., 2002). However, these pharmacological effects and especially the mechanism of the effects, have not been established yet, and the antidepressant effect of these extract, is still obscure. Thus, we evaluated the antidepressant activity of the LRC and BT for rats to get a better understanding of this extract.
The forced swimming test (FST) is a widely used screening test that can predict the efficacy of potential antidepressant compounds in rodents. The immobility induced by FST is well known as an animal model of the depressed state (Porsolt et al., 1977). This study examined the effect of LRC and BT on the immobility seen during the FST. Moreover, LRC and BT-induced alterations in the tissue levels of NE and 5-HT during FST were examined in the hippocampus and hypothalamus.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Samtaco, Kyungki-do, Korea), weighing 250-280 g were housed four per cage in a controlled environment (12:12-hour light:dark cycle, temperature 23 ± 3℃, humidity 50 ± 10%) for 7 days before starting experiments. Food and water were provided ad libitum. All procedure relating to the animals were approved by the Kyung Hee University Animal Care Committee for animal welfare [KHUASP(SE)-09-046] and their care conformed to Guidelines of the NIH and Korean Academy of Medical Sciences.
Preparation of the extract
The dried root of LRC was purchased from an oriental drug store (Jungdo Inc. Seoul, Korea). The voucher specimens documenting this purchase were deposited in the herbarium of the College of Oriental Medicine, Kyung Hee University. The LRC (300 g) was pulverized and extracted three times (3,000 ml each) in a reflux condenser at 100℃ for 4 h. The solution was combined, filtered through Whatman No. 2 filter paper, and concentrated using a rotary vacuum evaporator, this was followed by lyophilization. The yield of LRC was 8.7% (w/w).
Drug administration
BT (Betaine-HCl, Nacalai tesque, Inc., Japan) was used as major component from LRC and FLX (Fluoxetine-HCl, Eli Lilly Co., IN, USA) was used as standard drug for antidepressant effect. The effects of sub-chronic (23.5, 5 and 1 h after triple injections) drug administration were examined on the total duration of immobility for the rats during the FST. Rats were administered with LRC, BT, and FLX, respectively, 23.5, 5 and 1 h before the test swim session (Mancinelli et al., 1987). In all cases, the drugs were administered intraperitoneally in a volume equivalent to 2 ml/kg and the drug solutions were freshly prepared each morning. Doses were calculated as mg/kg of base and determined as described previously (Watanabe et al., 2002), and they were dissolved in isotonic saline solution (0.9% NaCl), except for the FLX that was solubilized in distilled water. All of the control rats received injections of saline solution only. The rats were assigned to one of six groups (n=4-6 rats per group): group 1: FST+vehicle (control); group 2: FST+BT 30 mg/kg (BT-30); group 3: FST+BT 100 mg/kg (BT-100); group 4: FST+LRC 100 mg/kg (LRC-100), group 5: FST+LRC 400 mg/kg (LRC-400); group 6: FST+FLX 10 mg/kg (FLX-10).
FST procedures
The procedure consisted of placing the rats in individual plexiglass cylinders (46 cm in height and 20 cm in diameter) that had previously been filled with the water (23-25℃) up to a depth of 30 cm from the bottom. The water level was deliberately chosen to be deeper than in the procedure described by Porsolt et al., in order to prevent the rats, from supporting themselves by touching the bottom with their hind limbs or tail (Porsolt et al., 1977). Clean water was used for each session, as use of the water previously swum in by another rat has been shown to alter behavior. In the pre-test session, rats were left to swim in the water for 15 min before being removed, dried with paper towels and then returned to their home cage. Twenty-four hours later the procedure was repeated during a 5 min test session. The drugs were administered in the period between the two swim sessions (23.5, 5, and 1 h prior to the start of the test session). Immobility was noted to occur, when animals remained in an immobile posture with making only minor movements to float. The time spent performing the behavior alteration test was measured using a video camera. The observer was blinded to the drug treatments.
Determination of brain monoamines concentrations
The animals were sacrificed by decapitation immediately after the FST. The brain was rapidly removed and then the hypothalamus and hippocampus were dissected out and placed onto an ice cold plate. All the tissue samples were quickly frozen and stored in a deep freezer at −80℃ until assayed. The samples weighed and then homogenized with a ultrasonic disruptor (Sonics Materials, INC, USA) in an ice cold 0.1 M perchloric acid (PCA) solution (600 μl) containing 0.1% sodium metabisulfate and 40 ng/ml of 3,4-dihydroxybenzylamine (DHBA) was used as an internal standard. After homogenization, the solution was centrifuged at 15,000 rpm in a micro 17R centrifuge (Micro 17R, Hanil Co. Korea) for 30 min at 4℃.
The levels of 5-HT and NE were determined by performing High Performance Liquid Chromatography (HPLC) coupled with electrochemical detection (ECD). Twenty μl sample of the supernatant was injected into a Bondapak C18 reverse-phase column (Waters Co., with a 300×3.9 mm internal diameter and a particle size of 5 μm). Determination of monoamines was done with an ECD (ESA, Coulochem II, Model 5200A), and a pump (ESA, Model 580). A guard cell (ESA, Model 5020) was set at +400 mV, the first and second electrodes of the analytical cell (ESA, Model 5011) were set at −40 and +200 mV, and the output of the second electrode was recorded as a chromatograph with using a HP 3395B printer (Hewlett Packard, Germany). The composition of the mobile phase was 150 mM sodium phosphate monobasic, 0.7 mM sodium octane sulfonate, 0.1 mM EDTA and 10% acetonitrile, and this was adjusted to pH 3.2 using 0.1 M phosphoric acid (flow rate of 1 ml/min). The tissue level of the monoamines was determined by performing a linear regression analysis for the peak heights obtained from a range of standard curves, and expressed as percentage above control level.
Statistical analysis
FST immobility time and monoamines data were analyzed by one-way analysis of variance (ANOVA) with drug administration. Post hoc comparisons were performed using the LSD test. The values are represented as group means with standard error of the mean (SEM). All data were considered statistical significant at p<0.05.
RESULTS
The effect of LRC and BT on the FST
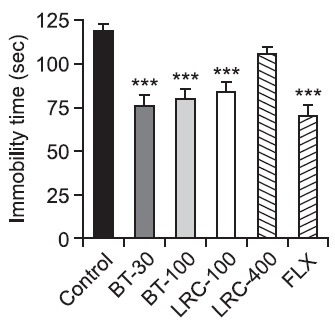
There was a significant effect of the drug administration for the immobility time in the FST as seen in Fig. 1. The immobility time during the 5 min of the FST was significantly different among the groups [F(5,30)=12.468, p<0.001]. Post hoc analysis revealed that the durations of immobility of the BT-30 and BT-100, the LRC-100 and the FLX-10 groups were significantly decreased compared with those of the control group. Both the BT-30 and the BT-100 groups had significantly decreased durations of immobility compared with the control group respectively (p<0.001). The LRC-100 group had a significantly decreased duration of immobility compared with the control group (p<0.001). The LRC-400 group had a slightly decreased duration of immobility but there was no significant difference among the groups (p>0.05). The FLX-10 group had a significantly decreased durations of immobility compared with the control group (p<0.001).
Fig. 1. Effects of LRC and BT on the immobility time during the FST. LRC (100 and 400 mg/kg, i.p.) and BT (30 and 100 mg/kg, i.p.) were sub-chronically (23.5, 5 and 1 h after three times) administered and FLX (10 mg/kg, i.p.) was used as a positive control. The data were analyzed by one way ANOVA and followed by LSD test. Values are represented as group means with standard errors of the mean (SEM) (n=6). ***p<0.001 vs. control group.
The effect of LRC and BT on brain monoamines
NE levels in the hypothalamus and hippocampus: There was a significant effect of the drug administration on the NE level in the hypothalamus (Fig. 2). The levels of NE during 5 min of the FST were significantly different among the groups [F(5,18)=3.168, p<0.05]. Post hoc analysis revealed that the LRC-100 group had a significantly increased NE level of the hypothalamus compared with the control group (p<0.01). The FLX-10 group had a slightly increased NE level of the hypothalamus, but there was not a significant difference among the groups (p>0.05). On the other hand, there was no significant effect of the drug administration on the NE level in the hippocampus (Fig. 3) [F(5,18)= 0.611, p>0.05].
Fig. 2. Effects of LRC and BT on the alteration of the NE level in the rat hypothalamus. LRC (100 and 400 mg/kg, i.p.) and BT (30 and 100 mg/kg, i.p.) were sub-chronically (23.5, 5 and 1 h after three times) administered and FLX (10 mg/kg, i.p.) was used as a positive control. The level of NE was expressed as percentage above control. The data were analyzed by one way ANOVA tests and followed by the LSD test. The values are represented as group means with SEM (n=4). **p<0.01 vs. control group.
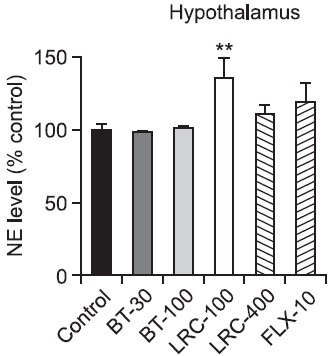
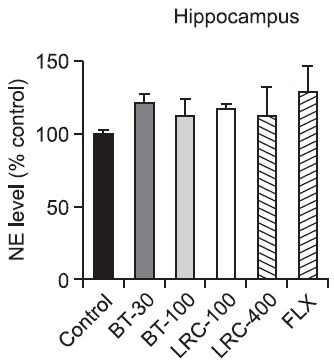
Fig. 3. Effects of LRC and BT on the alteration of the NE level in the rat hippocampus. LRC (100 and 400 mg/kg, i.p.) and BT (30 and 100 mg/kg, i.p.) were sub-chronically (23.5, 5 and 1 h after three times) administered and FLX (10 mg/kg, i.p.) was used as a positive control. The level of NE was expressed as percentage above control. Data were analyzed by one way ANOVA tests and this was followed by the LSD test. The values are represented as group means with SEM (n=4).
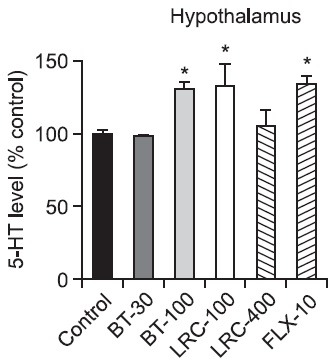
5-HT levels in the hypothalamus and hippocampus: There was a significant effect of drug administration on the 5-HT level in the hypothalamus (Fig. 4). The levels of 5-HT during the 5 min of the FST were significantly different among the groups in the hypothalamus [F(5,18)=4.192, p<0.05]. Post hoc analysis revealed that both the BT-100 group and the LRC-100 group had significantly increased 5-HT levels of the hypothalamus compared with the control group respectively (p<0.05). The FLX-10 group had also a significantly increased 5-HT level of the hypothalamus compared with the control group (p<0.05).
Fig. 4. Effects of LRC and BT on the alteration of the 5-HT level in the rat hypothalamus. LRC (100 and 400 mg/kg, i.p.) and BT (30 and 100 mg/kg, i.p.) were sub-chronically (23.5, 5 and 1 h after three times) administered and FLX (10 mg/kg, i.p.) was used as a positive control. The level of 5-HT was expressed as percentage above control. The data were analyzed by one way ANOVA tests and this was followed by LSD tests. The values are represented as group means with SEM (n=4). *p<0.05 vs. control group.
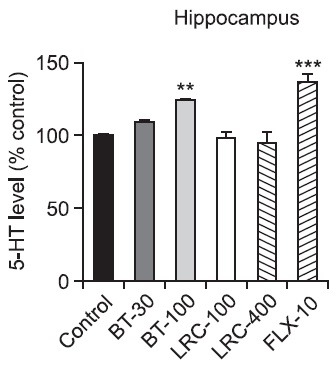
The levels of 5-HT were also significantly different among the groups in the hippocampus (Fig. 5) [F(5,18)=9.486, p<0.001]. Post hoc analysis revealed that the BT-100 group had significantly increased 5-HT levels of the hippocampus compared with the control group (p<0.01) and the FLX-10 group had also a significantly increased 5-HT level of the hippocampus compared with the control group (p<0.001).
Fig. 5. Effects of LRC and BT on the alteration of the 5-HT level in the rat hippocampus. LRC (100 and 400 mg/kg, i.p.) and BT (30 and 100 mg/kg, i.p.) were sub-chronically (23.5, 5 and 1 h after three times) administered and FLX (10 mg/kg, i.p.) was used as a positive control. The level of 5-HT was expressed as percentage above control. The data were analyzed by one way ANOVA tests and this was followed by LSD test. The values are represented as group means with SEM (n=4). **p<0.01, ***p<0.001 vs. control group.
DISCUSSION
Although the pathophysiology of depression is still unknown, there is a significant evidence for the abnormalities of the 5-HT and NE systems in depressive disorders. The pharmacological treatment of depression has been largely concerned with targeting the central monoaminergic system. Tricyclic antidepressants (TCAs), selective reversible inhibitors of monoamine oxidase A (RIMAs), selective serotonin reuptake inhibitors (SSRIs), selective-norepinephrine reuptake inhibitors (NRIs) and specific serotonin noradrenaline reuptake inhibitors (SNRIs) are all being clinically employed as effective antidepressant medications. Clinically effective antidepressant therapy includes drugs that have remarkable structural diversity that mostly affect monoamine re-uptake or metabolism. However, residual symptoms are relatively common among both the partial responders and the responders without remission, and the drugs inevitably produce a variety of undesirable adverse effects (Möller and Volz, 1996). In the present study, LRC (100 mg/kg, i.p.) and BT (30 and 100 mg/kg, i.p.) significantly decreased the immobility time compared with the vehicle-administered control rats, suggesting that these drugs have antidepressant-like activity. It was observed that fluoxetine (10 mg/kg) produced a decrease in immobility. Typically, the SSRIs increase swimming behavior and decrease the immobility during the FST (Page et al., 1999; Harkin et al., 2003). Data of FST showed that the activity of BT (30 mg/kg, i.p.) was quite close to that of the positive control fluoxetine. The observed behavioral changes were also accompanied by alterations in the rat brain’s monoaminergic activity. It is well known that an enhancement of level of 5-HT, NE, or both, underlies the antidepressant activity associated with most agents presently available to treat major depression (Blier and Abbott, 2001). In addition, recent studies have associated the depressive disorders with structural and functional dysfunctions such as decreases in volumes of the hippocampus (Sheline et al., 1999) and disruption of the neural connection within and between regions of the brain, including limbic, thalamic, and prefrontal cortical neuronal circuits (Liotti and Mayberg, 2001; Kosel and Schlaepfer, 2002). In this study, LRC and BT-induced alterations in 5-HT levels during FST were observed in the hippocampus and hypothalamus. An increase in 5-HT levels in the hypothalamus following a single acute FST exposure has been demonstrated in mice (Miura et al., 1993). The drug-induced increase in 5-HT level in rats exposed to the FST may reflect a decrease in 5-HT degradation during stress. The fact that these neurochemical alterations accompany LRC and BT-induced alterations in behavior during FST is certainly interesting. Indeed, the behavioral effects of LRC and BT during the FST are characteristic of the FST behavioral profile of antidepressant drugs that act predominantly on the central 5-HT system (Detke et al., 1995; Page et al., 1999; Cryan et al., 2005; Deltheil et al., 2008). It is clear that the central serotonergic system is particularly sensitive to both the administration of LRC, BT and exposure to the FST. Moreover, in the hypothalamus, LRC (100 mg/kg, i.p.) administration resulted in an increase in the tissue levels of NE in the rats exposed to the FST. Hwang et al. have reported an increase in NE uptake sites in the locus coeruleus of mice following restraint stress and exposure to the FST (Hwang et al., 1999). Furthermore, the reversible monoamine oxidase A inhibitor, moclobemide, also reduced NE turnover in a number of the brain regions in mice (Miura et al., 1993). LRC-induced increase in NE synthesis following its release combined with the inhibition of the metabolizing enzyme monoamine oxidase is a possible explanation for the increased NE levels that were observed, although conclusions to this effect cannot be drawn based solely on the present data. The response to stress varies depending upon the nature of the stressor, its duration and intensity, and the brain region or biochemical system under investigation. Thus, the monoamine systems respond with the production of a neurotransmitter profile that is individualized and characteristic for the particular stimulus applied. Differences between the nature, duration and intensity of the stress or the FST paradigm that is used, as well as species differences could account for these discrepancies. The mechanisms by which LRC produced its bimodal response still remain unclear. The present results were obtained with the LRC that may contain several active compounds. Further studies with using the pure compounds isolated from LRC are warranted and necessary to confirm the assumption that LRC probably contain several active compounds with possible antidepressant potential. Thus, in light of these data, we suggest the antidepressant effect could at least partly be linked to the improved effect of LRC on the functional deficiencies of the NE and 5-HT system at the neuron level.
A typical behavioral alteration was observed during the rats’ forced swimming posture of immobility and the neurochemical analysis of the rats’ brains revealed significant alterations in the monoamine levels. Both LRC and BT significantly decreased the duration of the rats’ immobility and increased level of monoamines in vivo brains of the depressed rats. These behavioral and neurochemical results indicate the antidepressant potentials of LRC and BT, which may be mediated by the serotonergic mechanisms in the rat brain. Further investigations aimed at characterization of these antidepressant compounds and at understanding their mode of action would be highly desirable. These drugs and their behavioral effects and pharmacological actions have been well characterized, and they may good candidates for further investigations that may ultimately result in their clinical use.
Acknowledgments
This work was supported by a post-doctoral fellowship grant from the Kyung Hee University in 2011 (KHU-20110693) and Science-based Korean medicine theory (KH12274) Project funded by Korea Institute of Oriental Medicine.
References
- 1.Barak A. J., Beckenhauer H. C., Tuma D. J. Betaine, ethanol, and the liver: a review. Alcohol. (1996);13:395–398. doi: 10.1016/0741-8329(96)00030-4. [DOI] [PubMed] [Google Scholar]
- 2.Bland R. C. Epidemiology of affective disorders: a review. Can. J. Psychiatry. (1997);42:367–377. doi: 10.1177/070674379704200403. [DOI] [PubMed] [Google Scholar]
- 3.Blier P., Abbott F. V. Putative mechanisms of action of antidepressant drugs in affective and anxiety disorders and pain. J. Psychiatry Neurosci. (2001);26:37–43. [PMC free article] [PubMed] [Google Scholar]
- 4.Blier P., de Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol. Sci. (1994);15:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- 5.Cryan J. F., Valentino R. J., Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci. Biobehav. Rev. (2005);29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Deltheil T., Guiard B. P., Cerdan J., David D. J., Tanaka K. F., Reperant C., Guilloux J. P., Coudore F., Hen R., Gardier A. M. Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice. Neuropharmacology. (2008);55:1006–1014. doi: 10.1016/j.neuropharm.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Detke M. J., Rickels M., Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) (1995);121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 8.Eikelboom J. W., Lonn E., Genest J. Jr., Hankey G., Yusuf S. Homocyst(e)ine and cardiovascular disease: a critical review of the epidemiologic evidence. Ann. Intern. Med. (1999);131:363–375. doi: 10.7326/0003-4819-131-5-199909070-00008. [DOI] [PubMed] [Google Scholar]
- 9.Han S. H., Lee H. H., Lee I. S., Moon Y. H., Woo E. R. A new phenolic amide from Lycium chinense Miller. Arch. Pharm. Res. (2002);25:433–437. doi: 10.1007/BF02976596. [DOI] [PubMed] [Google Scholar]
- 10.Harkin A., Shanahan E., Kelly J. P., Connor T. J. Methylenendioxyamphetamine produces serotonin nerve terminal loss and diminished behavioural and neurochemical responses to the antidepressant fluoxetine. Eur. J. Neurosci. (2003);18:1021–1027. doi: 10.1046/j.1460-9568.2003.02802.x. [DOI] [PubMed] [Google Scholar]
- 11.Hollister L. E. Problems in the search for cognition enhancers. Pharmacopsychiatry. (1990);23 Suppl 2:33–36. doi: 10.1055/s-2007-1014529. [DOI] [PubMed] [Google Scholar]
- 12.Hwang B. H., Kunkler P. E., Tarricone B. J., Hingtgen J. N., Nurnberger J. I. Jr. Stress-induced changes of norepinephrine uptake sites in the locus coeruleus of C57BL/6J and DBA/2J mice: a quantitative autoradiographic study using [3H]-tomoxetine. Neurosci. Lett. (1999);265:151–154. doi: 10.1016/s0304-3940(99)00241-4. [DOI] [PubMed] [Google Scholar]
- 13.Johnson J., Weissman M. M., Klerman G. L.. Service utilization and social morbidity associated with depressive symptoms in the community. JAMA. (1992);267:1478–1483. [PubMed] [Google Scholar]
- 14.Kim S. Y., Choi Y. H., Huh H., Kim J., Kim Y. C., Lee H. S. New antihepatotoxic cerebroside from Lycium chinense fruits. J. Nat. Prod. (1997);60:274–276. doi: 10.1021/np960670b. [DOI] [PubMed] [Google Scholar]
- 15.Kosel M., Schlaepfer T. E. Mechanisms and state of the art of vagus nerve stimulation. J. ECT. (2002);18:189–192. doi: 10.1097/00124509-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Liotti M., Mayberg H. S. The role of functional neuroimaging in the neuropsychology of depression. J. Clin. Exp. Neuropsychol. (2001);23:121–136. doi: 10.1076/jcen.23.1.121.1223. [DOI] [PubMed] [Google Scholar]
- 17.Mancinelli A., D'Aranno V., Borsini F., Meli A. Lack of relationship between effect of desipramine on forced swimming test and brain levels of desipramine or its demethylated metabolite in rats. Psychopharmacology (Berl) (1987);92:441–443. doi: 10.1007/BF00176475. [DOI] [PubMed] [Google Scholar]
- 18.Miura H., Naoi M., Nakahara D., Ohta T., Nagatsu T. Changes in monoamine levels in mouse brain elicited by forced-swimming stress, and the protective effect of a new monoamine oxidase inhibitor, RS-8359. J. Neural. Transm. Gen. Sect. (1993);94:175–187. doi: 10.1007/BF01277023. [DOI] [PubMed] [Google Scholar]
- 19.Möller H. J., Volz H. P. Drug treatment of depression in the 1990s. An overview of achievements and future possibilities. Drugs. (1996);52:625–638. doi: 10.2165/00003495-199652050-00001. [DOI] [PubMed] [Google Scholar]
- 20.Nishiyama R. Betaine of Lycium chinense. Nippon Shokuhin Kogyo Gakkaishi. (1963);10:517–519. [Google Scholar]
- 21.Page M. E., Detke M. J., Dalvi A., Kirby L. G., Lucki I. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology (Berl) (1999);147:162–167. doi: 10.1007/s002130051156. [DOI] [PubMed] [Google Scholar]
- 22.Ponasik J. A., Strickland C., Faerman C., Savvides S., Karplus P. A., Ganem B. Kukoamine A and other hydrophobic acylpolyamines: potent and selective inhibitors of Crithidia fasciculata trypanothione reductase. Biochem. J. (1995);311:371–375. doi: 10.1042/bj3110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porsolt R. D., Le Pichon M., Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. (1977);266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 24.Schwab U., Torronen A., Meririnne E., Saarinen M., Alfthan G., Aro A., Uusitupa M. Orally administered betaine has an acute and dose-dependent effect on serum betaine and plasma homocysteine concentrations in healthy humans. J. Nutr. (2006);136:34–38. doi: 10.1093/jn/136.1.34. [DOI] [PubMed] [Google Scholar]
- 25.Schwab U., Torronen A., Toppinen L., Alfthan G., Saarinen M., Aro A., Uusitupa M. Betaine supplementation decreases plasma homocysteine concentrations but does not affect body weight, body composition, or resting energy expenditure in human subjects. Am. J. Clin. Nutr. (2002);76:961–967. doi: 10.1093/ajcn/76.5.961. [DOI] [PubMed] [Google Scholar]
- 26.Sheline Y. I., Sanghavi M., Mintun M. A., Gado M. H. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J. Neurosci. (1999);19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sprince H., Parker C. M., Josephs J. A. Jr. Homocysteine-induced convulsions in the rat: protection by homoserine, serine, betaine, glycine and glucose. Agents Actions. (1969);1:9–13. doi: 10.1007/BF01990014. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi T. Neuroeconomics of suicide. Neuro. Endocrinol. Lett. (2011);32:400–404. [PubMed] [Google Scholar]
- 29.Tang W., Eisenbrand G. Chinese drugs of plant origin: Chemistry, Pharmacology, and Use in Traditional and Modern Medicine. Springer; New York: (1992). [Google Scholar]
- 30.Taylor D., Meader N., Bird V., Pilling S., Creed F., Goldberg D. Pharmacological interventions for people with depression and chronic physical health problems: systematic review and meta-analyses of safety and efficacy. Br. J. Psychiatry. (2011);198:179–188. doi: 10.1192/bjp.bp.110.077610. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe H., Kobayashi T., Tomii M., Sekiguchi Y., Uchida K., Aoki T., Cyong J. C. Effects of Kampo herbal medicine on plasma melatonin concentration in patients. Am. J. Chin. Med. (2002);30:65–71. doi: 10.1142/S0192415X02000077. [DOI] [PubMed] [Google Scholar]
- 32.Wong A. H., Liu F. Uncoupling the dopamine D1-D2 receptor complex: a novel target for antidepressant treatment. Clin. Pharmacol. Ther. (2012);91:298–302. doi: 10.1038/clpt.2011.311. [DOI] [PubMed] [Google Scholar]


