Abstract
Geniposide is an active product extracted from the gardenia fruit, and is one of the most widely used herbal preparations for liver disorders. This study examined the cytoprotective properties of geniposide and its metabolite, genipin, against hepatic ischemia/reperfusion (I/R) injury. C57BL/6 mice were subjected to 60 min of ischemia followed by 6 h of reperfusion. Geniposide (100 mg/kg) and genipin (50 mg/kg) were administered orally 30 min before ischemia. In the I/R mice, the levels of serum alanine aminotransferase and hepatic lipid peroxidation were elevated, whereas hepatic glutathione/glutathione disulfide ratio was decreased. These changes were attenuated by geniposide and genipin administration. On the other hand, increased hepatic heme oxygenase-1 protein expression was potentiated by geniposide and genipin administration. The increased levels of tBid, cytochrome c protein expression and caspase-3 activity were attenuated by geniposide and genipin. Increased apoptotic cells in the I/R mice were also significantly reduced by geniposide and genipin treatment. Our results suggest that geniposide and genipin offer significant hepatoprotection against I/R injury by reducing oxidative stress and apoptosis.
Keywords: Geniposide, Genipin, Ischemia, Reperfusion, Liver, Apoptosis
INTRODUCTION
Ischemia/reperfusion (I/R) injury to the liver is of clinical importance in humans after hemorrhagic and cardiogenic shock, liver surgery and liver transplantation (Sasaki et al., 1997). Although the nature of I/R has been widely studied, the mechanisms by which organ damage occurs are unclear. The initial hepatic I/R injury is known to be triggered by reactive oxygen species (ROS) with inflammation involving chemokines and cytokines, followed by neutrophil-mediated hepatic injury occurring in the late period of reperfusion (Jaeschke, 2006). Recent evidence has shown that ROS can also induce apoptosis, which is a mechanism for cell death following reperfusion of the ischemic liver (Yu et et al., 2011).
The fruit of Gardenia jasminoides Ellis (Rubiaceae) is a traditional Chinese medicinal source which has long been used for the treatment of inflammation, jaundice and hepatic disorders (Xu et al., 2008). Geniposide is one of the major iridoid glycosides of gardenia fruit. Geniposide has been shown to possess anti-inflammatory, anti-oxidant, anti-carcinogenic and anti-angiogenic activities (Liaw and Chao, 2001; Kuo et al., 2004). Oral administration of geniposide increased hepatic glutathione (GSH) content, which is responsible for hepatoprotection against aflatoxin B1-induced liver injury in rats (Kang et al., 1997). Furthermore, geniposide protected against hepatic steatosis in rats fed a high fat diet through its anti-oxidant actions and its regulation of adipocytokine release (Ma et al., 2011). Genipin, an aglycon of geniposide and a major component of gardenia fruit, not only demonstrated remarkable anti-inflammatory and anti-angiogenesis effects, but also inhibited lipid peroxidation and the production of nitric oxide (Koo et al., 2004b). Given that geniposide is hydrolyzed to genipin by intestinal bacteria (Akao et al., 1994), a detailed comparison of pharmacological activities of geniposide and genipin would be useful.
Therefore, we examined the protective effects of geniposide and genipin against I/R-induced hepatic injury, particularly on the oxidative stress and apoptosis.
MATERIALS AND METHODS
Hepatic I/R procedure
Male C57BL/6 mice weighing 23-25 g (Dae Han Biolink Co., Ltd., Eum-sung, Korea) were fasted for 18 h before the experiments but were provided with tap water ad libitum. All experiments were approved by the Animal Care Committee of Sungkyunkwan University and performed in accordance with the guidelines of the National Institute of Health. Mice were anesthetized intraperitoneally with ketamine (55 mg/kg body weight) and xylazine (7 mg/kg body weight), while body temperature was maintained at 37℃ throughout the anesthesia using heating pads. A transverse incision was made to the abdomen, and the left branches of the portal vein and hepatic artery were clamped with a micro-serrefine clip (Fine Science Tools Inc., Vancouver, BC, Canada) to induce complete ischemia of the median and left lobes of the liver. The right lobes remained perfused to prevent venous congestion of the intestine. After 60 min of ischemia, the clamp was removed to allow reperfusion. The wound was closed in layers using silk sutures and the animals were returned to their cages and allowed to recover from the anesthesia. The sham-operated animals underwent the same procedure but without vessel occlusion. The mice were sacrificed 6 h after reperfusion, and blood samples and ischemic liver tissues were collected. The liver tissues were immediately analyzed for histological changes (aliquots from the left lobe) and blood was examined for serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities. The remaining major portions of the liver tissues were immediately frozen in liquid nitrogen and kept at -75℃ for later analysis.
Administration of geniposide and genipin
Geniposide (ChromaDex Co., Irvine, CA, USA), dissolved in saline, and genipin (Wako Pure Chemical Industries., Ltd., Osaka, Japan), dissolved in 10% Tween 80 saline, were administered orally at 100 and 50 mg/kg, respectively, 30 min before the sustained ischemia. The dose and timing of geniposide and genipin treatments were selected based on previous reports (Koo et al., 2006) and our preliminary experiments. Mice were divided into six treatment groups: (1) vehicle-treated sham, (2) geniposide-treated sham, (3) genipin-treated sham, (4) vehicle-treated I/R, (5) geniposide-treated I/R, and (6) genipin-treated I/R. Since there were no differences in any of the parameters among the three sham groups, groups (1), (2) and (3) were pooled and are referred to as the sham group.
Serum aminotransferase activity
The serum ALT and AST activities were determined by standard spectrophotometric procedures using ChemiLab ALT and AST assay kits (IVDLab Co., Ltd., Uiwang, Korea), respectively.
Hepatic lipid peroxidation and glutathione content
The steady-state level of malondialdehyde (MDA), which is the end-product of lipid peroxidation, was analyzed in the liver homogenates by measuring the level of thiobarbituric acid reactive substances spectrophotometrically at a wavelength of 535 nm (Buege and Aust, 1978) using 1,1,3,3-tetraethoxypropane (Sigma-Aldrich, St. Louis, MO, USA) as the standard. After precipitation with 1% picric acid, the GSH level was determined in the liver homogenates using yeast-GSH reductase, 5,5’-dithio-bis(2-nitrobenzoic acid), and NADPH, at a wavelength of 412 nm (Tietze, 1969). The oxidized GSH (GSSG) level was measured by the same method in the presence of 2-vinylpyridine, and the GSH/GSSG ratio was calculated.
Western blot analysis
Cytosolic proteins were isolated from mice liver tissues using NE-PER® Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology, Rockford, IL, USA) according to the manufacturer’s instructions. A 20 μg sample of protein from each liver homogenate of cytosolic fraction was loaded per lane on various percent polyacrylamide gels for electrophoresis. The proteins were then transferred to nitrocellulose membranes and incubated at room temperature for 60 min in a buffer solution containing 5% dried skim milk to block non-specific binding. Bands were immunologically detected using polyclonal antibodies against mouse heme oxygenase-1 (HO-1) (Cell Signaling Technology Inc., Beverly., MA, USA; 1:1,000), truncated BH3 interacting domain death agonist (tBid) (Cell Signaling Technology Inc.; 1:1,000), cytochrome c (Cell Signaling Technology Inc.; 1:1,000) and monoclonal antibody against mouse β-actin (Sigma-Aldrich; 1:2,500). The binding of all antibodies was detected using an ECL detection system (iNtRON Biotechnology Co., Ltd., Sungnam, Korea) according to the manufacturer’s instruction. The visualized immunoreactive bands were evaluated densitometrically with ImageQuant TL software (Amersham Biosciences/GE Healthcare Life Science, Piscataway, NJ, USA).
Histological analysis
Liver tissues were fixed immediately in 10% neutral buffered formalin, embedded in paraffin and cut serially into 5 μm sections. For apoptotic cell detection, terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining, which labels the characteristic DNA double strand breaks, was performed using a commercially available kit (In situ Apoptosis Detection Kit; TaKaRa Co., Shiga, Japan). Under optical microscopy (OLYMPUS OPTICAL Co., Tokyo, Japan), the number of TUNEL-positive cells in ×400 histological fields were counted per liver section.
Caspase-3 activity
Caspase-3 activity was measured using a fluorogenic peptide substrate, N-acetyl-Asp-Glu-Val-Asp-7-amino-4-trifluoromethyl-cumarine (DEVD-AFC; Bio-Mol, Plymouth Meeting, PA, USA), according to a previous procedure (Morin et al., 2004). A 30 μg sample of the liver cytosolic protein was incubated in a buffer containing 30 mM N-[2-hydroxyethyl] piperazine-N’-[2-ethanesulfonic acid] (HEPES), 0.3 mM EDTA, 100 mM NaCl, 0.15% Triton X-100 and 10 mM dithiothreitol. The samples were incubated at room temperature for 15 min. The caspase reaction was then initiated by adding 200 μM DEVD-AFC, and the resulting mixture was incubated at 37℃. The change in fluorescence (excitation at 400 nm and emission at 490 nm) was monitored after 120 min.
Statistical analysis
All results are reported as the mean ± S.E.M. The overall significance of the data was examined by one-way analysis of variance (ANOVA). The differences between the groups were considered significant at p<0.05 with the appropriate Bonferroni correction made for multiple comparisons.
RESULTS
Serum aminotransferases activity
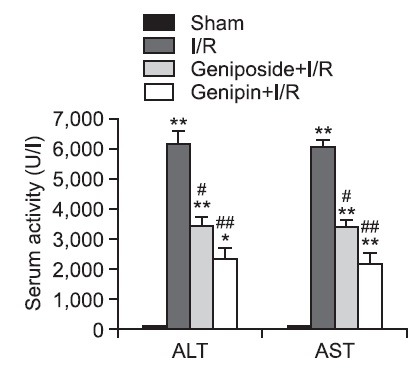
Serum ALT and AST activities were 97.4 ± 9.9 U/l and 107.9 ± 12.1 U/l in the sham group, respectively. After 6 h of reperfusion, the serum ALT and AST activities in the I/R group increased to approximately 63.4- and 56.1-fold of those in the sham group, respectively. These increases were significantly attenuated by geniposide and genipin treatment (Fig. 1).
Fig. 1. Effects of geniposide and genipin on serum ALT and AST activities. The results are presented as mean ± S.E.M. of eight animals per group. *,**Denote significant differences (p<0.05, p<0.01) compared with the sham group. #,##Denote significant differences (p<0.05, p<0.01) compared with the I/R group.
Hepatic lipid peroxidation, glutathione content and HO-1 protein expression
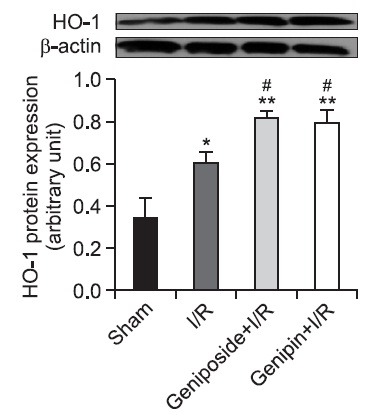
As shown in Table 1, the MDA level in the sham-operated liver tissues was 0.20 ± 0.01 nmol/mg protein. In the I/R group, however,evel increased approximately to two-fold of that seen in the sham group, which was attenuated by geniposide and genipin. In contrast, the GSH/GSSG ratio was significantly decreased to 2.87 ± 0.44 after 6 h of reperfusion compared to the sham-operated group of 8.87 ± 0.77. This decrease was also attenuated by geniposide and genipin pre-treatment. As shown in Fig. 2, the level of HO-1 protein expression increased after 6 h of reperfusion, by 177.1% compared to sham group. Protein expression was significantly potentiated to 240.1 and 233.7% by geniposide and genipin pre-treatment, respectively.
Table 1.
Effects of geniposide and genipin on hepatic MDA and GSH/GSSG ratio
| Group | Sham | I/R | Geniposide+I/R | Genipin+I/R |
|---|---|---|---|---|
| MDA (nmol/mg protein) | 0.20 ± 0.01 | 0.41 ± 0.03** | 0.31 ± 0.03# | 0.28 ± 0.04# |
| GSH/GSSG ratio | 8.87 ± 0.77 | 2.87 ± 0.44** | 5.88 ± 0.72# | 6.28 ± 0.29## |
The results are presented as mean ± S.E.M. of eight mice per group. **Denotes significant differences (p<0.01) compared with the sham group. #,## Denote significant differences (p<0.05, p<0.01) compared with the I/R group. GSH: Reduced glutathione, GSSG: Oxidized glutathione, I/R: Ischemia/reperfusion, MDA: Malondialdehyde.
Fig. 2. Effects of geniposide and genipin on HO-1 protein expression in hepatic I/R. After 6 h of reperfusion, HO-1 protein expression was measured from liver tissues. The results are presented as mean ± S.E.M. of eight animals per group. Blots shown are representative of three experiments with similar results. *,**Denote significant differences (p<0.05, p<0.01) compared with the sham group. #Denotes significant differences (p<0.05) compared with the I/R group.
Apoptotic cell detection and caspase-3 activity
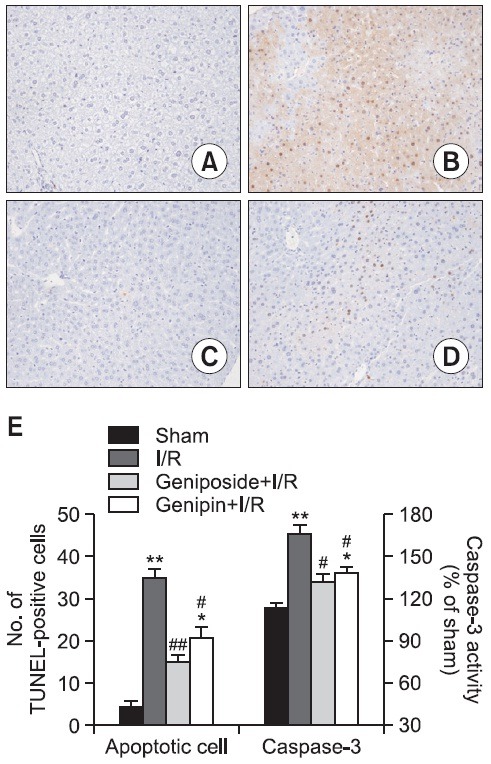
As shown in Fig. 3, a large number of TUNEL-positive hepatocytes were observed in the liver tissues obtained 6 h after reperfusion (B). However, few TUNEL positive hepatocytes were observed in the livers of mice treated with geniposide (C) and genipin (D). These results were confirmed with microscopic TUNEL-positive cell counting. Resembling the TUNEL assay result, caspase-3 activity in the cytosolic fraction of the ischemic liver was significantly higher than that from the sham-operated animals and was attenuated by geniposide and genipin pre-treatment (E).
Fig. 3. Anti-apoptotic effects of geniposide and genipin after hepatic I/R. TUNEL stained histology results of serial sections of mice liver after hepatic I/R (A-D, Original magnification × 400). (A) Sham group; (B) I/R group: a large number of TUNEL-positive hepatocytes were detected; (C) Geniposide (100 mg/kg)-treated I/R group and (D) Genipin (50 mg/kg)-treated I/R group: only a few TUNEL-positive cells were detected. (E) Number of TUNEL-positive hepatocytes and caspase-3 activity in I/R liver tissues. Numbers are apoptotic cells in randomly chosen ×400 histological fields and caspase-3 activities of I/R groups in comparison with sham-operated animals. The results are presented as mean ± S.E.M. of eight animals per group. *,**Denote significant differences (p<0.05, p<0.01) compared with the sham group. #,##Denote significant differences (p<0.05, p<0.01) compared with the I/R group.
tBID and cytosolic cytochrome c protein expression
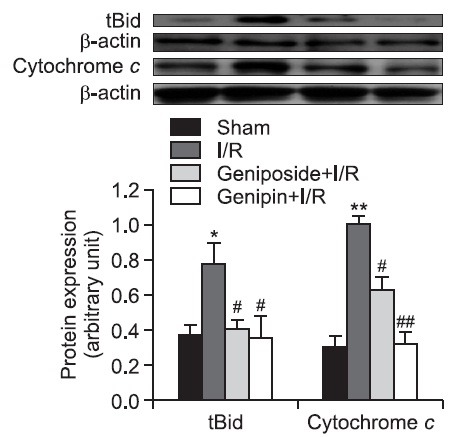
In the I/R group, the levels of tBid and cytochrome c protein expression in cytosol significantly increased after 6 h of reperfusion compared with those in the sham-operated animals. These increases were significantly attenuated by geniposide and genipin pre-treatment (Fig. 4).
Fig. 4. Effects of geniposide and genipin on tBid and cytochrome c protein expression in hepatic I/R. Levels of tBid and cytosolic cytochrome c protein expressions were measured from liver tissues. The results are presented as mean ± S.E.M. of eight animals per group. Blots shown are representative of three experiments with similar results. *,**Denote significant differences (p<0.05, p<0.01) compared with the sham group. #,##Denote significant differences (p<0.05, p<0.01) compared with the I/R group.
DISCUSSION
ROS plays an important role in hepatic injury associated with I/R and initiate lipid peroxidation, resulting in structural and functional damage to the organelles. Endogenous antioxidant levels decreased significantly during reperfusion (Marubayashi et al., 1987). Therefore, the administration of exogenous antioxidants, particularly from natural products, could possibly decrease the severity of I/R damage.
The bioactivities of geniposide have been identified in numerous studies, including anti-oxidant (Yin et al., 2010), antitumor (Peng et al., 2005), and anti-inflammation bioactivities, however, the most important aspect of geniposide concerns its anti-oxidative capacity. Geniposide protected rat hippocampal slice cultures against oxygen and glucose deprivation-induced neuronal cell death (Lee et al., 2006). Furthermore, geniposide was a novel agonist of glucagon-like peptide 1 receptor, which protected PC12 cells from oxidative stress (Liu et al., 2007). Recently, geniposide was reported to have a protective effect against hepatic steatosis via anti-oxidant actions (Ma et al., 2011).
Since most herbal medicines used in China, Korea and Japan are orally administered to humans, their components are inevitably brought into contact with intestinal microflora. Geniposide is hydrolyzed to genipin by β-D-glucosidases in the intestine and the liver (Akao et al., 1994). Therefore, to understand the biological effects of geniposide and genipin, the pharmacological activity should be studied in animal models. While geniposide and genipin showed similar tendencies to inhibit platelet aggregation (Suzuki et al., 2001), genipin, not geniposide, was shown to have an inhibitory effect on lipid peroxidation induced by Fe2+/ascorbate in rat brain homogenate (Koo et al., 2004b). Geniposide possessed a much stronger anti-angiogenic activity than genipin in a chick embryo chorioallantoic membrane assay (Koo et al., 2004a).
In the present study, mice challenged with I/R showed a significant increase in lipid peroxidation which coincided with a rapid decrease in the GSH/GSSG ratio. Reduced GSH plays an important role as a free radical scavenger to counteract the deleterious effect of ROS. These changes were attenuated by geniposide and genipin. Endogenous HO-1, which is ubiquitously expressed and highly induced by a number of stress stimuli, plays an important role in a host’s defense against oxidative injury. Our study demonstrated that the overexpression of HO-1 protects the liver against I/R injury by modulating oxidative stress (Yun et al., 2010). Furthermore, treatment with geniposide led to the activation of nuclear factor-E2-related factor 2 and its downstream target, HO-1, in primary hippocampal neurons (Yin et al., 2010). We found that geniposide and genipin augmented HO-1 protein expression after reperfusion. These observations paralleled the biochemical marker of hepatocellular damage. The increases in aminotransferase activities were attenuated in the geniposide- and genipin-treated animals. Our results suggest that geniposide and genipin protect against I/R injury by enhancing anti-oxidant capacity.
ROS play a central role in regulating cell signaling for survival or death. Levels that exceed defense capabilities or sustained production of ROS are potent inducers of apoptotic cell death (Eum et al., 2007). Death receptor signaling or cellular stresses activate many of the proteins involved in apoptosis, such as the proteases caspase-3 and -8. In the case of mitochondria-mediated apoptosis involving ROS, mitochondrial depolarization occurs due to membrane potential collapse and results in cytochrome c moving into cytosol, activating an apoptosis cascade (Green and Reed, 1998). When the liver was subjected to ischemia, the anti-apoptotic X-linked inhibitor of apoptosis protein decreased in the liver, and hepatocytes became sensitized to cell death. Inhibition of caspases also markedly reduced liver damage after I/R, suggesting that apoptosis plays a key role in organ damage induced by I/R (Cursio et al., 1999). Although geniposide and genipin have potent activities to regulate apoptotic signaling, the effects of geniposide and genipin on apoptosis are controversial in different experimental settings. In PC12 cells, geniposide induced the PI3 kinase signaling pathway and the expression of anti-apoptotic protein Bcl-2 (Liu et al., 2009). However, geniposide did not affect apoptotic signaling in HepG2 cells (Khanal et al., 2012). Genipin induced apoptosis with increased expression of caspase-3 and the pro-apoptotic protein, Bax, in HepG2 cells (Hong and Kim, 2007). In contrast, pre-treatment of mice with genipin protected them from hepatocyte apoptosis induced by lipopolysaccharide/galactosamine (Shang et al., 2009). In the present study, I/R increased the number of TUNEL-positive cells, caspase-3 activity and the cytosolic release of mitochondrial cytochrome c, which were suppressed by geniposide and genipin. The levels of tBid, one of the pro-apoptotic Bcl-2 proteins which amplifies apoptotic pathways by activating another pro-apoptotic molecules (Wei et al., 2001), significantly increased after reperfusion and this increase was attenuated by geniposide and genipin. Overall, these results indicate that the hepatoprotective effects of geniposide and genipin might be associated with anti-apoptotic properties.
In conclusion, we demonstrated that oral administration of geniposide and genipin offer significant hepatoprotection against I/R injury by reducing oxidative stress and apoptotic cell death. Since geniposide goes through intestinal metabolism before being converting into genipin, the protective effect revealed in this study is likely to be drawn from genipin itself. However considering the superior stability and solubility of geniposide, it should be given priority as a candidate for potential therapeutic agent on I/R injury.
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0028646).
References
- 1.Akao T., Kobashi K., Aburada M. Enzymic studies on the animal and intestinal bacterial metabolism of geniposide. Biol. Pharm. Bull. (1994);17:1573–1576. doi: 10.1248/bpb.17.1573. [DOI] [PubMed] [Google Scholar]
- 2.Buege J. A., Aust S. D. Microsomal lipid peroxidation. Methods Enzymol. (1978);52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 3.Cursio R., Gugenheim J., Ricci J. E., Crenesse D., Rostagno P., Maulon L., Saint-Paul M. C., Ferrua B., Auberger A. P. A caspase inhibitor fully protects rats against lethal normothermic liver ischemia by inhibition of liver apoptosis. FASEB J. (1999);13:253–261. doi: 10.1096/fasebj.13.2.253. [DOI] [PubMed] [Google Scholar]
- 4.Eum H. A., Cha Y. N., Lee S. M. Necrosis and apoptosis: sequence of liver damage following reperfusion after 60 min ischemia in rats. Biochem. Biophys. Res. Commun. (2007);358:500–505. doi: 10.1016/j.bbrc.2007.04.153. [DOI] [PubMed] [Google Scholar]
- 5.Green D. R., Reed J. C. Mitochondria and apoptosis. Science. (1998);281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 6.Hong H. Y., Kim B. C. Mixed lineage kinase 3 connects reactive oxygen species to c-Jun NH2-terminal kinase-induced mitochondrial apoptosis in genipin-treated PC3 human prostate cancer cells. Biochem. Biophys. Res. Commun. (2007);362:307–312. doi: 10.1016/j.bbrc.2007.07.165. [DOI] [PubMed] [Google Scholar]
- 7.Jaeschke H. Mechanisms of liver injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am. J. Physiol. Gastrointest. Liver Physiol. (2006);290:G1083–1088. doi: 10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- 8.Kang J. J., Wang H. W., Liu T. Y., Chen Y. C., Ueng T. H. Modulation of cytochrome P-450-dependent monooxygenases, glutathione and glutathione S-transferase in rat liver by geniposide from Gardenia jasminoides. Food Chem. Toxicol. (1997);35:957–965. doi: 10.1016/s0278-6915(97)87265-1. [DOI] [PubMed] [Google Scholar]
- 9.Khanal T., Kim H. G., Choi J. H., Do M. T., Kong M. J., Kang M. J., Noh K., Yeo H. K., Ahn Y. T., Kang W., Kim D. H., Jeong T. C., Jeong H. G. Biotransformation of geniposide by human intestinal microflora on cytotoxicity against HepG2 cells. Toxicol. Lett. (2012);209:246–254. doi: 10.1016/j.toxlet.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Koo H. J., Lee S., Shin K. H., Kim B. C., Lim C. J., Park E. H. Geniposide, an anti-angiogenic compound from the fruits of Gardenia jasminoides. Planta Med. (2004a);70:467–469. doi: 10.1055/s-2004-818978. [DOI] [PubMed] [Google Scholar]
- 11.Koo H. J., Lim K. H., Jung H. J., Park E. H. Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. J. Ethnopharmacol. (2006);103:496–500. doi: 10.1016/j.jep.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Koo H. J., Song Y. S., Kim H. J., Lee Y. H., Hong S. M., Kim S. J., Kim B. C., Jin C., Lim C. J., Park E. H. Antiinflammatory effects of genipin, an active principle of gardenia. Eur. J. Pharmacol. (2004b);495:201–208. doi: 10.1016/j.ejphar.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 13.Kuo W. H., Wang C. J., Young S. C., Sun Y. C., Chen Y. J., Chou F. P. Differential induction of the expression of GST subunits by geniposide in rat hepatocytes. Pharmacology. (2004);70:15–22. doi: 10.1159/000074238. [DOI] [PubMed] [Google Scholar]
- 14.Lee P., Lee J., Choi S. Y., Lee S. E., Lee S., Son D. Geniposide from Gardenia jasminoides attenuates neuronal cell death in oxygen and glucose deprivation-exposed rat hippocampal slice culture. Biol. Pharm. Bull. (2006);29:174–176. doi: 10.1248/bpb.29.174. [DOI] [PubMed] [Google Scholar]
- 15.Liaw J., Chao Y. C. Effect of in vitro and in vivo aerosolized treatment with geniposide on tracheal permeability in ovalbumin-induced guinea pigs. Eur. J. Pharmacol. (2001);433:115–121. doi: 10.1016/s0014-2999(01)01506-0. [DOI] [PubMed] [Google Scholar]
- 16.Liu J., Yin F., Zheng X., Jing J., Hu Y. Geniposide, a novel agonist for GLP-1 receptor, prevents PC12 cells from oxidative damage via MAP kinase pathway. Neurochem. Int. (2007);51:361–369. doi: 10.1016/j.neuint.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Liu J. H., Yin F., Guo L. X., Deng X. H., Hu Y. H. Neuroprotection of geniposide against hydrogen peroxide induced PC12 cells injury: involvement of PI3 kinase signal pathway. Acta Pharmacol. Sin. (2009);30:159–165. doi: 10.1038/aps.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma T., Huang C., Zong G., Zha D., Meng X., Li J., Tang W. Hepatoprotective effects of geniposide in a rat model of nonalcoholic steatohepatitis. J. Pharm. Pharmacol. (2011);63:587–593. doi: 10.1111/j.2042-7158.2011.01256.x. [DOI] [PubMed] [Google Scholar]
- 19.Marubayashi S., Dohi K., Ochi K., Kawasaki T. Protective effects of free radical scavenger and antioxidant administration on ischemic liver cell injury. Transplant. Proc. (1987);19:1327–1328. [PubMed] [Google Scholar]
- 20.Morin D., Pires F., Plin C., Tillement J. P. Role of the permeability transition pore in cytochrome C release from mitochondria during ischemia-reperfusion in rat liver. Biochem. Pharmacol. (2004);68:2065–2073. doi: 10.1016/j.bcp.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Peng C. H., Huang C. N., Wang C. J. The anti-tumor effect and mechanisms of action of penta-acetyl geniposide. Curr. Cancer Drug Targets. (2005);5:299–305. doi: 10.2174/1568009054064633. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki H., Matsuno T., Ishikawa T., Ishine N., Sadamori H., Yagi T., Tanaka N. Activation of apoptosis during early phase of reperfusion after liver transplantation. Transplant. Proc. (1997);29:406–407. doi: 10.1016/s0041-1345(96)00138-8. [DOI] [PubMed] [Google Scholar]
- 23.Shang Y., Liu Y., Du L., Wang Y., Cheng X., Xiao W., Wang X., Jin H., Yang X., Liu S., Chen Q. Targeted expression of uncoupling protein 2 to mouse liver increases the susceptibility to lipopolysaccharide/galactosamine-induced acute liver injury. Hepatology. (2009);50:1204–1216. doi: 10.1002/hep.23121. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki Y., Kondo K., Ikeda Y., Umemura K. Antithrombotic effect of geniposide and genipin in the mouse thrombosis model. Planta Med. (2001);67:807–810. doi: 10.1055/s-2001-18842. [DOI] [PubMed] [Google Scholar]
- 25.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal. Biochem. (1969);27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 26.Wei M. C., Zong W. X., Cheng E. H., Lindsten T., Panoutsakopoulou V., Ross A. J., Roth K. A., MacGregor G. R., Thompson C. B., Korsmeyer S. J. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. (2001);292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu M., Sun Q., Su J., Wang J., Xu C., Zhang T., Sun Q. Microbial transformation of geniposide in Gardenia jasminoides Ellis into genipin by Penicillium nigricans. Enzyme Microb. Technol. (2008);42:440–444. [Google Scholar]
- 28.Yin F., Liu J., Zheng X., Guo L., Xiao H. Geniposide induces the expression of heme oxygenase-1 via PI3K/Nrf2-signaling to enhance the antioxidant capacity in primary hippocampal neurons. Biol. Pharm. Bull. (2010);33:1841–1846. doi: 10.1248/bpb.33.1841. [DOI] [PubMed] [Google Scholar]
- 29.Yu H. C., Qin H. Y., He F., Wang L., Fu W., Liu D., Guo F. C., Liang L., Dou K. F., Han H. Canonical Notch pathway protects hepatocytes from ischemia/reperfusion injury in mice by repressing reactive oxygen species production through JAK2/STAT3 signaling. Hepatology. (2011);54:979–988. doi: 10.1002/hep.24469. [DOI] [PubMed] [Google Scholar]
- 30.Yun N., Eum H. A., Lee S. M. Protective role of heme oxygenase-1 against liver damage caused by hepatic ischemia and reperfusion in rats. Antioxid. Redox Signal. (2010);13:1503–1512. doi: 10.1089/ars.2009.2873. [DOI] [PubMed] [Google Scholar]


