Abstract
Objective of present study was to prepare and characterize self-nanoemulsifying drug delivery system (SNEDDS) of lutein and to evaluate its effect on bioavailability of warfarin. The SNEDDS was prepared using an oil, a surfactant, and co-surfactants with optimal composition based on pseudo-ternary phase diagram. Effect of the SNEDDS on the bioavailability of warfarin was performed using Sprague Dawley rats. Lutein was successfully formulated as SNEDDS for immediate self-emulsification and dissolution by using combination of Peceol as oil, Labrasol as surfactant, and Transcutol-HP or Lutrol-E400 as co-surfactant. Almost complete dissolution was achieved after 15 min while lutein was not detectable from the lutein powder or intra-capsule content of a commercial formulation. SNEDDS formulation of lutein affected bioavailability of warfarin, showing about 10% increase in Cmax and AUC of the drug in rats while lutein as non-SNEDDS did not alter these parameters. Although exact mechanism is not yet elucidated, it appears that surfactant and co-surfactant used for SNEDDS formulation caused disturbance in the anatomy of small intestinal microvilli, leading to permeability change of the mucosal membrane. Based on this finding, it is suggested that drugs with narrow therapeutic range such as warfarin be administered with caution to avoid undesirable drug interaction due to large amount of surfactants contained in SNEDDS.
Keywords: Self-nanoemulsifying drug delivery systems, Lutein, Warfarin, Dissolution, Bioavailability
INTRODUCTION
Carotenoids, poorly soluble lipophilic compounds, are a group of widely distributed plant pigments that have various physiological activities in human and animals. Lutein is one among several carotenoids that has been gaining attention lately because of its ability to prevent ocular diseases including age-related macular degeneration (AMD) and cataracts caused by complications of metabolic disorders such as diabetes (Hankinson et al., 1992; Seddon et al., 1994; Brown et al., 1999; Chasan-Taber et al., 1999; Lyle et al., 1999; Mares-Perlman et al., 2001; Gale et al., 2001). Lutein possesses an-tioxidant activity due to its conjugated double bonds that are highly effective in quenching reactive oxygen species that is involved in the pathogenesis of ocular diseases such as AMD and cataracts. Although lutein is a vital macular pigment with many beneficial activities, human is not capable of synthesizing lutein de novo and thus its presence in human tissues is entirely of dietary origin (Granado et al., 1996; Landrum and Bone, 2001; O’Neill et al., 2001; Johnson, 2004). Furthermore, bioavailability of lutein is reported to be very low (Chung et al., 2004; Granado-Lorencio et al., 2010). There have been many studies reported to improve its bioavailability by using various strategies such as mixed micelle and suspension formulations (Cha et al., 2011; Mamatha and Baskaran, 2011; Mitri et al., 2011; Shanmugam et al., 2011).
Lipid-based oral drug delivery system has been gaining attention recently with increasing application of lipid as a carrier for the delivery of poorly soluble lipophilic drugs (Pouton, 2006; Chakraborty et al., 2009). The unique properties of lipids, namely, their physicochemical diversity, biocompatibility, and ability to enhance oral bioavailability of poorly water soluble lipophilic drugs through selective lymphatic uptake have made them attractive candidates as carriers for oral formulations. Among those, self-nanoemulsifying drug delivery system (SNEDDS) is considered as a promising approach to improve solubility and absorption of poorly water soluble lipophilic drugs (Shao et al., 2010; Wu et al., 2011; Ma et al., 2012; Vithlani et al., 2012).
Although the exact mechanism is not yet fully understood, lutein has been thought to be absorbed through enterocytes by simple diffusion or receptor-mediated transport (Yonekura and Nagao, 2007). Primarily, lutein is emulsified into small lipid droplets in the stomach and further incorporated into mixed micelles by the action of bile salts and biliary phospholipids, after which mixed micelles are taken up by enterocytes with the aid of the scavenger receptor class B type I (SR-BI), a member of the ATP-binding cassette (ABC) transporter super-family. Accordingly, it is conceivable that the SNEDDS may enhance gastrointestinal absorption of lutein.
However, the SNEDDS may also affect the gastrointestinal absorption of other drugs. This is especially of concern to drugs with narrow therapeutic index or drugs used for the treatment of chronic diseases. Warfarin is a typical example of such drugs because it is often prescribed for a lifetime and prone to over- or under-coagulation due to pharmacokinetic and pharmacodynamic interaction with other drugs or supplements administered together. There have been many studies about drug interaction of warfarin with other drugs and supplements (Graefe-Mody et al., 2011; Malhotra et al., 2011; Zhou et al., 2012). However, the effect of lutein on the absorption of warfarin has not been reported.
We hypothesized that, although lutein may not cause drug interaction with warfain, large amount of surfactant and co-surfactant used to prepare SNEDDS formulation may affect gastrointestinal absorption of the drug. In this paper, we prepared SNEDDS of lutein to improve dissolution and investigated its effect on the bioavailability of warfarin using rats. Lutein as non-SNEDDS (lutein powder) was also tested to see the effect on the bioavailability of the drug.
MATERIALS AND METHODS
Materials
Lauroglycol FCC, Labrafil M1944, Labrafil 2125, Labrafac CC, Labrasol, Peceol, and Transcutol-HP were obtained from Gattefosse (Saint-Priest Cedex, France). Lutrol-E400 was obtained from BASF (Florham Park, NJ, USA). Saponified marigold extract and a commercial product (Eyelac®) were kindly donated by Korea Arlico Pharm (Seoul, Korea). Warfarin and naproxen were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were analytical grade and were used without further purification.
Extraction and purification of lutein
Lutein was extracted and purified from saponified marigold extract according to the previously reported methods (Khachik, 1995; Wei et al., 2003). The purified lutein was characterized by 1H NMR, 13C NMR, and HPLC. The 1H and 13C NMR data were in good agreement with the data from literatures (Aman et al., 2005).
Construction of pseudo-ternary phase diagram and preparation of SNEDDS
Construction of pseudo-ternary phase diagram and preparation of SNEDDS were performed as previously reported (Yoo et al., 2010). Tendency to form an emulsion was judged as ‘good’ when the droplets spread easily in distilled water resulting in a fine milky emulsion, and it was judged ‘bad’ when there was poor or no emulsion formation with immediate coalescence of oil droplets, especially when stirring was stopped. In this study, Peceol and Labrasol were selected as oil phase and surfactant, respectively, and Lutrol-E400 and Transcutol-HP were selected as co-surfactants. Concentration of lutein in the SNEDDS was fixed at 2% w/v.
Characterization of physicochemical properties of SNEDDS
Physicochemical properties evaluated in this study included zeta potential, droplet size, emulsification time, and dissolution of lutein from the SNEDDS. Procedures for the measurement of the properties followed previously reported method (Yoo et al., 2010).
Animal study
All animal treatment was carried out in accordance with Korea National Institute of Health. Seven week old male Sprague Dawley rats were supplied by Orient Bio (Seoul, Korea) and housed in groups not exceeding six per cage. The acclimation period was two weeks before the experimental procedure with a dark/light cycle of 12 h/12 h at a temperature of 23 ± 2℃ and 50-80% relative humidity. The animals had free access to food and water.
The animals were fasted for 10 h before the experiment and randomly assigned to three test groups and a control group (n=6 for each). On experiment day, rats in the three test groups received single doses of warfarin (per oral, 1.5 mg/kg) suspended in corn oil (2 ml/kg) and lutein as System A, System B, or non-SNEDDS (per oral, 5 mg/kg). Corn oil was used as suspending agent because it is physiologically inactive and has minimal solubility for both warfarin and lutein. For lutein as non-SNEDDS, lutein powder was administered as suspension in corn oil with warfarin. SNEDDS formulations containing lutein was diluted appropriately with distilled water before administration so that the volume of the formulation was 0.5 ml. Rats in the control group received warfarin only. Blood samples of 0.3 ml were serially withdrawn under anesthesia via the subclavian vein into small heparinized Eppendorf tubes at predetermined time intervals for 5 days. The blood samples were immediately centrifuged at 2,000 rpm for 7 min, and the plasma was removed and stored at -20℃ until HPLC assay of warfarin concentration.
HPLC assay of warfarin in plasma
The warfarin content of rat plasma samples was assayed by HPLC equipped with Class VP computer software, LC 10 AD VP pump, and SPD 10A UV-VIS detector at 310 nm using naproxen as internal standard. The column was Inertsil ODS-3 (4.6×150 mm, GL Science Inc, Japan) and the mobile phase consisted of a mixture of 0.02 M phosphate buffer and acetonitrile (50:50) adjusted to pH 3.5 with 1 N HCl. Flow rate was 1.0 ml/min and the injection volume of the sample was 20 μl. Before measuring the plasma level of warfarin by HPLC, validation of the assay was performed by repeating five times a day for five consecutive days using exactly the same conditions for warfarin concentration range of 50-15,000 ng/ml.
The frozen plasma samples were thawed at room temperature and 100 μl aliquots were spiked with 30 μl of internal standard (naproxen in methanol). After brief vortex mixing, 150 μl of phosphate buffer (pH 2.0) was added to acidify the plasma for effective extraction. One milliliter of diethyl ether was added for deproteination of the plasma sample followed by centrifugation for 1 min at 13,000 g to precipitate the proteins, and the clear supernatant was evaporated in a centrifugal vacuum evaporator. The residue obtained was reconstituted with 200 μl of mobile phase and injected to HPLC.
RESULTS
The solubility of lutein in various surfactants, co-surfactants and oils was presented in Table 1. Among surfactants and co-surfactants tested, lutein has the highest solubility in Labrasol with 56.54 ± 4.24 mg/ml followed by Lutrol-E400 (31.53 ± 3.27 mg/ml) and Transcutol-HP (16.27 ± 2.35 mg/ml). The lowest solubility of lutein among surfactants and co-surfactants was observed with Labrafil-2125 (3.77 ± 1.22 mg/ml). Peceol showed highest solubility of lutein (11.76 ± 1.38 mg/ml) among the oils screened, and solubility of lutein in all vegetable oils tested was less than 6 mg/ml. Therefore, Labrasol and Peceol were chosen as surfactant and oil, respectively, and Transcutol-HP and Lutrol-E400 were chosen as co-surfactants for the preparation of two different SNEDDS.
Table 1.
Composition, HLB value, and solubility profile of vehicles screened for selection of SNEDDS
| Vehicles | Composition | HLB | Solubility (mg/ml) |
|---|---|---|---|
| Surfactants/Co-surfactants: | |||
| Labrasol | Caprylocaproyl macrogolglycerides | 14 | 56.54 ± 4.24 |
| Lauroglycol-FCC | Propyleneglycol caprylate | 5 | 7.09 ± 1.18 |
| Transcutol-HP | Diethylene glycol monoethyl ether | 4.2 | 16.27 ± 2.35 |
| Lutrol-E400 | Polyethylene glycol; polyalkylene glycol; polyol | 4 | 31.53 ± 3.27 |
| Labrafil-M1944 | Oleoyl macrogolglycerides | 4 | 4.35 ± 1.02 |
| Labrafil-2125 | Linoleoyl macrogolglycerides | 4 | 3.77 ± 1.22 |
| Oils: | |||
| Labrafac CC | Caprylic/capric triglycerides | 1 | 7.83 ± 0.29 |
| Peceol | Glyceryl monooleate | 3 | 11.76 ± 1.38 |
| Corn oil | Linoleic acid 58%; oleic acid 28%; palmitic acid 11% | - | 1.86 ± 0.25 |
| Cotton seed oil | Linoleic acid 54%; oleic acid 19%; palmitic acid 22% | - | 2.01 ± 0.33 |
| Peanut oil | Linoleic acid 32%; oleic acid 48%; palmitic acid 11% | - | 1.46 ± 0.11 |
| Castor oil | Ricinoleic acid 95%; oleic acid 2%; linoleic acid 1% | - | 5.55 ± 0.36 |
Pseudo-ternary phase diagrams of the systems containing oil phase (Peceol), surfactant (Labrasol) and co-surfactants (Transcutol-HP and Lutrol-E400) were shown in Fig. 1. There was no significant difference in the emulsion formation with two different co-surfactants (Transcutol-HP and Lutrol-E400). Self-emulsification was achieved at greater than 50% surfactant concentration, and the self-emulsification efficiency was good when sum of the surfactant and co-surfactant concentration was more than 80% of SNEDDS formulation. In the pseudo-ternary phase diagrams, the ternary mixtures outside the solid line exhibited coalescence of the emulsion droplets.
Fig. 1. Pseudo-ternary phase diagrams of SNEDDS. (A) System A. (B) System B. Ternary mixtures inside the solid line exhibited self-emulsification, and the self-emulsification efficiency was good when sum of the surfactant and co-surfactant concentration was more than 80% of SNEDDS formulation.

In our study, we observed that increasing surfactant concentration up to 60% decreased the mean droplet size of emulsion formed (in the absence of co-surfactant). However, the droplet size increased when the surfactant concentration was above 60% (172.3 ± 18.1 nm at 60%, 323.5 ± 21.3 nm at 70%, 391.6 ± 24.7 nm at 80%). The effect of co-surfactants (Transcutol-HP and Lutrol-E400) concentration on the droplet size distribution in SNEDDS was similar to that of surfactant (Labrasol) in the ranges of 0-35% (Fig. 2). A decrease in droplet size was observed with an increase in the co-surfactant concentrations of Transcutol-HP and Lutrol-E400 from 5 to 25%, after which the droplet size was increased. The lowest size was observed at 25% of co-surfactant concentration for both Transcutol-HP and Lutrol-E400, showing 172.8 ± 14.8 nm and 93.2 ± 4.6 nm, respectively (Table 2). Among the two Systems tested, System B with component ratio of 60% Labrasol, 15% Peceol, and 25% Lutrol-E400 showed smallest mean droplet size (Fig. 2).
Fig. 2. Effect of co-surfactant concentration on droplet size of SNEDDS containing fixed surfactant concentration of 60%. Droplet size was decreased as concentration of Transcutol-HP and Lutrol-E400 increased up to 25%. However, when the concentration of the co-surfactants was beyond 25%, the size was increased. The lowest size was observed when the concentrations of Labrasol and Lutrol-E400 were 60% and 25%, respectively.
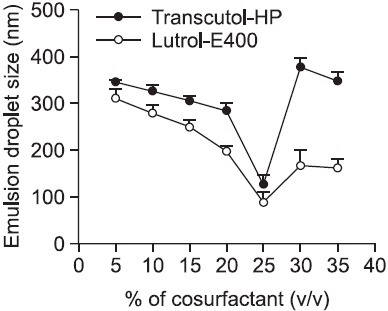
Table 2.
Vehicle composition, zeta potential, droplet size, and emulsification time of the two SNEDDS formulations
| Vehicle | System A | System B |
|---|---|---|
| Peceol (%) | 15 | 15 |
| Labrasol (%) | 60 | 60 |
| Transcutol-HP (%) | 25 | - |
| Lutrol-E400 (%) | - | 25 |
| Zeta potential (mV) | -3.02 | -2.17 |
| Droplet size (nm) | 172.8 ± 14.8 | 93.2 ± 4.6* |
| Emulsification time (sec) | 15 ± 3 | 18 ± 2 |
*p<0.01 compared to System A.
Emulsification time for both Systems was within 20 sec, showing relatively faster in the System A compared to System B (15 ± 3 vs 18 ± 2 sec). Dissolution profile of the two SNEDDS formulations was shown in Fig. 3. The dissolution of lutein was more than 90% accomplished within 10 min for the System B. Almost complete dissolution was achieved after 15 min while lutein was not detectable from the lutein powder or intra-capsule content of a commercial product (20 mg as of lutein in soft gelatin capsule) even after 4 h. No precipitation or aggregation was found for more than a week after dissolution study of the two SNEDDS formulations.
Fig. 3. Dissolution profile of lutein from SNEDDS. Each value represents the mean of three samples ± standard deviation. Dissolution of lutein from SNEDDS was very quick and accomplished within 10 min. System B exhibited faster and more complete dissolution than System A because of its smaller droplet size compared to System A. Undissolved amount of lutein is attributed to lutein in the emulsion droplets trapped by syringe filter (0.2 μm). In contrast, commercial formulation (Eyelac® soft gelatin capsule) and lutein powder did not dissolve lutein even at the end of the dissolution study.
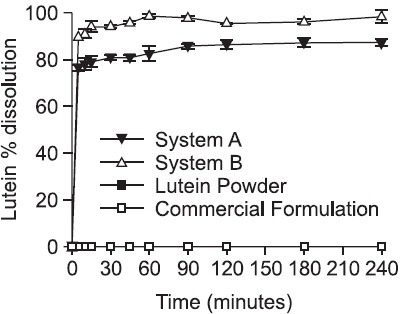
Table 3 summarized the pharmacokinetic parameters of warfarin in rats following a single oral dose of 1.5 mg/kg with lutein as non-SNEDDS (lutein powder) or SNEDDS formulations. Time to reach maximum plasma concentration (Tmax) of warfarin was 6 h when the drug was administered with or without 5 mg/kg of lutein as non-SNEDDS while it was 4 h when administered with lutein as SNEDDS formulations. Maximum plasma concentration (Cmax) of warfarin was significantly increased when the drug was administered with lutein as SNEDDS (p<0.01). Area under the curve (AUC) was also increased, but the increase was significant only for the System B (p<0.05). Elimination half life (t1/2) of warfarin was slightly shortened with concomitant administration with the SNEDDS formulations. Fig. 4 shows plasma level of warfarin as a function of time.
Table 3.
Pharmacokinetic parameters of warfarin in rats following a single oral dose of 1.5 mg/kg with lutein as non-SNEDDS or SNEDDS formulations (n=6)
| Warfarin only | With lutein as non-SNEDDS | With lutein as system A | With lutein as system B | |
|---|---|---|---|---|
| Tmax (h) | 6.00 | 6.00 | 4.00 | 4.00 |
| Cmax (µg/ml) | 5.62 ± 0.11 | 5.65 ± 0.15 | 6.14 ± 0.36** | 6.34 ± 0.22** |
| AUC (µg/ml×h) | 305.70 ± 9.42 | 308.89 ± 10.23 | 313.57 ± 12.24 | 324.10 ± 10.96* |
| t1/2 (h) | 22.91 ± 1.26 | 23.89 ± 1.74 | 21.12 ± 1.86 | 22.70 ± 1.67 |
Tmax: time to reach maximum plasma concentration, Cmax: maximum plasma concentration, AUC: area under the curve, t1/2: elimination half life, *p<0.05 and **p<0.01 compared to warfarin only.
Fig. 4. Plasma level of warfarin in rats following a single oral dose of 1.5 mg/kg with lutein as non-SNEDDS or SNEDDS formulations (n=6). Tmax of warfarin was reached at 4 h when the drug was administered with lutein as SNEDDS while it was 6 h when administered with lutein as non-SNEDDS. Cmax and AUC of warfarin was significantly increased when the drug was administered with lutein as SNEDDS (p<0.01). Reason for the enhanced bioavailability appears that SNEDDS formulation affected permeability of warfarin through the mucosal membrane of gastrointestinal tract.

DISCUSSION
Lutein is a very hydrophobic substance which follows same intestinal absorption path as dietary fat. When lutein is administered orally, the drug is emulsified in gastrointestinal tract and incorporated into mixed micelles in the presence of bile salts and biliary phospholipids (Baskaran et al., 2003; Lakshminarayana et al., 2006; Yonekura and Nagao, 2007). Therefore, absorption of lutein is affected by inappropriate bile juice secretion in patients with biliary diseases. For this reason, an alternative delivery system that relies on its own self-emulsification ability rather than the aid of bile juice would offer benefit to the elderly or patients who cannot eat food appropriately and have altered digestive functions. Hence, SNEDDS may be an ideal delivery system to improve solubilization and absorption of lutein because it spontaneously forms nanosized droplets of oil in water emulsion in aqueous environment without the aid of bile juice secretion.
There have been several strategies reported to improve bioavailability of lutein. Most widely used are mixed micelles and emulsion formulations with or without dietary fats (Marisiddaiah and Baskaran, 2009; Mamatha and Baskaran, 2011). Among the emulsion formulations, SNEDDS is considered promising approach because it distributes readily in the gastrointestinal tract, and digestive motility of the stomach and intestine provides sufficient agitation for spontaneous formation of emulsion. Furthermore, it does not require costly equipments in the manufacturing process and the emulsion droplet size is far less than a micron which is advantageous for gastrointestinal absorption.
Choosing the right combination of oil base, surfactant, and co-surfactant is one of the important points in designing SNEDDS formulations. Surfactant and co-surfactant molecules get preferentially absorbed at the liquid interface during the process of emulsion formation, reducing the interfacial energy of the system. This helps spontaneous emulsification without high energy input. Thus, well-designed SNEDDS formulation can ensure efficient self-emulsification as well as high solubilization capacity for the drug in the resultant dispersion.
In this study, we used Peceol, a glyceride-rich excipient mainly consisted of glyceryl monooleate, as oil base. Rationale for choosing Peceol was based on previous researcher’s finding that lipids consisted of monounsaturated fatty acid trended toward better absorption of lutein and other carotenoids in healthy subjects (Goltz et al., 2012). We used Labrasol as surfactant because it showed highest solubility for lutein (56.54 ± 4.24 mg/ml) among the vehicles screened and its HLB value was 14 (Table 1). Generally, surfactants with HLB 12-15 are regarded as being of good efficiency for self-emulsification (Singh et al., 2008; Thi et al., 2009; Shanmugam et al., 2011). Also, this vehicle is widely used in the pharmaceutical and food industries due to its excellent safety profile (Kommuru et al., 2001; Yan et al., 2011) Labrasol is composed of fatty acid esters of polyethyleneglycol and medium chain fatty acids of caproic acid (C6:0) and caprylic acid (C8:0) (mixture of mono-, di-, and tri-glycerides). We used two different co-surfactants in System A and System B (Transcutol-HP and Lutrol-E400, respectively). Reason for choosing Transcutol-HP and Lutrol-E400 was that they have similar HLB values (4.2 versus 4.0) but different solubility profile for lutein (16.27 versus 31.53 mg/ml, Table 1).
Dissolution profile of lutein from System B was faster and more complete than System A because of significantly smaller mean droplet size of System B. Although both Systems instantaneously formed nanoemulsion after introduction to dissolution medium (distilled water), the mean droplet size of System B was about half size of System A. Difference in the solubility of lutein to co-surfactants used in the two Systems might have also contributed to dissolution profile. Lutrol-E400 used as co-surfactant in System B showed about two-folds higher solubility for lutein compared to Transcutol-HP used in System A. Undissolved amount of lutein in the dissolution study is attributed to lutein in the emulsion droplets trapped by syringe filter (0.2 μm).
In contrast to SNEDDS, commercial formulation (Eyelac® soft gelatin capsule) and lutein powder did not dissolve lutein even at the end of the study. Inability of the commercial formulation to dissolve lutein may be due to oily nature of the vehicles that were used. Usually, such commercial product should be taken right after meal so that lutein can be emulsified by physiological emulsifiers such as bile juice. However, the SNEDDS that we have formulated does not need to be taken after meal as was evidenced by very rapid dissolution due to its self-emulsifying ability. This especially offers benefit to the elderly and patients who cannot eat food appropriately.
Previously, we reported that gastrointestinal absorption of lutein was significantly enhanced when administered to rabbits as SNEDDS formulation (Shanmugam et al., 2011). Cmax and AUC were enhanced as much as 21-fold and 12-fold compared to lutein powder, respectively. Compared to a commercial product (Eyelac®), relative bioavailability of the SNEDDS formulation was also significantly improved, showing about 3-folds increase based on AUC. Although this result was encouraging, there remains a concern that the SNEDDS formulation may affect gastrointestinal absorption of other drugs administered together. Especially if therapeutic index of the concomitantly administered drug is narrow, undesirable drug interactions could occur.
We hypothesized that surfactant and co-surfactant may affect absorption of concomitantly administered drugs because they are usually more than 80% of the SNEDDS formulation in weight basis. In this study, we investigated the effect of SNEDDS formulation of lutein on the pharmacokinetic parameters of warfarin using rats. Although there are many research conducted regarding the drug interaction issue of warfarin, interaction between warfarin and surfactants used in the SNEDDS has not been published so far (Graefe-Mody et al., 2011; Malhotra et al., 2011; Zhou et al., 2012). Since warfarin requires close therapeutic monitoring, bioavailability of the drug is of great concern. In the dispensing practice of warfarin prescription, even generic substitution is discouraged due to the bioavailability difference between commercially available brands (Ghate et al., 2011; Haines, 2011). Our result showed that Cmax and AUC of warfarin were increased about 10% when administered concomitantly with SNEDDS containing lutein. Tmax was also prolonged from 6 h to 4 h. These results clearly identified that SNEDDS formulation affected the absorption of warfarin, leading to bioavailability difference. Exact mechanism for this difference is not yet elucidated, but it appears that SNEDDS formulation affected permeability of warfarin through the mucosal membrane of gastrointestinal tract. This speculation is backed up by the lack of bioavailability change in rats administered with warfarin and lutein as non-SNEDDS. Limitation of this study may be that total amount of blood taken for bioavailability measurement was slightly more than 10% of total blood of the rats tested.
Finally, we hypothesized that surfactants in the SNEDDS formulation of lutein may affect bioavailability of warfarin and identified that the surfactants increased Cmax and AUC of warfarin. Based on this finding, it is suggested that SNEDDS formulations be administered before or after appropriate time interval when used together with narrow therapeutic range drugs.
Lutein was successfully formulated as SNEDDS for immediate self-emulsification and dissolution by using combination of oil base, surfactant, and co-surfactant. However, surfactants used in the SNEDDS affected bioavailability of warfarin, showing about 10% increase in Cmax and AUC of the drug in rats. Tmax was also changed from 6 h to 4 h. Although there is no strict consensus about how much bioavailability change is tolerable, concomitant administration of SNEDDS formulation and drugs with narrow therapeutic range appears to require attention for drug interaction.
Acknowledgments
This work was supported by the Gachon University research fund of 2012 (GCU 2012-M107).
References
- 1.Aman R., Biehl J., Carle R., Conrad J., Beifuss U., Schieber A. Application of HPLC coupled with DAD, APcI-MS and NMR to the analysis of lutein and zeaxanthin stereoisomers in thermally processed vegetables. Food Chem. (2005);92:753–763. [Google Scholar]
- 2.Baskaran V., Sugawara T., Nagao A. Phospholipids affect the intestinal absorption of carotenoids in mice. Lipids. (2003);38:705–711. doi: 10.1007/s11745-003-1118-5. [DOI] [PubMed] [Google Scholar]
- 3.Brown L., Rimm E., Seddon J., Giovannucci E., Chasan-Taber L., Spiegelman D., Willett W., Hankinson S. A prospective study of carotenoid intake and risk of cataract extraction in US men. Am. J. Clin. Nutr. (1999);70:517–524. doi: 10.1093/ajcn/70.4.517. [DOI] [PubMed] [Google Scholar]
- 4.Cha K. H., Lee J. Y., Song D. G., Kim S. M., Lee D. U., Jeon J. Y., Pan C. H. Effect of microfluidization on in vitro micellization and intestinal cell uptake of lutein from Chlorella vulgaris. J. Agric. Food Chem. (2011);59:8670–8674. doi: 10.1021/jf2019243. [DOI] [PubMed] [Google Scholar]
- 5.Chakraborty S., Shukla D., Mishra B., Singh S. Lipid: an emerging platform for oral delivery of drugs with poor bioavailability. Eur. J. Pharm. Biopharm. (2009);73:1–15. doi: 10.1016/j.ejpb.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Chasan-Taber L., Willett W., Seddon J., Stampfer M., Rosner B., Colditz G., Speizer F., Hankinson S. A prospective study of carotenoid and Vitamin A intakes and risk of cataract extraction in US women. Am. J. Clin. Nutr. (1999);70:509–516. doi: 10.1093/ajcn/70.4.509. [DOI] [PubMed] [Google Scholar]
- 7.Chung H. Y., Rasmussen H. M., Johnson E. J. Lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach in men. J. Nutr. (2004);134:1887–1893. doi: 10.1093/jn/134.8.1887. [DOI] [PubMed] [Google Scholar]
- 8.Gale C., Hall N., Phillips D., Martyn C. Plasma antioxidant vitamins and carotenoids and age-related cataract. Ophthalmology. (2001);108:1992–1998. doi: 10.1016/s0161-6420(01)00833-8. [DOI] [PubMed] [Google Scholar]
- 9.Ghate S. R., Biskupiak J. E., Ye X., Hagan M., Kwong W. J., Fox E. S., Brixner D. I. Hemorrhagic and thrombotic events associated with generic substitution of warfarin in patients with atrial fibrillation: a retrospective analysis. Ann. Pharmacother. (2011);45:701–712. doi: 10.1345/aph.1P593. [DOI] [PubMed] [Google Scholar]
- 10.Goltz S. R., Campbell W. W., Chitchumroonchokchai C., Failla M. L., Ferruzzi M. G. Meal triacylglycerol profile modulates postprandial absorption of carotenoids in humans. Mol. Nutr. Food Res. (2012);56:866–877. doi: 10.1002/mnfr.201100687. [DOI] [PubMed] [Google Scholar]
- 11.Graefe-Mody E. U., Brand T., Ring A., Withopf B., Stangier J., Iovino M., Woerle H. J. Effect of linagliptin on the pharmacokinetics and pharmacodynamics of warfarin in healthy volunteers. Int. J. Clin. Pharmacol. Ther. (2011);49:300–310. doi: 10.5414/cp201507. [DOI] [PubMed] [Google Scholar]
- 12.Granado F., Olmedilla B., Blanco I., Rojas-Hidalgo E. Major fruit and vegetable contributors to the main serum carotenoids in the Spanish diet. Eur. J. Clin. Nutr. (1996);50:246–250. [PubMed] [Google Scholar]
- 13.Granado-Lorencio F., Herrero-Barbudo C., Olmedilla-Alonso B., Blanco-Navarro I., Pérez-Sacristán B. Lutein bioavailability from lutein ester-fortified fermented milk: in vivo and in vitro study. J. Nutr. Biochem. (2010);21:133–139. doi: 10.1016/j.jnutbio.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Haines S. T. Substituting warfarin products: what's the source of the problem? Ann. Pharmacother. (2011);45:807–809. doi: 10.1345/aph.1Q063. [DOI] [PubMed] [Google Scholar]
- 15.Hankinson S., Stampfer M., Seddon J., Colditz G., Rosner B., Speizer F., Willett W. Nutrient intake and cataract extraction in women: a prospective study. BMJ. (1992);305:335–339. doi: 10.1136/bmj.305.6849.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson E. A biological role of lutein. Food Rev. Int. (2004);20:1–16. [Google Scholar]
- 17.Khachik F. Patent 5382714. U.S. Process for isolation, purification, and recrystallization of lutein from saponified marigolds oleoresin and uses thereof. (1995)
- 18.Kommuru T., Gurley B., Khan M., Reddy I. Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q10: formulation development and bioavailability assessment. Int. J. Pharm. (2001);212:233–246. doi: 10.1016/s0378-5173(00)00614-1. [DOI] [PubMed] [Google Scholar]
- 19.Lakshminarayana R., Raju M., Krishnakantha T., Baskaran V. Enhanced lutein bioavailability by lyso-phosphatidylcholine in rats. Mol. Cell. Biochem. (2006);281:103–110. doi: 10.1007/s11010-006-1337-3. [DOI] [PubMed] [Google Scholar]
- 20.Landrum J., Bone R. Lutein, zeaxanthin, and the macular pigment. Arch. Biochem. Biophys. (2001);385:28–40. doi: 10.1006/abbi.2000.2171. [DOI] [PubMed] [Google Scholar]
- 21.Lyle B., Mares-Perlman J., Klein B., Klein R., Greger J. Antioxidant intake and risk of incident age-related nuclear cataracts in the Beaver Dam Eye Study. Am. J. Epidemiol. (1999);149:801–809. doi: 10.1093/oxfordjournals.aje.a009895. [DOI] [PubMed] [Google Scholar]
- 22.Ma H., Zhao Q., Wang Y., Guo T., An Y., Shi G. Design and evaluation of self-emulsifying drug delivery systems of Rhizoma corydalis decumbentis extracts. Drug Dev. Ind. Pharm. (2012);38:1200–1206. doi: 10.3109/03639045.2011.643897. [DOI] [PubMed] [Google Scholar]
- 23.Malhotra B., Alvey C., Gong J., Li X., Duczynski G., Gandelman K. Effects of fesoterodine on the pharmacokinetics and pharmacodynamics of warfarin in healthy volunteers. Br. J. Clin. Pharmacol. (2011);72:257–262. doi: 10.1111/j.1365-2125.2011.03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mamatha B. S., Baskaran V. Effect of micellar lipids, dietary fiber and β-carotene on lutein bioavailability in aged rats with lutein deficiency. Nutrition. (2011);27:960–966. doi: 10.1016/j.nut.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Mares-Perlman J., Fisher A., Palta M., Block G., Millen A., Wright J. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third national health and nutrition examination survey. Am. J. Epidemiol. (2001);153:424–432. doi: 10.1093/aje/153.5.424. [DOI] [PubMed] [Google Scholar]
- 26.Marisiddaiah R., Baskaran V. Bioefficacy of beta-carotene is improved in rats after solubilized as equimolar dose of beta-carotene and lutein in phospholipid-mixed micelles. Nutr. Res. (2009);29:588–595. doi: 10.1016/j.nutres.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Mitri K., Shegokar R., Gohla S., Anselmi C., Müller R. H. Lutein nanocrystals as antioxidant formulation for oral and dermal delivery. Int. J. Pharm. (2011);420:141–146. doi: 10.1016/j.ijpharm.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 28.O’Neill M., Carroll Y., Corridan B., Olmedilla B., Granado F., Blanco I., Van den Berg H., Hininger I., Rousell A., Chopra M., Southon S., Thurnham D. A European carotenoid database to assess carotenoid intakes and its use in a five-country comparative study. Br. J. Nutr. (2001);85:499–507. doi: 10.1079/bjn2000284. [DOI] [PubMed] [Google Scholar]
- 29.Pouton C. W. Formulation of poorly water-soluble drugs for oral administration: physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. (2006);29:278–287. doi: 10.1016/j.ejps.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Seddon J., Ajani U., Sperduto R., Hiller R., Blair N., Burton T., Farber M., Gragoudas E., Haller J., Miller D., Yannuzzi L., Willett W. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. (1994);272:1413–1420. [PubMed] [Google Scholar]
- 31.Shanmugam S., Baskaran R., Balakrishnan P., Thapa P., Yong C. S., Yoo B. K. Solid self-nanoemulsifying drug delivery system (S-SNEDDS) containing phosphatidylcholine for enhanced bioavailability of highly lipophilic bioactive carotenoid lutein. Eur. J. Pharm. Biopharm. (2011);79:250–257. doi: 10.1016/j.ejpb.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Shao B., Tang J., Ji H., Liu H., Liu Y., Zhu D., Wu L. Enhanced oral bioavailability of Wurenchun (Fructus Schisandrae Chinensis extracts) by self-emulsifying drug delivery systems. Drug Dev. Ind. Pharm. (2010);36:1356–1363. doi: 10.3109/03639045.2010.480975. [DOI] [PubMed] [Google Scholar]
- 33.Singh A. K., Chaurasiya A., Singh M., Upadhyay S. C., Mukherjee R., Khar R. K. Exemestane loaded self-microemulsifying drug delivery system (SMEDDS): development and optimization. AAPS PharmSciTech. (2008);9:628–634. doi: 10.1208/s12249-008-9080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thi T. D., Van Speybroeck M., Barillaro V., Martens J., Annaert P., Augustijns P., Van Humbeeck J., Vermant J., Van den Mooter G. Formulate-ability of ten compounds with different physicochemical profiles in SMEDDS. Eur. J. Pharm. Sci. (2009);38:479–488. doi: 10.1016/j.ejps.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 35.Vithlani S., Sarraf S., Chaw C. S. Formulation and in vitro evaluation of self-emulsifying formulations of Cinnarizine. Drug Dev. Ind. Pharm. (2012);38:1188–1194. doi: 10.3109/03639045.2011.643895. [DOI] [PubMed] [Google Scholar]
- 36.Wei Y., Zhang T., Xu G., Ito Y. Application of CCC for the separation of lutein from a crude extract of marigold flower petals. J. Liq. Chromatogr. Relat. Technol. (2003);26:1659–1669. [Google Scholar]
- 37.Wu Z., Guo D., Deng L., Zhang Y., Yang Q., Chen J. Preparation and evaluation of a self-emulsifying drug delivery system of etoposide-phospholipid complex. Drug Dev. Ind. Pharm. (2011);37:103–112. doi: 10.3109/03639045.2010.495752. [DOI] [PubMed] [Google Scholar]
- 38.Yan Y., Kim J., Kwak M., Yoo B., Yong C., Choi H. Enhanced oral bioavailability of curcumin via a solid lipid-based self-emulsifying drug delivery system using a spray-drying technique. Biol. Pharm. Bull. (2011);34:1179–1186. doi: 10.1248/bpb.34.1179. [DOI] [PubMed] [Google Scholar]
- 39.Yonekura L., Nagao A. Intestinal absorption of dietary carotenoids. Mol. Nutr. Food Res. (2007);51:107–115. doi: 10.1002/mnfr.200600145. [DOI] [PubMed] [Google Scholar]
- 40.Yoo J. H., Shanmugam S., Thapa P., Lee E. S., Balakrishnan P., Baskaran R., Yoon S. K., Choi H. G., Yong C. S., Yoo B. K., Han K. Novel self-nanoemulsifying drug delivery system for enhanced solubility and dissolution of lutein. Arch. Pharm. Res. (2010);33:417–426. doi: 10.1007/s12272-010-0311-5. [DOI] [PubMed] [Google Scholar]
- 41.Zhou L., Wang S., Zhang Z., Lau B. S., Fung K. P., Leung P. C., Zuo Z. Pharmacokinetic and pharmacodynamic interaction of Danshen-Gegen extract with warfarin and aspirin. J. Ethnopharmacol. (2012);143:648–655. doi: 10.1016/j.jep.2012.07.029. [DOI] [PubMed] [Google Scholar]


