Abstract
Plasminogen activator inhibitor-1 (PAI-1) is a member of serine protease inhibitor family, which regulates the activity of tissue plasminogen activator (tPA). In CNS, tPA/PAI-1 activity is involved in the regulation of a variety of cellular processes such as neuronal development, synaptic plasticity and cell survival. To gain a more insights into the regulatory mechanism modulating tPA/PAI-1 activity in brain, we investigated the effects of proteasome inhibitors on tPA/PAI-1 expression and activity in rat primary astrocytes, the major cell type expressing both tPA and PAI-1. We found that submicromolar concentration of MG132, a cell permeable peptide-aldehyde inhibitor of ubiquitin proteasome pathway selectively upregulates PAI-1 expression. Upregulation of PAI-1 mRNA as well as increased PAI-1 promoter reporter activity suggested that MG132 transcriptionally increased PAI-1 expression. The induction of PAI-1 downregulated tPA activity in rat primary astrocytes. Another proteasome inhibitor lactacystin similarly increased the expression of PAI-1 in rat primary astrocytes. MG132 activated MAPK pathways as well as PI3K/Akt pathways. Inhibitors of these signaling pathways reduced MG132-mediated upregulation of PAI-1 in varying degrees and most prominent effects were observed with SB203580, a p38 MAPK pathway inhibitor. The regulation of tPA/PAI-1 activity by proteasome inhibitor in rat primary astrocytes may underlie the observed CNS effects of MG132 such as neuroprotection.
Keywords: MG132, Lactacystin, p38, Plasminogen activator inhibitor-1, Tissue plasminogen activator
INTRODUCTION
MG132 (N-(benzyloxycarbonyl)-Leu-Leu-Leu-al) is a cell permeable peptide-aldehyde molecules, which inhibits ubiquitin-proteasome pathway in reversible manner without affecting its ATPase and isopeptidase activity. The ubiquitin-proteasome pathway is a highly extralysosomal system for selective degradation of short-lived proteins. Although MG132 and other proteasome pathway inhibitors are regarded specific against ubiquitin-proteasome pathway resulting in up-regulation of rapidly turnovering proteins in cells, it can activate JNK to induce apoptosis and inhibits activation of NF-κB or prevents β-secretase cleavage at higher concentration. Recent studies also suggest that MG132 may also transcriptionally upregulate subsets of proteins such as monocyte chemoattractant protein-1, IL-8 and cyclooxygenase-2 by mechanism currently not fully understood, although involvement of ROS production, PI3K-Akt, PKC, Src and MAPKs activation as well as activation of C/EBP-δ and CREB binding protein has been suggested (Nakayama et al., 2001; Wu et al., 2002a; Wu et al., 2002b; Chen et al., 2005; Woo et al., 2006). More recently, it has been reported that MG132 induces nuclear translocation of a transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2), which modulates expression of COX-2 in human vascular endothelial cells (Sahni et al., 2008). The multitude of signaling effects of MG132 may underlie the diverse and sometimes seemingly contradictory array of cellular effects such as apoptosis, oxidant or antioxidant activity, neurite outgrowth and neuroprotection.
PAI-1 is an endogenous inhibitor of tissue plasminogen activator (tPA), which regulates a myriad array of neurophysiological and neuropathological processes in brain ranging from axonal outgrowth and synaptic plasticity to neuronal cell death and clearance of amyloid β. Several researchers including us reported that PAI-1 is mainly expressed in astrocytes (Kim et al., 2011) and various stimuli including inflammatory activation regulates PAI-1 expression and hence the activity of tPA by complex interplay of signaling pathways involving, for example, MAPKs, PKA, PKC and TGF-β-Smad and much more (Docagne et al., 2002; Higgins, 2006; Lee et al., 2008; Hultman et al., 2010; Kim et al., 2011). The expression level of tPA/PAI-1 is modulated by multiple levels of regulatory mechanisms including transcription, translation and stability control. Surprisingly, there is not much study available on the role of proteasome inhibitors on the level of PAI-1, especially focusing on the possible transcriptional control of gene expression.
In this study, we investigated the effects of MG132 on the expression of PAI-1 in cultured rat primary astrocytes, which is the major cell type expressing PAI-1 in CNS. MG132 increased the expression of PAI-1 by mechanism dependent on the activation of MAPKs and PI3K-Akt, which resulted in the downregulation of tPA activity. The regulation of PAI-1 by proteasome pathway inhibitor may provide additional tool for the delicate modulation of tPA/PAI-1 system in brain.
MATERIALS AND METHODS
Materials
Dulbecco’s Modified Eagle’s medium/F12 (DMEM/F12), Penicillin/Streptomycin, fetal bovine serum (FBS) and 0.25% Trypsin-EDTA were purchased from Invitrogen (Carlsbad, CA, USA). MG132, lactacystin, U0126, LY294002, and SB203580 were purchased from Calbiochem, EMD Biosciences (La Jolla, CA, USA), while SP600125 was purchased from Biomol (Plymouth Meeting, PA, USA).
Antibodies were purchased from the following companies: anti-PAI-1 from American Diagnostica Inc. (Stamford, CT, USA), anti-Akt, anti-JNK, anti-p38, anti-Erk, anti-phospho-Akt (Ser473), anti-phospho-JNK (Thr183/Tyr185), anti-phospho-Erk (Thr202/Tyr204) and anti-phospho-p38 (Thr180/Thr182) from Cell Signaling (Hitchln, UK), anti-β-actin from Sigma (St. Louis, MO, USA).
Primary astrocyte culture
Rat primary astrocytes were cultured from the frontal cortices of 2 day-old Sprague Dawley (SD) rat pups according to the methods previously described (Shin et al., 2001). Briefly, frontal cortex was dissected-out and digested with trypsin. Cells were seeded onto poly-d-lysine coated 75 cm2 flask. Confluent cells were subcultured with trypsin-EDTA and were grown in above media for 6-7 days before this study. The purity of astrocytes was about 95% which was determined by immunocytochemistry against specific markers for the specific cell types such as GFAP for astrocytes, OX-42 for microglia and MAP2 for neuron.
Assessment of cell viability
Cell viability was also assessed by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT; Sigma) assay. MTT (500 μg/ml) was added to the cell-culture medium and was incubated for 2 h. After color extraction with DMSO, the absorbance was read at 570 nm with a microplate reader (Molecular Devices, Palo Alto, CA, USA).
Measurement of intracellular level of ROS
Intracellular ROS formation was measured by fluorescence detection using 2',7'-dichlorodihydrofluorescein diacetate (H2DCF-DA; Molecular probe) (LeBel et al., 1990). After MG132 treatment, cultures were washed with cold PBS and loaded with 20 μM of H2DCF-DA for 30 min at 37℃, and then kept at room temperature for an additional 30 min to allow the complete de-esterification of the dye. The DCF fluorescence was analyzed using a fluorescence plate reader (Spectramax Gemini EM, Molecular Devices, Palo Alto, CA, USA) at an excitation of 490 nm and an emission of 530 nm.
Casein zymography
The tPA activity was assayed by direct casein zymography as described previously (Shin et al., 2004). Briefly, culture supernatant mixed with sample buffer was run on 10% polyacrylamide gel containing casein (1 mg/ml; Sigma, St. Louis, MO, USA) and plasminogen (13 μg/ml; American Diagnostica). The gel was washed with 2.5% Triton X-100 and incubated in 0.1 M Tris buffer for overnight at room temperature and then stained with Coomassie Brilliant Blue (G-250). To detect PAI-1 activity by one-phase inverse zymography, gels were incubated with uPA (0.5 IU/ml, American Diagnostica, Greenwich, CT, USA) after renaturation of the SDS-PAGE gel (Masos and Miskin, 1997; Kim et al., 2011).
Western blot analysis
Proteins were separated by 10% SDS-PAGE and electrically transferred to nitrocellulose membranes. The membranes were incubated with first antibody overnight at 4℃ and then with peroxidase-conjugated secondary antibody (Santa Cruz, CA, USA) for 2 h at room temperature. Specific bands were detected using the ECL system (Amersham, Buckinghamshire, UK) and exposed to LAS-3000 image detection system (Fuji, Japan).
PAI-1 promoter-luciferase reporter assay
Cells were transfected with hPAI-798 promoter-luciferase reporter gene (pGL-hPAI-798) and CMV-β-galactosidase plasmid (pCMV) using Lipofectamine as reported previously (Cho et al., 2006). After transfection, cells were incubated with increasing concentration of MG132. Luciferase and β-galactosidase activities were assayed using luciferase reporter assay system (Promega, Madison, WI, USA), respectively. Luciferase activity was normalized to the β-galactosidase activity in the cell lysates.
Semi-quantitative RT-PCR
Total RNA was extracted from cells using Trizol® reagent (Invitrogen, Carlsbad, CA, USA). The cDNA obtained from 1 μg total RNA was used as a template for PCR amplification of tPA (NM_013151), PAI-1 (NM_012620), iNOS (NM_012611), MMP-9 (NM_031055), IL-6 (NM_012589), IL-1β (NM_031512), TNF-α (NM_012675) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (NM_017008) mRNA as described previously (Cho et al., 2010). The PCR amplification consisted of 35 cycles (94℃, 5 min; 60℃, 1 min; 72℃, 1 min) with the following oligonucleotide primer sets (Table 1).
Table 1.
The sequence of primers used in this study
| Gene | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| tPA | TCAGATGAGATGACAGGGAAATGCC | ATCATACAGTTCTCCCAGCC |
| PAI-1 | GCTCCTGGTCAACCACCTTA | CCCCACAAAATTCAAGACCA |
| iNOS | CGTGTGCCTGCTGCCTTCCTGCTGT | GTAATCCTCAACCTGCTCCTCACTC |
| MMP-9 | AAAGGTCGCTCGGATGGTTA | AGGATTGTCATCTGGAGTCGA |
| IL-6 | TTGTGCAATGGCAATTCTGA | TGGAAGTTGGGGTAGGAAGG |
| IL-1β | AAAATGCCTCGTGCTGTCTG | CTATGTCCCGACCATTGCTG |
| TNF-α | TAGCCCACGTCGTAGCAAAC | GGAGGCTGACTTTCTCCTGG |
| GAPDH | TCCCTCAAGATTGTCAGCAA | AGATCCACAACGGATACATT |
Statistical analysis
Data are expressed as the mean ± standard error of mean (S.E.M.) and analyzed for statistical significance by one way analysis of variance (ANOVA) followed by Newman-Keuls test as a post hoc test; a p-value <0.05 was considered as statistically significant.
RESULTS
MG132 increases PAI-1 activity, protein and mRNA levels in rat primary astrocytes
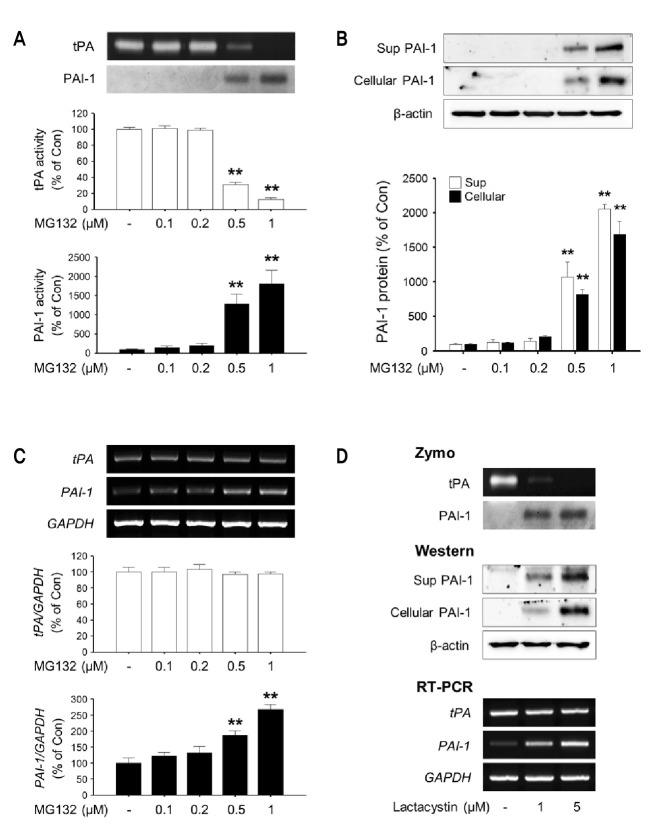
As shown in Fig. 1., 0.5 and 1 μM MG132 induced enzymatic activity as well as protein (Sup and Cellular) and mRNA expression of PAI-1 in rat primary astrocytes (Fig. 1A-C). Another proteasome inhibitor lactacystin (1 and 5 μM) also increased PAI-1 expression (Fig. 1D). The increased expression of PAI-1 inhibited tPA enzymatic activity in culture supernatant of rat primary astrocytes (Fig. 1A).
Fig. 1. The effects of MG132 and lactacystin on tPA/PAI-1 activity, protein and mRNA levels in rat primary astrocytes. Rat primary astrocytes were incubated in serum-free DMEM/F12 with increasing concentration of MG132 (0.1-1 μM) for 24 h. (A) The activity of tPA/PAI-1 in the culture supernatant was determined by casein zymographic procedure as described in Materials and Methods. (B) The level of PAI-1 protein secreted into culture spent media (Sup) and cell lysates (Cellular) were determined by Western blot using specific antibody against PAI-1. β-Actin levels were measured as a loading control. (C) Expression of total cellular tPA and PAI-1 mRNA were measured by RT-PCR. GAPDH levels were measured as an internal control. (D) Rat primary astrocytes were incubated in serum-free DMEM/F12 with Lactacystin (1, 5 μM) for 24 h. The activity of tPA/PAI-1 in the culture supernatant, the amount of PAI-1 protein secreted into culture spent media and cell lysates, expression of total cellular tPA and PAI-1 mRNA were determined as described. All graphs represent the mean ± S.E.M. of four independent experiments (**p<0.01 versus Control).
MG132 increases PAI-1 promoter activity in rat primary astrocytes
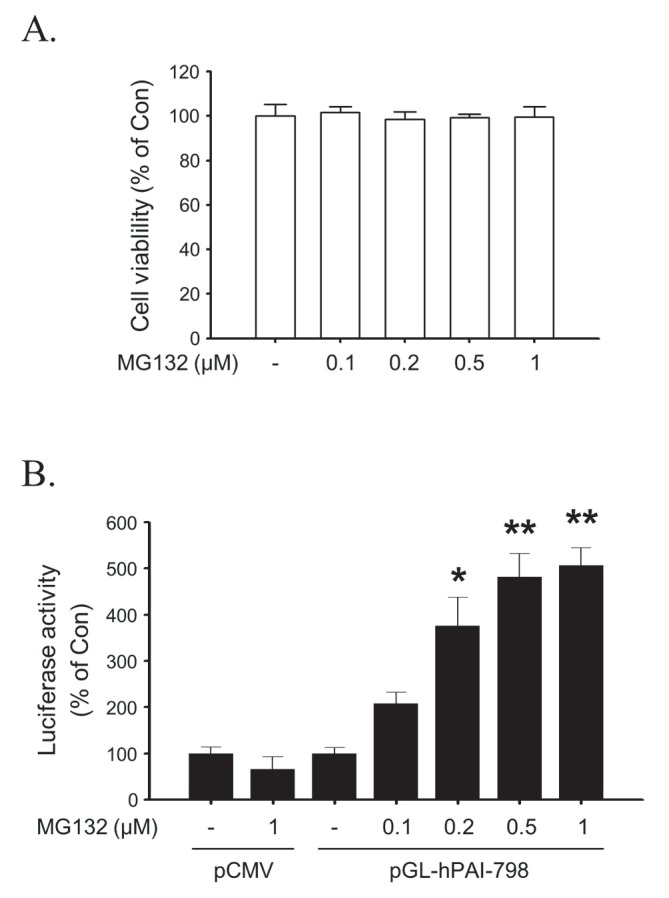
To verify MG132-induced expression of PAI-1 in rat primary astrocytes, a human PAI-1 promoter-luciferase reporter gene (pGL-hPAI-798) was transfected in rat primary astrocytes. MG132 increased transcriptional activity of PAI-1 expression reporter construct (Fig. 2B) in a concentration dependent manner without affecting cell viability (Fig. 2A).
Fig. 2. The effects of MG132 on cell viability and PAI-1 promoter activity in rat primary astrocytes. (A) Rat primary astrocytes were incubated in serum-free DMEM/F12 with increasing concentration of MG132 (0.1-1 μM) for 24 h. The viability of astrocytes was determined by the MTT reduction assay. (B) Rat primary astrocytes were in-cubated with MG132 (0.1-1 μM) for 24 h after transfection with pGL-hPAI-798 and pCMV-β-galactosidase plasmid. PAI-1 promoter activity was measured as described. All graphs represent the mean ± S.E.M. of four independent experiments (*p<0.05, **p<0.01 versus Control).
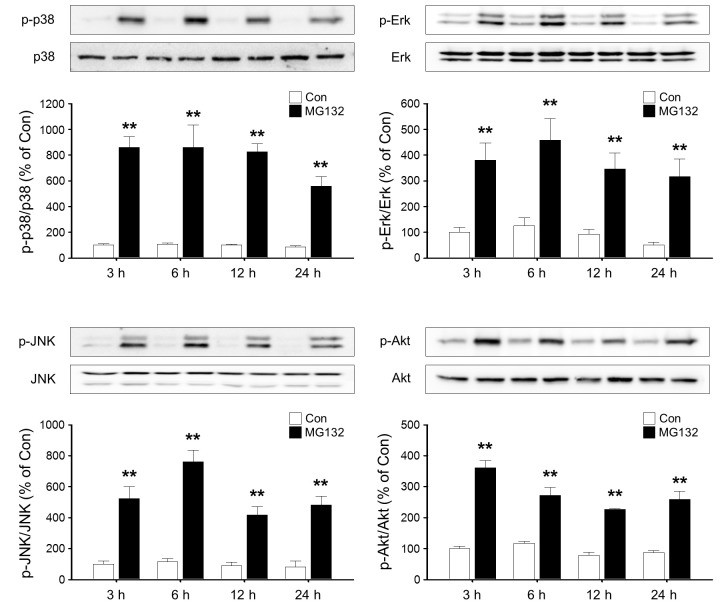
MG132 increases p38, Erk, JNK and Akt activities in rat primary astrocytes
We next examined the signaling pathway involved in the up-regulation of PAI-1 by MG132. MG132 resulted in sustained and robust increase in phosphorylation of p38, Erk and JNK. The phosphorylation of p38, Erk and JNK is peaked at 6 h and prolonged until 24 h. Treatment with MG132 also increased the phosphorylation of Akt (Fig. 3).
Fig. 3. Time course of p38, Erk, JNK and Akt activation by MG132 in rat primary astrocytes. Rat primary astrocytes were incubated in serum-free DMEM/F12 with 1 μM MG132 for 3-24 h. Cells were harvested for Western blot analysis and the activation of p38, Erk, JNK and Akt at different time points was assessed by measuring the respective phosphorylated forms of signaling molecules. Total level of p38, Erk, JNK and Akt were measured as controls. The graph represents quantification data which is the mean ± S.E.M. of four independent experiments (**p<0.01 versus Control).
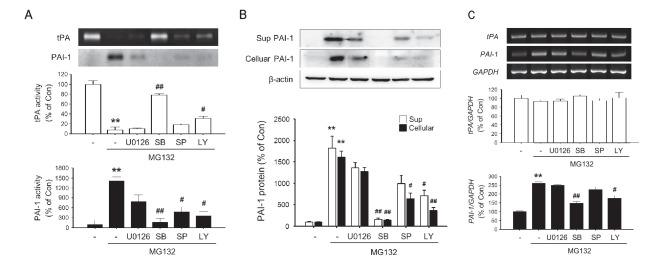
P38 inhibitor inhibited MG132-induced up-regulation of PAI-1 in rat primary astrocytes
To next investigated the effects of MAPK and PI3K inhibitors on PAI-1 induction by MG132. All three MAPK inhibitors, namely U0126, SB203580 and SP600125, which inhibits Erk, p38 and JNK and a PI3K inhibitor LY294002 inhibited MG132-induced upregulation of PAI-1 activity as well as the down-regulation of tPA acitivity (Fig. 4A). They also inhibited MG132 induced overexpression of PAI-1 protein (Sup and Cellular) and mRNA levels (Fig. 4B, C). The strongest inhibition was observed with p38 inhibitor SB203580.
Fig. 4. The effects of MAPK inhibitors on MG132-induced modulation of tPA/PAI-1 activity, protein, mRNA levels in rat primary astrocytes. Rat primary astrocytes were incubated in serum-free DMEM/F12 with 1 μM MG132 for 24 h with or without U0126 (10 μM), LY294002 (10 μM), SB203580 (10 μM), SP600125 (10 μM). (A) The activity of tPA/PAI-1 in the culture supernatant was determined by casein zymographic procedure as described. (B) The amount of PAI-1 protein secreted into culture spent media (Sup) and cell lysates (Cellular) were determined by Western blot using. β-Actin levels were measured as a loading control. (C) Expression of total cellular tPA and PAI-1 mRNA were measured by RT-PCR. GAPDH levels were measured as an internal control. All graphs represent the mean ± S.E.M. of four independent experiments (**p<0.01 versus Control, #p<0.05, ##p<0.01 versus MG132 1 μM treated group).
MG132 has no effects on immunological stimulation of rat primary astrocytes
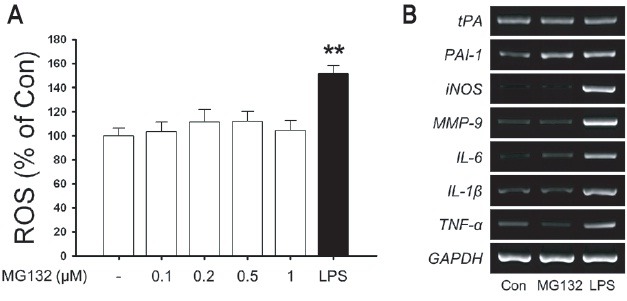
Immunological stimulation such as LPS strongly induces PAI-1 expression in rat primary astrocytes. Therefore, we examined whether MG132 induces oxidative stress and immunological stimulation in rat primary astrocytes. While LPS induced oxidative stress and up-regulation of inflammatory mediators such as iNOS, MMP-9, IL-6, IL-1β and TNF-α as well as PAI-1 mRNA, MG132 did not upregulated oxidative stress and iNOS, MMP-9, IL-6, IL-1β and TNF-α expression suggesting the specificity of MG132-induced upregulation of PAI-1 in rat primary astrocytes (Fig. 5).
Fig. 5. The effects of MG132 on immunological stimulation in rat primary astrocytes. (A) Rat primary astrocytes were incubated in serum-free DMEM/F12 with increasing concentration of MG132 (0.1-1 μM) and 100 ng/ml LPS for 24 h. Intracellular ROS was measured by H2DCF-DA fluorescence (490/530 nm) as described in Materials and Methods. (B) Rat primary astrocytes were incubated in serum-free DMEM/F12 with 1 μM MG132 and 100 ng/ml LPS for 24 h. Expression of total cellular tPA, PAI-1, iNOS, MMP-9, IL-6, IL-1β, TNF-α mRNA were measured by RT-PCR. GAPDH levels were measured as a loading control. Data are representative of four such experiments, which gave similar results (**p<0.01 versus Control).
DISCUSSION
In this study, submicromolar concentration of MG132 increased expression of PAI-1, which is related to the transcriptional activation of PAI-1 gene expression but possibly not by direct inhibition of PAI-1 degradation as evidenced by increased mRNA level determined by RT-PCR and increased PAI-1 promoter activity. Although some other researchers reported the transcriptional activation of specific gene expression by MG132, the concentration of MG132 used in those study is rather high, which may affects multiple of signaling pathways making the clear interpretation of the experimental data difficult (Nakayama et al., 2001; Wu et al., 2002a; Wu et al., 2002b). In case of MG132-induced transcriptional activation of COX-2 expression, the authors used micromolar concentration of MG132 and several signaling pathways such as PI3K, p38, Src, and PKC has been reported to induce C/EBP-δ binding to both CRE and NF-IL6 sites on the COX-2 promoter, resulting in the initiation of COX-2 transcription (Chen et al., 2005). Similar study conducted in Raw264.7 cells, showed submicromolar concentration of MG132 increased COX-2 expression in p38 but not Erk or JNK dependent manner, suggesting activation of p38 might be the crucial events regulating COX-2 expression by low concentration of MG132 (Woo et al., 2006), which is consistent with the results obtained from the present study.
Although the data obtained from the present study suggest that PAI-1 mRNA is upregulated by MG132, the inhibition of proteasomal degradation of PAI-1 by MG132 may also contribute to the increase in PAI-1 expression in our experimental system. To investigate the possible involvement of proteasomal pathway in the regulation of PAI-1 level, direct determination of ubiquitination on PAI-1 protein might be necessary. Interestingly, the extent of the fold-increase in PAI-1 promoter activity, mRNA level and protein activity is somewhat different in this study, which may suggest that not only the transcriptional control but also other mechanisms such as the increased protein stability, altered translational efficiency and stabilization of mRNAs by binding of RNA binding proteins such as HuR (Doller et al., 2009) are also involved in the MG132-induced up-regulation of PAI-1.
It has been suggested that MG132 may induce activation and nuclear translocation of a transcription factor Nrf2, which might be responsible for the MG132-induced upregulation of COX-2 (Sahni et al., 2008). However, Nrf2 overexpression effectively inhibited the TGF-β/Smad signaling and PAI-1 expression in an immortalized human hepatic stellate cell line (Oh et al., 2012) and the expression of Nrf2 is deregulated in many cancer cells results in Smad-dependent transcription of target genes including PAI-1 (Rachakonda et al., 2010). These results suggest that activation Nrf2 may not be a mechanism responsible for the MG132-induced PAI-1 upregulation. PAI-1 gene expression can be regulated by various transcription factors and regulators such as AP-1, Egr-1, IGF-1, HIF-1α, C/EBPα and TGF-β-Smad2/3α/4 in many different cell types including RAW264.7 murine macrophages, rat mesangial cells and HepG2 hepatoma cells. It is remained to be determined whether MG132 regulates the PAI-1 expression by regulating some of those factors in rat primary astrocytes.
MG132 and other proteasome inhibitors may induce apoptotic cell death in a variety of different cell types and one of the proteasome inhibitor such as Bortezomib (VELCADE) is in clinical use against relapsing multiple myeloma. MG132 induces caspase activity by upregulating the production of reactive oxygen species and modulating the expression of tumor suppressors, transcription factors and cell cycle regulators (Guo and Peng, 2012). However, MG132 did not affect cell viability in this study.
Although the physiological or clinical significance of current investigation is not certain at this moment, it is possible that proteasomal inhibitors like MG132 may protect neuron from excess tPA-induced cell death by upregulating PAI-1. Endogenous or exogenous tPA in excess amount in CNS may induce neurotoxicity either directly or by activating target molecules such as NMDA receptor. MG132 may also modulate neuronal migration or neurite outgrowth by modulating tPA/PAI-1 system. PAI-1 is one of the most robustly upregulated proteins upon proliferative/migratory stimuli and is regarded as a crucial regulator of cell migration along with uPA and uPAR (Czekay et al., 2011), which makes it an auxiliary target for cancer chemotherapy. PAI-1 binds to its endogenous substrate uPA and/or uPAR, ligand binding partner LRP-1 or extracellular matrix protein such as vitronectin and accumulates at the leading edge of migrating cells and is believed to fine tune the extracellular matrix and proteolytic activity for the optimal penetration of migrating cell processes. Interestingly, several researchers reported that MG132 induces neurite outgrowth in several different cell lines by diverse mechanism involving the induction of anti-oxidant enzymes, activation of MAPKs and PI3K (Giasson et al., 1999; Song et al., 2009; Duan et al., 2011; Song and Yoo, 2011). However, it should be remembered that the increase in PAI-1 expression resulted in the down-regulation of tPA activity, which is also implicated in cell migration as well as axonal growth during physiological development condition or regenerative condition after brain trauma such as stroke and seizure (Baranes et al., 1998; Seeds et al., 1999; Wu et al., 2000; Zhang et al., 2005). Therefore, the final effects of MG132 on neuronal migration or neurite outgrowth might be differentially regulated depending on the microenvironment affected in the brain, which needs empirical determination in each experimental models.
In conclusion, MG132 increased PAI-1 expression by transcriptional upregulation of PAI-1 gene expression in rat primary astrocytes, which might be regulated by activation of p38 MAPK pathway. Although it is possible that MG132 may regulate tPA expression in other cell types such as neuron in more classical way involving direct inhibition of proteasomal degradation of proteins, the upregulation of PAI-1 in astrocytes, which is the major cell types expressing PAI-1 in CNS, may contribute to the downregulation of tPA activity not only in astrocytes but also in neuron, which may results in neuroprotection in a condition where excess tPA activity may induce neurotoxic effects. The results from the present study may facilitate the understanding of the regulatory mechanism governing tPA/PAI-1 activity in CNS as well as provide the opportunity for pharmacological and cell biological dissection of neurobiological processes regulated by this intriguing neuromodulator system.
Acknowledgments
This paper was written as part of Konkuk University's research support program for its faculty on sabbatical leave in 2012 (CY, Shin).
References
- 1.Baranes D., Lederfein D., Huang Y. Y., Chen M., Bailey C. H., Kandel E. R. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. (1998);21:813–825. doi: 10.1016/s0896-6273(00)80597-8. [DOI] [PubMed] [Google Scholar]
- 2.Chen J. J., Huang W. C., Chen C. C. Transcriptional regulation of cyclooxygenase-2 in response to proteasome inhibitors involves reactive oxygen species-mediated signaling pathway and recruitment of CCAAT/enhancer-binding protein delta and CREB-binding protein. Mol. Biol. Cell. (2005);16:5579–5591. doi: 10.1091/mbc.E05-08-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cho I. J., Kim S. H., Kim S. G. Inhibition of TGFbeta1-mediated PAI-1 induction by oltipraz through selective interruption of Smad 3 activation. Cytokine. (2006);35:284–294. doi: 10.1016/j.cyto.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Cho K. S., Park S. H., Joo S. H., Kim S. H., Shin C. Y. The effects of IL-32 on the inflammatory activation of cultured rat primary astrocytes. Biochem. Biophys. Res. Commun. (2010);402:48–53. doi: 10.1016/j.bbrc.2010.09.099. [DOI] [PubMed] [Google Scholar]
- 5.Czekay R. P., Wilkins-Port C. E., Higgins S. P., Freytag J., Overstreet J. M., Klein R. M., Higgins C. E., Samarakoon R., Higgins P. J. PAI-1: An integrator of cell signaling and migration. Int. J. Cell Biol. (2011);2011:562481. doi: 10.1155/2011/562481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Docagne F., Nicole O., Gabriel C., Fernandez-Monreal M., Lesne S., Ali C., Plawinski L., Carmeliet P., MacKenzie E. T., Buisson A., Vivien D. Smad3-dependent induction of plasminogen activator inhibitor-1 in astrocytes mediates neuroprotective activity of transforming growth factor-beta 1 against NMDA-induced necrosis. Mol. Cell. Neurosci. (2002);21:634–644. doi: 10.1006/mcne.2002.1206. [DOI] [PubMed] [Google Scholar]
- 7.Doller A., Gauer S., Sobkowiak E., Geiger H., Pfeilschifter J., Eberhardt W. Angiotensin II induces renal plasminogen activator inhibitor-1 and cyclooxygenase-2 expression post-transcriptionally via activation of the mRNA-stabilizing factor human-antigen R. Am. J. Pathol. (2009);174:1252–1263. doi: 10.2353/ajpath.2009.080652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan W., Guo Y., Jiang H., Yu X., Li C. MG132 enhances neurite outgrowth in neurons overexpressing mutant TAR DNA-binding protein-43 via increase of HO-1. Brain. Res. (2011);1397:1–9. doi: 10.1016/j.brainres.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Giasson B. I., Bruening W., Durham H. D., Mushynski W. E. Activation of stress-activated protein kinases correlates with neurite outgrowth induced by protease inhibition in PC12 cells. J. Neurochem. (1999);72:1081–1087. doi: 10.1046/j.1471-4159.1999.0721081.x. [DOI] [PubMed] [Google Scholar]
- 10.Guo N., Peng Z. MG132, a proteasome inhibitor, induces apoptosis in tumor cells. Asia Pac. J. Clin. Oncol. (2012) doi: 10.1111/j.1743-7563.2012.01535.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Higgins P. J. The TGF-beta1/upstream stimulatory factor-regulated PAI-1 gene: potential involvement and a therapeutic target in Alzheimer's disease. J. Biomed. Biotechnol. (2006);2006:15792. doi: 10.1155/JBB/2006/15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hultman K., Blomstrand F., Nilsson M., Wilhelmsson U., Malmgren K., Pekny M., Kousted T., Jern C., Tjarnlund-Wolf A. Expression of plasminogen activator inhibitor-1 and protease nexin-1 in human astrocytes: Response to injury-related factors. J. Neurosci. Res. (2010);88:2441–2449. doi: 10.1002/jnr.22412. [DOI] [PubMed] [Google Scholar]
- 13.Kim J. W., Lee S. H., Ko H. M., Kwon K. J., Cho K. S., Choi C. S., Park J. H., Kim H. Y., Lee J., Han S. H., Ignarro L. J., Cheong J. H., Kim W. K., Shin C. Y. Biphasic regulation of tissue plasminogen activator activity in ischemic rat brain and in cultured neural cells: essential role of astrocyte-derived plasminogen activator inhibitor-1. Neurochem. Int. (2011);58:423–433. doi: 10.1016/j.neuint.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 14.LeBel C. P., Ali S. F., McKee M., Bondy S. C. Organometal-induced increases in oxygen reactive species: the potential of 2',7'-dichlorofluorescin diacetate as an index of neurotoxic damage. Toxicol. Appl. Pharmacol. (1990);104:17–24. doi: 10.1016/0041-008x(90)90278-3. [DOI] [PubMed] [Google Scholar]
- 15.Lee S. Y., Kim H. J., Lee W. J., Joo S. H., Jeon S. J., Kim J. W., Kim H. S., Han S. H., Lee J., Park S. H., Cheong J. H., Kim W. K., Ko K. H., Shin C. Y. Differential regulation of matrix metalloproteinase-9 and tissue plasminogen activator activity by the cyclic-AMP system in lipopolysaccharide-stimulated rat primary astrocytes. Neurochem. Res. (2008);33:2324–2334. doi: 10.1007/s11064-008-9737-2. [DOI] [PubMed] [Google Scholar]
- 16.Masos T., Miskin R. mRNAs encoding urokinase-type plasminogen activator and plasminogen activator inhibitor-1 are elevated in the mouse brain following kainate-mediated excitation. Brain. Res. Mol. Brain. Res. (1997);47:157–169. doi: 10.1016/s0169-328x(97)00040-5. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama K., Furusu A., Xu Q., Konta T., Kitamura M. Unexpected transcriptional induction of monocyte chemoattractant protein 1 by proteasome inhibition: involvement of the c-Jun N-terminal kinase-activator protein 1 pathway. J. Immunol. (2001);167:1145–1150. doi: 10.4049/jimmunol.167.3.1145. [DOI] [PubMed] [Google Scholar]
- 18.Oh C. J., Kim J. Y., Min A. K., Park K. G., Harris R. A., Kim H. J., Lee I. K. Sulforaphane attenuates hepatic fibrosis via NF-E2-related factor 2-mediated inhibition of transforming growth factor-β/Smad signaling. Free. Radic. Biol. Med. (2012);52:671–682. doi: 10.1016/j.freeradbiomed.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Rachakonda G., Sekhar K. R., Jowhar D., Samson P. C., Wikswo J. P., Beauchamp R. D., Datta P. K., Freeman M. L. Increased cell migration and plasticity in Nrf2-deficient cancer cell lines. Oncogene. (2010);29:3703–3714. doi: 10.1038/onc.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahni S. K., Rydkina E., Sahni A. The proteasome inhibitor MG132 induces nuclear translocation of erythroid transcription factor Nrf2 and cyclooxygenase-2 expression in human vascular endothelial cells. Thromb. Res. (2008);122:820–825. doi: 10.1016/j.thromres.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Seeds N. W., Basham M. E., Haffke S. P. Neuronal migration is retarded in mice lacking the tissue plasminogen activator gene. Proc. Natl. Acad. Sci. USA. (1999);96:14118–14123. doi: 10.1073/pnas.96.24.14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin C. Y., Choi J. W., Ryu J. R., Ryu J. H., Kim W., Kim H., Ko K. H. Immunostimulation of rat primary astrocytes decreases intracellular ATP level. Brain. Res. (2001);902:198–204. doi: 10.1016/s0006-8993(01)02385-x. [DOI] [PubMed] [Google Scholar]
- 23.Shin C. Y., Kundel M., Wells D. G. Rapid, activity-induced increase in tissue plasminogen activator is mediated by metabotropic glutamate receptor-dependent mRNA translation. J. Neurosci. (2004);24:9425–9433. doi: 10.1523/JNEUROSCI.2457-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song E. J., Hong H. M., Yoo Y. S. Proteasome inhibition induces neurite outgrowth through posttranslational modification of TrkA receptor. Int. J. Biochem. Cell Biol. (2009);41:539–545. doi: 10.1016/j.biocel.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 25.Song E. J., Yoo Y. S. Nerve growth factor-induced neurite outgrowth is potentiated by stabilization of TrkA receptors. BMB Rep. (2011);44:182–186. doi: 10.5483/BMBRep.2011.44.3.182. [DOI] [PubMed] [Google Scholar]
- 26.Woo K. J., Park J. W., Kwon T. K. Proteasome inhibitor-induced cyclooxygenase-2 expression in Raw264.7 cells is potentiated by inhibition of c-Jun N-terminal kinase activation. Biochem. Biophys. Res. Commun. (2006);342:1334–1340. doi: 10.1016/j.bbrc.2006.02.105. [DOI] [PubMed] [Google Scholar]
- 27.Wu H. M., Chi K. H., Lin W. W. Proteasome inhibitors stimulate activator protein-1 pathway via reactive oxygen species production. FEBS Lett. (2002a);526:101–105. doi: 10.1016/s0014-5793(02)03151-4. [DOI] [PubMed] [Google Scholar]
- 28.Wu H. M., Wen H. C., Lin W. W. Proteasome inhibitors stimulate interleukin-8 expression via Ras and apoptosis signal-regulating kinase-dependent extracellular signal-related kinase and c-Jun N-terminal kinase activation. Am. J. Respir. Cell Mol. Biol. (2002b);27:234–243. doi: 10.1165/ajrcmb.27.2.4792. [DOI] [PubMed] [Google Scholar]
- 29.Wu Y. P., Siao C. J., Lu W., Sung T. C., Frohman M. A., Milev P., Bugge T. H., Degen J. L., Levine J. M., Margolis R. U., Tsirka S. E. The tissue plasminogen activator (tPA)/plasmin extracellular proteolytic system regulates seizure-induced hippocampal mossy fiber outgrowth through a proteoglycan substrate. J. Cell Biol. (2000);148:1295–1304. doi: 10.1083/jcb.148.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y., Kanaho Y., Frohman M. A., Tsirka S. E. Phospholipase D1-promoted release of tissue plasminogen activator facilitates neurite outgrowth. J. Neurosci. (2005);25:1797–1805. doi: 10.1523/JNEUROSCI.4850-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]