Summary
Innovative strategies are needed to combat drug resistance associated with methicillin-resistant Staphylococcus aureus (MRSA). Here, we investigate the potential of wall teichoic acid (WTA) biosynthesis inhibitors as combination agents to restore β-lactam efficacy against MRSA. Performing a whole cell pathway-based screen we identified a series of WTA inhibitors (WTAIs) targeting the WTA transporter protein, TarG. Whole genome sequencing of WTAI resistant isolates across two methicillin-resistant Staphylococci spp. revealed TarG as their common target, as well as a broad assortment of drug resistant bypass mutants mapping to earlier steps of WTA biosynthesis. Extensive in vitro microbiological analysis and animal infection studies provide strong genetic and pharmacological evidence of the potential effectiveness of WTAIs as anti-MRSA β-lactam combination agents. This work also highlights the emerging role of whole genome sequencing in antibiotic mode-of-action and resistance studies.
Keywords: Staphylococcus aureus, MRSA, MRSE, imipenem, wall teichoic acid, antibiotic resistance, β-lactam potentiation, combination agent, chemical biology, Next Generation Sequencing
Introduction
Staphylococcus aureus remains the leading cause of hospital and community acquired infections by Gram-positive bacteria in much of the developed world (Boucher et al. 2009; Klevens et al. 2007; Johnson, 2011). This is attributed in large part to the emerging resistance of S. aureus to the entire armamentarium of β-lactam antibiotics, a broad and historically important class of antibiotics spanning penicillin, methicillin, and the more powerful carbapenems, including imipenem, which kill bacteria by inhibiting synthesis and chemical cross-linking of peptidoglycan (PG), a cell wall polymer, leading to weakening of the cell wall and cell lysis (Walsh, 2003).
The development of antibiotic combination agents has proven to be a highly successful therapeutic strategy to combat drug resistance, particularly against drug resistant Gram-negative bacteria (Drawz and Bonomo, 2010). Paramount to the rationale of combination agents is the increased potency and efficacy achieved by their combined effects. Ideally, this is achieved by the synergistic bioactivity of both agents affecting two interdependent cellular processes required for cell growth as well as the targeted inactivation of the resistance mechanism to the first agent by the combination agent (Tan et al., 2012). Applying a systems biology approach to discovering synergistic agents with this therapeutic potential is highly warranted; lethal or even growth-crippling chemical genetic interactions highlight a cellular network of interdependent biological processes and potential drug targets from which combination agents may be rationally discovered (Andrusiak et al., 2012; Costanzo et al., 2010; Nichols et al., 2011). We and others have adopted this approach to identify genetic mutations that restore β-lactam activity against MRSA, and as such, predict that cognate inhibitors of these β-lactam ‘potentiation’ targets may similarly restore the efficacy of the β-lactam (de Lencastre et al., 1999; Berger-Bachi and Rohrer, 2002, Huber et al., 2009; Lee et al., 2011; Tan et al., 2012). Indeed, several cellular processes contribute to buffering MRSA from the effects of β-lactams, including normal synthesis of a second cell wall polymer, wall teichoic acid (WTA) (Campbell et al., 2011; Lee et al., 2011). In support of this notion, target-specific inhibitors of this process, such as tunicamycin (Komatsuzawa et al., 1994; Campbell et al, 2011), an exquisitely selective inhibitor of TarO, responsible for the first step in WTA synthesis (Swoboda et al., 2009), was found to be highly synergistic in combination with β-lactams.
WTA is a Gram-positive specific anionic glycophosphate cell wall polymer of roughly equal abundance to PG. Unlike PG, however, WTA is not required for cell viability (Weidenmaier et al., 2004; D'Elia et al., 2009b) but plays important roles in cell growth, division, morphology, and as a virulence factor (Schirner et al., 2009; Swoboda et al., 2010; Atilano et al., 2010; Campbell et al., 2011; Dengler et al., 2012, Weidenmaier and Peschel, 2008). WTA polymers are sequentially synthesized on an undecaprenyl phosphate carrier lipid by a series of Tar enzymes localized on the inner face of the cytoplasmic membrane before being exported to the cell surface by a two component ATP-binding cassette (ABC) transporter system and covalently linked to PG (Brown et al., 2008; Swoboda et al., 2010; see also Figure S1). Interestingly, late steps in WTA biosynthesis in either S. aureus or Bacillus subtilis are essential for cell viability whereas early steps (encoded by tarO/tagO and tarA/tagA, respectively) are not (Weidenmaier et al., 2004; D'Elia et al., 2006a; D'Elia et al., 2006b; D'Elia et al., 2009a; D'Elia et al., 2009b). Further, late stage WTA genes are in fact conditionally essential since they are dispensable in either a tarO/tagO or tarA/tagA deletion background; this is referred to as the ‘essential gene paradox’ (D'Elia et al., 2006a; D'Elia et al., 2006b; D'Elia et al., 2009b). Two hypotheses have been given to explain these results; that toxic intermediate WTA precursors accumulate in late stage WTA mutants and/or sequestration of the essential biosynthetic precursor, bactoprenol, occurs and this leads to depletion of PG since its synthesis also requires bactoprenol as a carrier lipid (D'Elia et al., 2006b; D'Elia et al., 2009b).
Walker and colleagues have recently exploited this phenomenon by screening for late stage WTA inhibitors (WTAIs) that phenocopy the genetic characterization of the pathway. Such compounds should display intrinsic bioactivity against wild type S. aureus but lack activity against S. aureus strains in which flux into the WTA pathway is abolished either by genetic (e.g. tarO deletion) or pharmacological (e.g. tunicamycin) means (Swoboda et al., 2009). One compound they identified, 1835F03, was subsequently optimized for potency and named targocil (Lee et al., 2010; Suzuki et al., 2011). Drug resistance mutant isolation revealed that targocil inhibits TarG, an essential subunit of the WTA ABC transporter (Swoboda et al., 2009; Schirner et al., 2011). As expected, resistance to targocil is also achieved by loss-of-function mutations in tarO or tarA, which effectively bypass the mechanism of action (MOA) of targocil (Swoboda et al., 2009). Although the frequency of resistance (FOR) to targocil is high (Lee et al., 2010), the contribution of ΔtarO and ΔtarA mutants to targocil drug resistance could be eliminated in the presence of oxacillin (Campbell et al., 2011, Campbell et al., 2012). Collectively, these findings suggest that WTAIs could have significant potential as β-lactam combination agents against MRSA.
Despite extensive genetic and pharmacological studies of WTA biosynthesis in methicillin-sensitive S. aureus (MSSA) strains, relatively little is known about this pathway in MRSA strains in the context of a β-lactam potentiation target. Here we describe an analogous screening approach to identify and characterize three novel classes of late stage WTAIs that inhibit TarG. We show that multiple TarG inhibitors also display broad antibacterial spectrum and markedly reduced FOR in combination with imipenem. Interestingly, we demonstrate that the clinically acquired MRSA strain COL has a much broader repertoire of bypass mutations than previously identified. We show that these additional mutants are defective in late stage WTA biosynthesis and are attenuated in their virulence in a murine deep thigh infection model. Further, all mutants displayed striking hypersusceptibility phenotypes to β-lactam antibiotics both in vitro and in an animal model of MRSA infection, reinforcing the rationale for combining WTA inhibitors with β-lactam antibiotics. Co-administration of TarG inhibitors with imipenem displayed compound-specific synergistic or strongly additive effects that approached standard of care antibiotics and pharmacologically demonstrate the potential of WTAIs as effective β-lactam combination agents to treat MRSA.
Results
Pathway-based whole cell screening for late stage WTAIs
As first demonstrated with targocil, small molecule inhibitors of late stage WTA enzymes are predicted to display intrinsic growth inhibitory activity against S. aureus, which is specifically reversed in a ΔtarO strain background (Swoboda et al., 2009). Accordingly, a focused library of ∼20,000 S. aureus bioactive synthetic compounds (Huber et al., 2009) was screened in triplicate at two drug concentrations (3 and 16 μM) against the ΔtarO and wild type strains in tryptic soy broth (TSB) liquid medium in 1536 well plate format with growth measured by optical density. Compounds displaying greater than 80% reduced activity against the ΔtarO strain versus wild type were confirmed provided they displayed distinct dose responses against the two strains and an elevated minimum inhibitory concentration (MIC) against ΔtarO strain versus wild type (Figure 1). The confirmed compounds, their physicochemical properties (see Table S1) and chemical structures are shown (Figure 1A). Four compounds (L275, L638, L541, and L640) are analogs of the tricyclic indole structural class (note L541 and L640 follow-up was limited due to compound availability and structural redundancy with L275 and L638). L555 is a member of a large series of N-aryl triazoles in which each member contains two chiral centers but only L555 displays bioactive chemotypes consistent with inhibiting WTA biosynthesis (see Figure S2). The sixth compound, L524, is structurally a member of C-aryl triazoles. Interestingly, none of the compounds are structurally related to targocil (see Table S1).
Figure 1. Summary of WTAI Chemotypes.
(A) Dose response growth curves of MSSA wild type strain RN4220 (blue) and ΔtarO (red) at indicated drug concentrations for each putative WTAI. Chemical structure and structural class are indicated. (B). Minimum inhibitory concentrations (MIC; μg/ml) of WTAIs. Targocil bioactivity is included as control and comparator compound. Tunicamycin (Tuni) supplementation (2 μg/ml) is used to phenocopy ΔtarO and ΔtarA mutants. Imipenem (IPM) supplementation at 4 μg/ml reflects its clinical breakpoint concentration; highly reduced MIC values under such conditions reflect synergy.
Microbiological Characterization of late stage WTAIs
Based on extensive genetic characterization of WTA biosynthesis in S. aureus, inhibitory compounds acting on late stage WTA assembly are predicted to phenocopy targocil and phenotypes associated with loss-of-function mutations in late stage essential genes of the pathway (Swoboda et al., 2009). Accordingly, MIC determinations of these compounds were determined in comparison to targocil under a variety of conditions. Like targocil, each of the hit compounds displayed strong antimicrobial activity, with MIC values ranging from 1 – 8 μg/ml against MSSA strain RN4220 and MRSA strain COL, consistent with targeting an essential cellular process such as late stage WTA biogenesis (Figure 1B). Indeed, all compounds also display 4-32 fold higher MIC values against ΔtarO or ΔtarA null strains, analogous to genetic suppression of WTA late stage essential gene mutations in ΔtarO or ΔtarA strain backgrounds (D'Elia et al., 2006a; D'Elia et al., 2009a). S. aureus MIC values of each of these compounds were also noticeably antagonized (4-16 fold) by the addition of a sub-MIC level of tunicamycin (2 μg/ml) sufficient to specifically inhibit TarO activity (Campbell et al., 2011). Targocil is also reported to have a bacteriostatic mode of action (Swoboda et al., 2009; Campbell et al., 2012) and kill-curve analysis reveals that L275, L638, L524, and L555 all strongly arrest S. aureus cell division without compromising viability over an extended 24 hr time course (see Figure S3). Therefore, each of the compounds share striking microbiological characteristics with targocil that are consistent with their role as inhibitors of late stage WTA biogenesis.
Both a MRSA ΔtarO strain (Campbell et al., 2011) and an antisense interference-based depletion mutant of tarL (Lee et al., 2011) displays highly specific β-lactam hypersusceptibility phenotypes. Accordingly, we tested whether L275, L638, L524, and L555 are synergistic in combination with imipenem by scoring their fractional inhibitory concentration (FIC) index using the standard microdilution checkerboard assay, where FIC indices of ≤ 0.5, 1-2, or ≥ 4 indicate synergistic, additive, or antagonistic chemical interactions, respectively (Amsterdam, 2005). Each of the tested compounds tested against MRSA COL produced FIC indices between 0.516 and 0.625, indicating strong additivity in combination with imipenem (see Table S2). Only L524 demonstrated a strong synergistic activity with imipenem against methicillin-resistant Staphylococcus epidermidis (MRSE; see Table S2).
To further examine whether L275, L638, L524, and L555 are late stage WTA inhibitors, L275 was selected as a representative compound to isolate drug resistant mutants using a recently described S. aureus transposon (tsn) system (Wang et al., 2011). Applying this approach in MRSA COL, 10 independent tsn insertion mutants were identified, each specifically inactivating tarO (n=8) or tarA (n=2) (see Figure S4). These loss-of-function mutations recapitulate the demonstrated drug resistance compensatory mechanism of targocil (Swoboda et al., 2009). Further, L275R mutants independently reproduce the initial genetic basis which led to identification of L275 and other compounds.
B. subtilis Expressing TarGH is Selectively Sensitized to L275, L638, L524, and L555
Based on the observed phenocopy of targocil treatment, we hypothesized that our compounds may target the WTA transporter, TarGH. To address this, we determined compound MICs in both a wild type strain of B. subtilis and strain KS002, which is complemented with S. aureus TarGH in place of native TagGH (Schirner, et al. 2011). The complemented strain KS002 displayed 4-≥32-fold increased sensitivity to each of the compounds tested in comparison to wild type B. subtilis, suggesting TarGH as the likely target (see Figure S5). Activity against wild type B. subtilis also suggests an extended spectrum for these compounds in comparison to targocil, which is specific for S. aureus (Swoboda et al., 2009).
TargocilR Mutants are Cross-Resistant to L275, L638, L524, and L555
Fourteen independently derived targocil-resistant (targocilR, see Table S3 mutants were isolated in MRSA COL and those genes previously implicated in drug resistance (tarO, tarA, and tarG; Swoboda et al., 2009) were sequenced to identify causal mutations. Such mutations provide an important resource to test whether targocilR mutants are cross-resistant to the compounds we identified. Expectedly, 6 targocilR mutants mapped to either tarO or tarA and contained either missense, nonsense, or insertional loss-of-function mutations able to buffer the cell from the effects of targocil (see Figure S6A). As expected, all ΔtarO and ΔtarA mutants also exhibited a striking hypersusceptibility to imipenem with MIC values reduced 64-128 fold compared with the wild type MRSA COL strain. Importantly, 8 additional mutants mapped to TarG and correspond to the previously reported targocilR TarG-W73C or TarG-M80I mutants (Swoboda et al., 2009; Schirner et al., 2011) that despite conferring a 4-8 fold level of resistance to targocil, similar to ΔtarO and ΔtarA bypass mutants, lacked any change in their susceptibility to imipenem, implying that these are not loss-of-function mutations (see Figure S6A). Consistent with this view, TarG-W73C and TarG-M80I mutants lack an obvious growth phenotype and produce WTA as inferred by their full susceptibility to bacteriophage K – mediated cell lysis (see Figure S6B), which is a process dependent on phage recognition of WTA polymers that serve as a cell surface receptor for entry into the cell (Xia et al., 2010).
Employing the above described targocilR mutant set, we tested whether representative mutants are cross-resistant to L275, L638, L524, or L555. Interestingly, TarG-W73C or TarG-M80I MRSA COL strains are noticeably cross-resistant to each of the four compounds tested (Table 2). Further, the extent of cross resistance observed was comparable to that of the TarO-G48S loss-of-function mutant. As TarG-W73C and TarG-M80I mutants appear to produce WTA (see Figure S6B), these results strongly suggest that the tested compounds target TarG and that the suppression of their bioactivity is not due to the absence of WTA polymers.
Table 1.
Spontaneous Resistance Frequency of TarG inhibitors +/− Imipenem.
| Drug alone | Solubility issue | with 4 μg/mI IPM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2× | 4× | 6× | 8× | 2× | 4× | 6× | 8v | MIC | ||
| L275 | 6.2×10−8 | 1.9×10−8 | 3.6×10−10 | <3.6×10−10 | No | <3.6×10−10 | <3.6×10−10 | <3.6×10−10 | <3.6×10−10 | 1 |
| L638 | 2.1×10−7 | 1.3×10−7 | 4.2×10−8 | 1.9×10−8 | No | 4.6×10−8 | 1.5×10−8 | 2.7×10−10 | <2.7×10−10 | 2 |
| L524 | N/A | N/A | N/A | N/A | from 4× to 8× | N/A | N/A | N/A | N/A | 5 |
| L555 | 3.8×10−7 | 2.1×10−7 | 2.2×10−7 | 2.4×10−7 | 8× | 6.1×10−7 | 4.2×10−8 | 1.3×10−8 | 1.8×10−8 | 4 |
| targocil | 1.7×10−6 | 9.0×10−7 | N/A | N/A | from 4× to 8× | 1.8×10−7 | 1.9×10−7 | 1.7×10−7 | 1.6×10−7 | 1 |
colony forming units
N/A=Not Applicable (Too High to Count)
L275 and L638 Drug Resistance Mapping and Mechanism of Action Studies
L275R and L638R mutant selection and next generation whole genome sequencing (NGS) were performed in MRSA and MRSE strain backgrounds. As expected, fast growing L275R and L638R MRSA COL strains contained an extensive set of independently isolated TarG amino acid substitution mutations (n=20) (Figure 2). Indeed, several previously described targocilR mutations including TarG-F82L, TarG-C156Y, and TarG-Y190C (Swoboda et al., 2009; Schirner et al., 2011) confer resistance to L275 and/or L638. In addition, missense mutations yielding amino acid substitutions at 7 new positions in the TarG protein were identified. Many of these mutations map to the same predicted transmembrane domains as previously published targocilR mutations (Schirner et al., 2011; Figure 3A). Additional new TarG mutations map to predicted extracellular loop 1 (TarG-P64L) or extracellular loop 3 (TarG-H208Y, TarG-Y217C) of the protein. As NGS analysis failed to identify any additional non-synonymous mutations beyond tarG in 80% of L275R and L638R mutants examined and in the remaining instances where a second non-synonymous mutation was detected a TarG amino acid substitution mutation was also faithfully identified, we conclude that tarG mutations are causal for the observed drug resistance. In only one instance (two independently derived TarG-V54F mutations) was a growth phenotype observed (see Figure S7). Interestingly, an alternative amino acid substitution at this residue (TarG-V54L) grew indistinguishably from wild type, demonstrating the specificity of this mutation in effecting TarG function. Remarkably, all L275R and L638R TarG mutations displayed a common cross resistance to L524, L555, and targocil but not to other antibiotic classes tested (see Table S4).
Figure 2. Whole Genome Sequencing of L275R and L638R Mutants.
Heat map summary of all non-synonymous mutations identified by illumina-based whole genome sequencing (100× genome coverage) of 20 independently isolated L275R or L638R mutants in MRSA COL. Red, non-synonymous mutation; black, no change versus parental MRSA COL genome sequence. Genome position, base pair change, and resulting amino acid residue substitution are highlighted. Note, additional non-synonymous mutations (yellow) mapping to SACOL0539 (strains 707, 710, and 711) and SACCOL0687 (strain 663) are presumably unlinked to drug resistance as each strain also possesses a TarG amino acid substitution.
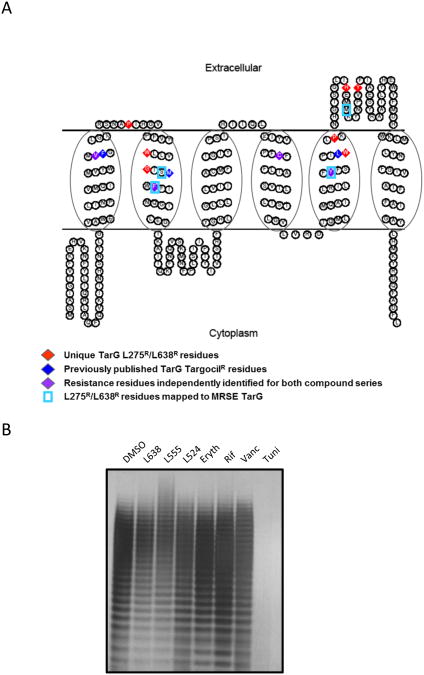
Figure 3. Predicted Structural Topology of TarG and Location of Drug Resistance Amino Acid Substitutions.
(A) Predicted topology of S. aureus TarG with previously identified targocilR mutations (blue diamonds), unique point mutations identified here conferring L275R or L638R (red diamonds), and resistance residues independently identified for both targocil and L275 or L638 (purple diamonds). Note, S. aureus and S. epidermidis TarG are 96% homologous and share identical length (see Figure S8). Accordingly, S. epidermidis TarG L275R or L638R mutations are also highlighted (boxed in blue). TarG transmembrane topology was predicted using the SOSUI program and diagram generated with TOPO2 software. (B) WTA extraction and analysis of WTA levels in MRSA COL challenged with 10× MIC of L638, L555, L524, or control compounds Erythromycin (Eryth), rifamicin (Rif), or Vancomycin (Vanc). Tunicamycin (4 μg/ml; Tuni) treatment at subMIC serves as a positive control for complete WTA depletion.
Similarly, L275R and L638R mutant selection and NGS analysis performed in MRSE identified single non-synonymous missense mutations mapping to the S. epidermidis TarG protein. Indeed, two of the drug resistant mutations correspond to the same residues identified in S. aureus TarG (TarG-F82L and TarG-Y190H). Additional new mutations, TarG-S79I and 4 independently derived mutations mapping to residue M211 (TarG-M211L and TarG-M211I), are predicted to localize within transmembrane 2 or extracellular loop 3 domains, respectively (Figure 3A, see also Figure S8). Collectively, these studies provide strong genetic evidence that TarG is the molecular target of L275 and L638 across methicillin-resistant Staphylococci and suggest that they may interfere with TarG function by binding either to the extracellular surface and/or substrate channel of the WTA transporter.
To further investigate inhibitory effects on WTA biogenesis by our compounds, WTA polymers were extracted from drug-treated MRSA COL, normalized according to cell biomass, and examined by polyacrylamide gel electrophoresis (PAGE) analysis (Meredith et al., 2008). As L638, L524, and L555 are highly potent and sufficient biomass is required to isolate WTA from drug treated cells, high initial innocula (1 × 109 cells/drug treatment) were required. WTA extracted from mock-treated control cells appears as a ladder of discrete size polymers (Figure 3B). As expected, WTA biogenesis was completely abolished by tunicamycin treatment and unaffected by antibiotic controls. Conversely, L638 and L555-treated cells displayed modest but reproducible changes in WTA polymer levels and/or size (Figure 3B). L524 treated cells, however, exhibited only a very minor decrease in WTA (as observed by reduced straining of higher molecular weight polymers), similar to repeated attempts with targocil under identical conditions (data not shown). Although no in vitro biochemical assay is available to test whether these compounds inhibit TarG directly, the incomplete depletion of WTA polymer levels by TarG inhibitors likely reflects the fact that such agents effectively inhibit growth. Consequently, pre-existing WTA comprising cells prior to TarG inhibitor treatment is not diluted as minimal cell doublings occur post drug treatment despite inhibition of new WTA biosynthesis, whereas under tunicamycin subMIC conditions where TarO-mediated WTA biosynthesis of new material is specifically inhibited, cells robustly grow and dilute pre-existing WTA through successive cell divisions.
Extended NGS Analysis of L638R mutations in MRSA COL
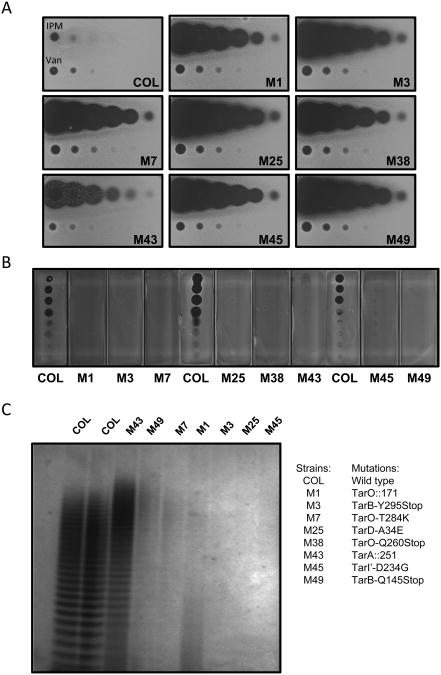
NGS analysis was also performed with a large number of slower growing MRSA COL L638R mutants (n=55) arising from this study to confirm expected presence of ΔtarO or ΔtarA mutations, and examine whether additional L638R compensatory drug resistance mechanisms exist. Indeed, several L638R mutants mapped to tarO or tarA and are presumed to oblate gene function (Figure 4). However, several additional mutants also mapped to late stage WTA genes, including tarB, tarD, and tarI' (Figure 4). Moreover, all isolated tarB, tarD, and tarI' mutants displayed similar phenotypes to ΔtarO and ΔtarA mutants. In addition to their L638R phenotype, tarB, tarD, and tarI' mutants displayed a striking cross resistance to targocil and other WTAIs (see Table S5) as well as restored susceptibility to imipenem and prominent resistance to phage K-mediated cell lysis, implying they are also loss-of-function mutations in these late stage WTA genes (Figure 4). Further, in numerous instances no additional non-synonymous mutations beyond those associated with tar genes were identified by NGS analysis of L638R mutants, thus ruling out the possibility that compensatory mutations are responsible for the observed phenotypes. To directly determine the severity of WTA phenotypes associated with tarO, tarA, tarB, tarD, and tarI' mutants, their WTA was extracted and analyzed by PAGE. All mutants examined displayed dramatic alterations in WTA abundance and/or polymer size (Figure 4C). Interestingly, these results demonstrate that MRSA COL is able to tolerate profound loss-of-function mutations in late stage WTA biosynthesis genes and that such mutations expand its repertoire of resistance to TarG inhibitors.
Figure 4. L638R tar Mutants Share β-Lactam Hypersusceptibility and WTA Depletion Phenotypes.
(A) Altered imipenem (IPM) susceptibility of MRSA COL tar mutants by agar susceptibility assay (see inset for description of specific mutations). Three-fold serial dilutions of IPM (starting with 8 μg material) spotted left to right across plate. Vancomycin (Van; 2 μg starting material) serves as control for specificity of β-lactam susceptibility phenotype. (B). Bacteriophage K resistance phenotype of MRSA COL tar mutants. (C) WTA extraction and PAGE analysis from L638R MRSA COL tar mutants. Note: WTA material normalized to cell biomass prior to loading.
tarB, tarD, and tarI' mutants Possess Temperature and Osmotic Sensitive Growth Phenotypes
Identifying viable mutations in late stage WTA genes with dramatic loss-of-function phenotypes was not expected since they were shown to be essential in both S. aureus and B. subtilis (D'Elia et al., 2006a; D'Elia et al., 2006b). To reconcile these findings, tarB, tarD, and tarI' mutant phenotypes were compared to ΔtarO and ΔtarA mutants under conditions of elevated temperature and osmotic stress. tarB and tarD mutants grown on LB agar displayed modest growth phenotypes at 37 °C and all tar loss-of-function mutants tested exhibited marked growth phenotypes under high osmotic pressure with 7.5% NaCl (Figure 5). Consistent with the expectation that these phenotypes are linked to the tar mutation, supplementing the medium with 2 μg/ml tunicamycin partially reversed their observed phenotypes. tarB, tarD, and tarI' mutants also displayed striking temperature sensitive growth phenotypes at 42°C whereas tarA and tarO mutants shared only mild growth phenotypes, as previously reported (D'Elia et al., 2006a; D'Elia et al., 2009a). Thus the relative severity of tar mutant growth phenotypes mirror that determined in a MSSA RN4220 strain background but is observed at higher temperature in MRSA COL. All temperature sensitive growth phenotypes were noticeably suppressed by tunicamycin or 4% NaCl supplementation and fully suppressed by adding both tunicamycin and 4% NaCl (Figure 5). Further, at 37°C under hypotonic growth conditions (no NaCl in LB medium), tarB and tarD mutants are also growth impaired and fully suppressed by co-supplementing tunicamycin and 4% NaCl (see Figure S9). At 42 °C under hypotonic conditions, all tar mutants (including tarO and tarA mutants) displayed extreme growth phenotypes fully reversed by tunicamycin and 4% NaCl (see Figure S9). These results corroborate the temperature sensitive and osmotic remedial growth phenotypes of ΔtarO mutants (Hoover and Gray, 1977; Vergara-Irigaray et al., 2008) and extend these finding to tarA as well as tarB, tarD, and tarI' loss-of-function mutants. Therefore relatively minor differences in growth conditions can profoundly impact the viability of S. aureus strains defective in late stage WTA biosynthesis.
Figure 5. Conditional Essentiality of MRSA COL tar mutants.
Growth phenotype of MRSA COL tar mutants spotted as 3–fold serial dilutions starting at 5 × 104 cells/spot (left to right) on LB medium, LB + 4% NaCl, LB + 7.5% NaCl and +/- tunicamycin (2 μg/ml) supplementation grown at 37°C or 42°C for 18 hr.
Attenuated Virulence Phenotypes of MRSA COL Strains Defective in Early or Late Stage WTA Biogenesis
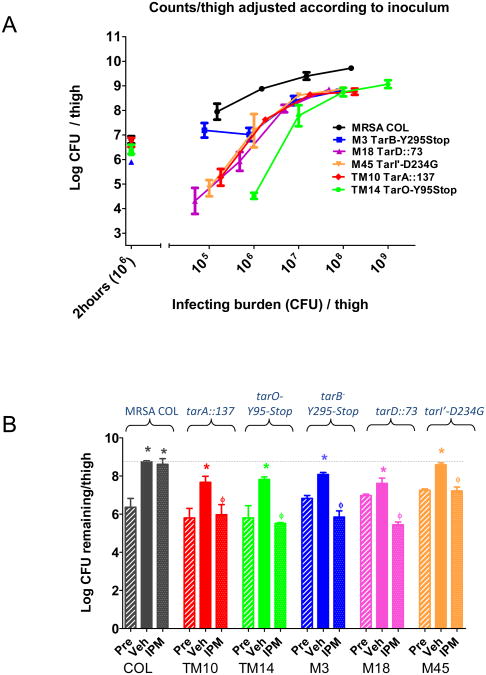
Identifying L275R and L638R loss-of-function mutants that map to early and late stage WTA biosynthetic genes enabled us to examine their possible virulence phenotypes in MRSA COL using a previously described murine thigh infection model (Gill et al., 2007). We reasoned that in vivo phenotypes may well be expected based on results obtained from extensive in vivo studies using MSSA-derived tarO mutants (Weidenmaier et al., 2004; Weidenmaier et al., 2005; Weidenmaier et al., 2010). Moreover, temperature and osmotic stress growth phenotypes of MRSA COL tarB, tarD, and tarI' mutants we observed under laboratory growth conditions may decrease S. aureus pathogenicity. As expected, tarO and tarA mutants typically displayed an approximate 1 log reduction in bacterial burden after 24 h versus the wild type parental strain over a broad range of infection doses in this animal model (Figure 6A, Table S6). Interestingly, at the lowest infection dose used (1×106 cells), the tarO mutant strain displayed an even more pronounced attenuated virulence, nearing as much as a 4 log reduction in bacterial burden compared to wild type MRSA COL. Similarly, tarD and tarI' mutants also displayed dramatic attenuated virulence phenotypes, particularly across lower infection doses where as much as 3 log reductions in bacterial burden were observed, while the tarB mutant more closely mirrored the tarA with bacterial burden after 24 h reproducibly reduced ∼ 1 log across all infection doses tested. These data demonstrate the relevance of WTA as a virulence determinant in S. aureus and suggest that their attenuated virulence phenotypes may further reduce the threat of drug resistance in a therapeutic context.
Figure 6. Attenuated Virulence and Imipenem Hypersusceptibility of MRSA COL tar Mutants in a Murine Deep thigh Infection Model.
(A) Attenuated virulence of tar mutants versus MRSA COL wild type (black line) across 4 log escalation in infection dose. See inset for description and color coding of specific mutants. Infection inocula of M14 (TarO-Y95stop) strain was one log great than other strains tested. 2 hour (106) bacterial burden control confirms infection dose (106/thigh) when enumerated from animals 2 h post infection. (B) Restored efficacy of Imipenem against MRSA COL tar mutants. Immune suppressed CD-1 mice (5 mice per group) were challenged intramuscularly with 1 × 106 CFU/thigh of MRSA COL or the indicated MRSA COL tar mutant. Imipenem (IPM; 10 mg/kg) or vehicle (Veh;10 mM MOPS) were dosed subcutaneously (SC) three times over a 24 hr period. Thigh homogenates were obtained 2 h post infection and prior to treatment (Pre), or post 24h treatment with Veh or IPM and serially plated to determine CFU/thigh remaining. Bacterial burden was enumerated and compared among three groups. *p<0.05 vs. respective 2h control; ϕ p<0.05 vs. respective 24h control.
Imipenem Efficacy against MRSA tar mutants Resistant to TarG Inhibitors
As tarO, tarA, tarB, tarD, tarI' loss-of-function mutations enhance MRSA COL susceptibility to β-lactam antibiotics in vitro, we tested whether this phenotype extends to the deep thigh murine infection model. Imipenem is ineffective in treating MRSA COL infected animals in this model when dosed at 10 mg/kg subcutaneously tid for 24 hrs versus the mock treated control group administered 10 mM MOPS as vehicle (Figure 6B). However, imipenem efficacy was restored in mice infected with tarO, tarA, tarB, or tarD mutant strains, producing a ∼3 log reduction in bacterial burden versus the parental COL strain identically treated with imipenem (Figure 6B, Table S7). Similarly, imipenem treatment achieved a ∼ 2 log reduction in bacterial burden with mice infected with a tarI' deficient strain versus wild type COL. Therefore, we expect that the drug resistance to TarG inhibitors, mediated by the selection for these compensatory bypass mutants will be reduced in a MRSA infection setting by combining such agents with a β-lactam antibiotic. These data also provide clear genetic demonstration that in addition to TarO and TarA, late stage WTA enzymes including TarB, TarD, or TarI' are also therapeutically relevant MRSA β-lactam potentiation targets.
TarG Inhibitors +/− Imipenem Frequency of Resistance
Targocil displays a high FOR of ∼7 × 10−7 cells at 8× MIC (Lee et al., 2010) which is substantially reduced in the presence of the β-lactam, oxacillin (Campbell et al., 2011). Accordingly, we examined the spontaneous rate of drug resistant mutants isolated amongst this extended set of TarG inhibitors. L524 and L555 displayed markedly higher or marginally lower FOR than targocil, respectively, over a range of MIC levels tested (Table 1). Whereas L638 tested at 2× MIC or 4× MIC only minimally reduced FOR (∼2 × 10−7) compared to targocil, a borderline acceptable FOR of 1.9 ×10−8 was achieved at 8× MIC. Interestingly, L275 displayed a highly favorable dose dependent FOR, with spontaneous drug resistant isolates recovered at a frequency of ∼4 × 10−8 at 2-4 × MIC, and ≤ 3.6 × 10−10 at 6-8× MIC levels (Table 1). Further, in combination with imipenem at a subMIC level of 4 μg/ml (its clinical breakpoint concentration against MRSA (Tan et al., 2012)), similar L275 FOR levels could be achieved at as low as 2× its MIC. Remarkably, resistance associated with L275 in combination with a sub inhibitory concentration of imipenem was therefore reduced ∼104-fold from the original FOR of targocil.
TarG Inhibitors +/− Imipenem In vivo Efficacy
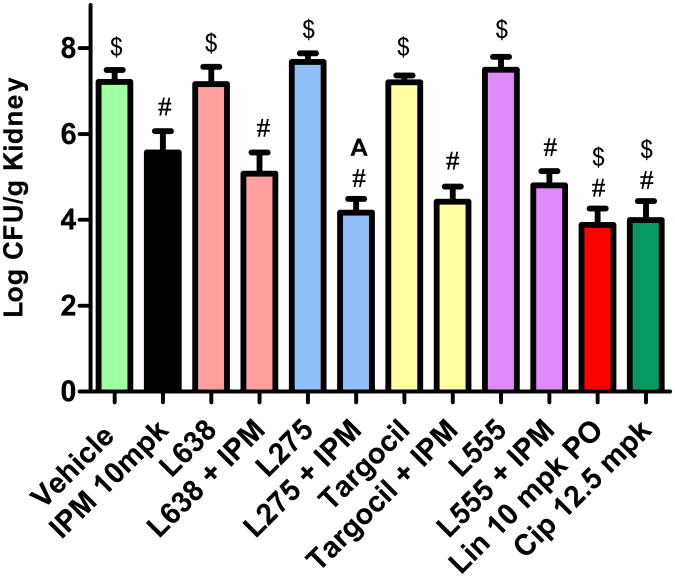
TarG inhibitors display favorable in vitro potency but possess high plasma protein binding which could diminish their efficacy in a treatment model of MRSA infection (see Table S8). As this issue may be particularly problematic if the site of infection is muscle tissue, such as the deep thigh infection model, we turned to an alternative animal efficacy model involving an intraperitoneal challenge and subsequent kidney colonization by MRSA COL (Gill et al., 2007). Neither targocil, L275, L638, or L555 administered subcutaneously at 200 mg/kg tid significantly reduced bacterial burden after 24 hrs treatment, as measured by colony forming units per gram kidney tissues (CFU/g) versus vehicle control treated animals (Figure 7, see also Table S9). Further, imipenem singly administered subcutaneously at 10 mg/kg tid in this animal model only modestly reduced bacterial burden ∼1.5 log CFU/g versus vehicle control group. However, a strongly additive or synergistic efficacy was achieved by co-administering TarG inhibitors with a sub-efficacious treatment of imipenem. For example, co-administering TarG inhibitors subcutaneous at 200 mg/kg tid and imipenem administration markedly reduced bacterial burden among treatment groups ranging from 2 -3 log CFU/g versus vehicle control group. Although only L275 displayed significant synergy in combination with imipenem versus imipenem alone (t-test p<0.05), co-administering these agents (albeit at relatively high drug concentrations) reduced bacterial burden to levels achieved by linezolid or ciprofloxacin treatment.
Figure 7. TarG inhibitors Augment Imipenem Efficacy.
Immune suppressed Balb/c mice (5 mice per group) were challenged intraperitoneally with ∼ 2 × 104 CFU of MRSA COL and dosed SC with the WTA inhibitor (200 mg/kg), imipenem (IPM; 10 mg/kg) co-formulated with 50 mg/kg cilastatin, or co-administration of the TarG inhibitor and imipenem/cilistatin at 2, 5 and 8 hr post infection challenge. Kidneys were aseptically collected, homogenized, and plated 24 hr after challenge to determine CFU remaining per gram kidney tissue. Linezolid (Lin; 10 mg/kg dosed orally) and ciprofloxacin (Cip; 12.5 mg/kg dosed SC) serve as positive control antibiotics for demonstrating efficacy. #, significant (p<0.05) versus vehicle; $, significant (p<0.05) versus IPM alone; A, significant (p<0.05) versus IPM alone.
Discussion
Applying a chemical biology screen designed to mimic the well characterized WTA gene dispensability phenotypes in S. aureus, we identify a series of synthetic compounds each chemically distinct from targocil. Extensive drug resistance mapping studies performed in MRSA and MRSE provide genetic evidence that like targocil, small molecules we report here inhibit TarG as their primary drug target. Corroborating this conclusion, WTA levels are partially depleted in TarG inhibitor-treated cells, particularly L638 and L555 structural classes, and bypass mutations to these new WTAIs correspond to loss-of-function mutations in tar genes which function upstream of TarG. Depending on their structural class, TarG inhibitors described here display either the selectivity and propensity for resistance as targocil or an improved antibacterial spectrum and markedly reduced frequency of drug resistance. As drug resistant bypass mutations isolated in MRSA COL are dramatically hypersensitive to β-lactam antibiotics, the effective FOR of these compounds can be dramatically reduced in combination with sub inhibitory concentrations of imipenem. These studies suggest TarG to be a highly druggable antibacterial target and demonstrate that cognate inhibitors display in vivo efficacy when paired with imipenem in treating an animal MRSA infection.
Essential versus Conditional Essential Phenotypes of Late Stage WTA Biosynthetic genes
Two hypotheses for the reported essentiality of late stage WTA biosynthetic genes and non-essentiality of early stage WTA genes have been widely considered (D'Elia et al., 2006a; D'Elia et al., 2006b; D'Elia et al., 2009c). Specifically, loss-of-function mutations in late stage WTA genes may accumulate toxic WTA intermediates and/or decrease available bactoprenol necessary for peptidoglycan synthesis to growth impairing levels. So why do multiple late stage WTA genes appear non-essential in MRSA COL when they have been demonstrated to be essential in MSSA strain RN4220? MRSA COL may simply produce more bactoprenol or possess greater flexibility in recycling bactoprenol from the WTA intermediates than S. aureus RN4220 or B. subtilis strains in which the terminal phenotype of WTA mutations have been examined (D'Elia et al., 2006a; D'Elia et al., 2006b). Alternatively, tarB, tarD, and tarI' mutants isolated here may be viable because they retain some minor level of residual activity which is required to reverse bactoprenol flux and such an activity would be absent amongst null mutations, rendering the latter inviable. Accordingly, the fact that such mutants make little to no WTA does not impair viability but some minimal level of WTA biosynthesis may be necessary to recycle bactoprenol. We also note that our tar mutant phenotypes are derived from amino acid substitution or C-terminal truncation mutations, all of which in the context of a postulated multimeric WTA enzyme complex (Formstone et al., 2008) may produce less severe phenotypes than complete gene deletion mutations. Importantly, MRSA COL mutations in late stage WTA biosynthesis are in fact conditionally essential at 42°C. Moreover, consistent with S. aureus WTA gene dispensability phenotypes (D'Elia et al., 2006a), MRSA COL tarB, tarD, and tarI' mutant phenotypes were noticeably suppressed by supplementing tunicamycin at levels that phenocopy deletion of tarO. Therefore, we conclude that the paradoxical severity of growth phenotypes amongst mutants in early versus late stage WTA synthesis is conserved in MRSA COL. However, the absolute strength of these phenotypes can vary contextually in different S. aureus genetic backgrounds as might be expected considering the extensive genomic diversity identified amongst multiple fully sequenced S. aureus strains (Feng et al., 2008).
Off target activity of L275, L638, L640, and L555
Unlike targocil, which is a highly specific inhibitor of S. aureus TarG activity (Swoboda et al., 2009; Schirner et al., 2011), several of the compounds we have identified likely possess an off-target activity. Firstly, bioactivity of each of the new TarG inhibitors (with the exception of L524) is not fully suppressed by genetic or chemical inhibition of TarO. Secondly, these TarG inhibitors display a notable antibacterial activity that exceeds the spectrum of bacteria possessing a TarG ortholog or WTA biosynthetic pathway, including Streptococcus pyogenes as well as Gram-negative bacteria including Moraxella catarrhalis and Haemophilus influenzae (see Table S10). Finally, L275, L638, and L555 display dramatically lower FOR against MRSA COL compared to targocil over a range of drug concentrations assessed either singly or in combination with imipenem. As all resistance mutants to these compounds isolated in both MRSA and MRSE shared either a TarG or the WTA bypass-mediated mechanism of resistance and these compounds lack anti- Candida albicans activity, we favor that this off-target activity reflects a secondary target rather than non-specific/general cellular toxicity of the compounds. Studies to identify a potential secondary target to these compounds are ongoing.
TarG is a Highly Druggable Target
Despite the opportunity for this pathway-based screen to identify inhibitors to any of the late stage WTA enzymes, only TarG WTAIs have been reported by us and the prior work (Swoboda et al. 2009). Interestingly, these inhibitors represent structurally distinct chemical series. We speculate that TarG is a particularly ‘druggable’ target when compared to other Tar enzymes. The S. aureus WTA transporter is predicted to be a heteromeric complex consisting of dimers of the cytoplasmic ATPase subunit TarH and a WTA substrate channel forming subunit, TarG (Cuthbertson et al., 2010; Schirner et al., 2011). Therefore, TarG is uniquely exposed to the extracellular milieu and potentially susceptible to inhibitors that need not enter the cytoplasm. Consistent with this, all drug resistant mutations to WTAIs we describe map to the presumed substrate channel or extracellular domains of TarG. Considering the substantial size of the substrate channel required to translocate WTA across the plasma membrane, structurally diverse compounds may have access to the channel and sterically interfere with WTA precursor export. In such a manner, TarG inhibitors would be mechanistically analogous to diverse protein synthesis inhibitors which bind to the ribosome polypeptide export tunnel to block release of nascent polypeptides (Walsh 2003).
WTAIs as β-lactam Combination Agents
Significant therapeutic advantages are anticipated by combining a WTAI with existing β-lactam antibiotics to treat MRSA infections. These include improved antibacterial potency by the strong additivity or synergistic activity of the two agents and reduced drug resistance in the context as dual agents. Unexpectedly, the degree of observed synergy between imipenem and TarG inhibitors is generally lower than genetic analysis would predict. Campbell et al (2012) have suggested this may reflect the fact that β-lactams are only effective against actively growing cells and the bacteriostatic nature of TarG inhibitors might antagonize β-lactam activity. Conflicting with this view however is the fact that β-lactams suppress the FOR of TarG inhibitors. Alternatively, the lack of striking in vitro synergy between these agents may reflect a limitation in the sensitivity of the standard microdilution checkerboard assay (Amsterdam 2005), particularly when testing potent bioactive agents. Whereas the checkerboard assay effectively scores synergy between non-bioactive agents, synergistic effects between bioactive agents may occur rapidly and possibly over only a few cell division cycles, obscuring such chemical – chemical interactions. Regardless, we demonstrate that their combined effects are pronounced in an animal model of MRSA infection. This exaggerated efficacy in a host environment reflects the dual manner in which these agents impair normal WTA synthesis, hence depleting an important S. aureus virulence determinant (Weidenmaier et al., 2004; Weidenmaier et al., 2008) and therefore disrupting the integrity of the cell wall to potentially expose otherwise masked cell surface immune recognition determinants required to activate an innate immune response (Guan et al., 2007; Pietrocola et al., 2011).
Importantly, reduced drug resistance is achieved by combining TarG inhibitors with imipenem, as recently demonstrated with targocil and oxicillin (Campbell et al., 2012) as well as a second β-lactam synergistic combination involving the FtsZ inhibitor, PC190723, and imipenem (Tan et al., 2012). Remarkably, L275 at 2× MIC combined with the breakpoint concentration of imipenem reduces the FOR of the combined agents to extremely low levels. This can likely be attributed to strong counter-selection of bypass resistance mutants in the presence of the β-lactam due to their dramatic hypersensitivity to imipenem, and the impact of L275 secondary target activity, particularly at higher drug concentrations.
Chemical Biology, Drug Resistance Analysis, and Whole Genome Sequencing
We demonstrate that combining a robust whole cell-based screen to identify pathway-specific bioactive compounds with drug resistance selection and NGS technology provides a powerful strategy to successfully identify target-specific inhibitors, their MOA and drug resistance mechanisms and to genetically predict the pharmacological effects of such agents. Drug resistant mutants identified in this study also provided a valuable resource to genetically validate other genes in the WTA pathway as new β-lactam potentiation targets. Further, the resulting mutations were valuable reagents to verify that genetic depletion of tarO, tarA, tarB, tarD, or tarI' in MRSA produce markedly attenuated virulence and β-lactam hypersusceptibility phenotypes in a host setting. Accordingly, the strategy can be used to draw strong target validation conclusions. Chemical biology studies on the effects of these compounds themselves provided a ‘surrogate’ genetic strategy to chemically select for a broad set of WTA pathway mutants, therefore bypassing molecular genetic methodologies necessary to evaluate such targets. We believe that combining resistance, suppression or other selection studies with NGS-based mapping of causal mutations will have an extraordinary impact, not only in antimicrobial discovery, but broadly in the fundamental pursuit of linking chemistry and biology.
Significance
Innovative strategies to combat drug resistance associated with methicillin-resistant Staphylococcus aureus (MRSA) and other β-lactam resistant Staphylococci are needed. Here, we have investigated the potential of wall teichoic acid (WTA) inhibitors as combination agents to effectively restore β-lactam susceptibility and reduce drug resistance amongst pre-existing drug resistant Staphylococci. Performing a whole cell pathway-based screen for WTA inhibitors (WTAIs) we identified three potent and structurally distinct classes of bacteriostatic agents with extended Gram-positive spectrum targeting TarG, the channel forming subunit of the WTA transporter. We use whole genome sequencing analysis in MRSA as well as in methicillin-resistant Staphylococcus epidermidis (MRSE) to map the drug resistance landscape. These analyses cross-validate a TarG-mediated mechanism of action for each of these WTAIs. Multiple drug resistant loss-of-function bypass mutations in earlier steps of WTA biosynthesis were also recovered, including tarO, and tarA, as well as tarB, tarD, and tarI', the latter of which are essential for growth in the methicillin-sensitive Staphylococcus aureus (MSSA) strain background RN4220 but not MRSA COL. Phenotypic characterization of tar mutants reveals their attenuated virulence and profound hypersusceptibility to β-lactam antibiotics in a murine infection model. Pharmacological evidence of their β-lactam potentiation activity is also achieved in an in vivo infection setting. Thus genetic and pharmacological studies demonstrate the potential of WTAIs as effective β-lactam combination agents to treat MRSA. This work also highlights the incredible robustness and efficiency of combining classical drug resistance selection and whole genome sequencing to identify drug targets and impact small molecule MOA and resistance studies.
Experimental Procedures
Microbiological studies
MRSA COL is a hospital-acquired penicillinase-negative strain extensively used in Staphylococcus aureus methicillin resistance and virulence studies (de Lencastre et al., 1999; Tan et al., 2012) and from which its genome has been fully sequenced and annotated (Gill et al., 2005). MRSE strain (MB6255) is a previously described methicillin-resistant S. epidermidis clinical isolate (CLB26329; Huber et al., 2009) isolated from a New York ICU in 2004. MICs were determined by the broth microdilution method according to the recommendations of the Clinical and laboratory Standards Institute. The standard checkerboard technique was used to quantify synergy between antibiotic agents (Amsterdam 2005). MRSA COL and MRSE MB6255 were grown in cation-adjusted Mueller Hinton broth (CAMHB) medium and assayed in a 96 well format using 2-fold dilutions of imipenem and TarG inhibitors. MIC determinations were assessed visually. FICI values were determined by adding the FIC value of each compound required to achieve a MIC when paired with the second agent. Bacteriophage K cell lysis assays (Swoboda et al., 2009; Xia et al., 2010), transposon-mediated selection of L275 drug resistant mutants (Wang et al., 2011), WTA extraction analyses (Meredith et al., 2008), and time kill studies (Tan et al., 2012) were performed as previously described. Agar susceptibility assay was performed as previously described (Lee et al., 2011).
Isolation of MRSA COL targocilR, L275R, and L638R mutants
MRSA and MRSE strains were grown to late-exponential phase (OD600 ∼1.0; approximately 109 CFU/ml) and spread on BHIA plates containing 2-fold escalating agar MIC levels of TarG inhibitors. To establish the number of viable cells in the starting inoculum, the culture was serially diluted and plated BHIA plates lacking the TarG inhibitor. Resistant isolates were re-streaked on plates containing the same TarG inhibitor concentration. The frequency of resistance (FOR) either in the absence or presence of 4 μg/ml imipenem was determined dividing the number of resistant isolates by the viable CFU in the late-exponential inoculum. See Table S11 for detailed description of mutants.
TarG inhibitor Efficacy Studies
Efficacy studies were performed using a previously described murine septicemia model of S. aureus infection where immune suppressed Balb/c mice (5 mice per group) were challenged with 1.8 × 104 CFU MRSA COL in 3% hog gastic mucin (Gill et al., 2007). Mice were dosed SC with the WTA inhibitor (200 mg/kg), imipenem (IPM; 10 mg/kg) co-formulated with 50 mg/kg cilastatin, or co-administration of the TarG inhibitor and imipenem/cilistatin at 2, 5 and 8 hr post infection challenge. Kidney homogenates were serially plated 24h after initiation of therapy to determine CFU/kidney remaining. Pharmacokinetic and plasma protein binding properties of TarG inhibitors are provided in Table S8. All animal experiments were performed according to Merck and AAALAC guidelines for the ethical treatment of animals.
In vivo virulence studies of MRSA COL tar mutants
Virulence phenotypes were assessed using a previously described murine deep thigh infection model (Gill et al., 2007; Tan et al., 2012). Bacterial cultures of MRSA COL (MB 5393), tarO, tarA, tarB, tarD, and tarI' were grown in TSB medium overnight to late-exponential phase (OD600 ∼1.0; approximately 109 CFU/ml). Five groups of mice were thigh inoculated (0.1 mL/injection) with an isolate, each at increasing inoculum concentrations ranging from ∼ 105 -109 CFU/ml. Thigh homogenates were serially plated 24h after infection to determine CFU/thigh remaining.
Restored efficacy of imipenem against MRSA COL tar mutants
Imipenem efficacy studies were performed using a previously described murine deep thigh infection model (Gill et al., 2007; Tan et al., 2012). Female CD-1 mice inoculated into the thighs with overnight cultures (∼ 106 CFU/thigh) of MRSA COL, or representative tar mutants were treated with vehicle or imipenem (10mg/kg per dose × 3 over 24h). Thigh homogenates were serially plated after 2 hour or 24h (vehicle, imipenem) to determine CFU/thigh remaining.
Supplementary Material
Acknowledgments
We thank BGI for whole genome sequencing support. All authors excluding John Santa Maria and Suzanne Walker are current or past employees of Merck as stated in the affiliations and potentially own stock and/or hold stock options in the company.
References
- Amsterdam D. Susceptibility Testing of Antimicrobials in Liquid Media. In: Lorian V, editor. Antibiotics in Laboratory Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. pp. 89–93. [Google Scholar]
- Andrusiak K, Piotrowski JS, Boone C. Chemical-genomic profiling: systematic analysis of the cellular targets of bioactive molecules. Bioorg Med Chem. 2012;20:1952–1960. doi: 10.1016/j.bmc.2011.12.023. [DOI] [PubMed] [Google Scholar]
- Atilano ML, Pereira PM, Yates J, Reed P, Veiga H, Pinho MG, Filipe SR. Teichoic acids are temporal and spatial regulators of peptidoglycan cross-linking in Staphylococcus aureus. Proc Natl Acad Sci USA. 2010;107:18991–18996. doi: 10.1073/pnas.1004304107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger-Bächi B, Rohrer S. Factors influencing methicillin resistance in Staphylococci. Arch Microbiol. 2002;178:165–171. doi: 10.1007/s00203-002-0436-0. [DOI] [PubMed] [Google Scholar]
- Brown S, Zhang YH, Walker S. A revised pathway proposed for Staphylococcus aureus wall teichoic acid biosynthesis based on in vitro reconstitution of the intracellular steps. Chem Biol. 2008;15:12–21. doi: 10.1016/j.chembiol.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Bad bugs, no drugs:no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- Campbell J, Singh AK, Santa Maria JP, Kim Y, Brown S, Swoboda JG, Mylonakis E, Wilkinson B, Walker S. Synthetic lethal compound combinations reveal a fundamental connection between wall teichoic acid and peptidoglycan biosyntheses in Staphylococcus aureus. ACS Chem Biol. 2011;6:106–116. doi: 10.1021/cb100269f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J, Singh AK, Swoboda JG, Gilmore MS, Wilkinson BJ, Walker S. An antibiotic that inhibits a late step in wall teichoic acid biosynthesis induces the cell wall stress stimulon in Staphylococcus aureus. Antimicrob Agents Chemother. 2012;56:1810–1820. doi: 10.1128/AAC.05938-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M, Baryshnikova A, Bellay J, Kim Y, Spear ED, Sevier CS, Ding H, Koh JL, Toufighi K, Mostafavi S, et al. The genetic landscape of a cell. Science. 2010;327:425–431. doi: 10.1126/science.1180823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson L, Kos V, Whitfield C. ABC transporters involved in export of cell surface glycoconjugates. Microbiol Mol Biol Rev. 2010;74:341–362. doi: 10.1128/MMBR.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Elia MA, Pereira MP, Chung YS, Zhao W, Chau A, Kenney TJ, Sulavik MC, Black TA, Brown ED. Lesions in teichoic acid biosynthesis in Staphylococcus aureus lead to a lethal gain of function in the otherwise dispensable pathway. J Bacteriol. 2006a;188:4183–4189. doi: 10.1128/JB.00197-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Elia MA, Millar KE, Beveridge TJ, Brown ED. Wall teichoic acid polymers are dispensable for cell viability in Bacillus subtilis. J Bacteriol. 2006b;188:8313–8316. doi: 10.1128/JB.01336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Elia MA, Henderson JA, Beveridge TJ, Heinrichs DE, Brown ED. The N-acetylmannosamine transferase catalyzes the first committed step of teichoic acid assembly in Bacillus subtilis and Staphylococcus aureus. J Bacteriol. 2009a;191:4030–4034. doi: 10.1128/JB.00611-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Elia MA, Pereira MP, Brown ED. Are essential genes really essential? Trends Microbiol. 2009b;17:433–438. doi: 10.1016/j.tim.2009.08.005. [DOI] [PubMed] [Google Scholar]
- D'Elia MA, Millar KE, Bhavsar AP, Tomljenovic AM, Hutter B, Schaab C, Moreno-Hagelsieb G, Brown ED. Probing teichoic acid genetics with bioactive molecules reveals new interactions among diverse processes in bacterial cell wall biogenesis. Chem Biol. 2009c;16:548–556. doi: 10.1016/j.chembiol.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Drawz SM, Bonomo RA. Three decades of beta-lactamase inhibitors. Clin Microbiol Rev. 2010;23:160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler V, Meier PS, Heusser R, Kupferschmied P, Fazekas J, Friebe S, Staufer SB, Majcherczyk PA, Moreillon P, Berger-Bächi B, McCallum N. Deletion of hypothetical wall teichoic acid ligases in Staphylococcus aureus activates the cell wall stress response. FEMS Microbiol Lett. 2012;333:109–120. doi: 10.1111/j.1574-6968.2012.02603.x. [DOI] [PubMed] [Google Scholar]
- de Lencastre H, Wu SW, Pinho MG, Ludovice AM, Filipe S, Gardete S, Sobral R, Gill S, Chung M, Tomasz A. Antibiotic resistance as a stress response: Complete sequencing of a large number of chromosomal loci in Staphylococcus aureus strain COL that impact on the expression of resistance to methicillin. Microb Drug Resist. 1999;5:163–175. doi: 10.1089/mdr.1999.5.163. [DOI] [PubMed] [Google Scholar]
- Feng Y, Chen CJ, Su LH, Hu S, Yu J, Chiu CH. Evolution and pathogenesis of Staphylococcus aureus: lessons learned from genotyping and comparative genomics. FEMS Microbiol Rev. 2008;32:23–37. doi: 10.1111/j.1574-6976.2007.00086.x. [DOI] [PubMed] [Google Scholar]
- Formstone A, Carballido-López R, Noirot P, Errington J, Scheffers DJ. Localization and interactions of teichoic acid synthetic enzymes in Bacillus subtilis. J Bacteriol. 2008;190:1812–1821. doi: 10.1128/JB.01394-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill CJ, Abruzzo GK, Flattery AM, Misura AS, Bartizal K, Hickey EJ. In vivo efficacy of a novel oxazolidinone compound in two mouse models of infection. Antimicrob Agents Chemother. 2007;51:3434–3436. doi: 10.1128/AAC.01567-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SR, Fouts DE, Archer GL, Mongodin EF, Deboy RT, Ravel J, Paulsen IT, Kolonay JF, Brinkac L, Beanan M, et al. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol. 2005;187:2426–2438. doi: 10.1128/JB.187.7.2426-2438.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan R, Mariuzza RA. Peptidoglycan recognition proteins of the innate immune system. Trends Microbiol. 2007;15:127–134. doi: 10.1016/j.tim.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Hoover DG, Gray RJ. Function of cell wall teichoic acid in thermally injured Staphylococcus aureus. J Bacteriol. 1977;131:477–485. doi: 10.1128/jb.131.2.477-485.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber J, Donald RG, Lee SH, Wang-Jarantow L, Salvatore MJ, Meng X, Painter R, Onishi RH, Occi J, Dorso K, et al. Chemical genetic identification of peptidoglycan inhibitors potentiating carbapenem activity against methicillin-resistant Staphylococcus aureus. Chem Biol. 2009;16:837–848. doi: 10.1016/j.chembiol.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Johnson AP. Methicillin-resistant Staphylococcus aureus: the European landscape. J Antimicrob Chemother. 2011;66(4):iv43–8. doi: 10.1093/jac/dkr076. [DOI] [PubMed] [Google Scholar]
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, et al. Active Bacterial Core surveillance (ABCs) MRSA Investigators. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- Komatsuzawa H, Suzuki J, Sugai M, Miyake Y, Suginaka H. Effect of combination of oxacillin and non-beta-lactam antibiotics on methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1994;33:1155–1163. doi: 10.1093/jac/33.6.1155. [DOI] [PubMed] [Google Scholar]
- Lee SH, Wang-Jarantow L, Wang H, Sillaots S, Cheng H, Meredith TC, Thompson J, Roemer T. Antagonism of Chemical Genetic Interaction Networks Resensitize MRSA to β-Lactam Antibiotics. Chem Biol. 2011;18:1379–1389. doi: 10.1016/j.chembiol.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Lee K, Campbell J, Swoboda JG, Cuny GD, Walker S. Development of improved inhibitors of wall teichoic acid biosynthesis with potent activity against Staphylococcus aureus. Bioorg Med Chem Lett. 2010;20:1767–1770. doi: 10.1016/j.bmcl.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols RJ, Sen S, Choo YJ, Beltrao P, Zietek M, Chaba R, Lee S, Kazmierczak KM, Lee KJ, Wong A, et al. Phenotypic landscape of a bacterial cell. Cell. 2011;144:143–156. doi: 10.1016/j.cell.2010.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith TC, Swoboda JG, Walker S. Late-stage polyribitol phosphate wall teichoic acid biosynthesis in Staphylococcus aureus. J Bacteriol. 2008;190:3046–3056. doi: 10.1128/JB.01880-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrocola G, Arciola CR, Rindi S, Di Poto A, Missineo A, Montanaro L, Speziale P. Toll-like receptors (TLRs) in innate immune defense against Staphylococcus aureus. Int J Artif Organs. 2011;34:799–810. doi: 10.5301/ijao.5000030. [DOI] [PubMed] [Google Scholar]
- Schirner K, Marles-Wright J, Lewis RJ, Errington J. Distinct and essential morphogenic functions for wall- and lipo-teichoic acids in Bacillus subtilis. EMBO J. 2009;28:830–842. doi: 10.1038/emboj.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirner K, Stone LK, Walker S. ABC transporters required for export of wall teichoic acids do not discriminate between different main chain polymers. ACS Chem Biol. 2011;6:407–412. doi: 10.1021/cb100390w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Swoboda JG, Campbell J, Walker S, Gilmore MS. In vitro antimicrobial activity of wall teichoic acid biosynthesis inhibitors against Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2011;55:767–774. doi: 10.1128/AAC.00879-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoboda JG, Campbell J, Meredith TC, Walker S. Wall teichoic acid function, biosynthesis, and inhibition. Chembiochem. 2010;11:35–45. doi: 10.1002/cbic.200900557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoboda JG, Meredith TC, Campbell J, Brown S, Suzuki T, Bollenbach T, Malhowski AJ, Kishony R, Gilmore MS, Walker S. Discovery of a small molecule that blocks wall teichoic acid biosynthesis in Staphylococcus aureus. ACS Chem Biol. 2009;4:875–883. doi: 10.1021/cb900151k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CM, Therien AG, Lu J, Lee SH, Caron A, Gill CJ, Lebeau-Jacob C, Benton-Perdomo L, Monteiro JM, Pereira PM, et al. Restoring methicillin-resistant Staphylococcus aureus susceptibility to β-lactam antibiotics. Sci Transl Med. 2012;4:126ra. doi: 10.1126/scitranslmed.3003592. [DOI] [PubMed] [Google Scholar]
- Vergara-Irigaray M, Maira-Litrán T, Merino N, Pier GB, Penadés JR, Lasa I. Wall teichoic acids are dispensable for anchoring the PNAG exopolysaccharide to the Staphylococcus aureus cell surface. Microbiology. 2008;154:865–877. doi: 10.1099/mic.0.2007/013292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C. Antibiotics: Actions, origins, resistance. Washington, DC: ASM Press; 2003. p. 345. [Google Scholar]
- Wang H, Claveau D, Vaillancourt JP, Roemer T, Meredith TC. High frequency transposition in Staphylococcus aureus via bacteriophage delivery for determining antibacterial mode of action. Nat Chem Biol. 2011;7:720–729. doi: 10.1038/nchembio.643. [DOI] [PubMed] [Google Scholar]
- Weidenmaier C, Kokai-Kun JF, Kristian SA, Chanturiya T, Kalbacher H, Gross M, Nicholson G, Neumeister B, Mond JJ, Peschel A. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat Med. 2004;10:243–245. doi: 10.1038/nm991. [DOI] [PubMed] [Google Scholar]
- Weidenmaier C, Peschel A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat Rev Microbiol. 2008;6:276–287. doi: 10.1038/nrmicro1861. [DOI] [PubMed] [Google Scholar]
- Xia G, Maier L, Sanchez-Carballo P, Li M, Otto M, Holst O, Peschel A. Glycosylation of wall teichoic acid in Staphylococcus aureus by TarM. J Biol Chem. 2010;285:13405–13415. doi: 10.1074/jbc.M109.096172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI document M7-A7. seventh. Clinical and Laboratory Standards Institute; Wayne, Pa: 2006. Methods for dilution antimicrobial susceptibility tests for bacterial that grow aerobically; Approved standard. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.