Abstract
This digest covers some of the most relevant progress in malaria drug disco very published betwe en 2010 and 2012. There is an urgent need to develop new antimalarial drugs. Such drugs can target the blood stage of the disease to alleviate the symptoms, the liver stage to prevent relapses, and the transmission stage to protect other humans. The pipeline for the blood stage is becoming robust, but this should not be a source of complacency, as the current therapies set a high standard. Drug disco very efforts directed towards the liver and transmission stages are in their infancy but are receiving increasing attention as targeting these stages could be instrumental in eradicating malaria.
Keywords: Malaria, Antimalarial, Plasmodium, Drugs, Review
Malaria remains one of the most prevalent and deadly infectious diseases across Africa, Asia, and the Americas. The World Health Organization (WHO) estimates 154–289 million malaria cases in 2010, with 660,000 associate deaths.1,2 An independent study suggests that the mortality is twice as high when including cases of malaria that are undiagnosed or untreated.3 Eighty percent of the estimated cases occur in sub-Saharan Africa and 86% of deaths occur in children less than 5 years of age.1 In Africa, the economic burden is estimated at $12 billion/year , but the sales of antimalarial drugs are orders of magnitude lower.4 Advances in malaria research are often reviewed2,5–16 and a recent monograph17 will prove useful to the medicinal chemist. This digest covers some of the most relevant progress in malaria drug discovery from 2010 to 2012, and limits itself to compounds with EC50 values <100 nM in a parasite proliferation assay. We cover the blood stage, the liver stage, and the transmission stage of the disease. Each section contains several scaffolds, and within each scaffold the compounds are arranged, when possible, with the marketed drugs first and the research compounds last.
Several species of Plasmodium cause malaria in humans: Plasmodium falciparum , Plasmodium vivax , Plasmodium ovale , Plasmodium malariae and the simian Plasmodium knowlesi . The most lethal species is P. falciparum , found predominantly in Africa.18 If left untreated, P. falciparum causes organ failures (severe malaria) and accumulates in the brain capillaries (cerebral malaria), leading to coma and eventually death. Furthermore, there is growing evidence that the lethality of P. vivax has been underestimated.19
The parasite has a complex life cycle and in order to eradicate the disease, every stage should be considered for treatment (Scheme 1):
Liver stage. Once the mosquito inoculates the parasites (sporozoites) into the blood stream, the parasites invade the liver within 30 min and start replicating there (schizonts). In addition, P. vivax and P. ovale can remain dormant in the liver (hypnozoites, not shown in Scheme 1) and cause relapses years after the initial infection. Drugs that target the liver stages are important to prevent the disease from developing (prophylactic treatment) and to provide what is known as a “radical cure” for P. vivax and P. ovale .
Blood stage. After approximately 5–10 days, the liver cells burst and merozoites invade the red blood cells where they rapidly proliferate, causing the symptomatic high fevers and the pathology. In their intraerythrocytic phase, the merozoites go through various forms (rings, trophozoites, schizonts) to form an average of 20 daughter merozoites that are released into the bloodstream and infect new red blood cells. Drugs that target the blood stages are important to control the symptoms of the disease and associated mortality.
Transmission stage. After several cycles of asexual reproduction, some parasites further differentiate into male and female gametocytes, which contain only a half set of chromosomes.
Mosquito stage. When ingested by mosquitoes , the male and female gametocytes fuse in the midgut to form a zygote that further develops into new sporozoites ready for the next human host.20 Drugs that target the transmission and mosquito stages are important to prevent the infection of other humans, and would benefit an eradication agenda.
Scheme 1.
Plasmodium life cycle. Liver, blood (= erythrocytic), transmission, and mosquito stages. See text for details.
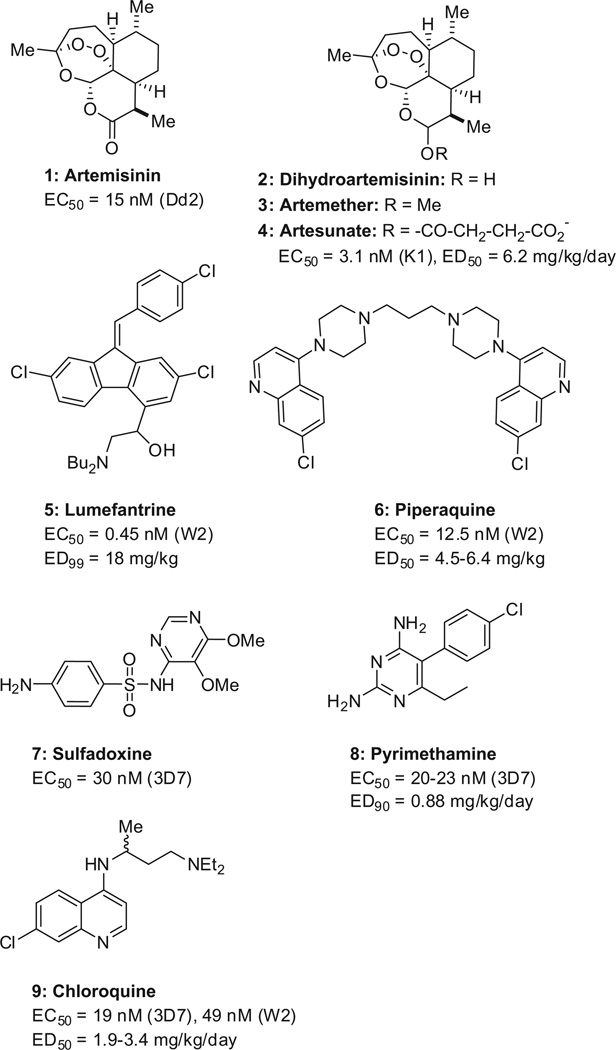
Artemisinin-based combination therapies (ACTs) are the current standard of care for uncompl icated malaria. Artemisinin (1, Scheme 2) and its derivatives (2–4) have a fast onset of action but are cleared rapidly (human t1/2 ~1 h),21 and are therefore combined with slow-clearing drugs to kill residual parasites. Typical partner drugs include lumefantrine (5, human t1/2 = 3–4 days)22 and piperaquine (6, human t1/2 = 8–16 days).23 The most popular combination consists of tablets containing artemether (3, 20 mg) and lumefantrine (5, 120 mg) sold as Coartem™ (Novartis).24 Adults take four tablets twice a day for 3 days,25 but compliance to this six-dose regimen is variable.26 In 2011, the European Medicines Agency (EMA) approved the combination of dihydroartemisinin (2) and piperaquine (6) which is taken once a day for 3 days (Eurartesim™, Sigma-Tau).27 The ACTs have supplanted the previously recommended sulfadoxine–pyrimethamine (7/8, Fansidar™, Roche), which in turn replaced chloroquine (9). Parenteral artesu-nate (4) is the drug of choice for severe malaria.28
Scheme 2.
Current standard of care (artemisinin derivatives, combined with lumefantrine or piperaquine) and previous first-line therapies (sulfadoxine–pyrimethamine and chloroquine). In vitro potency data (EC50 values) are reported for proliferation assays using different strains of P. falciparum that are either drug-sensitive (3D7) or multi-drug resistant (Dd2, K1, W2). The in vivo efficacy data (ED50 and ED90 values) are reported for rodent models of malaria. Data are reported for artemisinin,29 artesunate,29,30 lumefantrine,31,32 piperaquine,31,33 sulfadoxine,34 pyrimethamine,35,36 and chloroquine.29,37,38
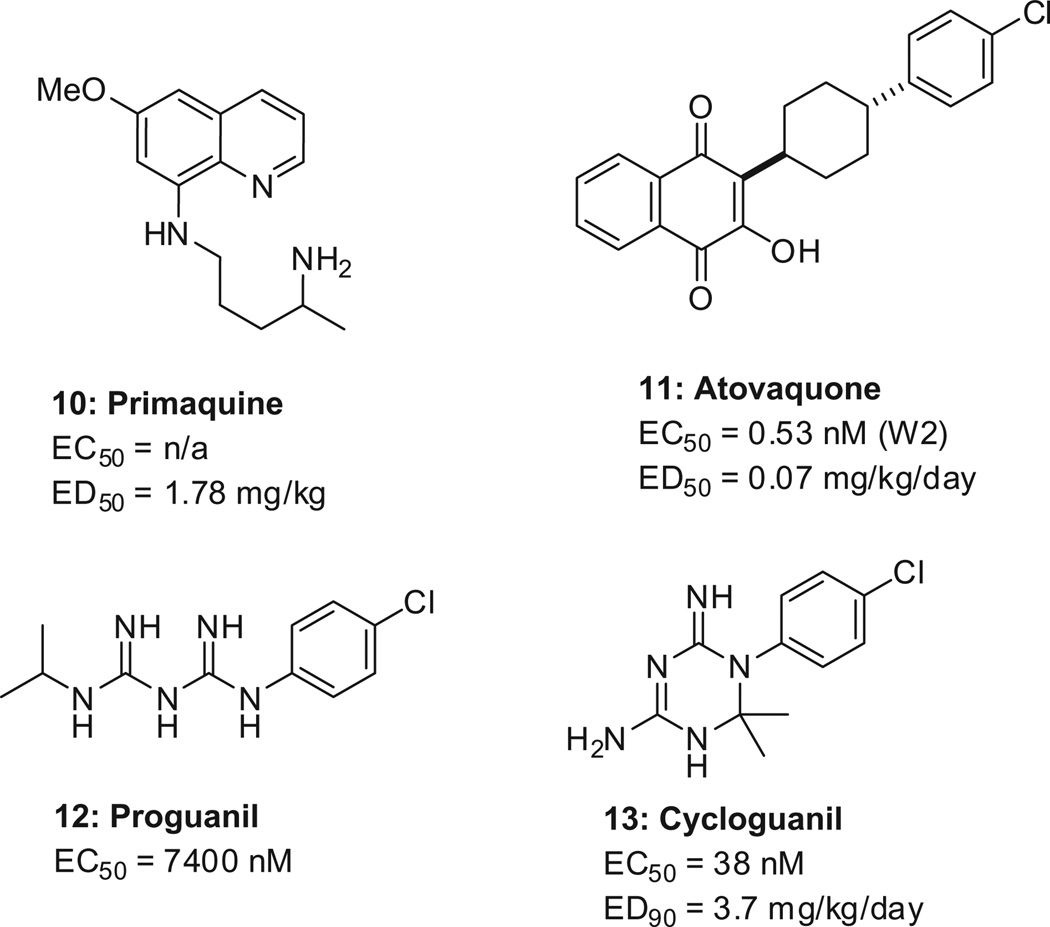
For the liver stages, primaquine (10, Scheme 3) is the only drug approved to eliminate hypnozoites. As for prophylactic treatment, atovaquone–proguanil (11/12) (Malarone, GlaxoSmith Kline) is usually preferred because it is well tolerated, but is expensive. Incidentally, proguanil is a pro-drug , of which cycloguanil (13) is the active metabolite. For the transmission stages, primaquine (10) is the only registered drug active against the mature gametocyte.39
Scheme 3.
Preferred drugs for the elimination of hypnozoites (primaquine), preferred combination for prophylaxis (atovaquone–proguanil), and the active metabolite of proguanil (cycloguanil). Data are reported for primaquine,40 ato-vaquone,41,42 proguanil,43 and its active metabolite cycloguanil.44
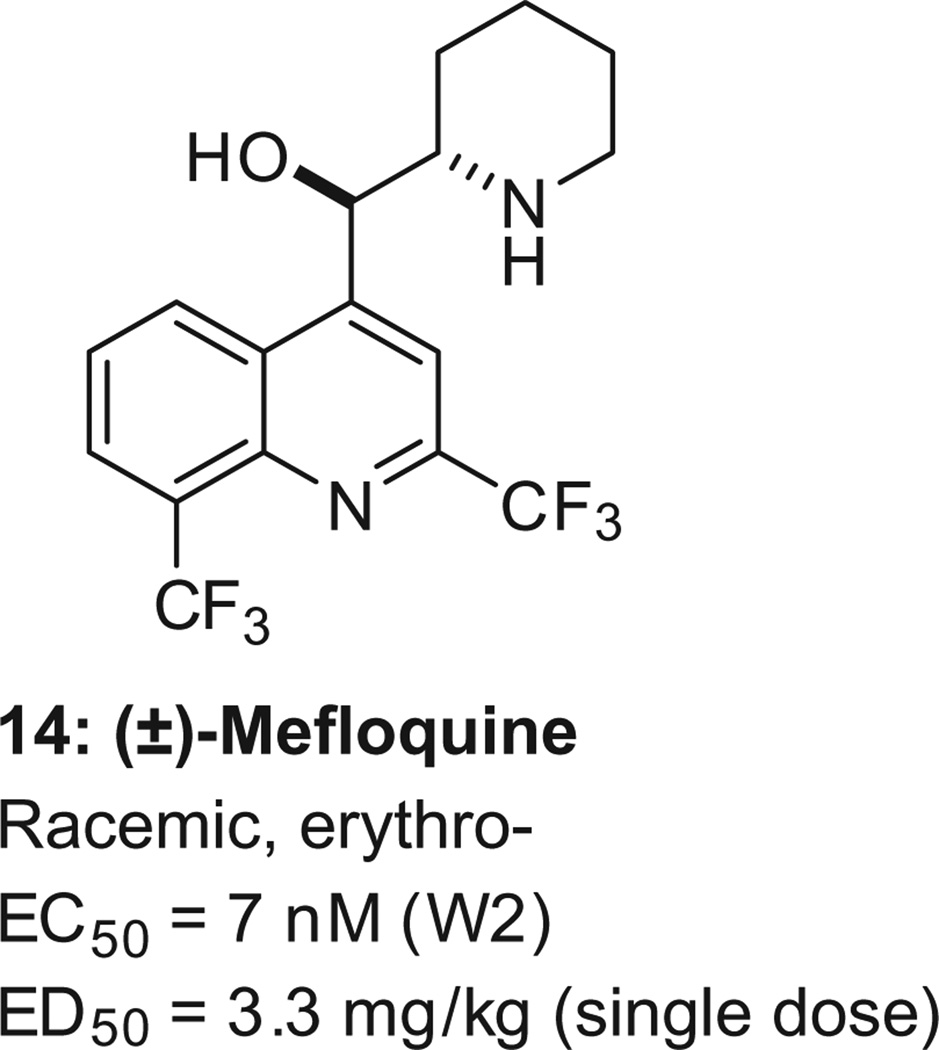
Resistance against the many existing antimalarials is well documented,45 and especially troubling is the emerging resistance to artemisinins. 45–48 Combining drugs can limit the emergence of resistance, but this technique is not infallible . For instance, in parts of Cambodia, the proportion of patients who were still parasitemic after 3 days of treatment with the dihydroartemis inin–piperaquine combination increased from 26% in 2008 to 45% in 2010.49 The problem of drug resistance requires new drugs. The challenge is that drug resistance is not the only feature. New, innovative drugs should also (i) be fast acting, (ii) be safe for children and pregnant women, and (iii) ideally be amenable to a single-dose administration. An example of how difficult it is to combine all these features is seen in mefloquine. It is the only registered drug effective in a single dose (14, Scheme 4, human t1/2 = 2–4 weeks, adult dose = 1250 mg);50 however , drug resistance is problematic. 51 Similarly, the only marketed antimalarial drug combination effective as a single dose is sulfadoxine–pyrimethamine, but it also suffers from drug resistance. 45,52,53
Scheme 4.
Mefloquine, the only single-agent antimalarial active as a single-dose in human. The EC50 value is for the multi-drug resistant strain W2, and the ED50 data for a single p.o. administration to mice.201 Mefloquine is a racemate, and the reported stereochemistry for mefloquine is relative, not absolute.
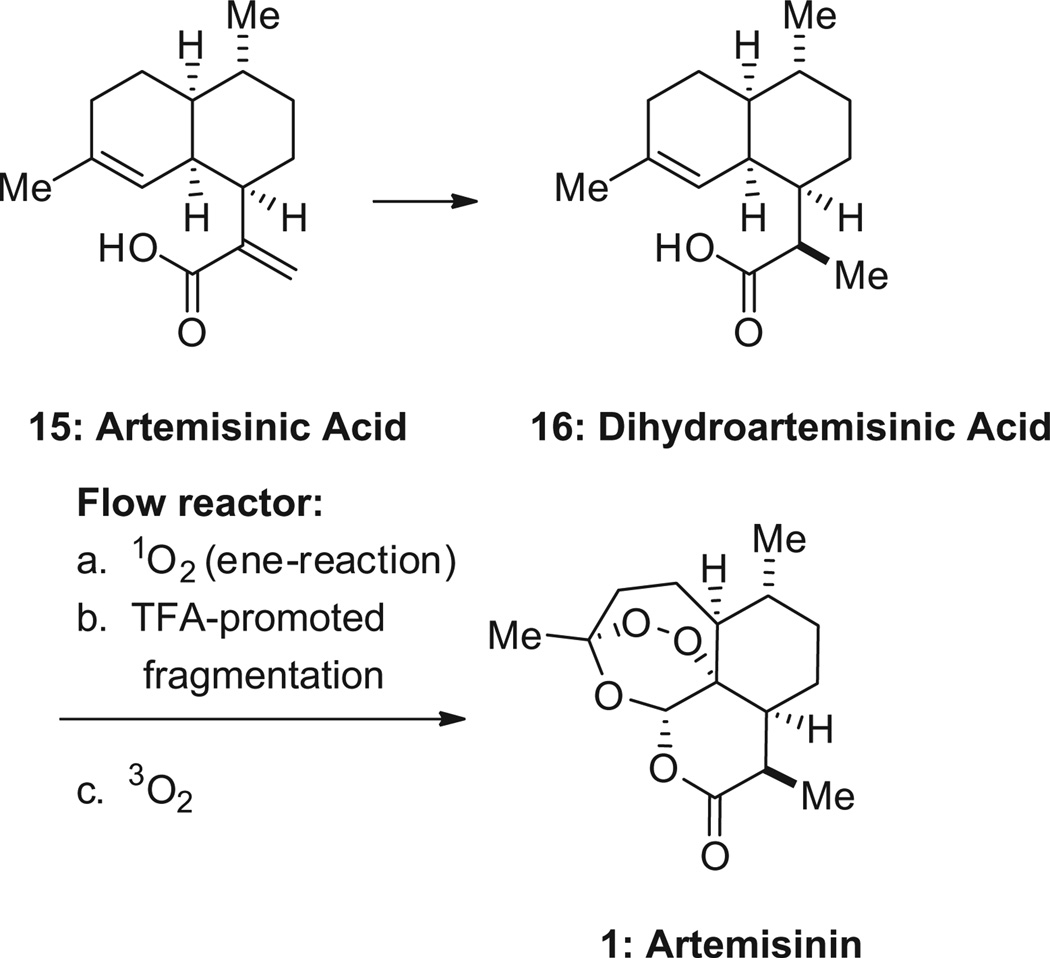
Artemisinin is commercially produced by extraction from sweet wormwood (Artemisia annua ) at a cost of $400–1100/kg. A recent alternative production method involves a yeast fermentation process that delivers the biosynthetic precursor artemisinic acid (15, Scheme 5, Amyris). The latter is converted to artemisinin in 62% yield using a photochemical oxidation process being implemented by Sanofi.54 An independent group adapted the process to a continuous flow reactor, better suited for conducting photoche mistry at an industrial scale, thus potentially reducing production costs. 55 In addition, Zhu and Cook published a remarkably concise synthesis of (+)-artemisinin, where cyclohexenone is converted in only five pots to the desired product, thus challenging the paradigm that total synthesis is not amenable to an industrially viable process.56
Scheme 5.
Conversion of artemisinic acid into artemisinin.
The combination of an artemisinin derivative with slow-clearing drugs can cure malaria in a single dose. The combination of artemisinin (1000 mg) and naphthoquine (17, Scheme 6, 400 mg) introduced by the Chinese army, appears to be effective as a single dose of eight tablets (ARCO, Phase III).57–63 Artemisone (18), a drug in Phase II trials, is 10 times more potent than artesunate (4) in vitro 64 and 4–10 times more potent in mice. 64 It provides a single-dose cure in Aotus monkeys infected with P. falciparum at 10 mg/kg when combined with mefloquine (5 mg/kg).65 Artemisone is also active in a murine model of cerebral malaria. 66 Combining artemisinin derivatives 1932 or 2067 (6 or 30 mg/kg, respectively) with the longer-acting mefloquine hydrochloride (18 mg/kg) was found to be curative in a single dose.
Scheme 6.
Active ingredients used in combinations that have potential for single-dose cure. EC50 values are reported for the drug-sensitive strains 3D7 and NF54.
The antimalarial action of artemisinin is thought to involve the cleavage of the peroxide bond by Fe(II) found in heme proteins, thus generating toxic oxygen radicals. Synthetic peroxides are proving to be useful substitutes for artemisinin . The first-generation ozonide OZ277 (21, Scheme 7), known as arterolane, inhibits the growth of chloroquine-resistant (K1) and chloroquine-sensitive (NF54) parasite strains with an IC50 = 1.6–1.8 nM.68 In 2012, Ranbaxy launched the combination of arterolane maleate and piperaquine phosphate as a 3-day treatment in India.
Scheme 7.
The ozonides OZ277 (Arterolane) and OZ439.
The second-generati on peroxide OZ439 (22, Scheme 7) (EC50 = 3.4–4.0 nM) is now in Phase IIa studies. It features an 8′-aryl rather than an 8′-alkyl group.30 This seemingly inconspicuous modification has far-reaching consequences . The stability of the O–O bond towards Fe(II) increases by 50-fold, presumably because of steric reasons. This in turn translates into a much longer half-life in both rats (t1/2 = 20 h for OZ439 vs. 1 h for OZ277) and humans (t1/ 2 = 25–30 h for OZ439).30,69 The improved pharmacokine tic profile renders OZ439 capable of completely curing mice of malaria in a single dose of 20 mg/kg, a feat that artemisinin derivatives cannot achieve without the addition of a second drug. Furthermore, OZ439 has significant prophylactic activity, and a single 30 mg/kg oral dose of OZ439 administere d 48 h prior to parasite inoculation is protective. In rat, multiple doses of OZ439 are tolerated up to 300 mg/kg.30 These ozonides are synthesized from an oxime (23) and a ketone (24) in the presence of ozone.
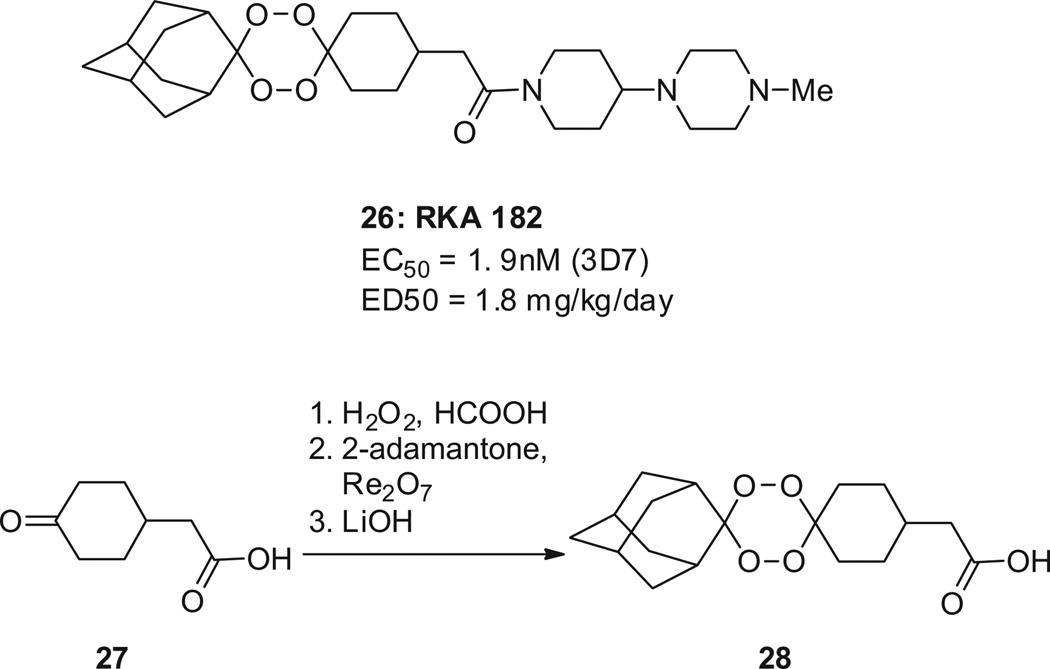
Another way of stabilizing the O–O bond is to form tetraoxanes, as was employed in the drug development candidate RKA 182 (26, Scheme 8),70 which displays IC 50 values of 4.9 nM against the P. falciparum 3D7 strain and of 1.9 nM against the K1 strain (chloroquine-sensitive and -resistant, respectively ). In a mouse model of malaria, RKA 182 inhibits parasite growth with an ED50 of 1.8 mg/kg/day. The oral bioavailability of its tosylate salt is 42% in mouse and 38% in rat. The tetraoxane ring is made via a Re2O7-catalyzed step (27→28).70 RKA 182 is not curative in a single dose.
Scheme 8.
The tetraoxane RKA182. EC50 values are reported for the drug-sensitive strain 3D7.
The Central Drug Research Institute, Lucknow, India, is investigating the trioxane CDRI-97/78 (29, Scheme 9) in Phase I studies.71–73 The key step in the construction of the trioxane core is an ene reaction between an allylic alcohol (30) and singlet oxygen, to give peroxide 31. Additional artemisin in derivatives, 74 ozonides,75 and 1,2,4-trioxanes72,76 have been reported to have potencies comparable to artemether, but were not shown to possess obvious advantages.
Scheme 9.
The trioxane CDRI-97/78.
The prototypical 4-aminoquinoline chloroquine has been widely used for treating malaria since World War II. Chloroquine exerts its antimalarial action by interfering with the formation of hemozoin within the parasite’s digestive vacuole. Hemozoin is a crystalline derivative of heme that the parasite makes as a way of disposing of toxic heme released upon hemoglobin digestion. Resistance to chloroquine is now found in all areas of the world, and involves multiple mutations in the P. falciparum chloroquine resistance transporter , PfCRT. These mutations result in an increased efflux of chloroquine from the acidic digestive vacuole to the cytosol of the parasite. Ferroquine (33, Scheme 10 ) was found to be active against chloroquine- resistant strains, and is currently undergoing Phase II clinical trials. Ferroquine, unlike chloroquine, accumulates in the digestive vacuole of the chloroquine-resistant parasites . This was demonstrated by X-ray fluorescence microscopy, a technique that allows visualizing the chlorine atom of both drugs.77 It was also shown that replacing the expensive ferrocene moiety of ferroquine with a simple and inexpensive benzene ring as in 34 and 35 retains activity against chloroquine-resistant strains (K1, W2).78 Because of its basicity, 35 is expected to accumulate like other 4-aminoquinolines in the acidic (pH = 5) environment of the food vacuole. In this organelle , the concentration of 35 is calculated to reach 370 µM at pharmacologically relevant doses, thus enabling PfCRT inhibition (IC50 = 69 µM) to become an operational second mode of action.79 The fused ‘dimeric quinoline’ 36 is active in vitro against drug-resistant strains, and in mouse when administered orally at 80 mg/kg.80
Scheme 10.
4-Aminoquinoline. EC50 values are reported for the drug-sensitive strain 3D7 and the multi-drug resistant strains W2 and K1.
Amodiaquine (37, Scheme 11 ) is also active against most chloroquine- resistant strains; however, hepatitis, myelotoxicity and agranulocytosis restrict its use to treating acute malaria. Amodiaquine is rapidly absorbed after oral administration in human, and rapidly metabolized, mostly, via N-deethylation. In addition, two reactive metabolites are formed, namely imine 38 and aldehyde 39, and are the likely cause of the hepatotoxicity and agranulocytosis, respectively .
Scheme 11.
Amodiaquine and derivatives. EC50 values are reported for the drug-sensitive strain 3D7 and the multi-drug resistant strains W2.
N-tert-Butyl isoquine (GSK369796, 40, Scheme 11 ) was designed to avoid the formation of quinone imines, and entered Phase I studies. It is potent in vitro, including in the chloroquine-resistant strain K1 (EC50 = 13 nM) and is active in vivo with an ED50 = 3.8 mg/kg/day, thus being comparable to amodia-quine.38,81,82 In spite of the excellent exposures and near quantitative oral bioavailabilities in animal models, its development was discontinue d due to exposures insufficient to demonstrate drug safety superior to chloroquine.17
Analogs exemplified by 41 (Scheme 11 ) were also designed to prevent the formation of quinone imines, and were found to retain activity against chloroquine-re sistant strains.83
Mefloquine (14, Scheme 12 ) has been widely used for malaria prevention due to a long half-life (2–4 weeks in human) that necessitates only a once-weekly dosing. Mefloquine is sold as a racemate (Lariam), and causes a relatively high incidence of depression, psychosis, and nightmares. While both enantiomers are active, the (−)-enantiomer 42 is believed to cause the neurological side effects by binding the adenosine receptors in the brain.84 In an effort to select the next generation of quinoline methanol derivatives that could serve as a replacement for mefloquine, the Walter Reed Army Institute of Research screened for analogs with a lower brain penetration, and identified WR621308 (44). 85,86 WR6213 08 has a substantially lower permeability across MDCK cell monolayers than mefloquine, suggesting lower brain exposures.
Scheme 12.
4-Hydroxymethylquinolines. Note that mefloquine is a racemate, and the reported stereochemistry for mefloquine is relative, not absolute. EC50 values are reported for the multi-drug resistant strain W2.86
Some of the most important malaria drugs, cycloguanil (13) and pyrimethamine (8), are inhibitors of dihydrofolate reductase (DHFR). DHFR converts 7,8-dihydrofolate (45, Scheme 13 ) into tetrahydrofolate (46), a cofactor involved in one-carbon transfer reactions and in the biosynthesis of nucleic acids. Inhibition of DHFR therefore arrests DNA replication,87 but resistance is widespread due to mutations in the enzyme.
Scheme 13.
DHFR inhibitors.
Structure-based drug design resulted in P218 (48, Scheme 13 ), a DHFR inhibitor active against all clinically relevant mutations. 88 P218 combines the pyrimidinering of pyrimethamine (8, Scheme 13 ), which brings potency, and the linker of the DHFR inhibitor WR99210 (47, Scheme 13 ), which tolerates mutations due to its flexibility. Furthermore, the terminal carboxylate group forms a salt bridge to the conserved Arg122 residue, thus mimicking the α-carboxylate of dihydrofolate (45). P218 is more potent than pyrimethamine against DHFR in the wild-type strain TM4 (IC50 = 4.6 and 58 nM, respectively ) as well as in the quadruple mutant strain V1/S (IC50 = 56 and >100,000 nM, respectively ). P218 is active against quadruple mutant P. falciparum in mice, with an ED50 = 0.3 mg/kg/day, orally. In rat, the oral bioavailability is 46%, and the oral t1/2 is 7.3 h.88
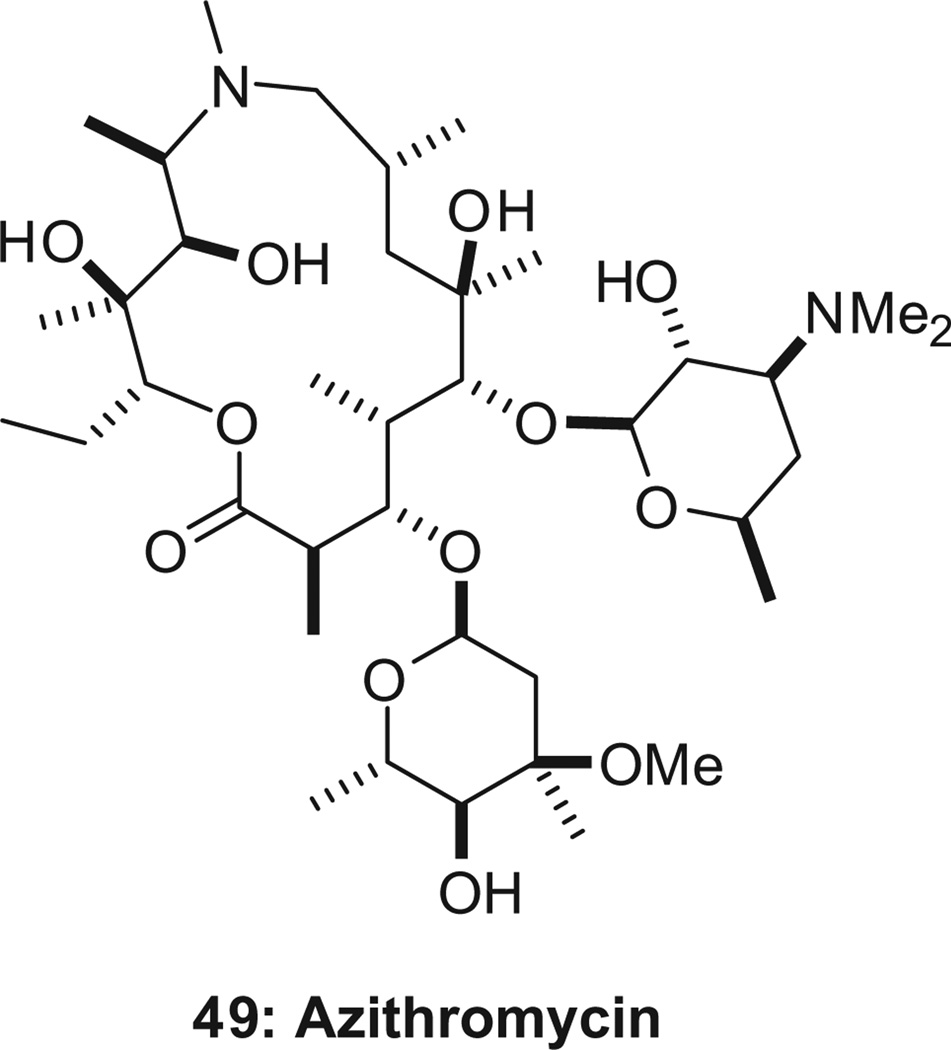
About 125 million pregnancies are at risk of malaria every year, and 10,000 women and 200,000 babies die as a result. An intermittent preventative treatment (IPT) is recommended for pregnant women, but drug-resistance to the currently adopted IPT (sulfa-doxine–pyrimethamine) necessitates new and effective regimens. Both azithromycin (49, Scheme 14 ) and chloroquine have demonstrated safety in children and pregnant women over a number of years. Notably, the azithromycin–chloroquine combination is designed to be synergistic against chloroquine-resistant strains of P. falciparum,89 and was shown to be synergistic in the treatment of symptomatic malaria in clinical trials. 89 By itself, azithromycin is a slow-acting antimalarial, with a maximum antiparasitic effect occurring only after two cycles of intraerythrocytic development (one cycle of invasion, development, and egress lasts 42–48 h).89,90 Finding azithromycin analogs with improved activity in mouse models of malaria has been challenging.90–92
Scheme 14.
Azithromycin.
The Novartis team screened its compound collection, identified spiroindolones as a novel chemotype, and optimized the series to deliver NITD-609 (52, Scheme 15 ), now in Phase II trials.29 The target was identified by genomics using clones with decreased susceptibility and was found to be the cation channel PfATPase4.29 In the medicinal chemistry route, the two enantiomers were separated by chiral chromatography93 NITD-609 has an excellent potency, with EC50 = 0.7 nM (3D7) and is 100% orally bioavailable in mouse and rat. Its oral t1/2 is 10 h (mouse) and 27 h (rat). In mouse, NITD-609 has an ED50 = 1.2 mg/kg, and is thus more potent than artesunate (ED50 = 6.2 mg/kg) or chloroquine (ED50 = 1.9 mg/kg). Three daily doses of 50 mg/kg, or a single dose of 100 mg/kg, afforded a complete cure. NITD-609 is also a potent inhibitor of gameto-cytogenesis, and blocks transmission to mosquitoes.94 The Medicines for Malaria Ventures (MMV) selected the spiroindolone project6,7 as the Project of the Year 2009.
Scheme 15.
The spiroindolone NITD-609. The EC50 value is for the drug-sensitive strain 3D7.
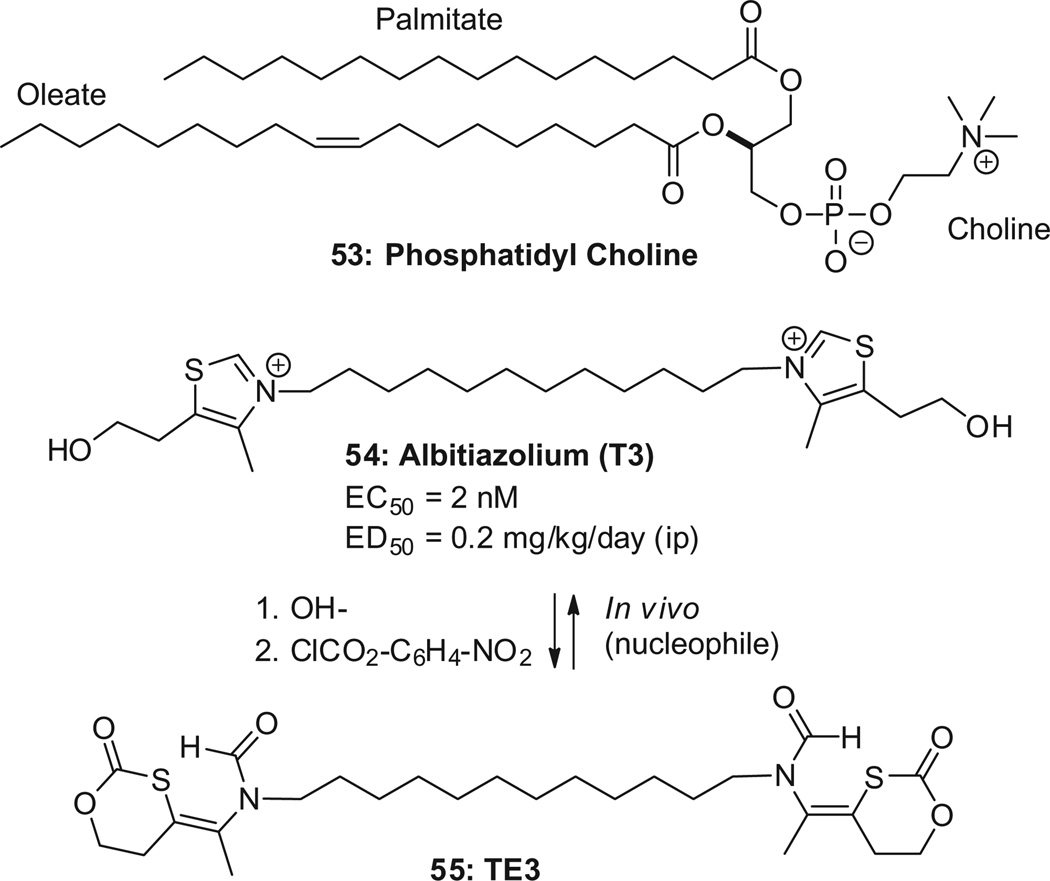
Albitiazolium (54, Scheme 16, also known as T3 or SAR97276) is a drug that has reached Phase II clinical trials (CNRS/University of Montpell ier/Sanofi).95 The understanding of its mechanism has recently been refined.96,97 Albitiazolium acts primarily by inhibiting the transport of choline into the parasite.97 The parasite requires choline to generate phosphatidylcholine (53), the main lipid of its cell membranes , as it replicates and forms new membranes . An important property of albitiazolium is that it accumulates irreversibly in the Plasmodium up to 1000-fold. Albitiazolium inhibits parasite growth (EC50 = 2 nM) and cures mice with an ED50 = 0.2 mg/kg/day (ip) without recrudescence. It is also active in severe conditions (ED50 ≤ 0.5 mg/kg ip at 5–10% parasitemia). Notably, a single injection is curative (ED50 = 1 mg/kg ip at 0.5–1% parasitemia ). A single-dose cure is observed even at high para-sitemia levels (ED50 = 2.5 mg/kg ip at 5–10% parasitemia).98,99
Scheme 16.
Albitiazolium and its pro-drug T3.
Albitiazolium is also efficacious when given orally, but with a much lower ED50 = 13 mg/kg/day,100 suggesting an oral bioavailability of the order of 2% (mouse). Because of its low oral bioavail- ability, the clinical trials have been conducted with intravenous or intramuscular injections. An oral version of albitiazolium would be highly desirable. The pro-drug TE3, reported in 2004–2005, has an oral ED50 = 5 mg/kg/day.98,101 This indicates a 2–3-fold improvement in mouse oral bioavailability; the rat bioavailability is 15%.100 In spite of numerous efforts made in the last few years,37,102–105 the bioavailability of these bis-cations has not been improved yet.
Unlike its human host, P. falciparum cannot salvage pyrimidines, and therefore depends on their de novo biosynthesis. Dihydrooro-tate dehydrogenase (DHODH) is the enzyme which catalyzes the rate-limiting step of the de novo pyrimidine biosynthetic pathway (56→59, Scheme 17 ). Factors that affect the human versus Plasmodium DHODH selectivity have been investigated by crystallogra-phy.106 The DHODH approach was awarded the MMV Project of the Year 2010.
Scheme 17.
DHODH inhibitors. EC 50 values are reported for the drug-sensitive strain 3D7.
A project coordinated by the MMV and involving the University of Texas Southwestern, the University of Washington , Monash University, and GlaxoSmithKline reported the preclinical candidate DSM265 (60, Scheme 17 ), expected to enter Phase I studies in 2013.107 DSM265 inhibits PfDHODH selectively over its human counterpart (IC50 = 33 nM and 2500 nM, respectively). It is orally bioavailable (rat: F = 57–68%, t1/2 = 12–28 h), and efficacious in vitro (EC50 = 43 nM (3D7)) and in mouse (ED50 = 2.8 mg/kg/ day). A related effort identified triazolopyrimidine DSM190 (61, Scheme 17 ), which is less potent in vitro (IC50 = 190 nM, EC50 = 1.1 µM (3D7)) and in mouse (ED50 = 10 mg/kg/day), but has a bioavailability of 100% in rat.36
Genzyme reported DHODH inhibitor 62 (Scheme 17 ) as a potential drug development candidate. 108 Benzimidazole 62 inhibits PfDHODH (IC50 = 40 nM) and parasite growth (EC50 = 7–10 nM, 3D7, Dd2). It is bioavailable in rat(49%) and dog(19%) and is eliminated with t1/2 = 0.85 h (rat) or 0.52 h (dog). In mouse, it is efficacious with an ED 50 of 13 mg/kg/day. 108
Most efforts use high-throughput phenotypic screens against the blood stage. GlaxoSmithKline, 109 Novartis,110 and the St. Jude Children’s Research Hospital111 performed such screens and made 20,000 hits publically available . The MMV narrowed the list down to 400 compounds representing a diverse dataset with low toxicity. The resulting “Malaria Box” can be downloaded, and is available in plates from the MMV. The targets are not known and will require deconvolution.112,113 Additional screens are reported below and a recent review of the patent literature is also available.114
Screening 70,000 compounds from the Broad Institute and the Harvard Medical School against the chloroquine resistant strain Dd2 led to Genzyme’s Genz-668764 (63, Scheme 18 , single enantiomer, absolute configuration not published).115 Genz-668764 inhibits P. falciparum in vitro (EC50 = 28 and 65 nM, 3D7 and Dd2 strains, respectively) and is active in mouse at doses of the order of 100 mg/kg/day. Allometric scaling predicts a human dose of 6 mg/day qd for 3 days, which would maintain plasma trough levels above the EC 50 against P. falciparum for at least 96 h after the last dose. The predicted human therapeutic index is approximately 3, on the basis of the exposure in rats at the no observable adverse effect level (NOAEL).
Scheme 18.
New scaffolds from phenotypic screens. EC 50 values are reported for the drug-sensitive strain 3D7 and the multi-drug resistant strain Dd2.
A similar screen of the 8000 compounds from the Broad Institute’s diversity-orien ted synthesis (DOS) library led to the discovery of the extremely potent ML238 (64, Scheme 18 , EC50 = 0.5 nM (Dd2)), which is also highly water soluble (120 µM) and not cytotoxic.116
Actelion reported ACT-213615 (65, Scheme 19 ).117,118 The compound is fast-acting against all asexual erythrocytic stages. Although 30 times less potent against the murine Plasmodium berghei than against P. falciparum , ACT-213615 completely cured P. berghei-infected mice with three consecutive oral daily doses of 750 mg/kg (ED90 = 54 mg/kg/day). ACT-213615 was efficacious in the recently established SCID mouse P. falciparum model (ED90 = 8.4 mg/kg/day) with potencies comparable to chloroquine (ED90 = 6.4 mg/kg/day). No acute toxicity was observed.
Scheme 19.
New scaffolds from phenotypic screens (continued). EC 50 values are reported for the drug-sensitive strain NF54 and the multi-drug resistant strain K1.
Anacor119 identified benzoxaborole (66, Scheme 19, EC50 = 26–44 nM) as a promising starting point (MW = 206, low clogP, solubility: 750 µg/mL at pH 7, and low to no cytotoxicity).120,121 SAR studies suggested that an acidic side-chain is favored for high anti-malarial potency, and a number of compounds with EC 50 values <100 nM were discovered, such as 67. Further optimization is ongoing.
The screening network funded by the WHO Special Programme for Research and Training in Tropical Diseases (TDR) reported the results of a 10,000 compound screen against seven whole organism pathogens responsible for tropical diseases, including the intra-erythrocytic forms of P. falciparum.122 The most potent screening hit was TDR84420 (68, Scheme 19 ) with an EC50 = 326 nM (K1).
The publically available screening data set from GSK has been analyzed to extract SAR trends 123 and GSK identified 47 high quality starting points for further follow-up.124 Further manual filtering led to the selection of five series. One of them was deprioritized due to resistance issues. TCMDC-134142 (69, Scheme 19 ) represents one of the four remaining series. 125
Amongst the targeted approaches, kinase inhibitors have been evaluated but so far are weak inhibitors (EC50 >100 nM)8,126 and are therefore outside of the scope of this digest.
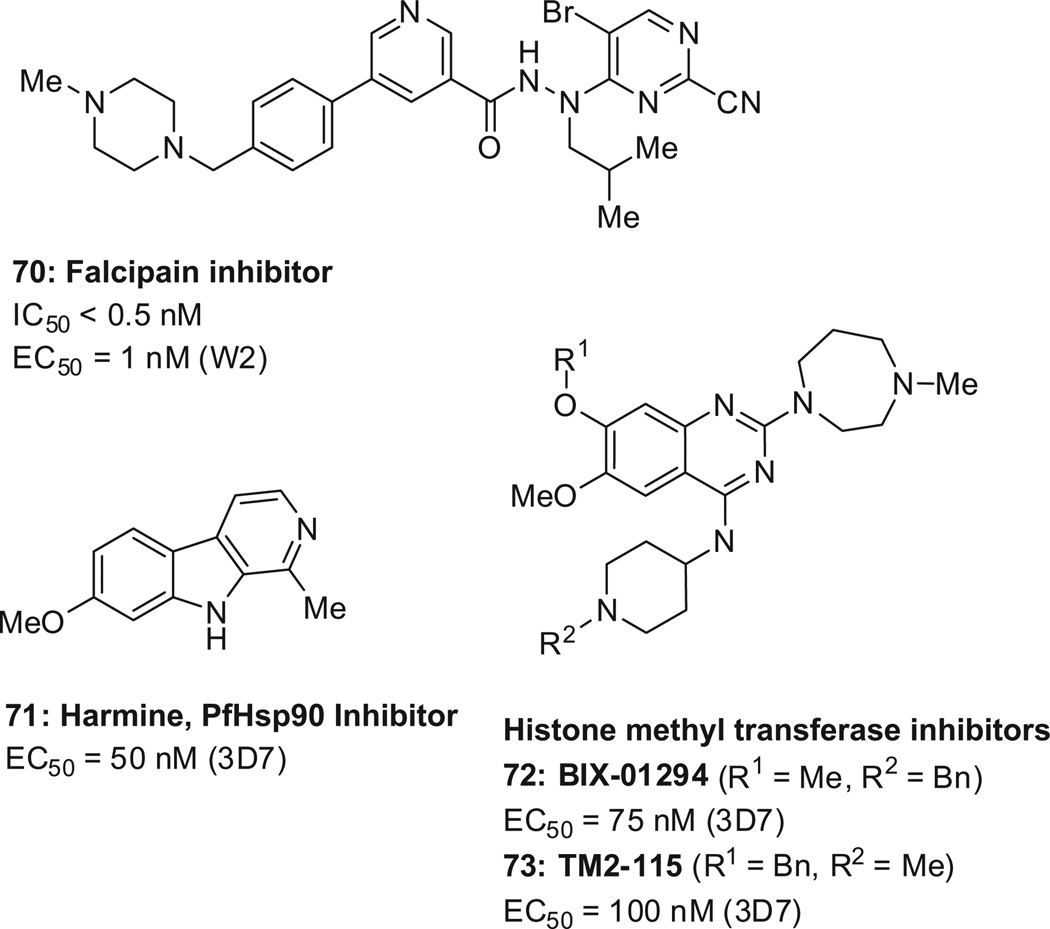
GSK reported a series of highly potent 2-pyrimidinecarbonitriles as inhibitors of falcipain-2 and falcipain-3, such as 70 (Scheme 20 , EC50 = 1nM).127 Falcipains are cysteine proteases that hydrolyze the host hemoglobin to provide amino acids for parasite protein synthesis.
Scheme 20.
New scaffolds from targeted approaches. EC50 values are reported for the drug-sensitive strain 3D7 and the multi-drug resistant strain W2.
Harmine (71, Scheme 20 ) was reported to inhibit the Plasmodium heat shock protein 90 (Hsp90) and some selectivity over the human Hsp90 was observed. Its cellular activity is EC50 = 50 nM (3D7).128 Hsp90 inhibitors have traditionally been pursued for cancer, and were found to have a limited therapeutic window.129
Epigenetic factors regulate the progression of the malaria parasite through its complex life cycle, and malarial histone acetyl-transferase (HAT)130 and histone deacetylase (HDAC)131,132 inhibitors have been reported. Recently, the targeting of P. falcipa-rum epigenetic factors was extended to include inhibitors of his-tone methyltransferases, such as BIX-01294133 and TM2-115 (72/ 73, Scheme 20 ).134 In an acute infection mouse model (highly virulent P. berghei ANKA strain), the compounds (dosed ip, 40 mg/kg) showed a rapid onset and a 2- and 18-fold parasite reduction.
Febrifugine (74, Scheme 21) is the active component of the Chinese herb Chang Shan (Dichroa febrifuga ), and is an appealing anti-malarial because of its rapid effect and availability. However, strong liver toxicity has precluded its use as a clinical drug. Radix Pharmaceuticals discovered febrifugine analog 75 with a much improved therapeutic index in mouse (TI = 132 vs 3), thus exceeding the therapeutic index of artesunate (TI = 37).135 The therapeutic index was defined as the ratio between the maximum tolerated dose (MTD), and the minimum clearance dose (MCD). Compound 75 is effective in mouse (EC50 = 0.3 mg/kg/day) and in Aotus monkeys (EC50 = 2 mg/kg/day).135
Scheme 21.
Febrifugine and improved analog. MCD = minimum clearance dose. MTD = maximum tolerated dose. TI = therapeutic index = MTD/MCD. EC 50 values are reported for the multi-drug resistant strain Dd2.
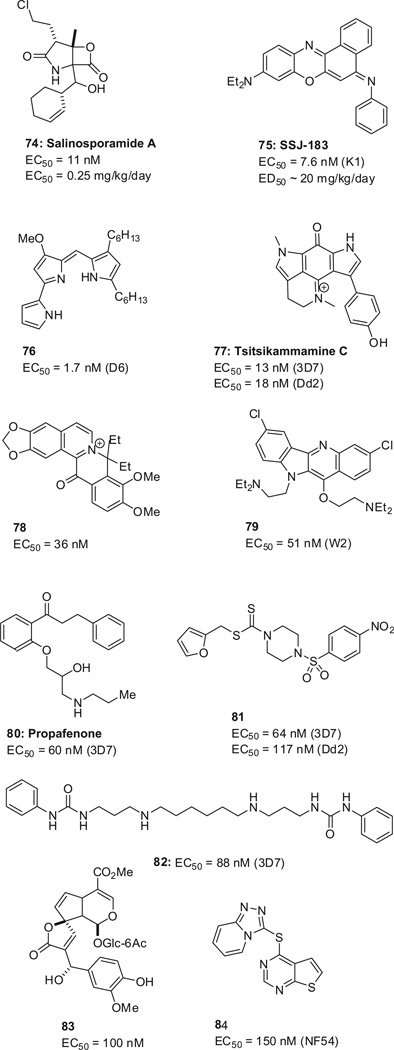
Several additional active compounds were identified by various approaches (Scheme 22 ), including the potent marine natural product salinosporamide A (74),136 SSJ-183 (75),137,138 prodiginine 74,139 tsitsikammamine C (77),140 the berberine analog 78,141 quin- dolone 79,142 propafenone 80,143 sulfonamide 81,144 diamine 82,145 the iridoid 83 extracted from traditional African herbal remedies,146 and iThemba’s 84.147
Scheme 22.
New scaffolds from various approaches. EC50 values are reported for drug-sensitive (3D7, NF54) and multi-drug resistant (Dd2, K1) strains.
Currently, most approved malaria drugs target only the blood stages of the disease. The two exceptions are the combination of atovaquone /proguanil which is also effective in clearing parasites from the liver, and primaquine.148 The latter clears not only liver schizonts but also hypnozoites , the dormant liver-stage parasites in P. vivax and P. ovale infections, thus providing what is known as a radical cure.149 Hypnozoites are long-lasting reservoirs responsible for recurring malaria episodes in the absence of mosquito bites, and are a major health concern, especially in the case of P. vivax .
The search for liver stage drugs has been severely hampered by the lack of culture techniques and by cumbersome primate animal models. It has been suggested that for prophylactic treatment, compounds without blood stage activity might be preferred in order to minimize the risk of the emergence of drug-resistant parasites.
Primaquine is a drug that acts slowly,150 and is therefore given together with other drugs, for example, chloroquine. Its mechanism of action is unclear, but is believed to be mediated by reactive metabolites which destroy the mitochondrial structure of the parasite. Primaquine, however , causes hemolytic anemia in people with glucose-6-phosphate dehydrogenase (G6PD) deficiencies, which occur in ~10% of the population,151 and are particularly prevalent in malaria endemic countries.202 In fact, the spatial extent of P. vivax malaria overlaps widely with that of G6PD deficiency.202 Additionally , compliance with the primaquine 14-day treatment regimen is difficult.
The primaquine analog tafenoquine (85, Scheme 23) is currently in Phase IIb/III clinical trials and has proven activity against hypnozoites . Tafenoquine has the same G6PD deficiency liability as primaquine, but has the advantage of being a single-dose treatment.
Scheme 23.
Primaquine and tafenoquine.
Recent drug discovery efforts have focused specifically on targeting the asymptomatic liver stage sporozoites and/or hypnozoites (possibly in addition to the blood stages) in order to provide novel, non-8-aminoqui noline drugs without the G6PD liability.149,152,153 A new imaging technique of Plasmodium liver stages, described by The Scripps Research Institute and Novartis, constitutes a breakthrough, and was applied to prioritize 4000 compounds already possessing blood-stage activity. 154 Imidazolopiperazines emerged as a hit series, exemplified by GNF-Pf-5069 (86, Scheme 24 ), which was then optimized to provide GNF179 (87) and GNF156 (88). The latter is currently in Phase I clinical trials.155,156
Scheme 24.
Imidazolopiperazines. EC50 values are reported for the drug-sensitive P. falciparum strain 3D7, and the hepatic stages of the murine P. yoelii
Most data was initially reported for the nonclinical compound GNF179,155 which inhibits both the blood stages of P. falciparum (EC50 = 6nM (3D7)) and the liver stages of the murine Plasmodium yoelii, (EC50 = 5nM). GNF179 is orally bioavailable in mouse (F = 58%, t1/2 = 8.9 h), and reduces P. berghei parasitemia in mice by 99.7% at 100 mg/kg. Importantly, a single dose of 15 mg/kg of GNF179 was shown to be completely protective in mice challenged with P. berghei sporozoites.
GNF156 was recently shown to be equally as potent as GNF179 against P. falciparum (EC50 = 6nM (3D7)). The potency against P. yoelii liver stages has not been disclosed.156 GNF156 is orally bioavailable in mouse (F = 72%, t1/2 = 2.2 h) and rat (F = 20–57%, t1/ 2 = 4.7–8.4 h).156 It is noteworthy that GNF156 not only inhibits the liver stages, but also transmission.156b
It is not yet known whether these compounds are active against the hypnozoites of P. vivax and P. ovale and would thus be able to provide a radical cure.154 The mechanism of action of the imidazolopiperazines remains unknown.
Atovaquone (11) targets the electron transport chain (ECT) of the mitochondrion , and specifically the cytochrome bc1 complex. Additional quinones have been reported, without obvious advan-tage,157,158 and most progress was made with pyridones.
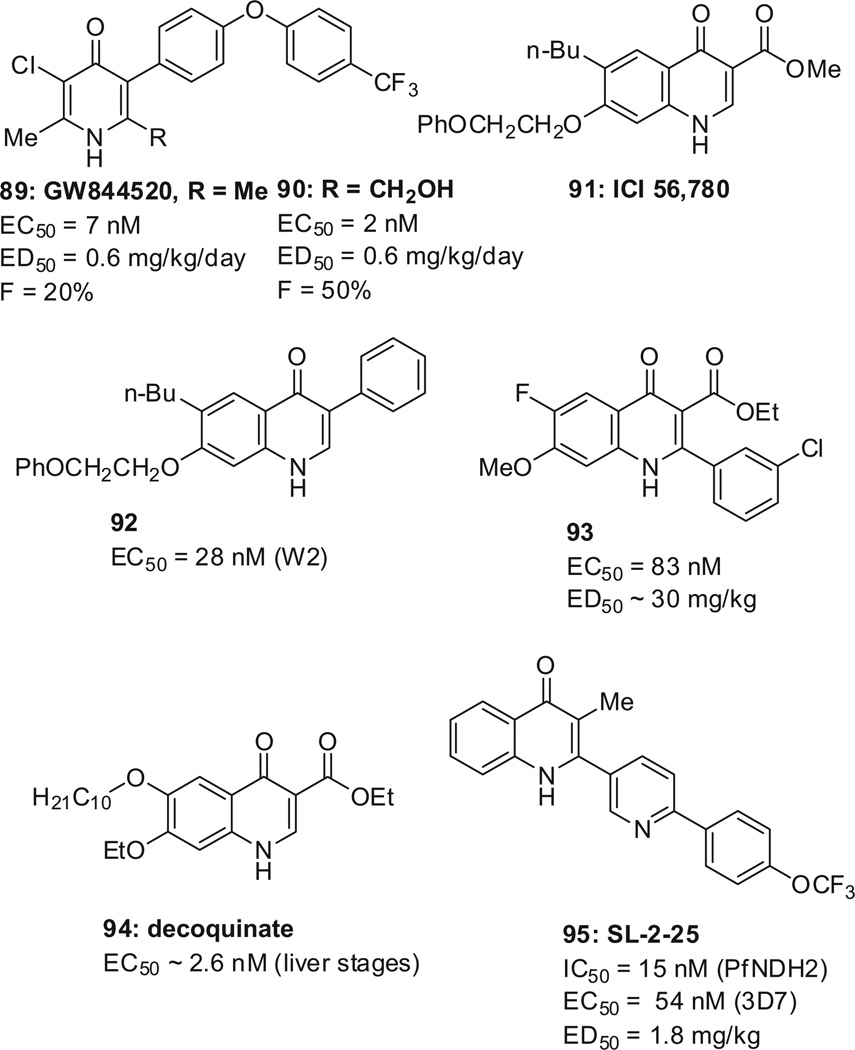
GSK reported a back-up to pyridone GW844520 (89, Scheme 25 ), a molecule targeting cytochrome bc1; hydroxymethyl derivative 90 remarkably improved the mouse oral bioavailability (50%) as compared to GW844520 (20%).159
Scheme 25.
Pyridones with activity against liver stages. EC 50 values are reported for the drug-sensitive strain 3D7 and multi-drug resistant strain W2.
The paucity of compounds with anti-relapse properties led to the reinvestigation of the quinolone ICI 56,780 (91, Scheme 25 ). The latter was discovered in the 1960s and a 7-day regimen of 91 prevents relapses for 120 days in rhesus monkeys infected with Plasmodium cynomolgi, suggesting potency against hypnozoites . It was abandon ed because resistance was obtained after only a single passage in P. berghei infected mice. The group of Manetsch at the University of South Florida, reported compound 92 with an EC50 = 28 nM against the W2 strain, and of 31 nM against the atovaquone- resistant TM90-C2B strain (chloroquine-, mefloquine-, pyrimeth amine-, and atovaquone-resistant).41,160,161 The Guy group at St. Jude Children’s Research Hospital, Memphis, reported compound 93 with an EC50 = 83 nM,162 which has suppression activity in mice comparable to that of amodiaquine (57% suppression of parasitemia at 30 mg/kg) but is not curative. It remains to be investigated if activity against hypnozoites is maintained , and if resistance against 92 and 93 develops as fast as with ICI 56,780.
In a screen including 1037 compounds which have reached at least Phase I clinical trials (veterinary and/or human), decoquinate (94, Scheme 25 ), an approved veterinary drug, was identified to potently inhibit the P. yoelii liver stage (estimated EC 50 of 2.6 nM) as well as the asexual (EC50 = 10 nM) and sexual (EC50 = 36 nM) P. falciparum blood stages.163 The decoquinate-induced reduction of mosquito transmission has not been reported. Decoquinate (administered p.o., 10 mg/kg) was found to completely protect P. berghei infected mice from developing disease when treated 24 h after the infection. The molecular target is the parasitic cytochrome bc1 complex (IC50 = 2 nM, >5000-fold selectivity over its human counterpar t).164 Unfortunately, decoquinate has so far only been tested in animals, which makes the repurposing of this drug challenging.
The O’Neill group, at the University of Liverpool, searched for compounds that would inhibit another enzyme involved in the ECT, namely NADH:ubiq uinone reductase (PfNDH2). A succession of in silico screens, HTS, and medicinal chemistry activities led to CK-2-25 (95, Scheme 25 ), which is specific for PfNDH2 over bc1, and is potent in mouse, with an ED50 = 1.8 mg/kg.42,165
The first screening examples have been reported, such as the imidazolopiperazines 86–88 described above.154 Additionally, a set of ~5300 biologically active compounds , which included 640 FDA-approved drugs, was screened and 37 structurally diverse compounds with varied known biological functions were identified to also inhibit the malarial liver stages.166 Screening a natural product library highlighted the secondary fungal metabolite cladosporin (96, Scheme 26 ), which potently inhibits the blood and liver stages in drug sensitive and multiple drug-resistant cell lines (IC50 ~40–90 nM).167 The molecular target was identified to be cytoplasmic lysyl-tRNA synthetase. (PfKrs1) Cladosporin is selective over human Krs1 (IC50 >20 µM).
Scheme 26.
Cladosporin and NITD-731 inhibit liver stages with novel mechanisms of action. EC 50 values are reported for the drug-sensitive strains 3D7 and D10.
In addition, there is a growing interest in signal peptide peptidases (SPP) such as NITD-731 (97, Scheme 26 ) which inhibits P. yoelii liver stages with EC 50 = 7.8 nM.168
Since many different proteins are expressed during the liver and blood stages of the parasite’s life cycle169,170 and since the necessary medium- to `high-throughput liver stages assays are continuously being developed and refined including assays which allow the assessment of hypnozoiticidal activities,171–174 it is reasonable to believe that in the near future many new chemotypes and biological targets will emerge from these efforts.
Drugs that can reduce the formation of gametocytes (gametocy-togenesis), or can kill them (gametocytocides), are highly desirable but have been underexplored because of the lack of quantitative high throughput assays.175,176 These transmission-blocking drugs could target endpoints such as:
The effective and complete killing of mature gametocytes once they are formed in the human host.
The inhibition of the onward development of gametocytes into ookinetes and ultimately into sporozoites in the mosquito. This assumes that enough drug from the blood sample reaches the gut of the mosquito.177
Gametocyte development goes through five stages of maturation, with stage V being the only form which can infect mosquitoes (Scheme 1). For P. falciparum , these mature gametocytes start to be present ~12 days after disease symptoms, circulate on average for 2.5–6.5 days,178 and persist for up to 22 days.179 Thus, circulating gametocytes can sustain malaria transmission well after drug treatment has caused disease symptoms to disappear. For the other Plasmodium parasites , mature gametocytes appear much earlier, closer to 1day after disease symptoms appear. This difference in biology represents an additional challenge when optimizing dosing regimens of transmission-blocking drugs in the clinic.
Most currently approved anti-malarial drugs, including ACTs, are only effective against blood stages and young gametocytes up to stage III and possibly stage IV of gametocyte maturation (stage III can be observed at day 4–6 and IV is observed at day 7–9; Scheme 1). This unfortunately does not result in complete clearance of mature gametocytes. To make matters worse, some drug treatments, for example, chloroquine180 and sulfadoxine–pyri-methamine,181 were found to induce gametocytogenes is, thus potentially contributing to increased numbers of transmissions and increased rates of new infections.182
The transmission-blocking potential of approved and clinical antimalarials has been reviewed.9,183,184 Currently, the only fully effective gametocytocidal drug is primaquine (10, Scheme 3), which acts against gametocytes of all malaria species and represents the WHO recommended treatment option against P. falciparum gametocytes. Until recently, the WHO recommendation was a single primaquine dose of 0.75 mg/kg,185 provided that the risk for acute hemolytic anemia (G6PD deficiency) had been evaluated prior to treatment. In 2012, the WHO recommended that the dose be lowered to 0.25 mg/kg, which is still effective at lowering transmission while being unlikely to cause serious toxicity in subjects with G6PD variants.186,187 Thus, a single dose of primaquine (0.25 mg base/kg) should be given to all patients with parasitologically-confirmed P. falciparum malaria on the first day of treatment in addition to an ACT, except for pregnant women and infants <1 year of age. 186
Because of the risks associated with primaquine, novel transmission-blocking drugs are being sought for achieving the goal of global malaria eradication. Large strides have recently been made in understanding the specifics of transmission-stage biology, and in developing in vitro assays focused on late-stage gametocyte development, lethality of mature gametocytes and the gametocyte–ookinete/sporozoite transition.188–192
Tafenoquine (84), NITD609 (52),94 and GNF156 (88) were shown to have transmission- blocking activities in vitro. Tafenoquine was also found to delay sporozoite formation in P. vivax . Interestingly, a recently developed gametocyte drug screening assay identified methylene blue (97, Scheme 27), as a potent inhibitor of gametocyte development across all stages. Methylene blue is an approved injectable monoamine oxidase inhibitor193 for methe-moglobinemia, which almost fully abolishes P. falciparum transmission to mosquitoes at concentrations readily achievable in humans, highlighting the potential of this chemical class to reduce the spread of malaria.194 Incidentally, methylene blue was the first antimalarial to be tested in man (1891), based on Ehrlich’s observation that it could stain the malaria parasite.
Scheme 27.
Compounds with transmission blocking properties.
Additional examples of compounds with transmission-blocking activities include, amongst others, trioxaquine DU1302 (98, Scheme 27), epoxomicin (99, nonselective over human cells),189 HIV protease inhibitors such as tipranavir (100),184,195 kinase inhibitor BKI1 (101),196 ketotifen (102),197,198 thiostrepton (103),195 and cycloheximide (104).199
Anti-malarial strategies are ideally a balanced use of mosquito control, anti-Plasmodium treatments, and a general improvement of sanitation and awareness.175,176 This is how malaria was eradicated from developed countries. Vaccines would also be extremely useful.200 Nonetheless , there is an urgent need for developing new anti-malarial drugs. The new drugs can target the blood stage of the disease to alleviate the symptoms, the liver stage to prevent relapses, and the transmission stage to protect other humans.
The pipeline for the blood stage is arguably the best in history, but still needs to be expanded. The last few years have seen an explosion of potent new chemotypes, and the new challenge is to assess the potential of these chemotypes. Ideally, the new drug should: (i) address drug-resistance issues, (ii) have a rapid onset of action, (iii) be safe, especially in children and pregnant women, and (iv) cure malaria in a single dose. The challenge is to find a drug that addresses all of these features. It is our hope that with the rich variety of new chemical entities, such a drug will be discovered. Nevertheless, drug discovery efforts should continue, as the artemisinins set a high standard of efficacy and safety.
Drugs that target the liver and transmission stages have the potential to be transformational, but research efforts have been hampered by the absence of high-throughput screens. New imaging techniques are beginning to solve this problem and open up novel avenues, with an innovative clinical compound having liver stage activity. The field of transmission-blocking agents is in its infancy, but may be the most transformative of all in achieving the ultimate goal of eradicating malaria.
Acknowledgements
KLR is supported by the National Institutes of Health (Grant R01 AI85077-01A1). The authors thank S. Cervantes for generating Scheme 1.
References and notes
- 1.WHO. World Malaria Report. 2012 http://www.who.int/malaria/publications/world_malaria_report_2012/en/index.html.
- 2.Shetty P. Nature. 2012;484:S14. doi: 10.1038/484S14a. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. Lancet. 2012;379:413. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 4.Kar S. Nat. Rev. Drug Disc. 2010;9:511. doi: 10.1038/nrd3207. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y-K, Ge M, Plattner JJ. Org. Prep. Proced. Int. 2012;44:340. [Google Scholar]
- 6.Wu T, Nagle AS, Chatterjee A. Curr. Top. Med. Chem. 2011;18:853. doi: 10.2174/092986711794927748. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee A, Yeung BKS. Curr. Top. Med. Chem. 2012;12:473. doi: 10.2174/156802612799362977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang VM, Chavchich M, Waters NC. Curr. Top. Med. Chem. 2012;12:456. doi: 10.2174/156802612799362922. [DOI] [PubMed] [Google Scholar]
- 9.Dechy-Cabaret O, Benoit-Vical FJ. Med. Chem. 2012;55:10328. doi: 10.1021/jm3005898. [DOI] [PubMed] [Google Scholar]
- 10.Grayson M. Nature. 2012;484:S13. doi: 10.1038/484S13a. [DOI] [PubMed] [Google Scholar]
- 11.Eisenstein M. Nature. 2012;484:S16. doi: 10.1038/484S16a. [DOI] [PubMed] [Google Scholar]
- 12.Maxmen A. Nature. 2012;484:S19. doi: 10.1038/484S19a. [DOI] [PubMed] [Google Scholar]
- 13.Crabb BS, Beeson JG, Amino R, Menard R, Waters A, Winzeler EA, Wahlgren M, Fidock DA, Nwaka S. Nature. 2012;484:S22. doi: 10.1038/484S22a. [DOI] [PubMed] [Google Scholar]
- 14.DeWeerdt S. Nature. 2012;484:S24. doi: 10.1038/484S24a. [DOI] [PubMed] [Google Scholar]
- 15.Gravitz L. Nature. 2012;484:S26. doi: 10.1038/484S26a. [DOI] [PubMed] [Google Scholar]
- 16.Wells TNC. In: Treatment and Prevention of Malaria. Staines HM, Krishna S, editors. Springer: Basel; 2012. p. 227. [Google Scholar]
- 17.Staines HM, Krishna S. Treatment and Prevention of Malaria: Antimalarial Drug Chemistry, Action and Use. Springer: Basel; 2012. [Google Scholar]
- 18.Gething PW, Patil AP, Smith DL, Guerra CA, Elyazar IR, Johnston GL, Tatem AJ, Hay SI. Malar. J. 2011;10:378. doi: 10.1186/1475-2875-10-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Battle KE, Gething PW, Elyazar IR, Moyes CL, Sinka ME, Howes RE, Guerra CA, Price RN, Baird KJ, Hay SI. Adv. Parasitol. 2012;80:1. doi: 10.1016/B978-0-12-397900-1.00001-3. [DOI] [PubMed] [Google Scholar]
- 20.Thus, in humans, the parasite replicates rapidly and asexually introducing replication errors and a small subset of genetic mutations; while in the mosquito, the sexual fusion of the gametocytes introduces large genetic variations, and increases the Darwinian fitness of the parasite prior to its invasion of another human.
- 21.Morris CA, Duparc S, Borghini-Fuhrer I, Jung D, Shin CS, Fleckenstein L. Malar. J. 2011;10:263. doi: 10.1186/1475-2875-10-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Djimde A, Lefevre G. Malar. J. 2009;8:S4. doi: 10.1186/1475-2875-8-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roshammar D, Hai TN, Hietala SF, Van Huong N, Ashton M. Eur. J. Clin. Pharmacol. 2006;62:335. doi: 10.1007/s00228-005-0084-9. [DOI] [PubMed] [Google Scholar]
- 24.Omari AA, Gamble C, Garner P. Cochrane Database Syst. Rev. 2005;19 doi: 10.1002/14651858.CD005564. CD005564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novartis Coartem International Package Leaflet. 2009 see also http://www.drugs.com/monograph/coartem.html).
- 26.Lemma H, Lofgren C, Lofgren C, San Sebastian M. Malar. J. 2011;10:349. doi: 10.1186/1475-2875-10-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keating GM. Drugs. 2012;72:937. doi: 10.2165/11203910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 28.Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A, Chhaganlal KD, Bojang K, Olaosebikan R, Anunobi N, Maitland K, Kivaya E, Agbenyega T, Nguah SB, Evans J, Gesase S, Kahabuka C, Mtove G, Nadjm B, Deen J, Mwanga-Amumpaire J, Nansumba M, Karema C, Umulisa N, Uwimana A, Mokuolu OA, Adedoyin OT, Johnson WB, Tshefu AK, Onyamboko MA, Sakulthaew T, Ngum WP, Silamut K, Stepniewska K, Woodrow CJ, Bethell D, Wills B, Oneko M, Peto TE, von Seidlein L, Day NP, White NJ. Lancet. 2010;376:1647. [Google Scholar]
- 29.Rottmann M, McNamara C, Yeung BK, Lee MC, Zou B, Russell B, Seitz P, Plouffe DM, Dharia NV, Tan J, Cohen SB, Spencer KR, Gonzalez-Paez GE, Lakshminarayana SB, Goh A, Suwanarusk R, Jegla T, Schmitt EK, Beck HP, Brun R, Nosten F, Renia L, Dartois V, Keller TH, Fidock DA, Winzeler EA, Diagana TT. Science. 2010;329:1175. doi: 10.1126/science.1193225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charman SA, Arbe-Barnes S, Bathurst IC, Brun R, Campbell M, Charman WN, Chiu FC, Chollet J, Craft JC, Creek DJ, Dong Y, Matile H, Maurer M, Morizzi J, Nguyen T, Papastogiannidis P, Scheurer C, Shackleford DM, Sriraghavan K, Stingelin L, Tang Y, Urwyler H, Wang X, White KL, Wittlin S, Zhou L, Vennerstrom JL. Proc. Natl. Acad. Sci. U.S.A. 2011;108:4400. doi: 10.1073/pnas.1015762108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nsobya SL, Kiggundu M, Nanyunja S, Joloba M, Greenhouse B, Rosenthal PJ. Antimicrob. Agents Chemother. 2010;54:1200. doi: 10.1128/AAC.01412-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slack RD, Mott BT, Woodard LE, Tripathi A, Sullivan D, Nenortas E, Girdwood SC, Shapiro TA, Posner GH. J. Med. Chem. 2012;55:291. doi: 10.1021/jm201214d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore BR, Batty KT, Andrzejewski C, Jago JD, Page-Sharp M, Ilett KF. Antimicrob. Agents Chemother. 2008;52:306. doi: 10.1128/AAC.00878-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng X, Nagle A, Wu T, Sakata T, Henson K, Chen Z, Kuhen K, Plouffe D, Winzeler E, Adrian F, Tuntland T, Chang J, Simerson S, Howard S, Ek J, Isbell J, Tully DC, Chatterjee AK, Gray NS. Bioorg. Med. Chem. Lett. 2010;20:4027. doi: 10.1016/j.bmcl.2010.05.095. [DOI] [PubMed] [Google Scholar]
- 35.Calderón F, Vidal-Mas J, Burrows J, de Rosa JC, Jiménez-Díaz MB, Mulet T, Prats S, Solana J, Witty M, Gamo FJ, Fernández E. ACS Med. Chem. Lett. 2012;3:373. doi: 10.1021/ml300008j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gujjar R, El Mazouni F, White KL, White J, Creason S, Shackleford DM, Deng X, Charman WN, Bathurst I, Burrows J, Floyd DM, Matthews D, Buckner FS, Charman SA, Phillips MA, Rathod PK. J. Med. Chem. 2011;54:3935. doi: 10.1021/jm200265b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caldarelli SA, Hamel M, Duckert JF, Ouattara M, Calas M, Maynadier M, Wein S, Perigaud C, Pellet A, Vial HJ, Peyrottes S. J. Med. Chem. 2012;55:4619. doi: 10.1021/jm3000328. [DOI] [PubMed] [Google Scholar]
- 38.O’Neill PM, Park BK, Shone AE, Maggs JL, Roberts P, Stocks PA, Biagini GA, Bray PG, Gibbons P, Berry N, Winstanley PA, Mukhtar A, Bonar-Law R, Hindley S, Bambal RB, Davis CB, Bates M, Hart TK, Gresham SL, Lawrence RM, Brigandi RA, Gomez-delas-Heras FM, Gargallo DV, Ward SA. J. Med. Chem. 2009;52:1408. doi: 10.1021/jm8012618. [DOI] [PubMed] [Google Scholar]
- 39.Dondorp AM. Clin. Infect. Dis. 2012;56:694. doi: 10.1093/cid/cis962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L, Sathunuru R, Luong T, Melendez V, Kozar MP, Lin A. J. Bioorg. Med. Chem. 2011;19:1541. doi: 10.1016/j.bmc.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 41.Cross RM, Namelikonda NK, Mutka TS, Luong L, Kyle DE, Manetsch R. J. Med. Chem. 2011;54:8321. doi: 10.1021/jm200718m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leung SC, Gibbons P, Amewu R, Nixon GL, Pidathala C, Hong WD, Pacorel B, Berry NG, Sharma R, Stocks PA, Srivastava A, Shone AE, Charoensutthivarakul S, Taylor L, Berger O, Mbekeani A, Hill A, Fisher NE, Warman AJ, Biagini GA, Ward SA, O’Neill PM. J. Med. Chem. 1844;2012:55. doi: 10.1021/jm201184h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohrt C, Willingmyre GD, Lee P, Knirsch C, Milhous W. Antimicrob. Agents Chemother. 2002;46:2518. doi: 10.1128/AAC.46.8.2518-2524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wangchuk P, Bremner JB, Rattanajak R, Kamchonwongpaisan S. Phytother. Res. 2010;24:481. doi: 10.1002/ptr.2893. [DOI] [PubMed] [Google Scholar]
- 45.Dondorp AM, Yeung S, White L, Nguon C, Day NP, Socheat D, von Seidlein L. Nat. Rev. Microbiol. 2010;8:272. doi: 10.1038/nrmicro2331. [DOI] [PubMed] [Google Scholar]
- 46.Dondorp AM, Fairhurst RM, Slutsker L, Macarthur JR, Breman JG, Guerin PJ, Wellems TE, Ringwald P, Newman RD, Plowe CVN. N. Engl. J. Med. 2011;365:1073. doi: 10.1056/NEJMp1108322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meshnick S. Am. J. Trop. Med. Hyg. 2012;87:783. doi: 10.4269/ajtmh.2012.12-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White NJ. Am. J. Trop. Med. Hyg. 2012;87:785. doi: 10.4269/ajtmh.2012.12-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fairhurst RM, Nayyar GM, Breman JG, Hallett R, Vennerstrom JL, Duong S, Ringwald P, Wellems TE, Plowe CV, Dondorp AM. Am. J. Trop. Med. Hyg. 2012;87:231. doi: 10.4269/ajtmh.2012.12-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. http://www.drugs.com/pro/mefloquine.html.
- 51.Cerutti C, Jr., Durlacher RR, de Alencar FE, Segurado AA, Pang LW. J. Infect. Dis. 1999;180:2077. doi: 10.1086/315141. [DOI] [PubMed] [Google Scholar]
- 52.Sibley CH, Hyde JE, Sims PF, Plowe CV, Kublin JG, Mberu EK, Cowman AF, Winstanley PA, Watkins WM, Nzila AM. Trends Parasitol. 2001;17:582. doi: 10.1016/s1471-4922(01)02085-2. [DOI] [PubMed] [Google Scholar]
- 53.Rieckmann K, Cheng Q. Trends Parasitol. 2002;18:293. doi: 10.1016/s1471-4922(02)02287-0. [DOI] [PubMed] [Google Scholar]
- 54.Dhainaut J, Dlublala A, Guevel R, Deard A, Oddon G, Raymond N, Turconi J. 2011 WO 2011/02685A1. [Google Scholar]
- 55.Levesque F, Seeberger PH. Angew. Chem., Int. Ed. 2012;51:1706. doi: 10.1002/anie.201107446. [DOI] [PubMed] [Google Scholar]
- 56.Zhu C, Cook SP. J. Am. Chem. Soc. 2012;134:13577. doi: 10.1021/ja3061479. [DOI] [PubMed] [Google Scholar]
- 57.Liu R, Dong HF, Jiang MS. Expert Rev. Clin. Pharmacol. 2012;5:521. doi: 10.1586/ecp.12.40. [DOI] [PubMed] [Google Scholar]
- 58.Tjitra E, Hasugian AR, Siswantoro H, Prasetyorini B, Ekowatiningsih R, Yusnita EA, Purnamasari T, Driyah S, Salwati E, Yuwarni E, Januar L, Labora J, Wijayanto B, Amansyah F, Dedang TA, Purnama A. Malar. J. 2012;11:153. doi: 10.1186/1475-2875-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Batty KT, Salman S, Moore BR, Benjamin J, Lee ST, Page-Sharp M, Pitus N, Ilett KF, Mueller I, Hombhanje FW, Siba P, Davis TM. Antimicrob. Agents Chemother. 2012;56:2472. doi: 10.1128/AAC.06250-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benjamin J, Moore B, Lee ST, Senn M, Griffin S, Lautu D, Salman S, Siba P, Mueller I, Davis TM. Antimicrob. Agents Chemother. 2012;56:2465. doi: 10.1128/AAC.06248-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hombhanje FW, Huang Q. Pharmaceuticals. 2010;3:3581. [Google Scholar]
- 62.Hombhanje FW, Linge D, Saweri A, Kuanch C, Jones R, Toraso S, Geita J, Masta A, Kevau I, Hiawalyer G, Sapuri M. Malar. J. 2009;8:196. doi: 10.1186/1475-2875-8-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tun T, Tint HS, Lin K, Kyaw TT, Myint MK, Khaing W, Tun ZW. Acta Trop. 2009;111:275. doi: 10.1016/j.actatropica.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 64.Vivas L, Rattray L, Stewart LB, Robinson BL, Fugmann B, Haynes RK, Peters W, Croft SL. J. Antimicrob. Chemother. 2007;59:658. doi: 10.1093/jac/dkl563. [DOI] [PubMed] [Google Scholar]
- 65.Obaldia N, 3rd, Kotecka BM, Edstein MD, Haynes RK, Fugmann B, Kyle DE, Rieckmann KH. Antimicrob. Agents Chemother. 2009;53:3592. doi: 10.1128/AAC.00471-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waknine-Grinberg JH, Hunt N, Bentura-Marciano A, McQuillan JA, Chan HW, Chan WC, Barenholz Y, Haynes RK, Golenser J. Malar. J. 2010;9:227. doi: 10.1186/1475-2875-9-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moon DK, Tripathi A, Sullivan D, Siegler MA, Parkin S, Posner GH. Bioorg. Med. Chem. Lett. 2011;21:2773. doi: 10.1016/j.bmcl.2010.09.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dong Y, Wittlin S, Sriraghavan K, Chollet J, Charman SA, Charman WN, Scheurer C, Urwyler H, Urwyler H, Santo Tomas J, Snyder C, Creek DJ, Morizzi J, Koltun M, Matile H, Wang X, Padmanilayam M, Tang Y, Dorn A, Brun R, Vennerstrom JL. J. Med. Chem. 2010;53:481. doi: 10.1021/jm901473s. [DOI] [PubMed] [Google Scholar]
- 69.Moehrle JJ, Duparc S, Siethoff C, van Giersbergen PL, Craft JC, Arbe-Barnes S, Charman SA, Gutierrez M, Wittlin S, Vennerstrom JL. Br. J. Clin. Pharmacol. 2013;75:524. doi: 10.1111/j.1365-2125.2012.04368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghorai P, Dussault PH. Org. Lett. 2009;11:213. doi: 10.1021/ol8023874. [DOI] [PubMed] [Google Scholar]
- 71.Singh C, Verma VP, Naikade NK, Singh AS, Hassam M, Puri SK. J. Med. Chem. 2008;51:7581. doi: 10.1021/jm801006v. [DOI] [PubMed] [Google Scholar]
- 72.Singh C, Verma VP, Naikade NK, Singh AS, Hassam M, Puri SK. Bioorg. Med. Chem. Lett. 2010;20:4459. doi: 10.1016/j.bmcl.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 73.Kushwaha HN, Gautam N, Misra A, Singh B, Kumar S, Siddiqui HH, Singh SK. Arzneimittelforschung. 2012;62:274. doi: 10.1055/s-0032-1306317. [DOI] [PubMed] [Google Scholar]
- 74.Singh C, Kanchan R, Chaudhary S, Puri SK. J. Med. Chem. 2012;55:1117. doi: 10.1021/jm2010699. [DOI] [PubMed] [Google Scholar]
- 75.Tang Y, Wittlin S, Charman SA, Chollet J, Chiu FC, Morizzi J, Johnson LM, Tomas JS, Scheurer C, Snyder C, Zhou L, Dong Y, Charman WN, Matile H, Urwyler H, Dorn A, Vennerstrom JL. Bioorg. Med. Chem. Lett. 2010;20:563. doi: 10.1016/j.bmcl.2009.11.088. [DOI] [PubMed] [Google Scholar]
- 76.Singh C, Hassam M, Naikade NK, Verma VP, Singh AS, Puri SK. J. Med. Chem. 2010;53:7587. doi: 10.1021/jm100678p. [DOI] [PubMed] [Google Scholar]
- 77.Dubar F, Bohic S, Dive D, Guérardel Y, Cloetens P, Khalife J, Biot C. ACS Med. Chem. Lett. 2012;3:480. doi: 10.1021/ml300062q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blackie MA, Yardley V, Chibale K. Bioorg. Med. Chem. Lett. 2010;20:1078. doi: 10.1016/j.bmcl.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 79.Zishiri VK, Joshi MC, Hunter R, Chibale K, Smith PJ, Summers RL, Martin RE, Egan TJ. J. Med. Chem. 2011;54:6956. doi: 10.1021/jm2009698. [DOI] [PubMed] [Google Scholar]
- 80.Opsenica I, Burnett JC, Gussio R, Opsenica D, Todorovic N, Lanteri CA, Sciotti RJ, Gettayacamin M, Basilico N, Taramelli D, Nuss JE, Wanner L, Panchal RG, Solaja BA, Bavari S. J. Med. Chem. 2011;54:1157. doi: 10.1021/jm100938u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O’Neill PM, Shone AE, Stanford D, Nixon G, Asadollahy E, Park BK, Maggs JL, Roberts P, Stocks PA, Biagini G, Bray PG, Davies J, Berry N, Hall C, Rimmer K, Winstanley PA, Hindley S, Bambal RB, Davis CB, Bates M, Gresham SL, Brigandi RA, Gomez-de-Las-Heras FM, Gargallo DV, Parapini S, Vivas L, Lander H, Taramelli D, Ward SA. J. Med. Chem. 1828;2009:52. doi: 10.1021/jm8012757. [DOI] [PubMed] [Google Scholar]
- 82.Lawrence RM, Dennis KC, O’Neill PM, Hahn DU, Roeder M, Struppe C. Org. Process Res. Dev. 2008;12:294. [Google Scholar]
- 83.Ongarora DS, Gut J, Rosenthal PJ, Masimirembwa CM, Chibale K. Bioorg. Med. Chem. Lett. 2012;22:5046. doi: 10.1016/j.bmcl.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 84.Schmidt M, Sun H, Rogne P, Scriba GK, Griesinger C, Kuhn LT, Reinscheid UM. J. Am. Chem. Soc. 2012;134:3080. doi: 10.1021/ja209050k. [DOI] [PubMed] [Google Scholar]
- 85.Milner E, McCalmont W, Bhonsle J, Caridha D, Carroll D, Gardner S, Gerena L, Gettayacamin M, Lanteri C, Luong T, Melendez V, Moon J, Roncal N, Sousa J, Tungtaeng A, Wipf P, Dow G. Bioorg. Med. Chem. Lett. 2010;20:1347. doi: 10.1016/j.bmcl.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 86.Milner E, Gardner S, Moon J, Grauer K, Auschwitz J, Bathurst I, Caridha D, Gerena L, Gettayacamin M, Johnson J, Kozar M, Lee P, Leed S, Li Q, McCalmont W, Melendez V, Roncal N, Sciotti R, Smith B, Sousa J, Tungtaeng A, Wipf P, Dow G. J Med. Chem. 2011;54:6277. doi: 10.1021/jm200647u. [DOI] [PubMed] [Google Scholar]
- 87.Huang H, Lu W, Li X, Cong X, Ma H, Liu X, Zhang Y, Che P, Ma R, Li H, Shen X, Jiang H, Huang J, Zhu J. Bioorg. Med. Chem. Lett. 2012;22:958. doi: 10.1016/j.bmcl.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 88.Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, Charman SA, McLennan DN, White KL, Vivas L, Bongard E, Thongphanchang C, Taweechai S, Vanichtanankul J, Rattanajak R, Arwon U, Fantauzzi P, Yuvaniyama J, Charman WN, Matthews D. Proc. Natl. Acad. Sci. U.S.A. 2012;109:16823. doi: 10.1073/pnas.1204556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pereira MR, Henrich PP, Sidhu AB, Johnson D, Hardink J, VanDeusen J, Lin J, Gore K, O’Brien C, Wele M, Djimde A, Chandra R, Fidock DA. Antimicrob. Agents Chemother. 2011;55:3115. doi: 10.1128/AAC.01566-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peric M, Fajdetic A, Rupcic R, Alihodzic S, Ziher D, Bukvic Krajacic M, Smith KS, Ivezic-Schonfeld Z, Padovan J, Landek G, Jelic D, Hutinec A, Mesic M, Ager A, Ellis WY, Milhous WK, Ohrt C, Spaventi R. J. Med. Chem. 2012;55:1389. doi: 10.1021/jm201615t. [DOI] [PubMed] [Google Scholar]
- 91.Bukvic Krajacic M, Peric M, Smith KS, Schonfeld ZI, Ziher D, Fajdetic A, Kujundzic N, Schonfeld W, Landek G, Padovan J, Jelic D, Ager A, Milhous WK, Ellis W, Spaventi R, Ohrt C. J. Med. Chem. 2011;54:3595. doi: 10.1021/jm2001585. [DOI] [PubMed] [Google Scholar]
- 92.Pesic D, Starcevic K, Toplak A, Herreros E, Vidal J, Almela MJ, Jelic D, Alihodzic S, Spaventi R, Peric M. J. Med. Chem. 2012;55:3216. doi: 10.1021/jm201676t. [DOI] [PubMed] [Google Scholar]
- 93.Yeung BK, Zou B, Rottmann M, Lakshminarayana SB, Ang SH, Leong SY, Tan J, Wong J, Keller-Maerki S, Fischli C, Goh A, Schmitt EK, Krastel P, Francotte E, Kuhen K, Plouffe D, Henson K, Wagner T, Winzeler EA, Petersen F, Brun R, Dartois V, Diagana TT, Keller TH. J. Med. Chem. 2010;53:5155. doi: 10.1021/jm100410f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.vanPelt-Koops JC, Pett HE, Graumans W, van der Vegte-Bolmer M, van Gemert GJ, Rottmann M, Yeung BK, Diagana TT, Sauerwein RW. Antimicrob. Agents Chemother. 2012;56:3544. doi: 10.1128/AAC.06377-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wengelnik K, Vidal V, Ancelin ML, Cathiard AM, Morgat JL, Kocken CH, Calas M, Herrera S, Thomas AW, Vial HJ. Science. 2002;295:1311. doi: 10.1126/science.1067236. [DOI] [PubMed] [Google Scholar]
- 96.Le Roch KG, Johnson JR, Ahiboh H, Chung DW, Prudhomme J, Plouffe D, Henson K, Zhou Y, Witola W, Yates JR, Mamoun CB, Winzeler EA, Vial H. BMC Genomics. 2008;9:513. doi: 10.1186/1471-2164-9-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wein S, Maynadier M, Bordat Y, Perez J, Maheshwari S, Bette-Bobillo P, Tran Van Ba C, Penarete-Vargas D, Fraisse L, Cerdan R, Vial H. Br. J. Pharmacol. 2012;8:2263. doi: 10.1111/j.1476-5381.2012.01966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vial HJ, Wein S, Farenc C, Kocken C, Nicolas O, Ancelin ML, Bressolle F, Thomas A, Calas M. Proc. Natl. Acad. Sci. U.S.A. 2004;101:15458. doi: 10.1073/pnas.0404037101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vial HJ. Personal communication. 2012 [Google Scholar]
- 100.Nicolas O, Margout D, Taudon N, Wein S, Calas M, Vial HJ, Bressolle FM. Antimicrob. Agents Chemother. 2005;49:3631. doi: 10.1128/AAC.49.9.3631-3639.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vial H, Ancelin ML, Vidal J, Calas M, Bourguignon J-J, Rubi E. US6. 2005;972 343 B1. [Google Scholar]
- 102.Caldarelli SA, Boisbrun M, Alarcon K, Hamze A, Ouattara M, Salom-Roig X, Maynadier M, Wein S, Peyrottes S, Pellet A, Calas M, Vial H. Bioorg. Med. Chem. Lett. 2010;20:3953. doi: 10.1016/j.bmcl.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 103.Caldarelli SA, El Fangour S, Wein S, Tran van Ba C, Perigaud C, Pellet A, Vial HJ, Peyrottes SJ. Med. Chem. 2013;56:496. doi: 10.1021/jm3014585. [DOI] [PubMed] [Google Scholar]
- 104.Ortial S, Denoyelle S, Wein S, Berger O, Durand T, Escale R, Pellet A, Vial H, Vo-Hoang Y. ChemMedChem. 2010;5:52. doi: 10.1002/cmdc.200900427. [DOI] [PubMed] [Google Scholar]
- 105.Degardin M, Wein S, Gouni S, Tran Van Ba C, Duckert JF, Durand T, Escale R, Vial H, Vo-Hoang Y. ChemMedChem. 2012;7:991. doi: 10.1002/cmdc.201200112. [DOI] [PubMed] [Google Scholar]
- 106.Bedingfield PT, Cowen D, Acklam P, Cunningham F, Parsons MR, McConkey GA, Fishwick CW, Johnson AP. J. Med. Chem. 2012;55:5841. doi: 10.1021/jm300157n. [DOI] [PubMed] [Google Scholar]
- 107.Coteron JM, Marco M, Esquivias J, Deng X, White KL, White J, Koltun M, El Mazouni F, Kokkonda S, Katneni K, Bhamidipati R, Shackleford DM, Angulo-Barturen I, Ferrer SB, Jimenez-Diaz MB, Gamo FJ, Goldsmith EJ, Charman WN, Bathurst I, Floyd D, Matthews D, Burrows JN, Rathod PK, Charman SA, Phillips MA. J. Med. Chem. 2011;54:5540. doi: 10.1021/jm200592f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Skerlj RT, Bastos CM, Booker ML, Kramer ML, Barker RH, Celatka CA, O’Shea TJ, Munoz B, Sidhu AB, Cortese JF, Wittlin S, Papastogiannidis P, Angulo-Barturen I, Jimenez-Diaz MB, Sybertz E. ACS Med. Chem. Lett. 2011;2:708. doi: 10.1021/ml200143c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gamo FJ, Sanz LM, Vidal J, de Cozar C, Alvarez E, Lavandera JL, Vanderwall DE, Green DV, Kumar V, Hasan S, Brown JR, Peishoff CE, Cardon LR, Garcia-Bustos JF. Nature. 2010;465:305. doi: 10.1038/nature09107. [DOI] [PubMed] [Google Scholar]
- 110.Plouffe D, Brinker A, McNamara C, Henson K, Kato N, Kuhen K, Nagle A, Adrian F, Matzen JT, Anderson P, Nam TG, Gray NS, Chatterjee A, Janes J, Yan SF, Trager R, Caldwell JS, Schultz PG, Zhou Y, Winzeler EA. Proc. Natl. Acad. Sci. U.S.A. 2008;105:9059. doi: 10.1073/pnas.0802982105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guiguemde WA, Shelat AA, Bouck D, Duffy S, Crowther GJ, Davis PH, Smithson DC, Connelly M, Clark J, Zhu F, Jimenez-Diaz MB, Martinez MS, Wilson EB, Tripathi AK, Gut J, Shadow ER, Bathurst I, El Mazouni F, Fowble JW, Forquer I, McGinley PL, Castro S, Angulo-Barturen I, Ferrer S, Rosenthal PJ, Derisi JL, Sullivan DJ, Lazo JS, Roos DS, Riscoe MK, Phillips MA, Rathod PK, Van Voorhis WC, Avery VM, Guy RK. Nature. 2010;465:311. doi: 10.1038/nature09099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McNamara C, Winzeler EA. Future Microbiol. 2011;6:693. doi: 10.2217/fmb.11.45. [DOI] [PubMed] [Google Scholar]
- 113.Crowther GJ, Napuli AJ, Gilligan JH, Gagaring K, Borboa R, Francek C, Chen Z, Dagostino EF, Stockmyer JB, Wang Y, Rodenbough PP, Castaneda LJ, Leibly DJ, Bhandari J, Gelb MH, Brinker A, Engels IH, Taylor J, Chatterjee AK, Fantauzzi P, Glynne RJ, Van Voorhis WC, Kuhen KL. Mol. Biochem. Parasitol. 2011;175:21. doi: 10.1016/j.molbiopara.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Svennas KL, Macdonald SJ, Willis PA. Expert Q-pin. Ther. Patents. 2012;22:607. doi: 10.1517/13543776.2012.691967. [DOI] [PubMed] [Google Scholar]
- 115.Barker RH, Jr., Urgaonkar S, Mazitschek R, Celatka C, Skerlj R, Cortese JF, Tyndall E, Liu H, Cromwell M, Sidhu AB, Guerrero-Bravo JE, Crespo-Llado KN, Serrano AE, Lin JW, Janse CJ, Khan SM, Duraisingh M, Coleman BI, Angulo-Barturen I, Jimenez-Diaz MB, Magan N, Gomez V, Ferrer S, Martinez MS, Wittlin S, Papastogiannidis P, O’Shea T, Klinger JD, Bree M, Lee E, Levine M, Wiegand RC, Munoz B, Wirth DF, Clardy J, Bathurst I, Sybertz E. Antimicrob. Agents Chemother. 2011;55:2612. doi: 10.1128/AAC.01714-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heidebrecht RW, Jr., Mulrooney C, Austin CP, Barker RH, Jr., Beaudoin JA, Cheng KC, Comer E, Dandapani S, Dick J, Duvall JR, Ekland EH, Fidock DA, Fitzgerald ME, Foley M, Guha R, Hinkson P, Kramer M, Lukens AK, Masi D, Marcaurelle LA, Su XZ, Thomas CJ, Weiwer M, Wiegand RC, Wirth D, Xia M, Yuan J, Zhao J, Palmer M, Munoz B, Schreiber S. ACS Med. Chem. Lett. 2012;3:112. doi: 10.1021/ml200244k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brunner R, Aissaoui H, Boss C, Bozdech Z, Brun R, Corminboeuf O, Delahaye S, Fischli C, Heidmann B, Kaiser M, Kamber J, Meyer S, Papastogiannidis P, Siegrist R, Voss T, Welford R, Wittlin S, Binkert CJ. Infect. Dis. 2012;206:735. doi: 10.1093/infdis/jis418. [DOI] [PubMed] [Google Scholar]
- 118.Aissaoui H, Boss C, Corminboeuf O, Heidmann B, Siegrist R. 2011 WO2011/083413. [Google Scholar]
- 119.Baker SJ, Ding CZ, Akama T, Zhang YK, Hernandez V, Xia Y. Future Med. Chem. 2009;1:1275. doi: 10.4155/fmc.09.71. [DOI] [PubMed] [Google Scholar]
- 120.Zhang YK, Plattner JJ, Freund YR, Easom EE, Zhou Y, Gut J, Rosenthal PJ, Waterson D, Gamo FJ, Angulo-Barturen I, Ge M, Li Z, Li L, Jian Y, Cui H, Wang H, Yang J. Bioorg. Med. Chem. Lett. 2011;21:644. doi: 10.1016/j.bmcl.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 121.Zhang YK, Plattner JJ, Freund YR, Easom EE, Zhou Y, Ye L, Zhou H, Waterson D, Gamo FJ, Sanz LM, Ge M, Li Z, Li L, Wang H, Cui H. Bioorg. Med. Chem. Lett. 2012;22:1299. doi: 10.1016/j.bmcl.2011.12.096. [DOI] [PubMed] [Google Scholar]
- 122.Nwaka S, Besson D, Ramirez B, Maes L, Matheeussen A, Bickle Q, Mansour NR, Yousif F, Townson S, Gokool S, Cho-Ngwa F, Samje M, Misra-Bhattacharya S, Murthy PK, Fakorede F, Paris JM, Yeates C, Ridley R, Van Voorhis WC, Geary T. PLoS Negl. Trap. Dis. 2011;5:e1412. doi: 10.1371/journal.pntd.0001412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wawer M, Bajorath JR. ACS Med. Chem. Lett. 2011;2:201. doi: 10.1021/ml100240z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Calderón F, Barros D, Bueno JM, Coterón JM, Fernández E, Gamo FJ, Lavandera JL, León ML, Macdonald SJF, Mallo A, Manzano P, Porras E, Fiandor JM, Castro J. ACS Med. Chem. Lett. 2011;2:741. doi: 10.1021/ml200135p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sanz LM, Jimenez-Diaz MB, Crespo B, De-Cozar C, Almela MJ, Angulo-Barturen I, Castaneda P, Ibanez J, Fernandez EP, Ferrer S, Herreros E, Lozano S, Martinez MS, Rueda L, Burrows JN, Garcia-Bustos JF, Gamo FJ. Antimicrob. Agents Chemother. 2011;55:5740. doi: 10.1128/AAC.05188-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rotella DP. Bioorg. Med. Chem. Lett. 2012;22:6788. doi: 10.1016/j.bmcl.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 127.Coteron JM, Catterick D, Castro J, Chaparro MJ, Diaz B, Fernandez E, Ferrer S, Gamo FJ, Gordo M, Gut J, de las Heras L, Legac J, Marco M, Miguel J, Munoz V, Porras E, de la Rosa JC, Ruiz JR, Sandoval E, Ventosa P, Rosenthal PJ, Fiandor JM. J. Med. Chem. 2010;53:6129. doi: 10.1021/jm100556b. [DOI] [PubMed] [Google Scholar]
- 128.Shahinas D, Liang M, Datti A, Pillai DR. J. Med. Chem. 2010;53:3552. doi: 10.1021/jm901796s. [DOI] [PubMed] [Google Scholar]
- 129.Biamonte MA, Van de Water R, Arndt JW, Scannevin RH, Perret D, Lee WC. J. Med. Chem. 2010;53:3. doi: 10.1021/jm9004708. [DOI] [PubMed] [Google Scholar]
- 130.Cui L, Miao J. Antimicrob. Agents Chemother. 2007;51:488. doi: 10.1128/AAC.01238-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Agbor-Enoh S, Seudieu C, Davidson E, Dritschilo A, Jung M. Antimicrob. Agents Chemother. 2009;53:1727. doi: 10.1128/AAC.00729-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wheatley NC, Andrews KT, Tran TL, Lucke AJ, Reid RC, Fairlie DP. Bioorg. Med. Chem. Lett. 2010;20:7080. doi: 10.1016/j.bmcl.2010.09.096. [DOI] [PubMed] [Google Scholar]
- 133.Kubicek S, O’Sullivan RJ, August EM, Hickey ER, Zhang Q, Teodoro ML, Rea S, Mechtler K, Kowalski JA, Homon CA, Kelly TA, Jenuwein T. Mol. Cell. 2007;25:473. doi: 10.1016/j.molcel.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 134.Malmquist NA, Moss TA, Mecheri S, Scherf A, Fuchter MJ. Proc. Natl. Acad. Sci. U.S.A. 2012;109:16708. doi: 10.1073/pnas.1205414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhu S, Chandrashekar G, Meng L, Robinson K, Chatterji D. Bioorg. Med. Chem. 2012;20:927. doi: 10.1016/j.bmc.2011.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Prudhomme J, McDaniel E, Ponts N, Bertani S, Fenical W, Jensen P, Le Roch K. PloS One. 2008;3:e2335. doi: 10.1371/journal.pone.0002335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ge J-F, Arai C, Yang M, Bakar Md A, Lu J, Ismail NSM, Wittlin S, Kaiser M, Brun R, Charman SA, Nguyen T, Morizzi J, Itoh I, Ihara M. ACS Med. Chem. Lett. 2010;1:360. doi: 10.1021/ml100120a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shi XL, Ge JF, Liu BQ, Kaiser M, Wittlin S, Brun R, Ihara M. Bioorg. Med. Chem. Lett. 2011;21:5804. doi: 10.1016/j.bmcl.2011.07.112. [DOI] [PubMed] [Google Scholar]
- 139.Mahajan DT, Masand VH, Patil KN, Ben Hadda T, Jawarkar RD, Thakur SD, Rastija V. Bioorg. Med. Chem. Lett. 2012;22:4827. doi: 10.1016/j.bmcl.2012.05.115. [DOI] [PubMed] [Google Scholar]
- 140.Davis RA, Buchanan MS, Duffy S, Avery VM, Charman SA, Charman WN, White KL, Shackleford DM, Edstein MD, Andrews KT, Camp D, Quinn RJ. J. Med. Chem. 2012;55:5851. doi: 10.1021/jm3002795. [DOI] [PubMed] [Google Scholar]
- 141.Bahar M, Deng Y, Zhu X, He S, Pandharkar T, Drew ME, Navarro-Vazquez A, Anklin C, Gil RR, Doskotch RW, Werbovetz KA, Kinghorn AD. Bioorg. Med. Chem. Lett. 2011;21:2606. doi: 10.1016/j.bmcl.2011.01.101. [DOI] [PubMed] [Google Scholar]
- 142.Lavrado J, Gani K, Nobre PA, Santos SA, Figueiredo P, Lopes D, Rosario V, Gut J, Rosenthal PJ, Moreira R, Paulo A. Bioorg. Med. Chem. Lett. 2010;20:5634. doi: 10.1016/j.bmcl.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 143.Lowes D, Pradhan A, Iyer LV, Parman T, Gow J, Zhu F, Furimsky A, Lemoff A, Guiguemde WA, Sigal M, Clark JA, Wilson E, Tang L, Connelly MC, Derisi JL, Kyle DE, Mirsalis J, Guy RK. J. Med. Chem. 2012;55:6087. doi: 10.1021/jm300286a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Martyn DC, Cortese JF, Tyndall E, Dick J, Mazitschek R, Munoz B, Clardy J. Bioorg. Med. Chem. Lett. 2010;20:218. doi: 10.1016/j.bmcl.2009.10.130. [DOI] [PubMed] [Google Scholar]
- 145.Verlinden BK, Niemand J, Snyman J, Sharma SK, Beattie RJ, Woster PM, Birkholtz LM. J. Med. Chem. 2011;54:6624. doi: 10.1021/jm200463z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tamura S, Kubata BK, Syamsurizal, Itagaki S, Horii T, Taba MK, Murakami N. Bioorg. Med. Chem. Lett. 2010;20:1520. doi: 10.1016/j.bmcl.2010.01.095. [DOI] [PubMed] [Google Scholar]
- 147.Edlin CD, Morgans G, Winks S, Duffy S, Avery VM, Wittlin S, Waterson D, Burrows J, Bryans J. ACS Med. Chem. Lett. 2012;3:570. doi: 10.1021/ml300091c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fernando D, Rodrigo C, Rajapakse S. Malar. J. 2011;10:351. doi: 10.1186/1475-2875-10-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mazier D, Renia L, Snounou G. Nat. Rev. Drug Disc. 2009;8:854. doi: 10.1038/nrd2960. [DOI] [PubMed] [Google Scholar]
- 150.Dow GS, Gettayacamin M, Hansukjariya P, Imerbsin R, Komcharoen S, Sattabongkot J, Kyle D, Milhous W, Cozens S, Kenworthy D, Miller A, Veazey J, Ohrt C. Malar. J. 2011;10:212. doi: 10.1186/1475-2875-10-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cappellini MD, Fiorelli G. Lancet. 2008;371:64. doi: 10.1016/S0140-6736(08)60073-2. [DOI] [PubMed] [Google Scholar]
- 152.Wells TN, Burrows JN, Baird JK. Trends Parasitol. 2010;26:145. doi: 10.1016/j.pt.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 153.Derbyshire ER, Mota MM, Clardy J. PLoS Pathog. 2011;7:e1002178. doi: 10.1371/journal.ppat.1002178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Meister S, Plouffe DM, Kuhen KL, Bonamy GM, Wu T, Barnes SW, Bopp SE, Borboa R, Bright AT, Che J, Cohen S, Dharia NV, Gagaring K, Gettayacamin M, Gordon P, Groessl T, Kato N, Lee MC, McNamara CW, Fidock DA, Nagle A, Nam TG, Richmond W, Roland J, Rottmann M, Zhou B, Froissard P, Glynne RJ, Mazier D, Sattabongkot J, Schultz PG, Tuntland T, Walker JR, Zhou Y, Chatterjee A, Diagana TT, Winzeler EA. Science. 2011;334:1372. doi: 10.1126/science.1211936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wu T, Nagle A, Kuhen K, Gagaring K, Borboa R, Francek C, Chen Z, Plouffe D, Goh A, Lakshminarayana SB, Wu J, Ang HQ, Zeng P, Kang ML, Tan W, Tan M, Ye N, Lin X, Caldwell C, Ek J, Skolnik S, Liu F, Wang J, Chang J, Li C, Hollenbeck T, Tuntland T, Isbell J, Fischli C, Brun R, Rottmann M, Dartois V, Keller T, Diagana T, Winzeler E, Glynne R, Tully DC, Chatterjee A. K.J. Med. Chem. 2011;54:5116. doi: 10.1021/jm2003359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156(a).Nagle A, Wu T, Kuhen K, Gagaring K, Borboa R, Francek C, Chen Z, Plouffe D, Lin X, Caldwell C, Ek J, Skolnik S, Liu F, Wang J, Chang J, Li C, Liu B, Hollenbeck T, Tuntland T, Isbell J, Chuan T, Alper PB, Fischli C, Brun R, Lakshminarayana SB, Rottmann M, Diagana TT, Winzeler EA, Glynne R, Tully DC, Chatterjee AK. J. Med. Chem. 2012;55:4244. doi: 10.1021/jm300041e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chatterjee A. Discovery of novel antimalarials through cell-based medicinal chemistry optimization of HTS hits, AntiMal Meeting. London, UK: Royal College of Surgeons; Mar 15–16, 2011. 2011. [Google Scholar]
- 157.Hussain H, Specht S, Sarite SR, Saeftel M, Hoerauf A, Schulz B, Krohn KJ. Med. Chem. 2011;54:4913. doi: 10.1021/jm200302d. [DOI] [PubMed] [Google Scholar]
- 158.Muller T, Johann L, Jannack B, Bruckner M, Lanfranchi DA, Bauer H, Sanchez C, Yardley V, Deregnaucourt C, Schrevel J, Lanzer M, Schirmer RH, Davioud-Charvet EJ. Am. Chem. Soc. 2011;133:11557. doi: 10.1021/ja201729z. [DOI] [PubMed] [Google Scholar]
- 159.Bueno JM, Manzano P, Garcia MC, Chicharro J, Puente M, Lorenzo M, Garcia A, Ferrer S, Gomez RM, Fraile MT, Lavandera JL, Fiandor JM, Vidal J, Herreros E, Gargallo-Viola D. Bioorg. Med. Chem. Lett. 2011;21:5214. doi: 10.1016/j.bmcl.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 160.Cross RM, Monastyrskyi A, Mutka TS, Burrows JN, Kyle DE, Manetsch R. J. Med. Chem. 2010;53:7076. doi: 10.1021/jm1007903. [DOI] [PubMed] [Google Scholar]
- 161.Lacrue AN, Saenz FE, Cross RM, Udenze KO, Monastyrskyi A, Stein S, Mutka TS, Manetsch R, Kyle DE. Antimicrob. Agents Chemother. 2013;57:417. doi: 10.1128/AAC.00793-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang Y, Clark JA, Connelly MC, Zhu F, Min J, Guiguemde WA, Pradhan A, Iyer L, Furimsky A, Gow J, Parman T, El Mazouni F, Phillips MA, Kyle DE, Mirsalis J, Guy RKJ. Med. Chem. 2012;55:4205. doi: 10.1021/jm201642z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nam TG, McNamara CW, Bopp S, Dharia NV, Meister S, Bonamy GM, Plouffe DM, Kato N, McCormack S, Bursulaya B, Ke H, Vaidya AB, Schultz PG, Winzeler EA. ACS Chem. Biol. 2011;6:1214. doi: 10.1021/cb200105d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.da Cruz FP, Martin C, Buchholz K, Lafuente-Monasterio MJ, Rodrigues T, Sonnichsen B, Moreira R, Gamo FJ, Marti M, Mota MM, Hannus M, Prudencio M. J. Infect. Dis. 2012;205:1278. doi: 10.1093/infdis/jis184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pidathala C, Amewu R, Pacorel B, Nixon GL, Gibbons P, Hong WD, Leung SC, Berry NG, Sharma R, Stocks PA, Srivastava A, Shone AE, Charoensutthivarakul S, Taylor L, Berger O, Mbekeani A, Hill A, Fisher NE, Warman AJ, Biagini GA, Ward SA, O’Neill PMJ. Med. Chem. 1831;2012:55. doi: 10.1021/jm201179h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Derbyshire ER, Prudencio M, Mota MM, Clardy J. Proc. Natl. Acad. Sci. U.S.A. 2012;109:8511. doi: 10.1073/pnas.1118370109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hoepfner D, McNamara CW, Lim CS, Studer C, Riedl R, Aust T, McCormack SL, Plouffe DM, Meister S, Schuierer S, Plikat U, Hartmann N, Staedtler F, Cotesta S, Schmitt EK, Petersen F, Supek F, Glynne RJ, Tallarico JA, Porter JA, Fishman MC, Bodenreider C, Diagana TT, Mowa NR, Winzeler EA. Cell Host Microbe. 2012;11:654. doi: 10.1016/j.chom.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Harbut MB, Patel BA, Yeung BK, McNamara CW, Bright AT, Ballard J, Supek F, Golde TE, Winzeler EA, Diagana TT, Greenbaum DC. Proc. Natl. Acad. Sci. U.S.A. 2012;109:21486. doi: 10.1073/pnas.1216016110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.LeRoch KG, Zhou Y, Blair PL, Grainger M, Moch JK, Haynes JD, De La Vega P, Holder AA, Batalov S, Carucci DJ, Winzeler EA. Science. 2003;301:1503. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- 170.Tarun AS, Peng X, Dumpit RF, Ogata Y, Silva-Rivera H, Camargo N, Daly TM, Bergman LW, Kappe SH. Proc. Natl. Acad. Sci. U.S.A. 2008;105:305. doi: 10.1073/pnas.0710780104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dembele L, Gego A, Zeeman AM, Franetich JF, Silvie O, Rametti A, Le Grand R, Dereuddre-Bosquet N, Sauerwein R, van Gemert GJ, Vaillant JC, Thomas AW, Snounou G, Kocken CH, Mazier D. PloS One. 2011;6:e18162. doi: 10.1371/journal.pone.0018162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Prudencio M, Mota MM, Mendes AM. Trends Parasitol. 2011;27:565. doi: 10.1016/j.pt.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 173.Chattopadhyay R, Velmurugan S, Chakiath C, Andrews Donkor L, Milhous W, Barnwell JW, Collins WE, Hoffman SL. PloS One. 2010;5:e14275. doi: 10.1371/journal.pone.0014275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gego A, Silvie O, Franetich JF, Farhati K, Hannoun L, Luty AJ, Sauerwein RW, Boucheix C, Rubinstein E, Mazier D. Antimicrob. Agents Chemother. 2006;50:1586. doi: 10.1128/AAC.50.4.1586-1589.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Alonso PL, Brown G, Arevalo-Herrera M, Binka F, Chitnis C, Collins F, Doumbo OK, Greenwood B, Hall BF, Levine MM, Mendis K, Newman RD, Plowe CV, Rodriguez MH, Sinden R, Slutsker L, Tanner M. PLoS Med. 2011;8:e1000406. doi: 10.1371/journal.pmed.1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.The malERA Consultative Group on Integration Strategies. PLoS Med. 2011;8:e1000404. doi: 10.1371/journal.pmed.1000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Delves MJ. Future Med. Chem. 2012;4:2251. doi: 10.4155/fmc.12.182. [DOI] [PubMed] [Google Scholar]
- 178.Bousema T, Okell L, Shekalaghe S, Griffin JT, Omar S, Sawa P, Sutherland C, Sauerwein R, Ghani AC, Drakeley C. Malar. J. 2010;9:136. doi: 10.1186/1475-2875-9-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Smalley ME, Sinden RE. Parasitology. 1977;74:1. doi: 10.1017/s0031182000047478. [DOI] [PubMed] [Google Scholar]
- 180.Buckling A, Ranford-Cartwright LC, Miles A, Read AF. Parasitology. 1999;118:339. doi: 10.1017/s0031182099003960. [DOI] [PubMed] [Google Scholar]
- 181.Puta C, Manyando C. Trap. Med. Int. Health. 1997;2:227. doi: 10.1046/j.1365-3156.1997.d01-267.x. [DOI] [PubMed] [Google Scholar]
- 182.Baker DA. Mol. Biochem. Parasitol. 2010;172:57. doi: 10.1016/j.molbiopara.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Burrows JN, Sinden RE. Neglected Diseases and Drug Discovery. 2012:112. RSC, Chapter 5. [Google Scholar]
- 184.Kiszewski AE. Pharmaceuticals. 2011;4:44. [Google Scholar]
- 185.WHO, Malaria Report 2009. Policies, Strategies and Targets for Malaria Control. Chapter 2. [Google Scholar]
- 186.WHO, Updated WHO Policy Recommendation: Single dose Primaquine as a gametocytocide in Plasmodium falciparum malaria. www.who.int/malaria/pq_updated_policy_recommendation_en_102012.pdf.
- 187.WHO Evidence Review Group: The Safety and Effectiveness of Single Dose Primaquine as a P. falciparum gametocytocide. http://www.who.int/malaria/mpac/sep2012/primaquine_single_dose_pf_erg_meeting_report_aug2012.pdf.
- 188.Delves MJ, Ramakrishnan C, Blagborough AM, Leroy D, Wells TN, Sinden RE. Int. J. Parasitol. 2012;42:999. doi: 10.1016/j.ijpara.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 189.Lelievre J, Almela MJ, Lozano S, Miguel C, Franco V, Leroy D, Herreros E. PloS One. 2012;7:e35019. doi: 10.1371/journal.pone.0035019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Roncales M, Vidal-Mas J, Leroy D, Herreros EJ. Parasitol. Res. 2012 doi: 10.1155/2012/927148. http://dx.doi.org/10.1155/2012/927148. Electronic publication ahead of print. [DOI] [PMC free article] [PubMed]
- 191.Delves M, Plouffe D, Scheurer C, Meister S, Wittlin S, Winzeler EA, Sinden RE, Leroy D. PLoS Med. 2012;9:e1001169. doi: 10.1371/journal.pmed.1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lucantoni L, Avery V. Future Med. Chem. 2012;4:2337. doi: 10.4155/fmc.12.188. [DOI] [PubMed] [Google Scholar]
- 193.Ramsay RR, Dunford C, Gillman PK. Br. J. Pharmacol. 2007;152:946. doi: 10.1038/sj.bjp.0707430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Adjalley SH, Johnston GL, Li T, Eastman RT, Ekland EH, Eappen AG, Richman A, Sim BK, Lee MC, Hoffman SL, Fidock DA. Proc. Natl. Acad. Sci. U.S.A. 2011;108:E1214. doi: 10.1073/pnas.1112037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Aminake MN, Schoof S, Sologub L, Leubner M, Kirschner M, Arndt HD, Pradel G. Antimicrob. Agents Chemother. 2011;55:1338. doi: 10.1128/AAC.01096-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ojo KK, Pfander C, Mueller NR, Burstroem C, Larson ET, Bryan CM, Fox AM, Reid MC, Johnson SM, Murphy RC, Kennedy M, Mann H, Leibly DJ, Hewitt SN, Verlinde CL, Kappe S, Merritt EA, Maly DJ, Billker O, Van Voorhis WCJ. Clin. Invest. 2012;122:2301. doi: 10.1172/JCI61822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Eastman RT, Pattaradilokrat S, Raj DK, Dixit S, Deng B, Miura K, Yuan J, Tanaka TQ, Johnson RL, Jiang H, Huang R, Williamson KC, Lambert LE, Long C, Austin CP, Wu Y, Su XZ. Antimicrob. Agents Chemother. 2013;57:425. doi: 10.1128/AAC.00920-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Milner E, Sousa J, Pybus B, Auschwitz J, Caridha D, Gardner S, Grauer K, Harris E, Hickman M, Kozar MP, Lee P, Leed S, Li Q, Melendez V, Moon J, Ngundam F, O’Neil M, Parriott S, Potter B, Sciotti R, Tangteung A, Dow GS. Eur. J. Drug Metab. Pharmacokinet. 2012;37:17. doi: 10.1007/s13318-012-0080-2. [DOI] [PubMed] [Google Scholar]
- 199.Toye PJ, Sinden RE, Canning EUZ. Parasitenkd. 1977;53:133. doi: 10.1007/BF00380457. [DOI] [PubMed] [Google Scholar]
- 200.Schwartz L, Brown GV, Genton B, Moorthy VS. Malar. J. 2012;11:11. doi: 10.1186/1475-2875-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ridley RG, Matile H, Jaquet C, Dorn A, Hofheinz W, Leupin W, Masciadri R, Theil FP, Richter WF, Girometta MA, Guenzi A, Urwyler H, Gocke E, Potthast JM, Csato M, Thomas A, Peters W. Antimicrob. Agents Chemother. 1997;41:677. doi: 10.1128/aac.41.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Howes RE, Battle KE, Satyagraha AW, Baird JK, Hay SI. Adv. Parasitol. 2013;81:133. doi: 10.1016/B978-0-12-407826-0.00004-7. [DOI] [PubMed] [Google Scholar]