Abstract
Superficial siderosis of the central nervous system is a rare neurological disorder caused by deposits of haemosiderin on subplial brain matter. Characterised by a thin dark layer surrounding the brain stem, cerebellum and cortical fissures on the T2-weighted MRI, symptoms include sensorineural hearing loss and progressive gait ataxia. A specific aetiology for the blood in the subarachnoid space is identified in less than 50% of cases. While identification of a specific vascular defect allows for vascular repair, treatment options are limited for idiopathic superficial siderosis. Recently, a pilot safety study demonstrated promising results using an iron chelator, deferiprone. While this approach is promising, we present a potential serious complication of this therapy—the first report of agranulocytosis in the treatment of superficial siderosis following deferiprone therapy.
Background
Animal experiments have suggested that chronic or recurrent blood exposure within the subarachnoid space results in superficial siderosis of the central nervous system (SSCNS).1 When plial astrocytes are exposed to blood products, they attempt to neutralise the toxic properties of free iron by generating ferritin and haemosiderin, which settle onto the surface of the brain and spinal cord. Over years of intermittent or chronic bleeding, astrocytes lose the ability to buffer free iron. This results in increased free radicals and oxidative stress in the CNS, leading to the neurological injury of the underlying tissue.2 3 The newly deposited haemosiderin contains unpaired electrons, resulting in a paramagnetic nucleus, which produces a dark signal on T2-weighted imaging. This dark signal is most visible along the periphery of the brainstem, cerebellum and along cortical fissures, areas with the largest amount of haemosiderin deposition.4
SSCNS is a rare finding as demonstrated by a literature review conducted in 2007. Evaluating the preceding 98 years, Levy et al5 noted only 270 cases published cases worldwide of SSCNS. Definitive causes of haemosiderin deposition were only noted in 65% of patients, with idiopathic causes noted in the remaining 35% of cases. In their evaluation, hearing loss and ataxia were present in 81% of patients and myelopathy in 53% of patients.5 Once symptoms have developed and superficial siderosis diagnosed, the goal of therapy focuses on identifying an underlying source of bleeding with imaging studies.6–8 It is hypothesised that by stopping the recurrent leakage of blood into the subarachnoid space; the deposition of haemosiderin can be curbed, halting disease progression.9
Often the underlying source remains unknown and treatment must focus on attempting to inhibit the formation of haemosiderin in the CNS.10 In experimental models, tin-protoporphyrin has shown limited effectiveness by inhibiting haeme-oxygenase and preventing the degradation of haeme products. Tin-protoporphyrin has poor penetration through the blood–brain barrier and therefore requires injection into the subarachnoid space. Although case studies have demonstrated positive results,11 there are only limited studies of its use in humans.12 Corticosteroids have also been evaluated; however, clinical response has been limited.13 Recently, a small clinical study demonstrated the utility of using deferiprone, an iron chelator currently with CNS penetration. Evaluated in 10 patients, 2 patients had MRI findings suggestive of improvement and 4 patients reported a subjective improvement, potentially offering hope for patients with superficial siderosis.14–16
Case presentation
Our patient is a 65-year-old man, whose clinical course presumably began following a motor accident in 1990, when he sustained a pelvic fracture requiring surgical fixation. He had residual right lower extremity (RLE) weakness due to a right L5 radiculopathy. His subsequent recovery was complicated by multiple episodes of deep vein thromboses and he was placed on long-term anticoagulation therapy (warfarin). In 2006 he was evaluated by a neurologist for low back pain and difficulty walking. A cervical MRI showed moderate cervical stenosis but there were no signs of myelopathy. Later that year, he reported hearing difficulty, and an audiology evaluation confirmed mild sensorineural hearing loss. An MRI of the brain in January 2010 demonstrated a thin dark rim lining the basilar cisterns, temporal lobe, occipital lobe and the opercular region on the T2-weighted images, consistent with superficial siderosis. The MRI were also notable for cervical stenosis. Searching for a reversible cause for his gait instability, he underwent surgery decompression of his cervical stenosis. Initially, there was improvement is his gait, but a few months later he experienced an increased number of falls and sustained a moderate bilateral subdural haematoma. He was hospitalised for medical management, not requiring surgery. At time of discharge, he required a walker for ambulation. Repeat MRI evaluation of the brain and spine 1 year later re-demonstrated the thin dark line of haemosiderin layering his CNS confirming the diagnosis of diffuse superficial siderosis. When he was examined by a neurologist in May 2011, he was found to have hyper-reflexia with an up-going left toe with persistent RLE weakness and sensory loss. His examination was also notable for an ataxic gait and he continued to require a walker. Subsequent extensive evaluation of the brain and spine in 2011 and 2012 demonstrated persistent findings of superficial siderosis as seen in figures 1 and 2. Workup for aetiology responsible for causing the patient's SSCNS, including spinal angiography, did not reveal a source for bleeding. In spring 2012, with a worsened functional status, his warfarin was discontinued and he was started on deferiprone therapy of 1000 mg twice a day for 5 days a week, with weekly monitoring of his blood count and liver function.
Figure 1.
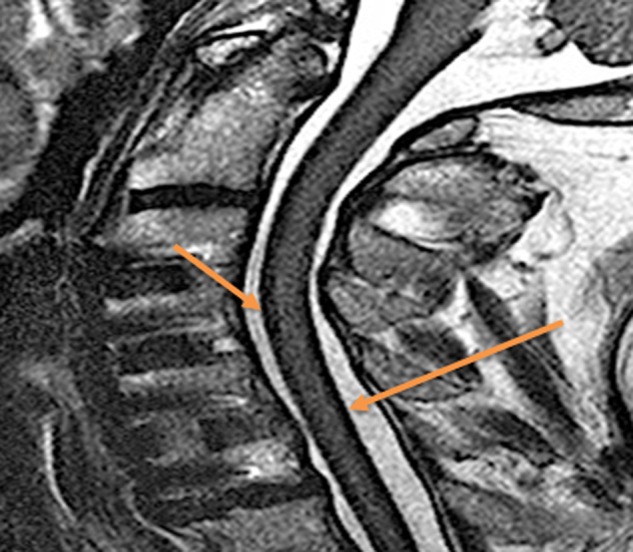
A sagittal T2-weighted MRI of the patient's cervical spine demonstrating a thin dark layer along the anterior and posterior aspect of the spinal cord. This thin dark line represents the haemosiderin deposition.
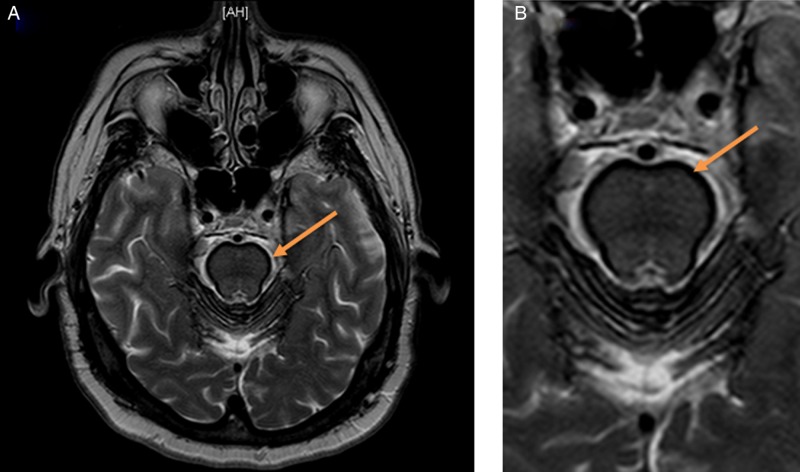
Figure 2.
An axial T2 with fat suppression (STIR) image through the brain at the level of the cerebellum (A and an enlargement of the cerebral peduncles and pons in B) demonstrating this thin dark layer of haemosiderin deposition along the cerebral peduncles and pons. Findings described above are highlighted by associated arrows.
Over the first 3 months of therapy, he tolerated deferiprone well. Repeat blood evaluations demonstrated a stable blood count without neutropenia or abnormal liver function tests. While repeat MRIs did not demonstrate change in haemosiderin deposition, he did note a subjective improvement in his symptoms. However, during month 4 of therapy, he presented to the emergency department with a fever, hypotension, and an absolute neutrophil count (ANC) of 0, a known potential serious adverse effect of deferiprone. Based on his presentation, he was admitted to the intensive care unit (ICU) for treatment of sepsis that included intensive respiratory and blood pressure support. Deferiprone was discontinued and he was started on daily growth-colony stimulating factor for recovery from the agranulocytosis. After 10 days of intensive care support, his fever had resolved and he was haemodynamically stable. He was transferred from the ICU with an ANC of 700 but had a prolonged hospitalisation, requiring multiple readmissions to the ICU. After a 2-month hospital course, he was discharged to a skilled nursing facility for physical rehabilitation therapy.
The patient's prior medical history is notable for vitamin B12 insufficiency, hypertension, hyperlipidaemia, chronic HCV infection and thoracic and lumbar degenerative disc disease.
Discussion
Deferiprone is a lipid-soluble iron chelation therapy currently approved by the Food and Drug Administration for the treatment of secondary haemochromatosis in thalassaemia major patients. With its lipid solubility, deferiprone is the only approved iron chelator able to penetrate the blood–brain barrier. Known serious adverse effects of deferiprone include agranulocytosis, neutropenia and elevation of liver function enzymes.17
The associated agranulocytosis or neutropenia can occur 6 weeks to 21 months after starting therapy. Per reports from 2003, deferiprone was associated with agranulatocytosis and/or neutropenia at rates of 0.2–2.8/100 patient-years.18 To date, no specific pathological mechanism responsible for agranulocytosis has been demonstrated. Suggested mechanisms include dose-based depletion of iron and copper or immune-mediated reactions.19–21 Current treatment guidelines suggest a weekly haematological assessment monitoring for a decreased neutrophil count, however, agranulocytosis has been demonstrated in spite of weekly surveillance.20 To date, there is no data suggesting alternative monitoring assessments offer improved outcomes compared to weekly haematological assessments.
In 2012, Levy et al14 evaluated deferiprone's safety profile in a study of 10 patients. Prescribed at a dose of 30 mg/kg/day for a 90-day course, four patients reported a subjective improvement in symptoms, four stated no change and two patients had worsening symptoms despite therapy. There was also a notable decrease in haemosiderin deposition in two of the patients on MRI evaluation. None of the patients experienced neutropenia or agranulocytosis in this small trial population.
Considering the progressive and debilitating nature of SSCNS, the authors recommended further study of the efficacy of deferiprone in superficial siderosis. While the study by Levy et al only treated patients for 90 days of therapy, our patient remained on therapy for 4 months. During routine re-evaluations, the patient stated a subjective improvement and his blood counts remained within normal limits. In the fourth month of therapy, he had a precipitous drop in his white blood cells count culminating with the emergent presentation and subsequent ICU support.
While the deferiprone safety profile study in superficial siderosis had no serious adverse events, our case suggests that the lower dose regimen does not protect against agranulocytosis. Adverse events observed in our patient are similar to those reported in clinical trials for patients receiving higher doses of deferiprone therapy. Potentially, the lack of serious adverse effects in the SSCNS safety profile study may be the result of the small sample size, as 4.9% and 1.1% of patients experienced neutropenia and agranulocytosis, respectively, with deferiprone use in larger studies.22 An alternative hypothesis is that the increased duration of exposure over 4 months rather than the previously evaluated 90 days, resulted in his agranulocytosis. However, longitudinal risk for agranulocytosis from deferiprone is not known.
Our case demonstrates that deferiprone dosing used for treatment of SSCNS does not exclude the hematological risks of agranulocytosis. Although the patient was monitored with weekly laboratory assessments, his precipitous drop in his absolute leucocyte count was not avoided. Further, larger studies may offer a better understanding of deferiprone's potential and limitations in the treatment of superficial siderosis.
Learning points.
Superficial siderosis superficial siderosis of the central nervous system (SSCNS) is a rare, debilitating neurological disorder characterised by haemosiderin deposition within the CNS and can manifest as a clinical triad of hearing loss, cerebellar ataxia and myelopathy.
There are few treatment options for SSCNS—however, there are early promising results for deferiprone, an iron chelation agent that crosses the blood–brain barrier.
Deferiprone dosing for the treatment of superficial siderosis can cause agranulocytosis despite close monitoring.
Larger studies may offer a better understanding of deferiprone's potential and limitations in the treatment of superficial siderosis.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Koeppen AH, Hurwitz CG, Dearborn RE, et al. Experimental superficial siderosis of the central nervous system: biochemical correlates. J Neurol Sci 1992;2013:38–45 [DOI] [PubMed] [Google Scholar]
- 2.Nanda S, Sharma SG, Longo S. Superficial siderosis—mechanism of disease: an alternative hypothesis. Ann Clin Biochem 2010, 2010;2013:275–8 [DOI] [PubMed] [Google Scholar]
- 3.Chinnery AMaPF Neuroferritinopathy: update on clinical features and pathogenesis. Curr Drug Targets 2012;2013:1200–3 [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto Y, Yamamoto S, Kuroki S. T2*-weighted MRI findings of superficial siderosis. Intern Med 2012;2013:1949. [DOI] [PubMed] [Google Scholar]
- 5.Levy M, Turtzo C, Llinas RH. Superficial siderosis: a case report and review of the literature. Nat Clin Pract Neuro . 2007;2013:54–8 [DOI] [PubMed] [Google Scholar]
- 6.Luetmer PH, Mokri B. Dynamic CT. myelography: a technique for localizing high-flow spinal cerebrospinal fluid leaks. AJNR Am J Neuroradiol 2003;2013:1711–14 [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar N, Lindell EP, Wilden JA, et al. Role of dynamic CT myelography in identifying the etiology of superficial siderosis. Neurology 2005;2013:486–8 [DOI] [PubMed] [Google Scholar]
- 8.Kumar N, Fogelson JL, Morris JM, et al. Superficial siderosis should be included in the differential diagnosis of motor neuron disease. Neurologist 2012;2013:139–45 [DOI] [PubMed] [Google Scholar]
- 9.Hoxworth JM, Trentman TL, Kotsenas AL, et al. The role of digital subtraction myelography in the diagnosis and localization of spontaneous spinal CSF leaks. Am J Roentgenol. 1 September2012, 2012;2013:649–53 [DOI] [PubMed] [Google Scholar]
- 10.Vale T GRTA IDiopathic superficial siderosis. Arch Neurol 2011;2013:1334–5 [DOI] [PubMed] [Google Scholar]
- 11.River Y, Honigman S, Gomori JM, et al. Superficial hemosiderosis of the central nervous system. Mov Disord 1994;2013:559–62 [DOI] [PubMed] [Google Scholar]
- 12.Koeppen AH, Dickson AC. Tin-protoporphyrin prevents experimental superficial siderosis in rabbits. J Neuropathol Exp Neurol 2002;2013:689–701 [DOI] [PubMed] [Google Scholar]
- 13.Scheid R, Frisch S, Schroeter ML. Superficial siderosis of the central nervous system—treatment with steroids? J Clin Pharm Ther 2009;2013:603–5 [DOI] [PubMed] [Google Scholar]
- 14.Levy M, Llinas R. Pilot safety trial of deferiprone in 10 subjects with superficial siderosis. Stroke 2012, 2012;2013:120–4 [DOI] [PubMed] [Google Scholar]
- 15.Levy M, Llinas RH. Update on a patient with superficial siderosis on deferiprone. Am J Neuroradiol 1 June2012, 2012;2013:E99–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy M, Llinas RH. Deferiprone reduces hemosiderin deposits in the brain of a patient with superficial siderosis. Am J Neuroradiol 2011, 2011;2013:E1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olivieri NF, Brittenham GM, Matsui D, et al. Iron-chelation therapy with oral deferiprone in patients with thalassemia major. N Engl J Med 1995;2013:918–22 [DOI] [PubMed] [Google Scholar]
- 18.Cohen AR, Galanello R, Piga A, et al. Safety and effectiveness of long-term therapy with the oral iron chelator deferiprone. Blood 2003;2013:1583–7 [DOI] [PubMed] [Google Scholar]
- 19.Masera N, Tavecchia L, Longoni DV, et al. Agranulocytosis due to deferiprone: a case report with cytomorphological and functional bone marrow examination. Blood Transfus 2011;2013:462–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henter J-I, Karlén J. Fatal agranulocytosis after deferiprone therapy in a child with Diamond-Blackfan anemia. Blood 2007, 2007;2013:5157–9 [DOI] [PubMed] [Google Scholar]
- 21.Cohen AR, Galanello R, Piga A, et al. Safety profile of the oral iron chelator deferiprone: a multicentre study. Br J Haematol 2000;2013:305–12 [DOI] [PubMed] [Google Scholar]
- 22.Brittenham GM. Iron-chelating therapy for transfusional iron overload. N Engl J Med 2011;2013:146–56 [DOI] [PMC free article] [PubMed] [Google Scholar]